Abstract
Background: Mammographic density defined by the conventional pixel brightness threshold, and adjusted for age and body mass index (BMI), is a well-established risk factor for breast cancer. We asked if higher thresholds better separate women with and without breast cancer.
Methods: We studied Australian women, 354 with breast cancer over-sampled for early-onset and family history, and 944 unaffected controls frequency-matched for age at mammogram. We measured mammographic dense area and percent density using the CUMULUS software at the conventional threshold, which we call Cumulus, and at two increasingly higher thresholds, which we call Altocumulus and Cirrocumulus, respectively. All measures were Box–Cox transformed and adjusted for age and BMI. We estimated the odds per adjusted standard deviation (OPERA) using logistic regression and the area under the receiver operating characteristic curve (AUC).
Results: Altocumulus and Cirrocumulus were correlated with Cumulus (r ∼ 0.8 and 0.6, respectively). For dense area, the OPERA was 1.62, 1.74 and 1.73 for Cumulus, Altocumulus and Cirrocumulus, respectively (all P < 0.001). After adjusting for Altocumulus and Cirrocumulus, Cumulus was not significant (P > 0.6). The OPERAs for percent density were less but gave similar findings. The mean of the standardized adjusted Altocumulus and Cirrocumulus dense area measures was the best predictor; OPERA = 1.87 [95% confidence interval (CI): 1.64–2.14] and AUC = 0.68 (0.65–0.71).
Conclusions: The areas of higher mammographically dense regions are associated with almost 30% stronger breast cancer risk gradient, explain the risk association of the conventional measure and might be more aetiologically important. This has substantial implications for clinical translation and molecular, genetic and epidemiological research.
Keywords: Breast cancer, case-control study, Australian women, mammography, mammographic density
Introduction
Mammographic density has been conventionally defined as the area of white or bright areas on a mammogram, and as such is a subjective concept.1,2 The current gold-standard measure is derived by using the CUMULUS software and a computer-assisted thresholding method, in which the observer selects a pixel brightness threshold to define the dense area for each mammogram.1–3
In establishing the evidence for mammographic density as a risk factor for breast cancer, considerable and warranted care has been taken to ensure that observers measure density in a similar and repeatable way.2–5 New observers have been trained to ensure comparability and repeatability with previous observers, to measure what has historically been referred to as the mammographically dense regions of the breast.
Multiple studies have shown that, after adjusting for age and body mass index (BMI), this measure of mammographic density predicts breast cancer risk.6–11 It is important to adjust for age and BMI because mammographic density decreases with both increasing age and increasing BMI, whereas breast cancer risk increases with these factors.
We asked if selecting pixel brightness thresholds of higher intensity better separates women with and without breast cancer. We did this by measuring mammographic density at three different thresholds: one based on the conventional approach, which we call Cumulus, and two based on successively higher thresholds. We call the latter two measures Altocumulus, when using a threshold of one level higher intensity, and Cirrocumulus when using a threshold of two levels higher intensity.12 We estimated and compared risk gradients on the scale of odds ratio per adjusted standard deviation (OPERA)13 and by the area under the receiver operating characteristic curve (AUC).
When we did this previously using digital mammograms and Korean women, we found that the transformed and adjusted Altocumulus measure gave the strongest risk prediction. But given that almost all the world’s evidence for the conventional mammographic density measure to be associated with risk of breast cancer comes from non-digital mammograms of Western women, it is essential that we try to replicate our findings in such a setting. In this paper we have done so using a case-control study of Australian women across a wide range of ages.
Methods
Sample
We studied 354 women with breast cancer (cases) over-sampled for early-onset disease or having a family history of breast cancer, and 944 women without breast cancer (controls), frequency-matched for age at mammogram in 5-year age groups. For the affected women, we used mammograms taken before diagnosis (by on average 4 years) for 32%, and for the other affected women we used the mammogram from the opposite side to that in which the cancer was diagnosed. For the unaffected women, we studied the mammogram of a randomly chosen breast. These women were selected from the Australian Breast Cancer Family Registry (ABCFR; 254 cases and 194 controls) and the Australian Mammographic Density Twins and Sisters Study (AMDTSS; 100 cases and 750 controls).
The ABCFR consists of families, a large proportion selected through women diagnosed with breast cancer of whom more than half were diagnosed before age 40 years.14–16 The AMDTSS includes: (i) twin pairs and their sisters, who were recruited through the Australian Twin Registry17,18 (48 cases and 704 controls); (ii) sisters, at least one of whom has had a diagnosis of breast cancer, recruited through the Breast Cancer Network Australia [https://www.bcna.org.au], the peak organization for women affected by breast cancer (49 cases and 33 controls); and (iii) women from Register4 [http://register4.org.au], a national online database of people willing to consider participating in cancer research19 (3 cases and 13 controls).
Participants in the ABCFR and AMDTSS completed similar interviewer-administered questionnaires assessing standard risk factors for breast cancer, including self-reported height, weight and reproductive history. Family cancer history was obtained from all participants.14
For all participants in this study we obtained at least one mammogram. For affected women, the mammograms we used were those taken at or before diagnosis. All participants gave informed consent. The study was approved by the human research ethics committees of the University of Melbourne and the Cancer Councils of Victoria and of New South Wales.
Measurement of mammographic density
Mammograms were retrieved from BreastScreen services across Australia, from screening clinics and from the women themselves. All mammograms were analog and were digitized (using 12-bit depth) by the Australian Mammographic Density Research Facility.
The CUMULUS software and a computer-assisted thresholding method were used to measure mammographic density. The observer selects pixel brightness thresholds using a sliding scale (ranged 0–4095) and the program draws a line around the regions on the digitized image for which the pixel density is above that threshold. For each mammogram, the observer first chooses a threshold to define the outer extent of the breast image. The observer then chooses a threshold to select the regions which he or she considers to be mammographically dense. The area within these region is called the dense area, and percent density is the dense area divided by the total breast area, multiplied by 100.
Four observers (T.L.N., Y.K.A., C.E., J.S.) independently measured mammographic density, blind to case-control status. First, C.E. and J.S. measured mammographic density using the conventional threshold.20,21 We call the average of measures taken using this approach Cumulus. The other two observers were trained to measure mammographic density at higher brightness thresholds and therefore in effect defined mammographic density at a higher threshold, as in Nguyen et al. (2015).12 Y.K.A. consistently measured using one level higher intensity based on what were considered to be the bright, as distinct from white, areas. We call this measure Altocumulus. T.L.N. measured using an even higher level of intensity based on what were considered to be the brightest regions. We call this measure Cirrocumulus. These new measures define mammographic density at successively higher pixel brightness thresholds.
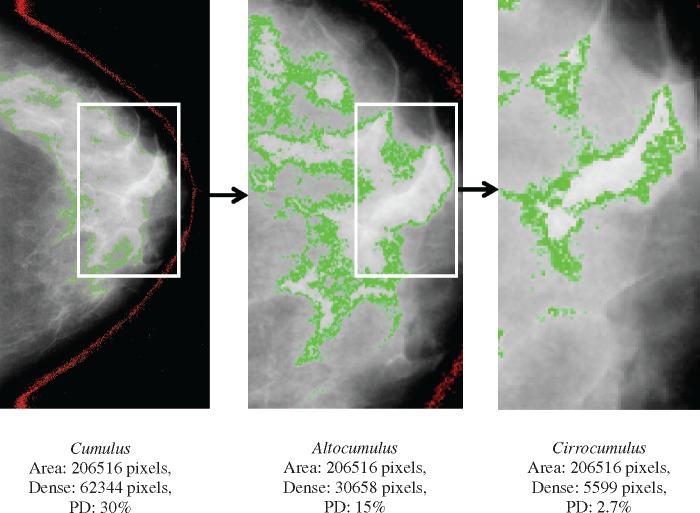
Figure 1 shows an example of Cumulus, Altocumulus and Cirrocumulus measures from the same mammogram. In the left-hand panel, the white or bright areas are chosen and outlined to give the Cumulus measure. In the centre panel, the bright areas are chosen and outlined within the white or bright area of the left-hand panel to give the Altocumulus measure. In the right-hand panel, the very bright areas are chosen within the bright areas of the centre panel to give the Cirrocumulus measure.
Figure 1.
Measurement of Cumulus (left), Altocumulus (centre) and Cirrocumulus (right) using the CUMULUS software from the same mammogram. Dense area (percent density) was 62 344 pixels (30%), 30 658 pixels (15%) and 5 599 pixels (2.7%) corresponding to Cumulus, Altocumulus and Cirrocumulus measures, respectively.
Repeatability was assessed, as in previous studies, by performing the measurements in sets of 100 mammograms with the same case-control ratio and including 10% repeat samples in each set. Observers were blinded to case-control status and blinded to any previous measures. The intraclass correlation coefficient was 0.99 and 0.93 for the transformed and adjusted Altocumulus and Cirrocumulus measures of dense areas, respectively. We also had two readers (T.L.N. and C.E.) measure the same 200 images for each of Cumulus, Altocumulus and Cirrocumulus. The correlation between the two readers was 0.95, 0.89 and 0.85 for dense areas, respectively.
Statistical methods
Logistic regression was used to estimate the associations between the mammographic density measures and breast cancer risk, adjusting for covariates. Each density measure was transformed using the Box–Cox power transformation to have an approximately normal distribution.22 The appropriate transformations were cube-root for the Cumulus measures and logarithm for both the Altocumulus and Cirrocumulus measures. For each fitted model, the means of the transformed measures were adjusted for age and BMI to derive the standard deviation of the residuals. From this we estimated the odds ratio per adjusted standard deviation (OPERA),13 and used maximum likelihood theory to determine the weighted combination of transformed, standardized and age- and BMI-adjusted measures that best predicted risk; see Appendix (available as Supplementary data at IJE online). The area under the receiver operating characteristic curve (AUC) was also estimated. All statistical analyses were conducted using the software package Stata.23 Following convention, P < 0.05 was considered to be statistically significant.
Results
Table 1 shows that cases did not differ from controls by more than 20% in terms of BMI or the other covariates. About 30% of cases and controls had a first-degree relative with breast cancer.
Table 1.
Characteristics of cases and controls
| Cases (354) | Controls (944) | |||
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | P* | ||
| Age at mammogram (years) | 47.51 (10.31) | 48.17 (9.75) | 0.3 | |
| Height (cm) | 163.57 (6.22) | 163.21 (6.69) | 0.4 | |
| Weight (kg) | 67.15 (12.08) | 68.96 (12.48) | 0.03 | |
| Body mass index (kg/cm2) | 25.14 (4.66) | 25.92 (5.03) | 0.01 | |
| Number of live births (n = 1032) | 2.56 (1.09) | 2.64 (1.02) | 0.3 | |
| Month of HRT use (n = 404) | 48.65 (52.12) | 67.77 (73.29) | 0.01 | |
| Menopausal status | ||||
| Pre (n, %) | 179 (50.56) | 564 (59.75) | 0.003 | |
| Post (n, %) | 175 (49.44) | 380 (40.25) | ||
| Family history of breast cancer | ||||
| Yes (n, %) | 102 (28.81) | 285 (30.19) | 0.6 | |
| No (n, %) | 252 (71.19) | 659 (69.81) | ||
| Mammographic measurements | ||||
| Cumulus | ||||
| Dense area (cm2) | 27.58 (21.20) | 18.74 (16.42) | < 0.001 | |
| Non-dense area (cm2) | 99.93 (59.01) | 103.04 (56.43) | 0.4 | |
| Percent density | 24.63 (16.73) | 18.00 (14.93) | < 0.001 | |
| Density threshold (0 to 4095) | 2683 (340) | 2567 (325) | < 0.001 | |
| Altocumulus | ||||
| Dense area (cm2) | 21.41 (15.08) | 15.51 (12.29) | < 0.001 | |
| Non-dense area (cm2) | 106.41 (59.19) | 105.57 (55.40) | 0.8 | |
| Percent density | 19.78 (13.83) | 15.28 (12.49) | < 0.001 | |
| Density threshold (0 to 4095) | 2786 (280) | 2640 (275) | < 0.001 | |
| Cirrocumulus | ||||
| Dense area (cm2) | 4.56 (4.66) | 2.88 (3.85) | < 0.001 | |
| Non-dense area (cm2) | 122.72 (58.42) | 117.58 (54.65) | 0.1 | |
| Percent density | 4.07 (4.02) | 2.74 (3.38) | < 0.001 | |
| Density threshold (0 to 4095) | 3083 (275) | 2955 (283) | < 0.001 | |
HRT, hormone replacement therapy.
*P-value for the difference between cases and controls.
Table 1 shows that for cases and controls, compared with the Cumulus measures, the corresponding Altocumulus measures of dense area and percent density were about 20% less, and the corresponding Cirrocumulus measures were substantially smaller. The mean pixel thresholds (standard deviation) were 2599 (333), 2680 (284) and 2990 (286) for Cumulus, Altocumulus and Cirrocumulus measures, respectively, therefore being 3% and 15% higher for Altocumulus and Cirrocumulus, respectively, compared with Cumulus. After transforming and adjusting, the correlations between dense area and percent density measures were 0.90, 0.85 and 0.95 for Cumulus, Altocumulus and Cirrocumulus, respectively. The correlations were between Cumulus and Altocumulus measures was 0.78 for dense area and 0.81 for percent density. The corresponding correlations between Cumulus and Cirrocumulus measures were 0.64 and 0.58, respectively, and the corresponding correlations between Altocumulus and Cirrocumulus measures were 0.54 and 0.50, respectively. Table 1 also shows that all mammographic density measures and density thresholds were higher for cases than for controls (all P < 0.001).
Table 2 shows that there were substantive risk associations for all three transformed and adjusted mammographic density measures (all P < 0.001). (These estimates changed by at most 3% on the log odds scale after adjustment for other potential confounders; data not shown). The OPERAs for both dense area and percent density were in general greater for the Altocumulus and Cirrocumulus measures than for the Cumulus measures. The OPERAs for the dense area measures were also greater than the OPERAs for the percent density measures.
Table 2.
Associations of mammographic density measures (as assessed by OPERA) with breast cancer risk, adjusting for age and body mass index
| Cumulus | |||||||
|---|---|---|---|---|---|---|---|
| Dense area | Cases (n) | Controls (n) | ORa | 95% CI | P* | AUC (95% CI) | |
| Q1 | 44 | 236 | 1.00 | – | |||
| Q2 | 71 | 236 | 1.59 | 1.05–2.42 | 0.03 | ||
| Q3 | 97 | 236 | 2.17 | 1.46–3.25 | < 0.001 | ||
| Q4 | 142 | 236 | 3.21 | 2.19–4.72 | < 0.001 | ||
| OPERA | 354 | 944 | 1.62 | 1.42–1.83 | < 0.001 | 0.64 (0.60–0.67) | |
| Percent density | |||||||
| Q1 | 45 | 236 | 1.00 | – | |||
| Q2 | 74 | 236 | 1.65 | 1.09–2.49 | 0.02 | ||
| Q3 | 97 | 236 | 2.13 | 1.43–3.17 | < 0.001 | ||
| Q4 | 138 | 236 | 3.06 | 2.09–4.49 | < 0.001 | ||
| OPERA | 354 | 944 | 1.52 | 1.34–1.73 | < 0.001 | 0.62 (0.58–0.65) | |
| Altocumulus | |||||||
| Dense area | ORa | 95% CI | P | ||||
| Q1 | 38 | 236 | 1.00 | – | |||
| Q2 | 58 | 236 | 1.52 | 0.97–2.37 | 0.07 | ||
| Q3 | 94 | 236 | 2.45 | 1.61–3.72 | < 0.001 | ||
| Q4 | 164 | 236 | 4.35 | 2.92–6.46 | < 0.001 | ||
| OPERA | 354 | 944 | 1.74 | 1.53–1.99 | < 0.001 | 0.66 (0.63–0.69) | |
| Percent density | |||||||
| Q1 | 46 | 236 | 1.00 | – | |||
| Q2 | 72 | 236 | 1.57 | 1.04–2.37 | 0.03 | ||
| Q3 | 108 | 236 | 2.35 | 1.59–3.47 | < 0.001 | ||
| Q4 | 128 | 236 | 2.79 | 1.90–4.09 | < 0.001 | ||
| OPERA | 354 | 944 | 1.47 | 1.30–1.68 | < 0.001 | 0.61 (0.58–0.64) | |
| Cirrocumulus | |||||||
| Dense area | ORa | 95% CI | P | ||||
| Q1 | 27 | 236 | 1.00 | – | |||
| Q2 | 62 | 236 | 2.29 | 1.40–3.72 | 0.001 | ||
| Q3 | 124 | 236 | 4.54 | 2.88–7.15 | < 0.001 | ||
| Q4 | 141 | 236 | 5.24 | 3.34–8.21 | < 0.001 | ||
| OPERA | 354 | 944 | 1.73 | 1.52–1.97 | < 0.001 | 0.66 (0.62–0.69) | |
| Percent density | |||||||
| Q1 | 35 | 236 | 1.00 | – | |||
| Q2 | 63 | 236 | 1.81 | 1.15–2.83 | 0.01 | ||
| Q3 | 117 | 236 | 3.33 | 2.19–5.06 | < 0.001 | ||
| Q4 | 139 | 236 | 3.97 | 2.63–6.00 | < 0.001 | ||
| OPERA | 354 | 944 | 1.59 | 1.40–1.80 | < 0.001 | 0.64 (0.60–0.67) | |
| Average transformed and adjusted Altocumulus and Cirrocumulus | |||||||
| Dense area | ORa | 95% CI | P | ||||
| Q1 | 28 | 236 | 1.00 | – | |||
| Q2 | 62 | 236 | 2.20 | 1.36–3.28 | 0.002 | ||
| Q3 | 94 | 236 | 3.61 | 2.09–5.24 | < 0.001 | ||
| Q4 | 170 | 236 | 6.07 | 3.91–9.41 | < 0.001 | ||
| OPERA | 354 | 944 | 1.87 | 1.64–2.14 | < 0.001 | 0.68 (0.65–0.71) | |
| Percent density | |||||||
| Q1 | 35 | 236 | 1.00 | – | |||
| Q2 | 65 | 236 | 1.77 | 1.13–2.78 | 0.02 | ||
| Q3 | 100 | 236 | 2.71 | 1.77–4.14 | < 0.001 | ||
| Q4 | 154 | 236 | 4.31 | 2.89–6.48 | < 0.001 | ||
| OPERA | 354 | 944 | 1.64 | 1.43–1.87 | < 0.001 | 0.64 (0.61–0.67) | |
HRT, hormone replacement therapy.
aOdds ratio per standard deviation after adjusting for age and body mass index.
*P-value for comparison with baseline category.
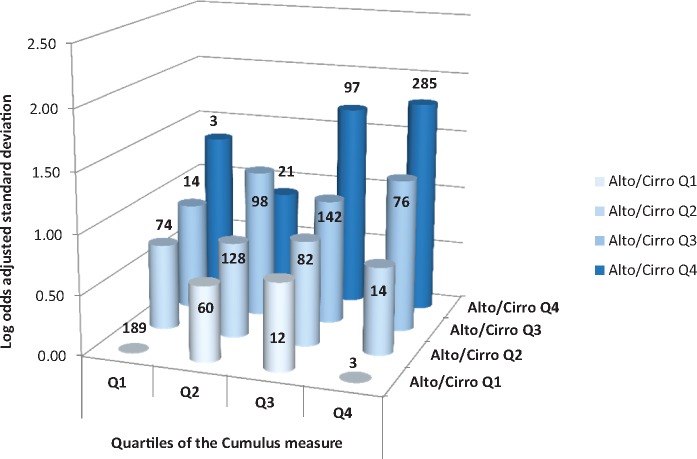
Table 3 shows that, for dense area, when the different measures were fitted together the risk associations for the Altocumulus and Cirrocumulus measures were little changed and always remained statistically significant, but the risk associations for the Cumulus measures were typically no longer significant. Figure 2 illustrates this confounding. It shows that, within every quartile of the Altocumulus measure, the risk associations did not increase substantially or consistently across quartiles of the Cumulus measure. On the other hand, for every quartile of the Cumulus measure, the risk associations increased substantially across quartiles of the Altocumulus measure.
Table 3.
Estimates and statistical significance (P) of odds ratio per standard deviation after adjusting for age and body mass index (OPERA) from fitting multiple mammographic density measures together
| Dense area | OPERAf (95% CI) | P* |
|---|---|---|
| Cumulusa | 1.18 (0.97–1.42) | 0.1 |
| Altocumulusa | 1.53 (1.25–1.87) | < 0.001 |
| Cumulusb | 1.24 (1.05–1.47) | 0.02 |
| Cirrocumulusb | 1.49 (1.25–1.77) | < 0.001 |
| Altocumulusc | 1.43 (1.22–1.68) | < 0.001 |
| Cirrocumulusc | 1.41 (1.21–1.65) | < 0.001 |
| Cumulusd | 0.95 (0.76–1.18) | 0.6 |
| Altocumulusd | 1.48 (1.21–1.81) | < 0.001 |
| Cirrocumulusd | 1.44 (1.20–1.72) | < 0.001 |
| Cumuluse | 0.96 (0.77–1.19) | 0.7 |
| Averagee* | 1.93 (1.54–2.42) | < 0.001 |
aCumulus and Altocumulus measures fitted together.
bCumulus and Cirrocumulus measures fitted together.
cAltocumulus and Cirrocumulus measures fitted together.
dCumulus, Altocumulus and Cirrocumulus measures fitted together.
eCumulus and Average = mean of the transformed and adjusted Altocumulus and Cirrocumulus measures fitted together.
fOdds ratio per standard deviation adjusted for age and body mass index.
*P-value for comparison with baseline category.
Figure 2.
For dense area, log odds ratio per adjusted standard deviation for quartiles of the average of the transformed and adjusted Altocumulus and Cirrocumulus measures by each quartile of the transformed and adjusted Cumulus measure. The number of women in each cell is also shown.
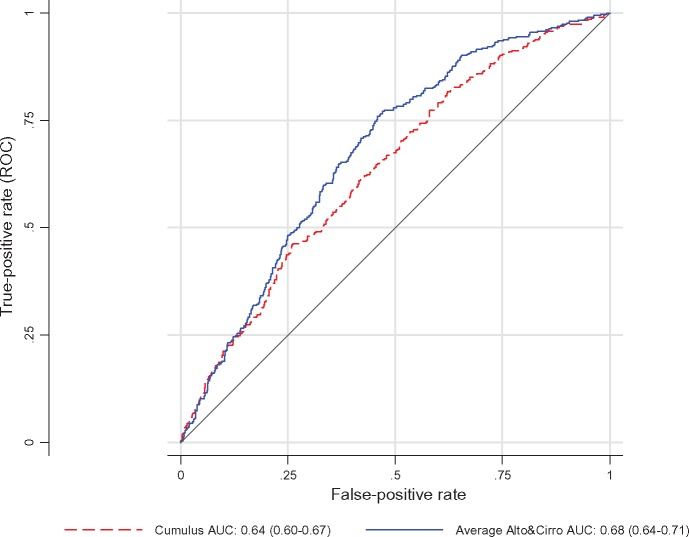
Figure 3.
Receiver operating characteristic curve and area under the curve (AUC) for dense area measures in terms of breast cancer risk (blue continuous line: average of Altocumulus and Cirrocumulus transformed and adjusted measures; red dashes: Cumulus transformed and adjusted measure; P-value for difference=0.0003): Australian Breast Cancer Family Registry and Australian Mammographic Density Twins and Sisters Study.
The OPERAs for the Altocumulus and Cirrocumulus measures, when fitted together, were similar. Before or after excluding the Cumulus measures, the Altocumulus and Cirrocumulus measures had about the same risk association, as measured by OPERA. When weighted linear combinations of these two measures were considered, the maximum likelihood estimate of the weight was 0.5 [90% confidence interval (CI): 0.3–0.7]. When the average of the two transformed, adjusted and standardized measures was fitted, the OPERA was 1.87 (95% CI: 1.64–2.14). The AUC was 0.68 (95% CI: 0.65–0.71), compared with 0.64 (95% CI: 0.60–0.67) when the Cumulus risk measure was fitted on its own (P = 0.0001).
Similar conclusions arose from considering the percent density associations, in that the best fitting model involved the average of the two transformed, adjusted and standardized measures. In particular, for the best fitting model the OPERA was 1.64 (95% CI: 1.43–1.87) and the AUC was 0.64 (95% CI: 0.61–0.67).
Discussion
This study has found that, by in effect defining mammographic density at higher pixel brightness thresholds than have traditionally been used, greater separation between women with and without breast cancer can be achieved. We studied two new measures based on different thresholds for defining mammographic density which, for the purposes of this paper, we call Altocumulus and Cirrocumulus. For Altocumulus, we defined the mammographically dense regions as being bright, rather than just white, and therefore at a higher level of pixel brightness threshold than has been conventionally used. For Cirrocumulus, we defined the mammographically dense regions as being the brightest regions, another level higher. When considered as risk factors, mammographic density measures are adjusted for age and BMI, so we used the OPERA concept which adjusts for these factors to compare the relative strengths of the risk factor associations on the same scale.
Both Altocumulus and Cirrocumulus risk measures (i.e. transformed, adjusted for age and BMI and standardised) separated cases from controls better than the Cumulus measure, despite these three measures being moderately correlated with one another. Given that the difference between the means of the upper and lower quartiles of a normal distribution is 2.54 standard deviations, the estimated OPERA risk gradients for the adjusted prediction mean of the Altocumulus and Cirrocumulus risk measures were equivalent to an interquartile risk ratio of 1.872.54 = 4.9 (95% CI: 3.5–6.9) for dense area and 1.642.54 = 3.5 (95% CI: 2.5–4.9) for percent density. These were more than the corresponding equivalent interquartile risk ratio estimates based on the Cumulus measures of 1.622.54 = 3.4 (95% CI: 2.4–4.6) and 1.522.54 = 2.9 (95% CI: 2.1–4.0). When considering AUC as a measure of risk discrimination, comparison has to be made with 0.5 as that is what would be expected if there was no information on risk. The AUC for the standardized mean of the Altocumulus and Cirrocumulus transformed and adjusted risk measures was 0.68, which is almost 30% greater than 0.5 than is the AUC of 0.64 for the Cumulus risk measure alone. In terms of risk gradient, log (OPERA) was 0.49 for Cumulus and 0.63 for the Altocumulus and Cirrocumulus risk, a 29% increase.
Most importantly, when the different density measures were fitted together, there was no evidence that the conventional measure added information on risk. That is, the information on risk comes from the amount or percent of breast tissue above the higher thresholds. There was no evidence that the white but not bright areas of the breast are associated with risk of breast cancer, though they might well be associated with risk of masking of breast cancers.
Our finding that measuring density at higher pixel brightness thresholds captures considerably more risk-predicting information than measuring at the usual threshold is important for several reasons. First, the mammographically denser regions might be more aetiologically important for breast cancer than the regions currently being studied. The relevant tissues and biological processes involved in explaining why mammographic density is a risk factor for breast cancer are more likely to be in the higher density areas of the breast. If confirmed, this is a critical observation for molecular, genetic and other studies trying to determine the underlying biological processes behind this phenomenon.24 It is also important for research and translation on the prospect of using mammographic density to better predict women as candidates for interventions or targeted screening.
Second, on the basis of this study, the Altocumulus and Cirrocumulus measures would be among the strongest known risk factors for breast cancer when viewed from a population, as distinct from an individual, perspective. OPERA is an omnibus measure of the strength of a risk factor that is similar to the change in AUC. OPERA has the advantage of explicitly taking into account other risk factors, and therefore being independent across measures. The OPERA we estimated here of ∼ 2.0 for the combination of Altocumulus and Cirrocumulus measures is greater than the OPERA of 1.55 for the current common genetic markers recently found to be associated with risk.25 The OPERA for rare mutations in BRCA1 and BRCA2, combined, is about 1.2.13 Therefore, these new mammographic density measures might do at least as well in predicting risk on a population basis as all the genetic risk factors identified in the past two decades.
Third, these new measures substantially reclassify women in terms of risk. For the sake of argument, suppose that women in the top quartile of the transformed and adjusted Cumulus measure are designated as ‘high-risk’ (this is about the proportion of women designated as such using BI-RADS). The risk gradient for the average of the transformed and adjusted Altocumulus and Cirrocumulus measures is 30% steeper, so the absolute risk for women in the upper quartile of the Cumulus measure will be about the same as for those in the top tertile of the combined Altocumulus/Cirrocumulus risk measure. Using the 944 controls, we tabulated the quartiles of Cumulus risk score against the tertiles of the Altocumulus/Cirrocumulus risk measure. There were 314 ‘high-risk’ women in the upper tertile of the Altocumulus/Cirrocumulus risk measure, of whom only 192 would be in same absolute risk category based on the Cumulus risk measure (upper quartile). So 122 (39%) of these high-risk women by Altocumulus/Cirrocumulus would not have been called high-risk by Cumulus. Similarly, of the 236 women classified as ‘high-risk’ by Cumulus, 44 (19%) were not high-risk by the Altocumulus/Cirrocumulus. Therefore, for Cumulus, the false-positive proportion is 39% and the false-negative proportion is 19%. Of all controls, 18% were reclassified. Only 192 (20%) were considered high-risk by both measures. So more women would be put into different risk categories than would be maintained in the high-risk category. This is of substantial clinical relevance.
Possible strengths of this study are that we studied cases across a wide range of age at diagnosis and included a larger proportion diagnosed at a younger age than most studies of mammographic density, which are usually based on women attending screening programmes (55% of our cases were under the age of 50 years at diagnosis). We also, in effect, frequency-matched cases and controls on family history, and had deliberately over-sampled for subjects for whom a higher proportion than otherwise had a first-degree relative with breast cancer (∼ 30%). The referent Cumulus measures were taken by multiple experienced trained observers, and the resulting risk gradient estimates were if anything better than found from other studies. Given that epidemiological studies make inference about risk associations for unaffected individuals (i.e. the population from which the controls had been sampled), we have found that these new mammographic density measures are potentially strong risk factors for younger as well as older women, and for women with a family history.
One of the potential weaknesses of this study, as with all studies that have used the CUMULUS software to study conventionally-defined mammographic density, is that the new measures are observer dependent. But, as with the traditional Cumulus measure, the Altocumulus and Cirrocumulus measures have high repeatability and the trained observers were blind to case-control status. We also demonstrated that there were strong correlations across two observers measuring the same concept.
We are not trying to conclude from this study alone what the optimal definition of density is for risk prediction, but we have made the observation that by going to higher pixel brightness thresholds, better risk prediction can be achieved. There must be an optimal pixel brightness threshold, at least for a given population measured on a given machine, and this study suggests that it is at a higher level than has been conventionally used in the literature.
We are not in a position to speculate about what the differences between the white and brighter areas are, at least not in terms of tissue composition for which studies other than observational epidemiological designs are needed. We do, however, believe that this is an important observation that should inspire and inform biological studies of the causes and mechanisms.
Our finding obviously needs to be tested by others and in other settings. We have been measuring mammographic density across different thresholds in different populations to try to clarify what might be the best mammographic density predictors of risk. Despite the measurement issues also implicit in previous mammographic density research, we are finding a stronger risk prediction gradient for the new measures and little or no residual impact of the conventional measure. If our conjecture is true, and if measurement error could be reduced by, for example, measuring all three measurements on the same mammogram at the same time, we would expect the risk gradient to be even steeper. We have already found that Altocumulus and Cirrocumulus measures are better predictors than Cumulus measures for Korean women, using full-field digital mammograms.12 We are also measuring the familial aggregation of Altocumulus and Cirrocumulus measures using twin and family studies, as we have done for Cumulus,17,18,20 and studying their associations with genetic variants known to be associated with breast cancer risk as has been done for the Cumulus measures.26
In conclusion, this case-control study of women over-sampled for early onset and having a family history of breast cancer, has found that the new definitions of mammographic density at higher pixel brightness thresholds are associated with almost 30% stronger breast cancer risk gradient than the conventional measure of mammographic density. They also explain the risk association of the conventional measure. This suggests that the mammographically denser (brighter) regions might be more aetiologically important for breast cancer, with substantial implications for biological, molecular, genetic and epidemiological research and clinical translation. Our findings are now relevant not only to digital mammography and Korean women, but also to the decades of mammographic density research on Western women using non-digital mammography.
Supplementary Data
Supplementary data (Appendix) are available at IJE online.
Funding
This research was supported by the National Health and Medical Research Council (NHMRC), the Victorian Health Promotion Foundation, Cancer Council Victoria, Cancer Council NSW, Cancer Australia and the National Breast Cancer Foundation. It has also been supported by the Breast Cancer Network Australia, and Register4 and its members. The ABCFR was also supported by grant UM1 CA164920 from the National Cancer Institute. The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centres in the Breast Cancer Family Registry (BCFR), nor does mention of trade names, commercial products or organizations imply endorsement by the US Government or the BCFR. The work was also supported by the National Institutes of Health (CA102659) and Canadian Breast Cancer Research Alliance (016442). T.L.N. has been supported by an NHMRC Post-Graduate Scholarship and the Richard Lowell Travelling Scholarship, the University of Melbourne. Y.K.A. has been supported by the Australian Agency for International Development (AusAID). J.S. has been supported by the National Breast Cancer Foundation Post-Doctoral Training Fellowship. J.L.H. is an NHMRC Senior Principal Research Fellow and a Distinguished Visiting Professor at Seoul National University. M.C.S. and M.A.J. are NHMRC Senior Research Fellows.
Conflict of interest: None declared.
Key Messages
By in effect defining mammographic density at higher pixel brightness thresholds than has traditionally been used, greater separation between women with and without breast cancer was achieved.
When the density measures based on higher pixel brightness thresholds were fitted, there was no evidence that the conventional measure added information on risk.
The mammographically denser (brighter) regions might be more aetiologically relevant for breast cancer risk, with implications for biological, molecular, genetic and epidemiological research and clinical translation.
Supplementary Material
References
- 1. Byng JW, Yaffe MJ, Jong RA et al. . Analysis of mammographic density and breast cancer risk from digitized mammograms. Radiographics 1998;18:1587–98. [DOI] [PubMed] [Google Scholar]
- 2. Yaffe MJ, Boyd NF, Byng JW et al. . Breast cancer risk and measured mammographic density. Eur J Cancer Prev 1998;7(Suppl 1):S47–55. [DOI] [PubMed] [Google Scholar]
- 3. Byng JW, Boyd NF, Fishell E et al. . The quantitative analysis of mammographic densities. Phys Med Biol 1994;39:1629–38. [DOI] [PubMed] [Google Scholar]
- 4. Boyd NF, Lockwood GA, Byng JW et al. . Mammographic densities and breast cancer risk. Cancer Epidemiol Biomarkers Prev 1998;7:1133–44. [PubMed] [Google Scholar]
- 5. Ursin G. [Mammographic density as indicator of breast cancer risk.]. Tidsskr Nor Laegeforen 2003;123:3373–76. [PubMed] [Google Scholar]
- 6. Heusinger K, Loehberg CR, Haeberle L et al. . Mammographic density as a risk factor for breast cancer in a German case-control study. Eur J Cancer Prev 2011;20:1–8. [DOI] [PubMed] [Google Scholar]
- 7. Kim BK, Choi YH, Nguyen TL et al. . Mammographic density and risk of breast cancer in Korean women. Eur J Cancer Prev 2015;24:422–29. [DOI] [PubMed] [Google Scholar]
- 8. Kotsuma Y, Tamaki Y, Nishimura T et al. . Quantitative assessment of mammographic density and breast cancer risk for Japanese women. Breast 2008;17:27–35. [DOI] [PubMed] [Google Scholar]
- 9. McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2006;15:1159–69. [DOI] [PubMed] [Google Scholar]
- 10. Razzaghi H, Troester MA, Gierach GL et al. . Mammographic density and breast cancer risk in White and African American Women. Breast Cancer Res Treat 2012;135:571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ursin G, Ma H, Wu AH et al. . Mammographic density and breast cancer in three ethnic groups. Cancer Epidemiol Biomarkers Prev 2003;12:332–38. [PubMed] [Google Scholar]
- 12. Nguyen TL, Aung YK, Evans CF et al. . Mammographic density defined by higher than conventional brightness threshold better predicts breast cancer risk for full-field digital mammograms. Breast Cancer Res 2015;17:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hopper JL. Odds per adjusted standard deviation: comparing strengths of associations for risk factors measured on different scales and across diseases and populations. Am J Epidemiol 2015;182:863–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dite GS, Jenkins MA, Southey MC et al. . Familial risks, early-onset breast cancer, and BRCA1 and BRCA2 germline mutations. J Natl Cancer Inst 2003;95:448–57. [DOI] [PubMed] [Google Scholar]
- 15. John EM, Hopper JL, Beck JC et al. . The Breast Cancer Family Registry: an infrastructure for cooperative multinational, interdisciplinary and translational studies of the genetic epidemiology of breast cancer. Breast Cancer Res 2004;6:R375–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hopper JL, Bishop DT, Easton DF. Population-based family studies in genetic epidemiology. Lancet 2005;366:1397–406. [DOI] [PubMed] [Google Scholar]
- 17. Boyd NF, Dite GS, Stone J et al. . Heritability of mammographic density, a risk factor for breast cancer. N Engl J Med 2002;347:886–94. [DOI] [PubMed] [Google Scholar]
- 18. Nguyen TL, Schmidt DF, Makalic E et al. . Explaining variance in the cumulus mammographic measures that predict breast cancer risk: a twins and sisters study. Cancer Epidemiol Biomarkers Prev 2013;22:2395–403. [DOI] [PubMed] [Google Scholar]
- 19. Hopper JL, Apicella C, Butt AJ. Register4: an Australian web-enabled resource created by the National Breast Cancer Foundation to facilitate and accelerate cancer research. Med J Aust 2014;200:460. [DOI] [PubMed] [Google Scholar]
- 20. Stone J, Dite GS, Gunasekara A et al. . The heritability of mammographically dense and nondense breast tissue. Cancer Epidemiol Biomarkers Prev 2006;15:612–17. [DOI] [PubMed] [Google Scholar]
- 21. Stone J, Gurrin LC, Byrnes GB et al. . Mammographic density and candidate gene variants: a twins and sisters study. Cancer Epidemiol Biomarkers Prev 2007;16:1479–84. [DOI] [PubMed] [Google Scholar]
- 22. Box GEP, Cox DR. An analysis of transformations. J R Stat Soc BB 1964;26:211–52. [Google Scholar]
- 23. StataCorp. Stata Statistical Software. College Station, TX: StataCorp LP, 2009. [Google Scholar]
- 24. Martin LJ, Boyd NF. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast Cancer Res 2008;10:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mavaddat N, Pharoah PD, Michailidou K et al. . Prediction of breast cancer risk based on profiling with common genetic variants. J Natl Cancer Inst 2015;107 doi: 10.1093/jnci/djv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Odefrey F, Stone J, Gurrin LC et al. . Common genetic variants associated with breast cancer and mammographic density measures that predict disease. Cancer Res 2010;70:1449–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.