Abstract
Introduction
High blood pressure (BP) is associated with adverse cardiovascular outcomes.1 A number of highly effective pharmacological therapies are available to treat hypertension but a substantial proportion of affected subjects remain inadequately controlled world-wide.2 There are many reasons for this situation, such as lack of access to treatments, physician inertia, inadequate dosing or combinations of treatments, suboptimal patient adherence to treatment, the use of interfering drugs or substance of abuse, the presence of undiagnosed secondary hypertension or of treatment-resistant hypertension. A large number of patients with hypertension are reluctant to take or adhere to pharmacotherapeutic regimens, because of interference with their daily lives, fear/experience of side effects, preferences for alternative medications, or other reasons.3 Treatment-resistant hypertension is commonly defined as BP level above target [office systolic BP (SBP) ≥140 mmHg or diastolic BP ≥90 mmHg] despite treatment with at least 3 antihypertensive medications in adequate doses, one of which should be a diuretic.4
The difficulty of treating hypertension, its high prevalence and severe consequences, and the absence of novel antihypertensive drugs on the horizon and the limitations of purely pharmacologic approaches have prompted the development of interventional approaches to provide complementary treatments. Several device-based approaches have been invented and subsequently tested; one which has received much positive as well as skeptical attention is catheter-based renal denervation (RDN).5,6 The method uses radiofrequency energy, alternatively ultrasound or chemical ablation, to disrupt renal nerves within the renal artery wall, thereby reducing sympathetic efferent and sensory afferent signalling to and from the kidneys.7 Historical observations showed that surgical sympathectomy can reduce BP as well as morbidity and mortality in patients with uncontrolled hypertension.8 Even though based on strong pathophysiological rationale,8 catheter-based RDN has not conclusively demonstrated its value for the treatment of resistant hypertension and its place in the therapeutic armamentarium remains uncertain.9–12 Other device-based approaches under investigation include the creation of a central iliac arteriovenous (AV)–anastomosis with a coupler, the stimulation of the carotid sinus, the ablation of the carotid body, and stent-based expansion of the carotid bulb.13,14
The multidisciplinary European Expert Group has previously published proceedings from their 2014 clinical consensus conference aiming at exploring the gaps in our knowledge about RDN and making recommendations of future randomized controlled trial design.6 A follow-up conference was convened in October 2016 to evaluate the position of device therapies for hypertension in the light of the latest clinical developments. This article presents the main conclusions from this event. We first present a survey of the changing clinical environment surrounding hypertension and its implications for clinical trials in the field. This is followed by an update on currently on-going clinical trials of device-based hypertension therapy and design considerations for further trials. Finally, needs and recommendations on the standardised assessment of emerging device therapies are discussed.
What is the impact of recent hypertension trials on the design of device-based hypertension studies?
Since the last consensus conference in 2014, several clinical trials have been published, which may influence how device-based therapies are viewed and investigated. The results raise questions around treatment regimens, BP targets, and the most appropriate way to measure BP.
Spironolactone and PATHWAY-2
In patients with resistant hypertension, the option of adding a fourth antihypertensive drug has been investigated. The crossover trial PATHWAY-2 recently showed spironolactone to be superior to placebo as an add-on in patients with resistant hypertension who had been identified by renin profiling as potential responders to the therapy.15 Despite the positive results from PATHWAY-2, the Expert Group has discussed whether these results should prompt adding spironolactone as a fourth line treatment in the management of resistant hypertension to define a trial population for device-based proof of concept studies. In the PATHWAY-2 trial, patients were on a low-dose of bendroflumethiazide, a drug less effective than chlorthalidone or indapamide, which may have favoured the BP response to spironolactone; BP response was not analysed per aldosterone levels, body mass index or BP at baseline; the short time (6 weeks) of exposure to the maximum dose of spironolactone (50 mg/d) was insufficient for an accurate assessment of the long-term tolerability of this drug; and there are no data on efficacy and safety in patients with eGFR <45 mL/min/1.73 m2 since they were excluded from the trial. Moreover, in clinical practise spironolactone has a challenging tolerability profile with higher rates of intolerance than in PATHWAY-2, including gynecomastia and erectile dysfunction, which may cause treatment interruption or termination at the request of the healthcare provider or patient.16 Indeed, an unexpectedly low rate of side effects occurred in the PATHWAY-2 study, possibly because of the relatively short treatment duration.
Based on these reservations, the European Expert Group felt that there isno needto mandate failure to control BP on spironolactone as an inclusion criteria for resistant hypertension patients ina proof-of-concepttrial of device-based hypertension therapies.
Blood pressure targets
The results from Systolic Blood Pressure Intervention Trial (SPRINT) have generated a vivid discussion around treatment targets and the most appropriate way to measure office BP. SPRINT reported a significantly lower risk for cardiovascular disease outcomes and all-cause mortality by targeting SBP <120 mmHg compared with <140 mmHg in a population of hypertensive persons with ≥1 additional cardiovascular risk factor.17 The SPRINT results have been interpreted as supporting a lower recommended target SBP than the currently widely accepted 140 mmHg. An expedited review of the SPRINT study was undertaken by the Canadian Hypertension Education Programme, which led to the recommendation that in selected high-risk patients, intensive BP reduction to target SBP ≤120 mmHg should be considered to lower the risk of cardiovascular events.18 From a safety standpoint, it is generally accepted that there is very low risk for harm from further SBP reductions below 140 mmHg.19,20 However, several design idiosyncrasies in the SPRINT study are relevant to the discussion on the most appropriate method to measure BP in clinical trials. SPRINT is the only outcomes trial to date to have used automated, unattended BP measurements with a dedicated device. This was done to reduce the influence of the presence of physicians or other healthcare professionals, or ‘white coat hypertension’. Though two other major blood pressure trials, Secondary Prevention of Small Subcortical Strokes (SPS3)21 and Effects of Intensive Blood-Pressure Control in Type 2 Diabetes Mellitus (ACCORD),22 also used automated BP devices but with less standardized unattended BP measurement than in SPRINT. It has been noted that unobserved measures of SBP may be 5–15 mmHg lower than BP measured manually, or when patients are being observed.23 Previous studies in treated hypertensive subjects have shown that automatic unattended office BP measurements may be even lower than daytime ambulatory SBP, and up to 20 mmHg lower than conventional attended auscultatory office SBP.24 Finally, a recent meta-analysis of intervention trials on the effects of more or less intense BP lowering on outcome, which included SPRINT data, has provided evidence that a significant reduction in the absolute risk of events occurred whenever systolic BP was reduced below 150, 140, or even 130 mmHg, although the absolute reduction in risk of events was smaller when aiming at a systolic BP reduction <130 mmHg.25 In parallel, however, another recent meta-analysis of intervention trials by the same group has clearly shown that the lower the systolic BP achieved by treatment, the higher the number of patients who discontinued their drug therapy because of treatment-related side effects, which indicates a failure in achieving patients’ protection through pharmacological hypertension management.26
As target BP in a clinical trial of device-based therapy in hypertension, the European Expert Group recommended anattended target seated office BP < 140 mmHg using the conventional method and a validated device.Whichever method is used, it is critical that consistency is maintained in all centres and at all visits.
The recommended target is closely related to clinical practice, in line with current guidelines as well as with our previous recommendations. It is also the target used in many currently on-going clinical trials, although their primary efficacy endpoint is the ambulatory BP (see below). The European Expert Group further pointed out that having too ambitious targets may cause trials to count as failed even if they achieve substantial and significant BP reductions.26 The alternative of using BP measurements at home was also discussed. Complementary to ABPM, home BP monitoring may favour BP control and patient adherence to treatment and is included in the most recent guidelines from the European Society for Hypertension.27 A number of electronic tools and smartphone apps are emerging to simplify the procedure for patients and its interpretation by physicians.28
Considering the less well established standardization of home compared with ABPM, the European Expert Group does not recommend using home BP measurement as a primary endpoint in clinical trials, while there is large agreement that it can be used as a secondary endpoint. Furthermore, in device trials home BP monitoring may conceivably influence adherence if the trial includes hypertensive individuals attracted to the therapy as a non-drug solution.
Clinically meaningful blood pressure reduction
A related question to calculating the power and sample size of clinical trials is what degree of BP reduction associated with a clinically meaningful response. Two recent meta-analyses support a reduction of 8.4 and 10 mmHg in office BP as clinically meaningful, respectively.29,30 These numbers have long been used in the power calculation for clinical studies and is comparable to what can typically be achieved with one antihypertensive drug. These numbers are intended for power calculations only. A smaller, but statistically relevant reduction in BP would still constitute a proof of concept in a controlled study of device therapies. It is also highly desirable to reduce BP variability among trial populations.
For the purpose ofpower calculationsin hypertension device trials the European Expert Group considered a10 mmHg reduction in office SBPto be a clinically meaningful outcome. This corresponds to 6-7 mmHg in ABP.31For all BP targets themethod of measurementshould be specified. The group reinforced that ABP should be the primary efficacy parameter in device trials of hypertension.
Which device-based randomized controlled trials are ongoing and currently recruiting patients?
Renal denervation
After the publication of the neutral results with RDN in the sham-controlled randomized Symplicity-HTN-3 trial in 20149 there was a transient gap in clinical trials of RDN. Trialists have learned from the earlier trials (Table 1) and the activity has picked up again in recent years. Thus, a number of randomized trials are investigating RDN in patients with resistant hypertension as well as in untreated hypertensive patients (Table 2). Since BP-lowering is a long-accepted surrogate marker, there is no need for a mortality/morbidity trial with RDN a priori, as long as the technology is efficient in lowering BP in a randomized controlled trial with a good safety profile.
Table 1.
Current trials for device-based therapies in patients with hypertension
| TRIAL | Procedure | Study design | Sample size/Geography | Patient population | Condition | Medication adherence | Primary end points |
|---|---|---|---|---|---|---|---|
SPYRAL HTN ON |
RF renal denervation | Double-blind, randomized (1:1) sham controlled |
|
|
1–3 drugs | Toxicological analysis |
|
SPYRAL HTN OFF |
RF renal denervation | Double-blind, randomized (1:1) sham controlled |
|
|
No drugs | Toxicological analysis |
|
REINFORCE |
RF renal denervation | Double-blind, randomized (2:1), sham controlled |
|
|
No drugs | — |
|
RADIANCE HTN/REQUIRE |
Ultrasound renal denervation | Double-blind, randomized (1:1), sham controlled |
|
|
|
Toxicological analysis |
|
WAVE IV (Stopped) |
External ultrasound renal denervation | Double-blind, randomized (1:1) sham controlled |
|
|
≥3 drugs | Toxicological analysis |
|
EnligHTNed IDE |
RF renal denervation | Double-blind, randomized (2:1), sham-controlled |
|
|
3 drugs | Toxicological analysis |
|
TARGET BP I |
Chemical (Alcohol) renal denervation | Double-blind, randomized (2:1), sham-controlled |
|
|
2-5 drugs | Urine analysis | Primary efficacy: ASBP at 8 weeks Multiple secondary outcomes |
ROX II |
AV-fistula creation | Double-blind, randomized (1:1 stratified by race), sham controlled |
|
|
≥3 drugs | — | Primary efficacy: office and ASBP at 6 months |
CALM-2 |
Carotid body restoration | Double-blind, randomized (2:1), sham-controlled | N = 300 | 24-h ASBP ≥135 mmHg to ≤ 180 mmHg | 3–5 drugs | Toxicological analysis |
|
OSBP, office systolic BP; ASBP, ambulatory systolic BP; MACE, major adverse cardiovascular events; RDN, renal denervation; RF, radiofrequency; AV, arteriovenous; M, months; US, United States of America; IDE, investigational device exemption; IND, investigational new drug. Source: clinicaltrials.gov.
Table 2.
Differences between a first generation (SYMPLICITY HTN-3) and latest generation clinical trial (SPYRAL HTN)
| First generation RCT (SYMPLICITY HTN-3) | Latest generation RCT (SPYRAL HTN Global Clinical Trial Programme) | |
|---|---|---|
| Technology | Single-electrode catheter | Multi-electrode catheter |
| Ablation pattern | Main vessels | Main, accessory, and branch vessels |
| Proceduralist’s experience | Varied level of experience | Experienced proceduralists, including many sites with significant familiarity with the procedure |
| Regimen | Range of medication regimens allowed for enrolment | Medication regimen required for enrolment is standardized |
| Absence of medications | Data only obtained for patients taking anti-hypertensive medications | Data also obtained for patients not taking antihypertensive medications |
| Maximum dose | Patients required to be on maximum tolerated dose, with an average of >5 anti-hypertensive medications | Patients not required to be on maximum tolerated dose |
| Adherence | No medication adherence protocol | Witnessed intake (on medication arm) and medication adherence analysis (both arms) |
| Disease severity | Patients with severe hypertension with an average of >10 years of treatment without control | Patients with severe to moderate hypertension due to lower OSBP entry criteria and no maximum tolerated drug requirement |
| BP measurement | Office blood pressure at 6 months as primary endpoint; may have included white coat population | ABPM at 3 months as primary measure |
| Geography | US patients only | Global study |
The Symplicity Spyral multi-electrode RDN system is studied in patients with uncontrolled hypertension in the absence (SPYRAL HTN OFF-MED; NCT02439749) and presence (SPYRAL HTN ON-MED; NCT02439775) of antihypertensive medications (Table 2).32 These trials have a primary efficacy endpoint of change in 24-h SBP from baseline to 3-month post-procedure. The control groups receive sham treatment with renal angiography.
RADIANCE-HTN (NCT02649426) compares the ReCor Medical Paradise ultrasound system to a sham procedure with the primary endpoint change in average daytime ambulatory SBP from baseline to 2 months post-procedure in two separate on- (TRIO) and off-medication (SOLO) cohorts of patients with uncontrolled hypertension. In the TRIO cohort, participants with resistant hypertension will discontinue their current antihypertensive drugs and switch to standardised single-pill triple therapy. REQUIRE (NCT02918305, n = 140) is designed to evaluate resistant hypertension patients on standard of care medication in Japan, and South Korea.
REDUCE HTN: REINFORCE (NCT02392351) studies the performance of the balloon-based bipolar Vessix system over a 2-month period comparing the effects with those from a sham procedure of percutaneous renal angiography on mean reduction in daytime ambulatory SBP.
The design of the EnligHTNed IDE Trial in resistant hypertensive patients is being finalized in discussion with the US Food and Drug Administration. The trial is randomized, double-blinded and with sham control. Participants will discontinue their current antihypertensive drugs and switch to standardized single-pill triple therapy to be maintained at least 12 months after randomization. The primary efficacy endpoint is reduction in 24-h SBP at 6 months compared with baseline.
WAVE-IV (NCT02029885) was a sham controlled, double blind study with the non-invasive ultra-sound based Kona Medical Surround Sound System for bilateral RDN. This trial used change in office SBP from screening to 6 months post-randomization as the primary efficacy end point. The trial was stopped for futility on 19 July 2016 as it had by this time point not demonstrated any differences between the groups in either office BP or 24-h ABP. There had been no safety concerns.
Arteriovenous–anastomosis creation with a coupler
The ROX II pivotal study will be performed in the USA with the aim of enrolling 500 patients who will be randomized to coupler implantation (with a shunt volume of approximately 800 mL/min) or sham treatment (NCT NCT02895386). Patients should be stable on three antihypertensive drugs. The primary endpoint is change from baseline in 24 h systolic ABP at 6 months. The study will be conducted using a Bayesian approach with the first safety analysis at 100 and first efficacy look at 250 randomized patients.
Carotid bulb expansion, carotid body ablation, and baroreflex stimulation
Carotid bulb expansion (using a dedicated carotid stent, NCT02804087) and carotid body ablation (using a transvenous catheter, NCT02099851) are two different approaches, that are currently being investigated in first-in-man studies. Pending positive results, randomized-controlled trials will be conducted. In the Rheos Pivotal Trial, baroreflex stimulation failed to meet its early safety endpoint. Open label, non-randomized follow-up of the whole cohort reported that office systolic BP reduction of > 30 mmHg was sustained up to 53 months with no important safety concerns.33,34 The Barostim NeoTM system has CE Mark approval for the treatment of RHTN and for heart failure. The Economic Evaluation of Baroreceptor STIMulation for the Treatment of Resistant HyperTensioN (ESTIM-rHTN) trial, funded by the French Ministry of Health, is ongoing and aims to study baroreceptor activation in patients with resistant hypertension and eGFR 30 mL/min per 1.73 m2 or higher (NCT02364310).35
What factors should be considered in future clinical studies for emerging device therapies in hypertension?
A sham control group is a prerequisite for a successful proof-of-concept trial of device-based therapies in hypertension. The majority of the trials listed above include a sham treatment. There was an initial controversy around the need for a sham group in trials investigating the BP lowering efficacy of device-based treatments for hypertension, but at present, this requirement met near-unanimous support. However, the use of a sham procedure is associated with certain degree of complexity, including the ethics of performing a procedure conferring an immediate risk of adverse event in those trials recruiting especially untreated patients with Grade I to II hypertension who are at low immediate cardiovascular and cerebrovascular risk. A blinding index should be used to assess the efficacy of blinding in clinical device trials.36 The index can be used for any blinded group, not only study subjects and researchers. An assessment of appropriate blinding is particularly important in randomized controlled device trials where proper blinding can be highly challenging.
A run-in phase with repeated BP assessments (office and ABP measurements) should be mandatory in clinical device trials in hypertension to reduce bias introduced by regression to the mean.37 For trials of treatment resistant populations, therapeutic regimens should be consistent between the groups. The need for consistency of pharmacotherapeutic regimens was emphasized in the earlier consensus document.6 For trials in drug-treated populations, a standardized, stepped titration scheme used in trials with antihypertensive drugs, e.g. The LIFE study38 or the DENER-HTN trial10 is a highly desirable design component to reduce heterogeneity and ensure that all patients receive appropriate cardiovascular protection. No established and validated tool exists to adjust BP changes after an intervention, if dose or drug regimen has been changed.
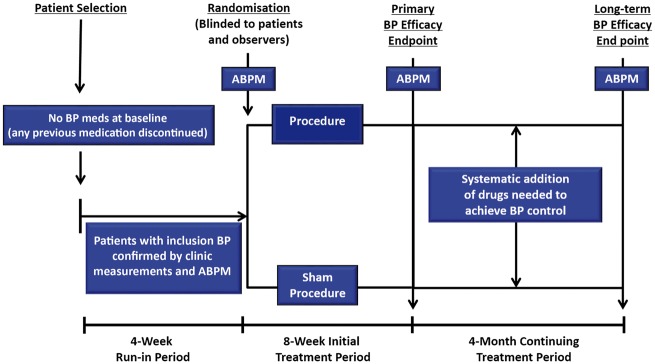
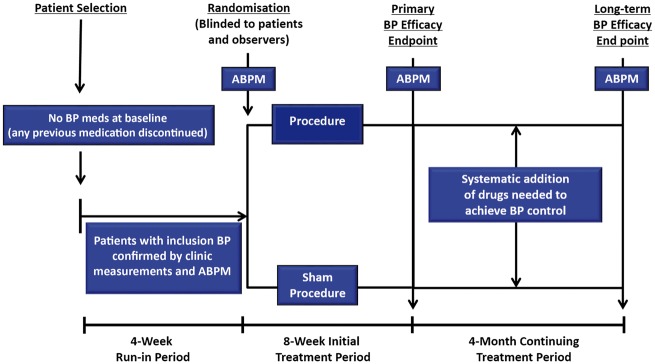
Several trials currently investigate patients with Stage 1 or 2 hypertension but not treated with pharmacotherapy. This may be a more suitable population than treated resistant patients to demonstrate proof of concept for new technologies. It would also show whether device-based interventions can reduce or eliminate the need for antihypertensive drugs in achieving BP control and whether it may affect the efficacy of drugs. However, these trials may face potential ethical objections, as the patients would forgo treatment with long-established pharmacotherapies and treatment regiments would not be in accordance with the recommendations of international Guidelines. The European Expert group acknowledged this objection in the earlier consensus publication.6 However, studies in untreated hypertensive patients can be adapted to meet ethical considerations. A follow-up period of 3 months would reduce the risk to patients from uncontrolled hypertension and may be sufficient to demonstrate efficacy in a proof of concept trial. If the results are positive, further studies will be performed in settings applicable to clinical practice to investigate the persistence of the effects. An appealing study design is to follow patients until the primary efficacy endpoint is reached and then to introduce a stepped antihypertensive drug regimen in both groups (Take home figure). Such a design would provide the best possible therapy to all patients as well as provide data on whether the intervention affects the response to pharmacotherapy.
By considering patients’ preference in the study design, it may be possible preferentially to enrol patients who are actively demanding a non-pill based therapy. In the experience of the group members, a number of hypertensive individuals, particularly those of younger age, are reluctant to start on a potentially lifelong pharmacotherapy regimen. In such situations, ethical concerns would be less of an impediment to enrolment as long as patients provide informed consent to randomization, including the possibility of undergoing a sham procedure.
It has to be considered that device-based therapies may lower BP more effectively in the presence of antihypertensive drugs in patients with resistant hypertension, which implies the need for clinical studies with standardized concomitant drug treatment.
It would be desirable to maximize adherence to oral antihypertensive treatment and lifestyle modification measures as much as possible. Modern technologies such as mass spectrometry have made it easier to measure drug adherence in simple urinanalyses39 and the methods have been used to assess adherence as a factor in resistant hypertension before and after RDN.40,41 The method would deserve to be explored for use in trials as an alternative to electronic monitoring of pill packages. However, adherence should not be the main focus of a trial of device therapies as the topic itself needs more research. Nevertheless, the European Expert Group considered that urinalysis/toxicological testing is desirable and should, therefore, be encouraged in all trials of device therapies for hypertension. Alternative methods include directly observed medication intake and simultaneous ABP recording.
The European Expert Group further emphasized that the large number of on-going studies in the field provides a unique opportunity to perform prospectively designed meta-analyses, and economic evaluations, not only on the therapies but also on the effects of sham procedures. Since current trials have similar inclusion and exclusion criteria, a patient based meta-analysis increases the power to precisely assess, which patient group benefits most from RDN. The group strongly recommends the establishment of an independent research collaboration which should be granted access to all data from all sponsors to conduct a meta-analysis based on individual data. Country-specific economic evaluations, using fully pooled data on the use of healthcare resources and country-specific values for unit costs should be encouraged to inform policy makers. Whenever possible, the results should be analysed according to adherence to therapy.
Take home figure.
Suggested flow chart for trial of renal denervation in hypertensive patients initially off drugs.
What information will the ongoing studies provide and what are potential outcome scenarios?
| On medication | Off medication | Comments |
|---|---|---|
| Positive | Positive |
The concept works. The devices definitely reduce BP.
|
| Negative | Negative |
The concept does not work in hypertension with the current technology
|
| Positive | Negative |
The concept partially works. RDN reduces BP in specific settings.
New trials may be considered to:
|
| Negative | Positive |
The concept partially works. RDN reduces BP in specific settings.
New trials may be considered to:
|
Why are the trials recruiting slowly and how to improve recruitment rates?
The problems with slow recruitment and difficulties finding appropriate patients need to be overcome to run sufficiently large trials within a reasonable time frame. Most trials of device therapies in hypertension are bedevilled by slow recruitment. In one by no means unique example, the DENERHTN trial screened 1416 patients over a 17-month period to identify 106 patients who were enrolled in the trial.10 For trials in treatment resistant patients, the need to show insufficient response to a large number of medications before qualifying complicates patient selection. Patients are often reluctant to be potentially assigned to a sham arm, which may be particularly relevant in those patients who are not on pharmacotherapy. To increase the attractiveness of clinical trials it would be necessary to counter the prevailing negative image of sham treatment. Sham is known to reduce BP, as seen in all device trials. A meta-analysis found average SBP reductions of around 9 mmHg in the placebo/sham arms in trials of resistant hypertension.42 In the experiences of the members of the European Expert Group, there is no placebo effect from everyday renal angiography in hypertensive patients, but the same procedure reduces BP when used as sham in a clinical trial.
To increase recruitment patients should be informed about the nature and the rationale of the sham procedure. In addition, all patients should be offered the active treatmentif the trial eventually shows to be positive.
A pro-active information strategy should be implemented with respect to referrals, communications and screening. A more patient-centric approach should be considered, taking advantage of electronic media and information technology when possible. Recruitment campaigns and newsletters need to target patients in addition to the current focus on investigators. Today’s patients are highly connected and informed, and should be approached as partners, or e-patients (Table 3) in an effort to bring the trial to the patient. Young, hypertensive but otherwise healthy people are mainly treated by general practitioners, not cardiologists or hypertension specialists. To identify, contact and recruit these individuals, innovative approaches may be necessary. On the side of the investigators, less complex trials with fewer recorded variables, limited number of focused end points and simplified case report forms would reduce the workload and may increase the willingness to take part in the trial.
Thehelp of patient’s organisationsshould be actively solicited. As has been seen in e.g. cancer or HIV, if a study is attractive patients actively seek opportunities for participation. The appropriate positioning of device studies is necessary to achieve similar effects.
Table 3.
Characterization of e-patients
| Equipped | Possessing skills to manage their own condition. |
| Enabled | Make choices about self-care and finding those choices respected. |
| Excellent patient-care | Promote centre of excellence, centre of clinical trials, networks. |
| Engaged | Engaged in their own care. |
| Expert patients | Able to improve their self-rated health status; cope with generic features of chronic disease and dependence on hospital care; able to share their experience and convince other patients. |
| Evaluating | Evaluate not only the information found but also the source of that information; establishing trust in sources at an early stage (website of the trial, information by and the patient during a trial). |
| Equal | The e-patient expects to be an equal member of the team in partnership with professionals involved in their care. |
What are unmet needs in the assessment of device therapies for hypertension?
There is an urgent need to develop simple and reproducible intraprocedural technologies to evaluate the extent of nerve ablation. Promising markers have been suggested, such as the periprocedural veno-arterial noradrenaline gradient (the veno-arterial difference). Greater periprocedural renal veno-arterial noradrenaline gradient reduction during RDN was associated with greater BP responses at 3 and 6 months post-procedure.43 Another suggested predictor of response are BP changes induced by renal nerve stimulation before vs. after the procedure, which correlate with changes in 24-h ABPM 3–6 months post-procedure.44 Whether any of these methods can be adapted for routine clinical use is unclear, however. Imaging techniques such as transluminal imaging of renal nerves using optical coherence tomography have been suggested as a potential tool to assess the geometry and state of the renal artery and optimise the denervation procedure.45 All of these tools have yet to be investigated in controlled trials. If they can be successfully validated, tools for procedural guidance should be consistently integrated into future study protocols. At present the European Expert Group considers that no tool for intraprocedural assessment has demonstrated sufficient usefulness to warrant a recommendation for generalized use.
The first systems for RDN employed radiofrequency energy in a manner analogous to ablation for cardiac conditions such as atrial fibrillation. This approach is still widely employed, but a number of alternative technologies are being developed, for instance based on alcohol injection, high-frequency ultrasound or low-intensity focused ultrasound.14 Even within the same approach, electrode designs vary, with spiral, basket or helical radiofrequency multi-electrodes and other designs for non-radiofrequency technologies. Since the space is crowded, it is critical to uphold consistent criteria for the evaluation of emerging technologies. Too little is known about the clinical effects of the different devices. A class effect from RDN remains to be demonstrated. It is unlikely that all devices in development will be equally effective, let alone successful. Based on these considerations, the European Expert group strongly recommend that all devices be tested preclinically, more appropriately in hypertensive animal models (obese dog or swine), as a prerequisite for first-in-man evaluation and market approval.
Most preclinical studies have been performed in healthy, normotensive porcine models and there remains a need for a model that is more closely related to human hypertension. One interesting model of modern human hypertension may be the Ossabaw breed, which appears to represent a translationally relevant model of hypertension with its associated comorbidities. RDN reduces diet-induced hypertension in this model.46 A suitable, hypertensive animal model with long-term follow-up would greatly help to further assess the BP effects and other surrogate markers of efficacy, e.g., histological denervation and renal noradrenaline content. However, the peri-arterial renal nerve anatomy in preclinical models differs from that in humans and it is unclear how applicable these results are to diseased vessels of patients with hypertension and atherosclerosis.
It has been shown that arterial microanatomy determines the success of energy-based RDN.47 In the future, assessment of the morphology of the arterial wall and the adventitia may be required before deciding on the most suitable device for each patient. The optimal degree of contact against the renal artery wall and the depth, duration and intensity of energy delivery to provide the best procedural results will need to be investigated and optimized for each specific renal-denervation technology. As proper dose-response studies are lacking, there is no reliable information available to guide these efforts and there is no simple way to assess dose-response in human subjects currently.
A consistent and appropriate follow-up period between the procedure and histological examination is critical for the correct assessment of nerve injury. The correlation between the duration of follow-up on nerve injury and arterial wall injury is unknown. A related question is that of nerve regeneration, which has been observed in animal studies48 but data in humans are very limited. The possibility and clinical implications of nerve regeneration in humans need to be clarified with long-term follow-up of patients.
The safety of new devices requires careful monitoring. To enable accurate comparisons of risk-benefit profiles between different technologies, complications should be documented and evaluated using consistent terms and methods in all trials with emerging devices. The European Expert Group considers a 3-6-month follow-up sufficient to reveal safety signals, as for most other vascular interventions. For progression of stenosis and long-term vascular safety, longer time frames need to be considered, which may be different from one device to another.
Better tools are urgently needed to guide clinical decisions. It is likely that patient’s characteristics and the underlying pathophysiology of hypertension contributes to success of the procedure. Patients with isolated systolic hypertension, e.g. show limited response to RDN therapy,49,50 probably due to increased aortic stiffness and pressure wave reflection.51 Whether these patients benefit more from other device-therapies remains to be proven.52
All guidelines and recommendations should be device-specific. The correlation between morphology and the efficacy of different devices needs to be systematically assessed before specific recommendations can be issued. The group strongly believes that efficacy and safety data acquired with a certain device cannot be transferred to any other device or intervention.
Summary and outlook
The interest in RDN for hypertension has fluctuated recently, with a flurry of initial enthusiasm followed by sudden loss of interest by researchers and device manufacturers, with an almost as sudden resurgence in clinical trials activity and device innovation more recently. There is widespread consensus that this therapeutic strategy can be effective, at least for some of the technologies available. Major uncertainties remain as to the clinical role of RDN, and whether any of the emerging technologies such as AV–anastomosis formation, carotid body ablation, carotid bulb expansion, or baroreflex stimulation will have a future as effective treatment options in patients with hypertension. In our first consensus report in 2015, the European Expert Group pointed to the major unmet need of standardization of measurements, trial design and procedural performance.6 With the large number of different technologies currently in the pipeline, this need has even increased. Only through high-quality, collaborative research and openness to new methods for recruitment, patient selection, and assessment of outcomes will it be possible to establish incontrovertibly whether device therapies for hypertension are effective and what are preferred patient populations. Once the proof of concept is established, further studies with a design relevant to clinical reality will be needed to establish the place of new devices in the treatment armoury. The clinical and research community has a large responsibility to prove or disprove the value of new therapies, in order to ensure that antihypertensive devices provide future patients with the greatest benefit and the smallest risk.
Acknowledgements
We thank Pelle Stolt (Basel, Switzerland) for his valuable help with this manuscript. Organisation Committee: Michel Azizi, Sebastian Ewen, Felix Mahfoud, Atul Pathak, Roland E. Schmieder, Costas Tsioufis, Thomas Zeller, WilliamWijns.
Funding
The European Expert meeting was supported by an unrestricted educational from Europa Organisation, Toulouse, France.
Conflicts of interest: M.A. has received honoraria for advisory board meetings from Vessix, Boston Scientific Corporation, Cordis, Actelion, has received speakers’ honoraria from Cordis, CVRx, Servier; was involved as investigator in Symplicity HTN-2 (Ardian/Medtronic) and Reduce-HTN (Vessix/Boston Scientific Corporation) trials; is involved as PI in the Radiance-HTN trial; and has received a research grant from Servier.
S.B. has received financial support for travel expenses and is a co-investigator in the European post-market study by Ablative Solutions Inc. He is co-owner of Cardiorenal LLC, a company that has developed a catheter for renal denervation. In addition, the institution he works for has ownership interest in or has received consulting fees, travel expenses or study honoraries from the following companies: Abbott, Access Closure, AGA, Angiomed, Arstasis, Atritech, Atrium, Avinger, Bard, Boston Scientific, Bridgepoint, Cardiac Dimensions, CardioKinetix, CardioMEMS, Coherex, Contego, CSI, EndoCross, EndoTex, Epitek, Evalve, ev3, FlowCardia, Gore, Guidant, Guided Delivery Systems, Inc., InSeal Medical, Lumen Biomedical, HLT, Kensey Nash, Kyoto Medical, Lifetech, Lutonix, Medinol, Medtronic, NDC, NMT, OAS, Occlutech, Osprey, Ovalis, Pathway, PendraCare, Percardia, pfm Medical, Rox Medical, Sadra, SJM, Sorin, Spectranetics, SquareOne, Trireme, Trivascular, Velocimed, Veryan.
P.J.B. has received research funds from Metronic and SJM, travel payments and consultancy from Medtronic, and consultancy fees from SJM.
M.B. has received honoraria for scientific advice and lectures from Astra-Zeneca, Boehringer Ingelheim, Medtronic, St. Jude, Novartis, Servier and Vifor.
M.B. has received research support from Medtronic.
I.D.Z. has received honoraria for advisory board meetings from Medtronic, Abbvie, Janssen, BMS, MSD, Pfizer.
S.E.K. is supported by the Norwegian Government and the Norwegian Council for Cardiovascular Diseases, and has received speaker honoraria and consultancy fees from ABDiiBRAHiM, Bayer, MSD and Takeda within the past 3 years.
M.D.L. is supported by the Barts Charity and has received speaker honoraria and consultancy fees from CVRx, St Jude Medical, ROX Medical and Cardiosonic.
C.L. has received consultancy fees from Medtronic.
F.M. is supported by Deutsche Hochdruckliga and Deutsche Gesellschaft für Kardiologie, and has received speaker honoraria and consultancy fees from St. Jude Medical, and Medtronic.
G.P. has received received honoraria for lectures from CVRx.
A.P. has received honoraria for advisory board meetings from Abbott, Ablative Solution CVRx, Recor, Medtronic, Saint Jude Medical, has received speakers’ honoraria from, Abbott, CVRx, Recor, Medtronic, Saint Jude Medical.
P.R. has received personal fees from CVRx, Novartis, Relypsa, AstraZeneca, Vifor Fresenius Medical Care Renal Pharma, Stealth Peptides, Bayer, and CTMA and is co-founder of CardioRenal.
L.R. has received honoraria for advisor and speaker activities from Medtronic.
H.S. has received consulting fees, travel expenses, and study honoraria from Abbott, Ablative Solutions, Acoredis, Atrium, Biosense Webster, Bioventrix, Boston Scientific, Carag, Cardiac Dimensions, CardioKinetix, Celonova, Cibiem, CGuard, Coherex, Comed B.V., Contego, CSI, CVRx, ev3, FlowCardia, Gardia, Gore, GTIMD Medical, Guided Delivery Systems, Hemoteq, InspireMD, Kona Medical, Lumen Biomedical, Lifetech, Medtronic, Occlutech, pfm Medical, Recor, SentreHeart, Svelte Medical Systems, Terumo, Trivascular, Valtech, Vascular Dynamics, Venus Medical, Veryan and holds stocks options from Cardiokinetix, Access Closure, Coherex, SMT.
R.E.S. has received speaker fees, honoraria, consultancy, advisory board fees, investigator, committee member activities from Astra Zeneca, Berlin Chemie AG, Boehringer-Ingelheim, Medtronic, Kona Medical, Recor, Servier, Novartis and research funding (departmental or institutional) from BMBF—German Ministry of Research and Education, Novartis, Boehringer Ingelheim, Vascular Dynamics, Medtronic, Recordati International, Vasopharm, Recor, Ablative Solution, and Rox Medical.
F.S. has received consultancy fees for Medtronic.
C.T. has received speaker honoraria and consultancy fees from St. Jude Medical, and Medtronic.
S.W. has received institutional research grants from Abbott, Boston Scientific, Biotronik, Medtronic, Edwards. T.Z. The institution is receiving research grants from Medtronic.
References
- 1.Freis ED. Effects of treatment on morbidity in hypertension: results in patients with diastolic blood pressures averaging 115 through 129 mmHg. JAMA 1967;202:1028–1034. [PubMed] [Google Scholar]
- 2. Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, Chen J, He J.. Global disparities of hypertension prevalence and control. Circulation 2016;134:441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marshall IJ, Wolfe CDA, McKevitt C.. Lay perspectives on hypertension and drug adherence: systematic review of qualitative research. BMJ 2012;345:e3953.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, Backer GD, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Burnier M, Ambrosioni E, Caufield M, Coca A, Olsen MH. et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Hear J 2013;34:2159–2219. [DOI] [PubMed] [Google Scholar]
- 5. Mahfoud F, Lüscher TF, Andersson B, Baumgartner I, Cifkova R, DiMario C, Doevendans P, Fagard R, Fajadet J, Komajda M, LeFèvre T, Lotan C, Sievert H, Volpe M, Widimsky P, Wijns W, Williams B, Windecker S, Witkowski A, Zeller T, Böhm M.. Expert consensus document from the European Society of Cardiology on catheter-based renal denervation. Eur Heart J 2013;34:2149–2157. [DOI] [PubMed] [Google Scholar]
- 6. Mahfoud F, Böhm M, Azizi M, Pathak A, Durand Zaleski I, Ewen S, Tsioufis K, Andersson B, Blankestijn PJ, Burnier M, Chatellier G, Gafoor S, Grassi G, Joner M, Kjeldsen SE, Lüscher TF, Lobo MD, Lotan C, Parati G, Redon J, Ruilope L, Sudano I, Ukena C, Leeuwen EV, Volpe M, Windecker S, Witkowski A, Wijns W, Zeller T, Schmieder RE.. Proceedings from the European clinical consensus conference for renal denervation: considerations on future clinical trial design. Eur Heart J 2015;36:2219–2227. [DOI] [PubMed] [Google Scholar]
- 7. Böhm M, Linz D, Ukena C, Esler M, Mahfoud F.. Renal denervation for the treatment of cardiovascular high risk-hypertension or beyond? Circ Res 2014;115:400–409. [DOI] [PubMed] [Google Scholar]
- 8. Smithwick RH. Surgical treatment of hypertension. Am J Med 1948;4:744–759. [DOI] [PubMed] [Google Scholar]
- 9. Bhatt DL, Kandzari DE, O’neill WW, D’agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen S. A, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL.. A controlled trial of renal denervation for resistant hypertension. N Eng J Med 2014;370:1393–1401. [DOI] [PubMed] [Google Scholar]
- 10. Azizi M, Sapoval M, Gosse P, Monge M, Bobrie G, Delsart P, Midulla M, Mounier-Véhier C, Courand PY, Lantelme P, Denolle T, Dourmap-Collas C, Trillaud H, Pereira H, Plouin PF, Chatellier G.. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): A multicentre, open-label, randomised controlled trial. Lancet 2015;385:1957–1965. [DOI] [PubMed] [Google Scholar]
- 11. Krum H, Schlaich MP, Sobotka PA, Böhm M, Mahfoud F, Rocha-Singh K, Katholi R, Esler MD.. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet 2014;383:622–629. [DOI] [PubMed] [Google Scholar]
- 12. Persu A, Jin Y, Azizi M, Baelen M, Volz S, Elvan A, Severino F, Rosa J, Adiyaman A, Fadl Elmula FE, Taylor A, Pechere-Bertschi A, Wuerzner G, Jokhaji F, Kahan T, Renkin J, Monge M, Widimsky P, Jacobs L, Burnier M, Mark PB, Kjeldsen SE, Andersson B, Sapoval M, Staessen JA.. Blood pressure changes after renal denervation at 10 European expert centers. J Hum Hypertens 2014;28:150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ng FL, Saxena M, Mahfoud F, Pathak A, Lobo MD.. Device-based therapy for hypertension. Curr Hypertens Rep 2016;18:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lobo MD, Sobotka PA, Pathak A.. Interventional procedures and future drug therapy for hypertension. Eur Heart J 2016; doi: 10.1093/eurheartj/ehw303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williams B, Macdonald TM, Morant S, Webb DJ, Sever P, McInnes G, Ford I, Cruickshank JK, Caulfield MJ, Salsbury J, Mackenzie I, Padmanabhan S, Brown MJ.. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): A randomised, double-blind, crossover trial. Lancet 2015;386:2059–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosa J, Widimský P, Waldauf P, Lambert L, Zelinka T, Táborský M, Branny M, Toušek P, Petrák O, Čurila K, Bednář F, Holaj R, Štrauch B, Václavík J, Nykl I, Krátká Z, Kociánová E, Jiravský O, Rappová G, Indra T, Widimský J.. Role of adding spironolactone and renal denervation in true resistant hypertension: one-year outcomes of randomized PRAGUE-15 study. Hypertension 2016;67:397–403. [DOI] [PubMed] [Google Scholar]
- 17. Sprint Research Group Wright JT, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT.. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leung AA, Nerenberg K, Daskalopoulou SS, McBrien K, Zarnke KB, Dasgupta K, Cloutier L, Gelfer M, Lamarre-Cliche M, Milot A, Bolli P, Tremblay G, McLean D, Tobe SW, Ruzicka M, Burns KD, Vallée M, Prasad GVR, Lebel M, Feldman RD, Selby P, Pipe A, Schiffrin EL, McFarlane PA, Oh P, Hegele RA, Khara M, Wilson TW, Penner SB, Burgess E. et al. Hypertension Canada’s 2016 Canadian hypertension education program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol 2016;32:569–588. [DOI] [PubMed] [Google Scholar]
- 19. Kjeldsen SE, Berge E, Bangalore S, Messerli FH, Mancia G, Holzhauer B, Hua TA, Zappe D, Zanchetti A, Weber MA, Julius S.. No evidence for a J-shaped curve in treated hypertensive patients with increased cardiovascular risk: The VALUE trial. Blood Press 2016;25:83–92. [DOI] [PubMed] [Google Scholar]
- 20. Verdecchia P, Reboldi G, Angeli F, Trimarco B, Mancia G, Pogue J, Gao P, Sleight P, Teo K, Yusuf S.. Systolic diastolic blood pressure changes in relation with myocardial infarction and stroke in patients with coronary artery disease. Hypertension 2015;65:108–114. [DOI] [PubMed] [Google Scholar]
- 21. SPS-3 Study Group. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013;382:507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. ACCORD Study Group, Cushman WC, Evans GW, Byington RP, Goff DC, Grimm RH, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F.. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kjeldsen SE, Lund-Johansen P, Nilsson PM, Mancia G.. Unattended blood pressure measurements in the systolic blood pressure intervention trial: implications for entry and achieved blood pressure values compared with other trials. Hypertension 2016;67:808–812. [DOI] [PubMed] [Google Scholar]
- 24. Filipovský J, Seidlerová J, Kratochvíl Z, Karnosová P, Hronová M, Mayer O.. Automated compared to manual office blood pressure and to home blood pressure in hypertensive patients. Blood Press 2016;25:228–234. [DOI] [PubMed] [Google Scholar]
- 25. Thomopoulos C, Parati G, Zanchetti A.. Effects of blood pressure lowering on outcome incidence in hypertension: 7. Effects of more vs. less intensive blood pressure lowering and different achieved blood pressure levels—updated overview and meta-analyses of randomized trials. J Hypertens 2016;34:613–622. [DOI] [PubMed] [Google Scholar]
- 26. Thomopoulos C, Parati G, Zanchetti A.. Effects of blood pressure lowering treatment in hypertension: 8. Outcome reductions vs. discontinuations because of adverse drug events—meta-analyses of randomized trials. J Hypertens 2016;34:1451–1463. [DOI] [PubMed] [Google Scholar]
- 27. Parati G, Stergiou G, O’brien E, Asmar R, Beilin L, Bilo G, Clement D, la Sierra A, de Leeuw P, de Dolan E, Fagard R, Graves J, Head GA, Imai Y, Kario K, Lurbe E, Mallion JM, Mancia G, Mengden T, Myers M, Ogedegbe G, Ohkubo T, Omboni S, Palatini P, Redon J, Ruilope LM, Shennan A, Staessen JA, vanMontfrans G, Verdecchia P. et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens 2014;32:1359–1366. [DOI] [PubMed] [Google Scholar]
- 28. Goldberg EM, Levy PD.. New approaches to evaluating and monitoring blood pressure. Curr Hypertens Rep 2016;18:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K.. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016;387:957–967. [DOI] [PubMed] [Google Scholar]
- 30. Thomopoulos C, Parati G, Zanchetti A.. Effects of blood pressure lowering on outcome incidence in hypertension. 1. Overview, meta-analyses, and meta-regression analyses of randomized trials. J Hypertens 2014;32:2285–2295. [DOI] [PubMed] [Google Scholar]
- 31. Schmieder RE, Schmidt ST, Riemer T, Dechend R, Hagedorn I, Senges J, Messerli FH, Zeymer U.. Disproportional decrease in office blood pressure compared with 24-hour ambulatory blood pressure with antihypertensive treatment: dependency on pretreatment blood pressure levels. Hypertension 2014;64:1067–1072. [DOI] [PubMed] [Google Scholar]
- 32. Kandzari DE, Kario K, Mahfoud F, Cohen SA, Pilcher G, Pocock S, Townsend R, Weber MA, Böhm M.. The SPYRAL HTN Global Clinical Trial Program: rationale and design for studies of renal denervation in the absence (SPYRAL HTN OFF-MED) and presence (SPYRAL HTN ON-MED) of antihypertensive medications. Am Hear J 2016;171:82–91. [DOI] [PubMed] [Google Scholar]
- 33. Bisognano JD, Bakris G, Nadim MK, Sanchez L, Kroon AA, Schafer J, Leeuw PW, de ica DA.. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double-blind, randomized, placebo-controlled rheos pivotal trial. J Am Coll Cardiol 2011;58:765–773. [DOI] [PubMed] [Google Scholar]
- 34. Bakris G, Nadim M, Haller H, Lovett E, Bisognano J.. Baroreflex activation therapy safely reduces blood pressure for at least five years in a large resistant hypertension cohort. J Am Soc Hypertens 2014;8:e9. [Google Scholar]
- 35. Rossignol P, Massy ZA, Azizi M, Bakris G, Ritz E, Covic A, Goldsmith D, Heine GH, Jager KJ, Kanbay M, Mallamaci F, Ortiz A, Vanholder R, Wiecek A, Zoccali C, London GM, Stengel B, Fouque D.. The double challenge of resistant hypertension and chronic kidney disease. Lancet 2015;386:1588–1598. [DOI] [PubMed] [Google Scholar]
- 36. Bang H, Ni L, Davis CE.. Assessment of blinding in clinical trials. Control Clin Trials 2004;25:143–156. [DOI] [PubMed] [Google Scholar]
- 37. Pocock SJ, Bakris GL, Bhatt DL, Brar S, Fahy M, Gersh BJ.. Regression to the mean in SYMPLICITY HTN-3: implications for design and reporting of future trials. J Am Coll Cardiol 2016;68:2016–2025. [DOI] [PubMed] [Google Scholar]
- 38. Dahlof B, Devereux R, Faire U, De Fyhrquist F, Hedner T, Ibsen H, Julius S, Kjeldsen S, Kristianson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H.. The Losartan Intervention For Endpoint reduction (LIFE) in Hypertension study rationale, design, and methods. Am J Hypertens 1997;10:705–713. [PubMed] [Google Scholar]
- 39. Jung O, Gechter JL, Wunder C, Paulke A, Bartel C, Geiger H, Toennes SW.. Resistant hypertension? Assessment of adherence by toxicological urine analysis. J Hypertens 2013;31:766–774. [DOI] [PubMed] [Google Scholar]
- 40. Schmieder RE, Ott C, Schmid A, Friedrich S, Kistner I, Ditting T, Veelken R, Uder M, Toennes SW.. Adherence to antihypertensive medication in treatment-resistant hypertension undergoing renal denervation. J Am Heart Assoc 2016;5. doi: 10.1161/JAHA.115.002343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ewen S, Meyer MR, Cremers B, Laufs U, Helfer AG, Linz D, Kindermann I, Ukena C, Burnier M, Wagenpfeil S, Maurer HH, Böhm M, Mahfoud F.. Blood pressure reductions following catheter-based renal denervation are not related to improvements in adherence to antihypertensive drugs measured by urine/plasma toxicological analysis. Clin Res Cardiol 2015;104:1097–1105. [DOI] [PubMed] [Google Scholar]
- 42. Patel HC, Hayward C, Ozdemir BA, Rosen SD, Krum H, Lyon AR, Francis DP, Mario di C.. Magnitude of blood pressure reduction in the placebo arms of modern hypertension trials: implications for trials of renal denervation. Hypertension 2015;65:401–406. [DOI] [PubMed] [Google Scholar]
- 43. Tiroch K, Sause A, Szymanski J, Nover I, Leischik R, Mann JFE, Vorpahl M, Seyfarth M.. Intraprocedural reduction of the veno-arterial norepinephrine gradient correlates with blood pressure response after renal denervation. EuroIntervention 2015;11:824–834. [DOI] [PubMed] [Google Scholar]
- 44. Jong MR, De Adiyaman A, Gal P, Smit JJJ, Delnoy Pphm Heeg JE, Hasselt Baam Van Lau EOY, Persu A, Staessen JA, Misier ARR, Steinberg JS, Elvan A.. Renal nerve stimulation-induced blood pressure changes predict ambulatory blood pressure response after renal denervation. Hypertension 2016;68:707–714. [DOI] [PubMed] [Google Scholar]
- 45. Ikeno F, Lambert D, Arjun MD.. Transluminal imaging of renal nerves using optical coherence tomography. J Am Coll Cardiol 2014;64:B124. [Google Scholar]
- 46. Mahfoud F, Moon LB, Pipenhagen CA, Jensen JA, Pathak A, Papademetriou V, Ewen S, Linz D, Böhm M.. Catheter-based radio-frequency renal nerve denervation lowers blood pressure in obese hypertensive swine model. J Hypertens 2016;34:1854–1862. [DOI] [PubMed] [Google Scholar]
- 47. Tzafriri AR, Keating JH, Markham PM, Spognardi AM, Stanley JR, Wong G, Zani BG, Highsmith D, O’fallon P, Fuimaono K, Mahfoud F, Edelman ER.. Arterial microanatomy determines the success of energy-based renal denervation in controlling hypertension. Sci Transl Med 2015;7:285ra65.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Booth LC, Nishi EE, Yao ST, Ramchandra R, Lambert GW, Schlaich MP, May CN.. Reinnervation of renal afferent and efferent nerves at 5.5 and 11 months after catheter-based radiofrequency renal denervation in sheep. Hypertension 2015;65:393–400. [DOI] [PubMed] [Google Scholar]
- 49. Ewen S, Ukena C, Linz D, Kindermann I, Cremers B, Laufs U, Wagenpfeil S, Schmieder RE, Böhm M, Mahfoud F.. Reduced effect of percutaneous renal denervation on blood pressure in patients with isolated systolic hypertension. Hypertension 2015;65:193–199. [DOI] [PubMed] [Google Scholar]
- 50. Mahfoud F, Bakris G, Bhatt DL, Esler M, Ewen S, Fahy M, Kandzari D, Kario K, Mancia G, Weber M, Böhm M.. Reduced blood pressure-lowering effect of catheter-based renal denervation in patients with isolated systolic hypertension: data from SYMPLICITY HTN-3 and the Global SYMPLICITY Registry. Eur Heart J 2017;38:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Logan AG. Hypertension in aging patients. Expert Rev Cardiovasc Ther 2011;9:113–120. [DOI] [PubMed] [Google Scholar]
- 52. Ott C, Lobo MD, Sobotka PA, Mahfoud F, Stanton A, Cockcroft J, Sulke N, Dolan E, Giet M, van der Hoyer J, Furniss SS, Foran JP, Witkowski A, Januszewicz A, Schoors D, Tsioufis K, Rensing BJ, Saxena M, Scott B, Ng GA, Achenbach S, Schmieder RE.. Effect of arteriovenous anastomosis on blood pressure reduction in patients with isolated systolic hypertension compared with combined hypertension. J Am Heart Assoc 2016;5:e004234.. [DOI] [PMC free article] [PubMed] [Google Scholar]