Abstract
Aims
A genetic variant in LILRB5 (leukocyte immunoglobulin-like receptor subfamily-B) (rs12975366: T > C: Asp247Gly) has been reported to be associated with lower creatine phosphokinase (CK) and lactate dehydrogenase (LDH) levels. Both biomarkers are released from injured muscle tissue, making this variant a potential candidate for susceptibility to muscle-related symptoms. We examined the association of this variant with statin intolerance ascertained from electronic medical records in the GoDARTS study.
Methods and results
In the GoDARTS cohort, the LILRB5 Asp247 variant was associated with statin intolerance (SI) phenotypes; one defined as having raised CK and being non-adherent to therapy [odds ratio (OR) 1.81; 95% confidence interval (CI): 1.34–2.45] and the other as being intolerant to the lowest approved dose of a statin before being switched to two or more other statins (OR 1.36; 95% CI: 1.07–1.73). Those homozygous for Asp247 had increased odds of developing both definitions of intolerance. Importantly the second definition did not rely on CK elevations. These results were replicated in adjudicated cases of statin-induced myopathy in the PREDICTION-ADR consortium (OR1.48; 95% CI: 1.05–2.10) and for the development of myalgia in the JUPITER randomized clinical trial of rosuvastatin (OR1.35, 95% CI: 1.10–1.68). A meta-analysis across the studies showed a consistent association between Asp247Gly and outcomes associated with SI (OR1.34; 95% CI: 1.16–1.54).
Conclusion
This study presents a novel immunogenetic factor associated with statin intolerance, an important risk factor for cardiovascular outcomes. The results suggest that true statin-induced myalgia and non-specific myalgia are distinct, with a potential role for the immune system in their development. We identify a genetic group that is more likely to be intolerant to their statins.
Keywords: Statins, Pharmacogenetics, Immunogenetics, Precision medicine, Adverse drug reactions, Myalgia
Introduction
Statins are first choice lipid-modifying medications for prevention and management of cardiovascular diseases (CVD).1,2 The UK is one of the largest users of statins worldwide,3 and with revised NICE guidelines approximately 12 million UK individuals will be prescribed statins.4,5 While statins are generally well tolerated, neurological,6 gastro-intestinal, or muscle-based7,8 adverse drug reactions are reported. Adverse reactions to statins are likely to manifest as muscle aches (myalgia) along with elevated creatine phosphokinase (CK). Adherence to statin treatment is often negatively impacted in response to adverse reactions.9,10 The inability to adhere to statin treatment, whether due to statin-induced myalgia or more general forms of statin intolerance result in poor on-statin outcomes.11 Therefore, examining risk factors predisposing to statin intolerance is crucial from a public health perspective.
A genome-wide association study (GWAS) by Dubé et al.,12 reported a missense variant Asp247Gly in the leukocyte immunoglobulin-like receptor subfamily B member 5 gene, LILRB5 on chromosome 19, base position 54759361 (Human Genome Build GRCh37), was associated with circulating serum CK levels. The mean CK levels of Asp247 homozygotes (T/T) were significantly higher. This association was found to be independent of statin use, however there is no known biological mechanism for the variant in determining CK levels. A GWAS by Kristjansson et al.13 of over 60 000 Icelanders replicated the association of the variant and CK levels. The same study also reported the association of the variant with serum lactate dehydrogenase (LDH) levels in a population of over 90 000 Icelanders.13 The LILRB5 variant showed the same direction of effect, i.e. Asp247 homozygotes had higher LDH and CK levels. LDH is often used in conjunction with CK as a marker of tissue damage. The findings suggest the variant might impart a statin independent susceptibility to muscle-based events. This makes LILRB5 a potential marker for susceptibility to the commonly noted muscle-based symptoms attributed to statin intolerance.
These discoveries warrant an investigation into the role of the LILRB5 variant in statin intolerance. Population-based studies use surrogate markers of intolerance, such as elevations in CK, trends in statin treatment, dose reductions, switching or the discontinuation of therapy. Therefore, a priori, we considered two definitions of statin intolerance, one dependent on and one independent of elevated CK levels. We hypothesize that carriers of variant associated with higher muscle enzyme levels (CK and LDH) will also be predisposed to forms of statin intolerance independent of CK levels.
The principal cohort used was the Genetics of Diabetes Audit and Research, Tayside Scotland (GoDARTS). GoDARTS has been previously used to establish pharmacogenetic associations of genes such as the hepatic influx transporter SLCO1B1 and statin intolerance.14 At present, GoDARTS contains 11 912 statin users and provides approximately 98 000 person-years of statin exposure, providing an ideal cohort to examine the association of this genetic variant with statin intolerance. Replication was examined in the Clinical Practice Research Datalink (CPRD) STAGE study15 and clinically adjudicated cases of statin-induced myopathy (SIM) in the European PREDICTION-ADR consortium study.9 The interaction of this effect with statin use was then studied among participants who developed myalgia in the JUPITER (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) randomized clinical trial (RCT) where individuals were allocated rosuvastatin or placebo to assess the relative reduction in vascular events.16,17
Methods
GoDARTS cohort
Study population
Tayside Medical Ethics Committee approved the GoDARTS study and informed consent was obtained for all participants. The dataset contains complete electronic medical records (EMR), prescription information and laboratory results from 18 306 Scottish Caucasian individuals. Prescribing data were available from 1 January 1990 to 31 July 2013. In all, 10 149 study participants had type 2 diabetes (T2D) at recruitment and the remainder (8157) were recruited as non-diabetic controls. We performed a case-control study for statin intolerance in this population. We found 11 947 statin users from GoDARTS who had at least two prescriptions of statins. The prescription patterns indicating intolerance used in this study are similar to those used by Donnelly et al.14 to establish the association between statin intolerance and SLCO1B1 genotypes in the GoDARTS study. For features used to define statin intolerance and tolerance, see Supplementary Material online, Methods S1.
Statin intolerance in GoDARTS
General statin intolerance
Cases of general statin intolerance (GSI) were defined as users with CK raised above the ULN (Upper Limit of Normal), after start of statin therapy, who either switched statin therapies two or more times (not including systemic shifts to atorvastatin after patent expiration in the UK) or discontinued therapy (n = 588). Controls (statin tolerant individuals—ST1) had over 90% coverage with statin prescriptions, for a minimum of 5 years, at a minimum average daily dose of 40 mg of simvastatin (or equivalent dose of another statin), had consistently normal CKs while on statins, had never switched their statin therapy (except the systematic switch to atorvastatin) and had not discontinued therapy (n = 356).
Lowest approved daily starting dose statin intolerance
Since the LILRB5 Asp247Gly variant was known to be associated with CK levels, a phenotype independent of CK elevations was created in order to determine if the association of the variant with statin intolerance was confounded by the variant‘s association with CK levels. The European Society of Cardiology and European Atherosclerosis Society joint recommendations on the management of dyslipidaemias suggest cessation of statin treatment if the user presents with normal CK, but persistent symptoms of intolerance.18 This intolerance definition was derived from the GAUSS-2 trial, the consensus definition based on recommendations by Banach et al. and recommendations of the National Lipid Association (NLA) in 2014.19–22
Cases of low dose intolerance (LDI) had used two or more different statins, and at least one statin that was discontinued would have to be at the lowest approved daily starting dose (NLA, 2014) before discontinuation, irrespective of their CK levels (n = 591). Controls (statin tolerant individuals—ST2) met all the criteria of the previous statin tolerant (ST1) group, except the definition was independent of the CK elevation criteria (n = 443). A higher proportion of controls had CK levels in the normal range, specifically, 354 of the 443 (80%) controls compared to 335 of 591 (57%) cases. Therefore, association tests for this phenotype were adjusted for (log-transformed) CK levels, in addition to other covariates.
For genotyping methods, see Supplementary material online, Methods S1.
Validation of statin intolerance phenotypes in GoDARTS
These phenotypic definitions of statin intolerance were validated against known SLCO1B1 genotype risk score14 and the outcome of major adverse cardiovascular event (see Supplementary material online, Methods S2). They were significantly associated with both.
Replication studies
CPRD-STAGE study: statin-induced myopathy
Replication was sought from the CPRD-STAGE study.15 Data were available for 129 cases of SIM and 2501 population controls from the Wellcome Trust Case Control Consortium (WTCCC1).15 Cases of statin myopathy were identified using CPRD and tertiary muscle clinics and conformed to SIM classification standards.9 Analysis presented is unadjusted for covariates due lack of available data for the WTCCC population controls. For additional cohort information, see Supplementary material online, Methods S3.
PREDICTION-ADR: statin-induced myopathy
Cases and controls for SIM were contributed by the consortium‘s study centres in Uppsala (Sweden), Dundee and Liverpool (UK). Cases met criteria for classification of SIM.9 Identification of SIM from population cohorts: GoDARTS, Genetics of Scottish Health Registry (GoSHARE)23 and CPRD using EMRs was based on on-statin CK levels raised ≥4 times ULN. Subsequently, clinical adjudication was undertaken by physicians and specialists. Factors considered were resolution of CK after de-challenge, post-event prescribing changes (e.g. switching or total discontinuation), medical history of kidney disease, trauma, falls, myocardial infarction, thyroid disease and tests for HMGCR (3-Hydroxy-3-Methylglutaryl-CoA Reductase) antibodies, muscle biopsy and physical activity, if available. Cases were also identified from CVD clinics, general practitioners (GP) practices, and muscle disease clinics where adjudication was performed directly by physicians. Swedish cases were selected from Swedegene Biobank, which recruits patients reported to the adverse drug reaction registry at Medical Products Agency, Uppsala. Statin tolerant controls were on therapy for a minimum of 1 year with no recorded adverse events. Analysis was performed on 229 cases of SIM and 432 adjudicated controls of Caucasian ethnicity. Whole-exome sequencing was undertaken in laboratories in Liverpool, Dundee and Uppsala. For details of sequencing methods, see Supplementary material online, Methods S4. There were no sample overlaps from GoDARTS in the discovery and replication cohorts.
JUPITER trial: myalgia
The replication in JUPITER focused on 8749 study participants of verified European ancestry with available genetic data among whom 4381 were randomized to receive statin treatment and 4368 were randomized to placebo. The population demographics of the genotyped sub-population of the JUPITER trial has been previously described.16 The median follow-up period in the trial was 1.9 years, during which traits such as CK, therapy compliance, and myalgia were recorded.17 Myalgia was ascertained by physicians blinded to trial-allocation arm24 and 837 participants in the study sample were recorded as developing myalgia. Due to possible association between CK measures and diagnosis of myalgia, log-transformed final CK levels were included in analyses as a potential confounder. For genotyping methods, see Supplementary material online, Methods S1.
Statistical analyses
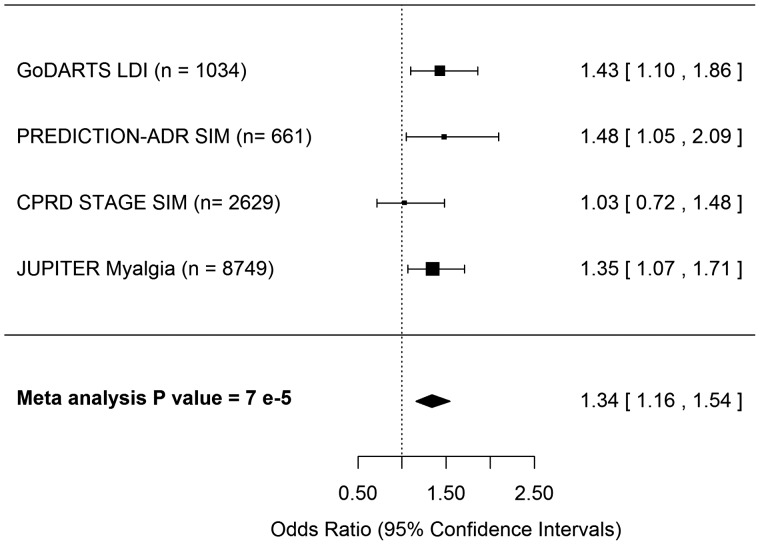
All statistical analyses on GoDARTS, CPRD-STAGE, and PREDICTION-ADR data were performed in SAS 9.3 (SAS Institute, Cary, NC, USA). Statistical analyses in JUPITER were performed using R.25 Binary logistic regression was used to test the association between the variant and each phenotype of intolerance. Covariates associated with intolerance such as gender, age, co-medication usage, type of statin, dose of statin, and CK levels were added to models where appropriate and available. A backwards step-wise approach was used to eliminate covariates that were not significant predictors in adjusted models. Finally, a fixed-effects meta-analysis on results from the discovery and replication cohorts was performed. Only one phenotype from GoDARTS could be selected since the two groups contained overlapping individuals. LDI phenotype was selected as the phenotype definition did not include the CK levels and the model was adjusted for CK measures. The analysis was performed using the metafor package in R26 and results are presented in a Forest plot (Figure 1).
Figure 1.
Forest plot representing meta-analysis of the association between LILRB5 Asp247Gly and outcomes observed across GoDARTS, CPRD-STAGE, PREDICTION-ADR, and JUPITER studies. Study sample size is in parentheses. LDI, low-dose intolerance; SIM, statin-induced myopathy.
The minor allele frequency in GoDARTS, CPRD, PREDICTION-ADR, and JUPITER study populations were 0.37, 0.39, 0.37, and 0.40, respectively. The effect of the LILRB5 variant was considered dominant based on large-scale analyses with serum CK in GoDARTS (see Supplementary material online, Results S1). Therefore all association tests compared those homozygous for Asp247 (T/T) with carriers of 247GlyX (T/C or C/C).
Results
Baseline characteristics of general statin intolerance and low dose intolerance
Covariates associated with statin use or with the development of adverse drug reactions (ADR) were tested. Specifically, mean age at start and end of statin therapy, sex, diagnosis of T2D, first and last statin used, starting and ending doses, use of interacting co-medications, statin use for the secondary prevention of CVD, CK levels, and LDL levels prior to statin use. The Comparison of GSI with statin tolerance (ST1) and of LDI with statin tolerance (ST2) is presented (Table 1).
Table 1.
Contrasting general statin intolerance with raised CK and statin tolerance (ST1) and low dose intolerance with statin tolerance (ST2)
| Variables | GSI |
P-value | LDI |
P-value | ||
|---|---|---|---|---|---|---|
| Cases (n = 588) | Controls (n = 356) | Cases (n = 591) | Controls (n = 443) | |||
| Mean age start therapy (SD) | 60 (10) | 62 (10) | 0.005 | 60 (10) | 60 (10) | 0.9 |
| Years on statin therapy (SD) | 10.4 (5) | 9.3 (3) | <0.0001 | 10 (5) | 9.5 (3) | 0.007 |
| Sex (% females) | 50 | 43 | 0.007 | 48 | 46 | 0.16 |
| Type 2 diabetics (%) | 78 | 79 | 0.68 | 92 | 90 | 0.33 |
| First statin as simvastatin (%) | 64 | 71 | <0.0001 | 59 | 65 | <0.0001 |
| Last statin as simvastatin (%) | 31 | 41 | <0.0001 | 31 | 36 | <0.0001 |
| Starting dose as ‘low’ (<20 mg/day) (%) | 85 | 53 | <0.0001 | 94 | 37 | <0.0001 |
| Ending dose as ‘high’ (≥80 mg/day) (%) | 22 | 36 | <0.0001 | 23 | 50 | <0.0001 |
| Interacting co-medications (yes %) | 52 | 44 | 0.0025 | 51% | 42% | <0.0001 |
| Statin use for secondary prevention of CVD (%) | 27 | 23 | 0.18 | 28% | 25% | 0.3 |
| CK levels (IU/L)a | ||||||
| Median | 200 | 76 | <0.0001 | 98 | 85 | <0.0001 |
| Mean (minimum, maximum) | 306 (120, 12, 700) | 81 (17, 179) | 170 (13, 12 735) | 107 (19, 1369) | ||
| LDL levels (mmol/L)a | ||||||
| Median | 3.5 | 3.2 | 0.07 | 3.2 | 3.1 | 0.38 |
| Mean (minimum, maximum) | (3.5) (1.1, 5.5) | 3.2 (0.4, 8.7) | 3.1 (1.1, 6.4) | 3.2 (0.5, 8.7) | ||
Indicates associations were tested using log 10 transformed values.
SD, standard deviation; GSI, general statin intolerance; LDI, Low dose intolerance.
Covariates associated with GSI included younger age at start and longer duration of therapy, female gender, lower incidence of simvastatin use at the start and end of therapy, lower starting and ending dose, the use of interacting co-medications and, by phenotype definition, CK levels at time of diagnosis.
Covariates associated with LDI included longer duration of statin therapy, lower frequency of simvastatin use at the start and end of therapy, lower starting and ending dose, the use of interacting co-medications and CK levels. A concern with a phenotype that is dose dependent is that differences might arise from LDL cholesterol reduction required between tolerant and intolerant individuals, however LDL levels prior to statin therapy were not significantly different across the groups.
Association between statin intolerance and the LILRB5 Asp247Gly variant in GODARTS
The variant effect was observed in both unadjusted and adjusted models for both phenotypes (Table 2). Individuals homozygous for Asp247 had 1.96 [(95% confidence interval (CI): 1.25–3.07] times the odds of having GSI compared with carriers of the 247Gly variant, in a model adjusted for first statin on therapy, dose, age, sex, and concurrent use of interacting medications (Table 2). For LDI, individuals homozygous for LILRB5 Asp247 genotype had 1.43 (95% CI: 1.10–1.86) times the odds of being intolerant compared to carriers of the 247Gly variant, in a model adjusted for the first and last statin on therapy, CK levels and concurrent use of interacting medications (Table 2).
Table 2.
Association of phenotypes of statin intolerance with LILRB5 Asp247Gly
| Statin intolerance phenotype description |
LILRB5 |
|||
|---|---|---|---|---|
| Asp247Gly (T/C) | ||||
| Unadjusted model |
Adjusted model |
|||
| Asp247Asp vs. Gly247X: OR (95% CI) | P-value | Asp247Asp vs. Gly247X: OR (95% CI) | P-value | |
| GSI | 1.62 (1.24, 2.12) | 4 × 10−4 | 1.96 (1.25–3.07) | 3 × 10−3 |
| LDI | 1.36 (1.07, 1.73) | 0.013 | 1.43 (1.10–1.86) | 7 × 10−3 |
Odds of intolerance for those homozygous for the ancestral allele (Asp247: T/T) are being contrasted to carriers of the minor allele (Gly247X: T/C or C/C) first in main effects or unadjusted models and later in models adjusted for significant covariates. For GSI, the covariates were first statin on therapy, dose of the statin, age, sex and concurrent use of interacting medications. For LDI, the covariates were the first and last statin while on therapy, CK levels and concurrent use of interacting medications.
GSI, general statin intolerance; LDI, Low dose intolerance; CK, creatine phosphokinase; OR, odds ratio; CI, confidence interval.
Results of replication studies
Replication of the genotypic effect was noted in two out of three studies of statin-related adverse outcomes. In the CPRD-STAGE study, the association was found to be non-significant [odds ratio (OR) 1.03, 95% CI: 0.72–1.49] in a main effects model. However, in the PREDICTION-ADR cohort of SIM, individuals homozygous for LILRB5 Asp247 had higher odds of developing SIM (OR 1.48, 95% CI: 1.02–2.10) compared to those carrying the 247Gly variant (Table 3). Cases and controls were matched for sex, type of statin at time of event during recruitment. The analysis was adjusted for study centre.
Table 3.
Distribution of LILRB5 Asp247Gly genotypes by clinically adjudicated SIM status
| Group | Asp247Asp (T/T) | Gly247X (T/C or C/C) | Total | Association |
|---|---|---|---|---|
| Statin-induced myopathy | 96 | 133 | 229 | Odds ratio = 1.48 |
| Statin tolerant controls | 161 | 271 | 432 | 95% CI: 1.05–2.10 |
| Total | 257 | 404 | 661 | P-value 0.025 |
Cases and controls were selected from PREDICTION-ADR in Dundee, Liverpool and Uppsala. Model was adjusted for study center.
CI, confidence interval.
In the JUPITER trial, LILRB5 Asp247Gly had a statistically significant effect on development of myalgia. In a model adjusted for final CK levels and treatment allocation arm, individuals homozygous for Asp247 had 1.35 (95% CI: 1.07–1.71) times the odds of developing myalgia compared to 247Gly variant carriers (Table 4). Interestingly, interaction between genotype and statin use was significant (P-value 0.04). An analysis of effect stratified by genotype (Table 5), showed that a statin-specific myalgia effect is only seen among 247Gly variant carriers. Therefore, while Asp247 homozygotes have an overall higher risk of myalgia, statin-induced myalgia is only observed in 247Gly variant carriers. Similar results are observed in survival analysis (see Supplementary material online, Results S2).
Table 4.
Odds ratios of developing myalgia in the JUPITER trial
| Variables | β | Odds ratio 95% confidence interval | P-value |
|---|---|---|---|
| Main effects model | |||
| Asp247Asp vs. 247GlyX Gly247X | 0.16 | 1.17 (1.02–1.35) | 0.03 |
| Adjusted model | |||
| Asp247Asp vs. 247GlyX | 0.30 | 1.35 (1.07–1.71) | 0.01 |
| Rosuvastatin vs. placebo | −0.13 | 0.88 (0.68–1.13) | 0.34 |
| Final CK measure (log transformed) | 0.19 | 1.21 (0.87–1.69) | 0.26 |
| Asp247Gly*rosuvastatin | −0.34 | 0.71 (0.51–0.99) | 0.04 |
The adjusted model accounts for treatment allocation arm and final CK measures.
CK, creatine phosphokinase.
Table 5.
Association of rosuvastatin treatment with myalgia stratified by genotype in the JUPITER trial
| Comparison groups | Hazards ratio (95% CI) | P-value |
|---|---|---|
| Asp247 (T/T) | ||
| Rosuvastatin vs. placebo | 0.87 (0.67–1.12) | 0.31 |
| 247GlyX (C/T or C/C) | ||
| Rosuvastatin vs. placebo | 1.23 (1.01–1.50) | 0.04 |
The model was adjusted for log-transformed final CK measures.
CK, creatine phosphokinase; CI, confidence interval.
Meta-analysis of results
A meta-analysis of the observed effects across GoDARTS, CPRD-STAGE, PREDICTION-ADR and JUPITER revealed that overall, Asp247 homozygotes have 1.34 (95% CI: 1.16–1.54) times the odds of having outcomes associated with statin intolerance compared with carriers of the 247Gly variant (P-value 7 × 10−5) (Figure 1).
Discussion
We report that the LILRB5 Asp247 homozygous genotype is associated with increased risk of outcomes associated with statin intolerance across observational, clinically adjudicated and clinical trial datasets. We observe a consistent relationship of increased risk of statin intolerance and the LILRB5 Asp247 genotype using two definitions of intolerance in the GoDARTS population. There was congruent association with adjudicated cases and controls of SIM in the PREDICTION-ADR study and with myalgia in the JUPITER trial, but no significant association in the CPRD-STAGE study.
A possible mechanism for the role of LILRB5 in muscle pathology is suggested by a recent study by Kuswanto et al.27 who highlight a role for the immune system in the repair and regeneration of skeletal muscles. They report that the presence and rapid accumulation of T regulatory (Treg) cells is crucial in the repair of damaged skeletal muscles. It is also reported that statins increase both the number and suppressive function of CD4+ Foxp3+ Treg cells28; Foxp3 is a transcription factor that is the master regulator of Treg immune-suppressive activity.29 The same study shows sustained increased expression of Foxp3 with statin use. Therefore, mechanisms that induce Foxp3 expression and sustain Treg function are of great interest in understanding muscle homeostasis. We examined mRNA expression data from the GTEx portal and found that the LILRB5 Asp247 variant is associated with FOXP3 mRNA expression in the spleen (see Supplementary material online, Figure S6).30,31FOXP3 lies on the X chromosome, indicating a trans-eQTL effect, with the LILRB5 variant having an indirect immunomodulatory effect on FOXP3 expression. These findings underscore a role for immunogenetics in understanding muscle damage and repair, with LILRB5 Asp247Gly being the first candidate to be found for common statin intolerance. An illustration of the potential mechanism is provided in Supplementary material online, Figure S7.
Previously proposed mechanisms for SIM include an association between statins and reduced mitochondrial function, attenuated energy production, and altered muscle protein degradation contributing to muscle symptoms.32,33 Studies have shown evidence of structural perturbations in skeletal muscle cells associated with statin use.34,35 However, other studies failed to observe such abnormalities.36 There are other known immune-mediated forms of statin-related myopathies such as idiopathic inflammatory myositis or immune-mediated necrotizing myopathy, however these are very rarely observed.37,38 Overall, however, there remains a lack of clarity about the underlying pathophysiology of statin intolerance.18
While the current variant results in an amino acid substitution, it is not clear that the phenotype results from this protein change. This is especially true as this variant is also associated with LILRB5 expression in certain tissues in the GTEx portal database (see Supplementary material online, Figure S8).30,31 We used GTEx to identify the strongest cis-eQTLs for LILRB5 expression, and found SNPs rs1408812 and rs3852892 showed much stronger effects in skeletal muscles and whole blood, respectively (see Supplementary material online, Figures S9 and S10). However, these polymorphisms have no known associations with CK, LDH or statin intolerance and were not in linkage with the Asp247Gly variant. This demonstrates that the observed phenotypes are not associated with genetically driven variation in LILRB5 expression and therefore the association with the Asp247Gly variant would appear to be mediated by a functional change in the protein due to the amino acid substitution. However, this has to be confirmed by direct experimentation.
Previous studies have shown that CK levels were associated with LILRB5 variant12,13 and with all definitions of intolerance, including diagnoses of myalgia, making it a potential confounder. We have ruled out any artefactual associations by including CK measures as a covariate in analyses, where appropriate. Since the GSI phenotype in GoDARTS and diagnoses of myopathy in CPRD-STAGE and PREDICTION-ADR were entirely dependent on elevated CK, this adjustment was not possible. Additionally, the findings of this study could be impacted by confounders that were unmeasured in the cohorts used. Indeed, the non-significant finding in CPRD-STAGE might be due to the use of population controls with no available clinical or demographic information, limiting inclusion of important covariates in the analysis.
Another source of bias in non-randomized studies could be drug interactions that are known to increase risk of SIM.39 Inhibition of cytochrome P450 (CYP) 3A4 can increase exposure to simvastatin and atorvastatin several fold. On the other hand, inhibition of hepatic influx transporter OATP1B1 by drugs such as gemfibrozil can increase plasma concentrations of all statins.40 Due to the increased risk of muscle toxicity with combination therapy,41 fibrates are not generally recommended for concurrent use with statins for primary lipid control,42 but are co-prescribed to patients with T2D to control triglyceride levels. Since GoDARTS is primarily a population of T2 Diabetics, fibrates are widely prescribed in the study population. We observe comedications, especially fibrates consistently increased the risk of statin intolerance. However, in adjusted models, the association of the LILRB5 Asp247Gly variant was independent of the effect of these co-medications. Additionally, the effect is observed in the RCT setting, which confirms that the results from our observational data are not biased.
This finding, in addition to other previously reported genetic associations can facilitate the development of gene risk scores for the prevention of adverse outcomes to statin therapy. Preliminary analyses in GoDARTS reveal a significant interaction between the known SLCO1B1 polymorphisms and LILRB5 Asp247Gly (see Supplementary material online, Results S4). This would suggest that we are making progress in dissecting 3the complex genetic architecture of statin intolerance, but future studies would be required to examine the corresponding pharmacokinetics of specific statins such as rosuvastatin that are not affected by SLCO1B1.
The JUPITER trial allowed us to examine the association of the LILRB5 Asp247Gly variant in the presence and absence of statins, revealing that homozygosity of Asp247 is associated with increased odds of myalgia regardless of statin allocation. This supports the concept that LILRB5 Asp247 homozygous genotype modulates CK and LDH levels through statin-independent muscle damage. The association of 247Gly carriers with the development of myalgia in JUPITER is suggestive of a more complex biological gene–drug interaction. The observation that 247Gly carriers showed statin-specific myalgia, suggests a subpopulation of individuals who are inherently protected from myalgia, are susceptible to true ‘statin-induced’ myalgia. In observational data such as GoDARTS it is impossible to determine if intolerance to statins is occurring due to statin-specific or non-specific side effects. However, occurrence of intolerance is associated with increased risk of adverse CV outcomes (see Supplementary material online, Methods S2). We believe a recruit-by-genotype trial would be the ideal platform to examine the statin-dependency by LILRB5 Asp247Gly genotypes, and to further explore the underlying immune mechanisms.
Clinical trials have consistently found no evidence of statin-specific myalgia, just as there is no difference in incidence of myalgia between placebo and rosuvastatin arms of the JUPITER trial.17,24 The lack of association of statins with muscle pain in RCTs has led to a debate regarding the existence of statin-related muscle symptoms. Indeed the difficulty of ascribing causality in statin related muscle symptoms is highlighted by the Goal Achievement after Utilizing an anti-PCSK9 Antibody in Statin-Intolerant Subjects -3 (GAUSS-3) trial.43 Data from this trial were the first systematic evaluation of statin-specific myalgia with rechallenge and provided an estimate of 43% of individuals having statin-specific myalgia, and also demonstrated that 37% of intolerant individuals have statin-independent or non-specific myalgia. The LILRB5 Asp247Gly genotype presents a unique opportunity to probe the immune mechanisms behind the phenomena of muscle pain specific to statins compared to ‘constitutive’ muscle pain that appears in LILRB5 Asp247 homozygotes.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
We are grateful to all the participants in this study, the GoDARTS and SHARE team for their help in recruiting the participants, and to the whole team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. The study complies with the Declaration of Helsinki. We acknowledge the support of the Health Informatics Centre, University of Dundee for managing and supplying the anonymised data and NHS Tayside, the original data owner. M.P. and A.A. also thank the MRC Centre for Drug Safety Science for infrastructure support.
Funding
European Community‘s Seventh Framework Programme (FP7/2007-2013) [Grant no. 602108] through the PREDICTION-ADR project to M.K.S., C.M., D.C., K.B., M.P., M.W., A.A., A.H.M., and C.N.A.P. PREDICTION-ADR cohort used individuals from the SHARE Bioresource (GoSHARE) and SHARE has ongoing funding from NHS Research Scotland and established by funding from The Wellcome Trust Biomedical Resource [Grant No. 099177/Z/12/Z]. The GoDARTS study was funded by The Wellcome Trust (Award 072960 and 084726) and the UK Medical Research Council (Award G0601261). Genetic analysis in the JUPITER trial was supported by a research grant from AstraZeneca jointly to D.I.C. and P.M.R. Recruitment of Swedish patients was supported by the Swedish Research Council (Medicine 521-2011-2440 and 521-2014-3370), Swedish Heart and Lung Foundation (20120557 and 20140291), the Medical Products Agency, Selander‘s Foundation, Thureus’ Foundation, and the Clinical Research Support ‘Avtal om Läkarutbildning och Forskning’ at Uppsala University, Sweden. CPRD-STAGE was funded by a grant from the e-Health Initiative funded jointly by the Medical Research Council (reference: MC_qA137929), the Wellcome Trust, the Engineering and Physical Sciences Research Council, and The Economic and Social Research Council. M.P. is a National Institute for Health Research senior investigator.
Declaration of Helsinki: The studies in this article comply with the Declaration of Helsinki, and locally appointed ethics committee have approved respective research protocols and informed consent has been obtained from the subjects.
Conflict of interest: none declared.
References
- 1. Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, Dallongeville J, De Backer G, Ebrahim S, Gjelsvik B, Herrmann-Lingen C, Hoes A, Humphries S, Knapton M, Perk J, Priori SG, Pyorala K, Reiner Z, Ruilope L, Sans-Menendez S, Scholte Op Reimer W, Weissberg P, Wood D, Yarnell J, Zamorano JL, Walma E, Fitzgerald T, Cooney MT, Dudina A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Funck-Brentano C, Filippatos G, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Hellemans I, Altiner A, Bonora E, Durrington PN, Fagard R, Giampaoli S, Hemingway H, Hakansson J, Kjeldsen SE, Larsen ML, Mancia G, Manolis AJ, Orth-Gomer K, Pedersen T, Rayner M, Ryden L, Sammut M, Schneiderman N, Stalenhoef AF, Tokgozoglu L, Wiklund O, Zampelas A; European Society of Cardiology (ESC) Committee for Practice Guidelines (CPG) . European guidelines on cardiovascular disease prevention in clinical practice: executive summary: Fourth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by representatives of nine societies and by invited experts). Eur Heart J 2007;28:2375–2414. [DOI] [PubMed] [Google Scholar]
- 2. Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R.. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90, 056 participants in 14 randomised trials of statins. Lancet 2005;366:1267–1278. [DOI] [PubMed] [Google Scholar]
- 3. ÓNeill P, Sussex J.. International Comparison of Medicines Usage: Quantitative Analysis. London: Office of Health Economics; 2014. [Google Scholar]
- 4. Sathasivam S. Statin induced myotoxicity. Eur J Intern Med 2012;23:317–324. [DOI] [PubMed] [Google Scholar]
- 5. Law M, Rudnicka AR.. Statin safety: a systematic review. Am J Cardiol 2006;97:52C–60C. [DOI] [PubMed] [Google Scholar]
- 6. Gregoor PJHS. Atorvastatin may cause nightmares. BMJ 2006;332:950.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lansberg PJ, Mitchel YB, Shapiro D, Kastelein JJP, Altman R, Jerums G, Bolzano K, Giannini S, Davignon J, Dewailly P, Darioli R, Mancini M, Scott R, Hayden MR.. Long-term efficacy and tolerability of simvastatin in a large cohort of elderly hypercholesterolemic patients. Atherosclerosis 1995;116:153–162. [DOI] [PubMed] [Google Scholar]
- 8. Dam M. V, Zwart M, Beer F. D, Smelt AHM, Prins MH, Trip MD, Havekes LM, Lansberg PJ, Kastelein JJP.. Long term efficacy and safety of atorvastatin in the treatment of severe type III and combined dyslipidaemia. Heart 2002;88:234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alfirevic A, Neely D, Armitage J, Chinoy H, Cooper RG, Laaksonen R, Carr DF, Bloch KM, Fahy J, Hanson A, Yue QY, Wadelius M, Maitland-van Der Zee AH, Voora D, Psaty BM, Palmer CN, Pirmohamed M.. Phenotype standardization for statin-induced myotoxicity. Clin Pharmacol Ther 2014;96:470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maningat P, Gordon BR, Breslow JL.. How do we improve patient compliance and adherence to long-term statin therapy? Curr Atheroscler Rep 2013;15:291.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rannanheimo PK, Tiittanen P, Hartikainen J, Helin-Salmivaara A, Huupponen R, Vahtera J, Korhonen MJ.. Impact of statin adherence on cardiovascular morbidity and all-cause mortality in the primary prevention of cardiovascular disease: a population-based cohort study in Finland. Value Health 2015;18:896–905. [DOI] [PubMed] [Google Scholar]
- 12. Dube MP, Zetler R, Barhdadi A, Brown AM, Mongrain I, Normand V, Laplante N, Asselin G, Zada YF, Provost S, Bergeron J, Kouz S, Dufour R, Diaz A, Denus S. D, Turgeon J, Rheaume E, Phillips MS, Tardif JC.. CKM and LILRB5 are associated with serum levels of creatine kinase. Circ Cardiovasc Genet 2014;7:880–886. [DOI] [PubMed] [Google Scholar]
- 13. Kristjansson RP, Oddsson A, Helgason H, Sveinbjornsson G, Arnadottir GA, Jensson BO, Jonasdottir A, Jonasdottir A, Bragi Walters G, Sulem G, Oskarsdottir A, Benonisdottir S, Davidsson OB, Masson G, Th Magnusson O, Holm H, Sigurdardottir O, Jonsdottir I, Eyjolfsson GI, Olafsson I, Gudbjartsson DF, Thorsteinsdottir U, Sulem P, Stefansson K.. Common and rare variants associating with serum levels of creatine kinase and lactate dehydrogenase. Nat Commun 2016;7:10572.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Donnelly LA, Doney AS, Tavendale R, Lang CC, Pearson ER, Colhoun HM, McCarthy MI, Hattersley AT, Morris AD, Palmer CN.. Common nonsynonymous substitutions in SLCO1B1 predispose to statin intolerance in routinely treated individuals with type 2 diabetes: a go-DARTS study. Clin Pharmacol Ther 2011;89:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O’Meara H, Carr DF, Evely J, Hobbs M, McCann G, Staa T, van, Pirmohamed M.. Electronic health records for biological sample collection: feasibility study of statin-induced myopathy using the clinical practice research datalink. Br J Clin Pharmacol 2014;77:831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chasman DI, Giulianini F, MacFadyen J, Barratt BJ, Nyberg F, Ridker PM.. Genetic determinants of statin-induced low-density lipoprotein cholesterol reduction: the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial. Circ Cardiovasc Genet 2012;5:257–264. [DOI] [PubMed] [Google Scholar]
- 17. Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM, Kastelein JJP, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ.. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 18. European Association for Cardiovascular Prevention & Rehabilitation, Reiner Z, Catapano AL, Backer G, De Graham I, Taskinen M-R, Wiklund O, Agewall S, Alegria E, Chapman MJ, Durrington P, Erdine S, Halcox J, Hobbs R, Kjekshus J, Filardi PP, Riccardi G, Storey RF, Wood D; ESC Committee for Practice Guidelines (CPG) 2008-2010 and 2010-2012 Committees. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J 2011;32:1769–1818. [DOI] [PubMed] [Google Scholar]
- 19. Stroes E, Colquhoun D, Sullivan D, Civeira F, Rosenson RS, Watts GF, Bruckert E, Cho L, Dent R, Knusel B, Xue A, Scott R, Wasserman SM, Rocco M.. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol 2014;63:2541–2548. [DOI] [PubMed] [Google Scholar]
- 20. Cho L, Rocco M, Colquhoun D, Sullivan D, Rosenson RS, Dent R, Xue A, Scott R, Wasserman SM, Stroes E.. Design and rationale of the GAUSS-2 study trial: a double-blind, ezetimibe-controlled phase 3 study of the efficacy and tolerability of evolocumab (AMG 145) in subjects with hypercholesterolemia who are intolerant of statin therapy. Clin Cardiol 2014;37:131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Banach M, Rizzo M, Toth PP, Farnier M, Davidson MH, Al-Rasadi K, Aronow WS, Athyros V, Djuric DM, Ezhov MV, Greenfield RS, Hovingh GK, Kostner K, Serban C, Lighezan D, Fras Z, Moriarty PM, Muntner P, Goudev A, Ceska R, Nicholls SJ, Broncel M, Nikolic D, Pella D, Puri R, Rysz J, Wong ND, Bajnok L, Jones SR, Ray KK, Mikhailidis DP.. Statin intolerance—an attempt at a unified definition. Position paper from an International Lipid Expert Panel. Arch Med Sci 2015;11:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jacobson TA. NLA task force on statin safety–2014 update. J Clin Lipidol 2014;8:S1–S4. [DOI] [PubMed] [Google Scholar]
- 23. McKinstry B, Sullivan FM, Vasishta S, Armstrong R, Hanley J, Haughney J, Philip S, Smith BH, Wood A, Palmer CNA.. Cohort profile: the Scottish Research register SHARE. A register of people interested in research participation linked to NHS data sets. BMJ Open 2017;7:e013351.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hsia J, MacFadyen JG, Monyak J, Ridker PM.. Cardiovascular event reduction and adverse events among subjects attaining low-density lipoprotein cholesterol <50 mg/dl with rosuvastatin: the JUPITER trial (justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin). J Am Coll Cardiol 2011;57:1666–1675. [DOI] [PubMed] [Google Scholar]
- 25. R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 26. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010;36:1–48. [Google Scholar]
- 27. Kuswanto W, Burzyn D, Panduro M, Wang KK, Jang YC, Wagers AJ, Benoist C, Mathis D.. Poor repair of skeletal muscle in aging mice reflects a defect in local, interleukin-33-dependent accumulation of regulatory T cells. Immunity 2016;44:355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rodriguez-Perea AL, Montoya CJ, Olek S, Chougnet CA, Velilla PA.. Statins increase the frequency of circulating CD4+ FOXP3+ regulatory T cells in healthy individuals. J Immunol Res 2015;2015:762506.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fontenot JD, Gavin M. A, Rudensky AY.. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003;4:330–336. [DOI] [PubMed] [Google Scholar]
- 30. Carithers LJ, Moore HM.. The Genotype-Tissue Expression (GTEx) project. Biopreserv Biobank 2015;13:307–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science 2015;348:648–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mikus CR, Boyle LJ, Borengasser SJ, Oberlin DJ, Naples SP, Fletcher J, Meers GM, Ruebel M, Laughlin MH, Dellsperger KC, Fadel PJ, Thyfault JP.. Simvastatin impairs exercise training adaptations. J Am Coll Cardiol 2013;62:709–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, Roden M, Stein E, Tokgozoglu L, Nordestgaard BG, Bruckert E, Backer GD, Krauss RM, Laufs U, Santos RD, Hegele RA, Hovingh GK, Leiter LA, Mach F, Marz W, Newman CB, Wiklund O, Jacobson TA, Catapano AL, Chapman MJ, Ginsberg HN.. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J 2015;36:1012–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Draeger A, Monastyrskaya K, Mohaupt M, Hoppeler H, Savolainen H, Allemann C, Babiychuk EB.. Statin therapy induces ultrastructural damage in skeletal muscle in patients without myalgia. J Pathol 2006;210:94–102. [DOI] [PubMed] [Google Scholar]
- 35. Mohaupt MG, Karas RH, Babiychuk EB, Sanchez-Freire V, Monastyrskaya K, Iyer L, Hoppeler H, Breil F, Draeger A.. Association between statin-associated myopathy and skeletal muscle damage. CMAJ 2009;181:E11–E18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lamperti C, Naini AB, Lucchini V, Prelle A, Bresolin N, Moggio M, Sciacco M, Kaufmann P, DiMauro S.. Muscle coenzyme q10 level in statin-related myopathy. Arch Neurol 2005;62:1709–1712. [DOI] [PubMed] [Google Scholar]
- 37. Limaye V, Bundell C, Hollingsworth P, Rojana-Udomsart A, Mastaglia F, Blumbergs P, Lester S.. Clinical and genetic associations of autoantibodies to 3-hydroxy-3-methyl-glutaryl-coenzyme a reductase in patients with immune-mediated myositis and necrotizing myopathy. Muscle Nerve 2015;52:196–203. [DOI] [PubMed] [Google Scholar]
- 38. Bosch X, Poch E, Grau JM.. Rhabdomyolysis and acute kidney injury. N Engl J Med 2009;361:62–72. [DOI] [PubMed] [Google Scholar]
- 39. Neuvonen PJ, Niemi M, Backman JT.. Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther 2006;80:565–581. [DOI] [PubMed] [Google Scholar]
- 40. Tornio A, Neuvonen PJ, Niemi M, Backman JT.. Role of gemfibrozil as an inhibitor of CYP2C8 and membrane transporters. Expert Opin Drug Metab Toxicol 2017;13:83–95. [DOI] [PubMed] [Google Scholar]
- 41. Amend KL, Landon J, Thyagarajan V, Niemcryk S, McAfee A.. Incidence of hospitalized rhabdomyolysis with statin and fibrate use in an insured US population. Ann Pharmacother 2011;45:1230–1239. [DOI] [PubMed] [Google Scholar]
- 42. Joint Formulary Committee. British National Formulary. London: BMJ Gr. Pharm. Press; 2014. http://www.medicinescomplete.com (25 November 2015). [Google Scholar]
- 43. Nissen SE, Stroes E, Dent-Acosta RE, Rosenson RS, Lehman SJ, Sattar N, Preiss D, Bruckert E, Ceska R, Lepor N, Ballantyne CM, Gouni-Berthold I, Elliott M, Brennan DM, Wasserman SM, Somaratne R, Scott R, Stein EA.. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA 2016;315:1580–1590. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.