Translational Perspective
Congenital anomalies of arterial valves and valvar aortic stenosis are a common, serious birth defect without effective medicine. We have developed animal models presenting localized congenital anomalies of arterial valves and valvar aortic stenosis similar to that of humans. Our study with these models reveals a key molecular signalling between valvar endothelium and interstitial tissue that underlies the aetiology of congenital anomalies of arterial valves and subsequent valvar aortic stenosis. Our results suggest a potential therapy for preventing the stenotic progression of congenital anomalies of arterial valves.
Keywords: Congenital anomalies of arterial valves, Valvar aortic stenosis, Notch, Tnf
Abstract
Aims
Congenital anomalies of arterial valves are common birth defects, leading to valvar stenosis. With no pharmaceutical treatment that can prevent the disease progression, prosthetic replacement is the only choice of treatment, incurring considerable morbidity and mortality. Animal models presenting localized anomalies and stenosis of congenital arterial valves similar to that of humans are critically needed research tools to uncover developmental molecular mechanisms underlying this devastating human condition.
Methods and results
We generated and characterized mouse models with conditionally altered Notch signalling in endothelial or interstitial cells of developing valves. Mice with inactivation of Notch1 signalling in valvar endothelial cells (VEC) developed congenital anomalies of arterial valves including bicuspid aortic valves and valvar stenosis. Notch1 signalling in VEC was required for repressing proliferation and activating apoptosis of valvar interstitial cells (VIC) after endocardial-to-mesenchymal transformation (EMT). We showed that Notch signalling regulated Tnfα expression in vivo, and Tnf signalling was necessary for apoptosis of VIC and post-EMT development of arterial valves. Furthermore, activation or inhibition of Notch signalling in cultured pig aortic VEC-promoted or suppressed apoptosis of VIC, respectively.
Conclusion
We have now met the need of critical animal models and shown that Notch-Tnf signalling balances proliferation and apoptosis for post-EMT development of arterial valves. Our results suggest that mutations in its components may lead to congenital anomaly of aortic valves and valvar stenosis in humans.
Translational perspective.
Congenital anomalies of arterial valves and valvar aortic stenosis are a common, serious birth defect without effective medicine. We have developed animal models presenting localized congenital anomalies of arterial valves and valvar aortic stenosis similar to that of humans. Our study with these models reveals a key molecular signalling between valvar endothelium and interstitial tissue that underlies the aetiology of congenital anomalies of arterial valves and subsequent valvar aortic stenosis. Our results suggest a potential therapy for preventing the stenotic progression of congenital anomalies of arterial valves.
Introduction
Congenital anomalies of arterial valves are common birth defects and often progress to degenerative valvar stenosis with significant mortality and morbidity.1 Developing arterial valves originate from the formation of endocardial cushions at the cardiac outflow tract through a process of endocardial-to-mesenchymal transformation (EMT), in which valvar endothelial cells (VEC) delaminate and invade the matrix-enriched endocardial cushions and become the precursors to valvar interstitial cells (VIC).2 Once cellularized, endocardial cushions elongate and become thin leaflets, conceivably through a balanced cell proliferation and apoptosis.3,4 Previous studies have discovered key molecular signalling pathways regulating EMT and endocardial cushion formation.5 In contrast, molecular mechanisms underlying post-EMT development of valves are largely unknown.
Notch signalling plays a critical role in cell proliferation and apoptosis.6 Binding of Notch ligands to Notch receptors initiates sequential cleavage of Notch receptors and releases their intracellular domain (NICD), which shuttles to the nucleus to activate gene transcription through its nuclear partner Rbpj. In humans, mutations in NOTCH1 are associated with bicuspid aortic valves (BAV) and stenosis.7 In mice, Notch1 is predominately expressed in the cardiac endocardium and vascular endothelium during embryogenesis,8,9 and it plays critical roles in the formation of endocardial cushions8 and cardiac chambers,9 and vasculogenesis.10 We have yet to understand how Notch1 signalling participates in post-EMT development of valves, which is needed to explain human BAV and stenosis due to NOTCH1 mutations.
In this study, we addressed this gap by employing valvar tissue-specific Cre mice to alter Notch1 signalling in VEC or VIC during post-EMT development of valves. This approach allowed us to identify a previously unknown Notch1-Tnf signalling axis that regulates post-EMT development of arterial valves. This finding suggests that genetic and epigenetic alterations in this axis may contribute to the pathogenesis of congenital anomalies of arterial valves and valvar aortic stenosis in humans.
Methods
Detailed methods are included in Supplementary material online.
Mouse experiments were performed according to the guidelines of the National Institute of Health and the protocols approved by the Institutional Animal Care and Use Committee of Albert Einstein College of Medicine and Cincinnati Children's Medical Center. Noontime on the day of detecting vaginal plugs was designated as embryonic day (E) 0.5. Echocardiography was performed to measure cardiac or aortic valve function. Movat's Pentachrome, Sirius Red, Von Kossa, X-gal, antibody staining, and RNA in situ hybridization were carried out using standard protocols. Quantitative polymerase chain reaction (qPCR) analyses were performed on cDNA generated from total RNA extracted from the arterial valves of E14.5 hearts or cultured pig aortic VEC and VIC with gene-specific primers (Supplementary material online, Tables S1 and S2).
Student's t-test or one-way analysis of variance with Tukey's post-test was used for statistical analysis. All data were presented as mean ± standard deviation, and P-value of <0.05 was considered as significant.
Results
Attenuated Notch1 expression in valvar endothelial cells of post-endocardial to mesenchymal transformation valves results in stenosis of arterial valves
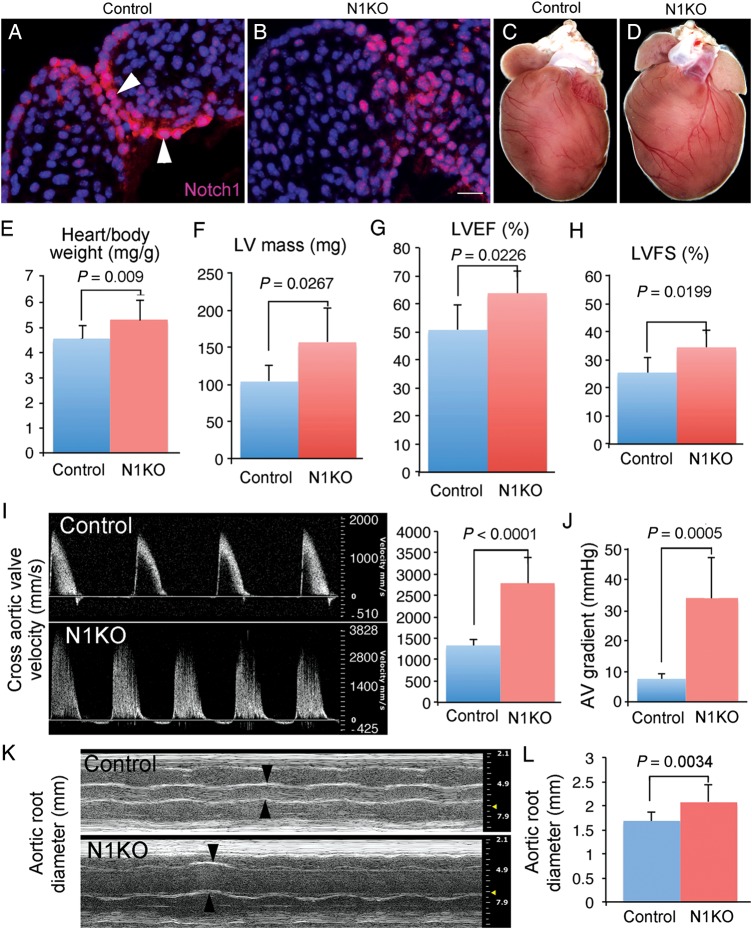
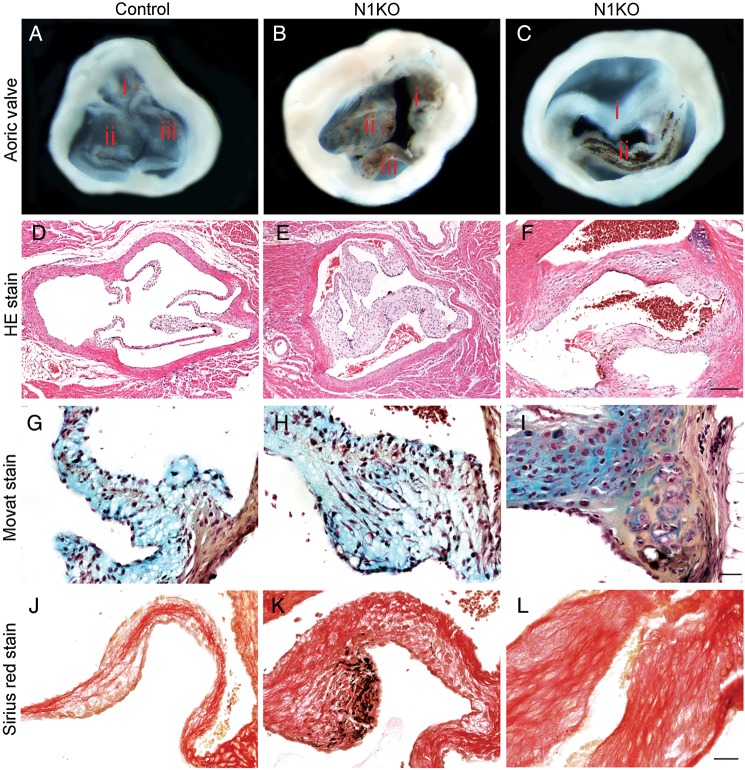
To understand how and where attenuated NOTCH activities in the developing valves give rise to congenital aortic valvar stenosis, we modelled and studied the disease in mice. We first inactivated Notch1 in VEC of post-EMT valves (Figure 1A and B) using a VEC-specific Cre, Nfatc1enCre.4 Valvar endothelial cells Notch1 deletion mice (N1KO, hereafter) survived to birth but developed cardiac hypertrophy (Figure 1C–E). Echocardiography showed that N1KO hearts had increased left ventricular mass, ejection fraction, fraction shortening (Figure 1F–H), blood velocity and pressure gradient across the aortic valves, and dilated aortic roots (Figure 1I–L). Gross pathology of N1KO hearts showed thickened aortic valve leaflets with underdeveloped interleaflet triangles (Supplementary material online, Figure S1), and 30% of them were bicuspid (Figure 2A–C). Histology revealed that N1KO mice developed a full spectrum of characteristics of human valvar aortic stenosis, including thickened valve leaflets (Figure 2D–F), increased collagen and glycosaminoglycan deposition (Figure 2G–L), cartilage formation (Supplementary material online, Figure S2), and calcific nodules (Supplementary material online, Figure S3). Calcification was seen at the edge of valvar leaflets of N1KO mice at 4 months of age and later in the body of the leaflets at 10 months of age (Supplementary material online, Figure S3), suggesting an age-dependent progression. We examined concomitantly the pulmonary valves of N1KO mice, which were also hypertrophic with increased velocity, although we did not find any calcific nodules (Supplementary material online, Figure S4, data not shown).
Figure 1.
Inactivation of Notch1 in valvar endothelial cells leads to hypertrophic left ventricle and stenotic aortic valves. Immunostaining of Notch1 on E14.5 heart sections shows a high level of Notch1 proteins present in valvar endothelial cells of control hearts (A) and a much reduced level in valvar endothelial cells of N1KO hearts (B). Front view of hearts from 4-month-old control (C) and N1KO mice (D) shows that N1KO hearts are hypertrophic. (E) Four-month-old N1KO mice (n = 16) have significantly increased ratio of heart/body weight (5.293 ± 0.764 vs. 4.521 ± 0.491 mg/g) compared with age-matched controls (n = 10). Echocardiography shows that N1KO mice (n = 8) have significantly increased left ventricular mass (157.003 ± 46.365 vs. 104 ± 21.801 mg) (F), ejection fraction (63.763 ± 8.092 vs. 50.695 ± 8.706%) (G), and fractional shortening (34.456 ± 5.970 vs. 25.469 ± 5.262%) (H), compared with age-matched controls (n = 6). Doppler analysis shows that 4-month-old N1KO mice (n = 8) have significantly increased across aortic valves systolic velocity (2788.883 ± 586.584 vs. 1332.224 ± 132.996 mm/s) (I), pressure gradient (33.737 ± 13.078 vs. 7.121 ± 1.446 mmHg) (J), and aortic root diameter (2.068 ± 0.364 vs. 1.681 ± 0.172 mm) (K and L), compared with the controls (n = 6). Bar = 20 µm.
Figure 2.
Inactivation of Notch1 in valvar endothelial cells results in congenital anomalies of aortic valves and valvar aortic stenosis. Gross view of aortic valves from 4-month-old mice shows that, compared with the controls (A), N1KO mice have thickened aortic valves with pigmented nodules at the edge of leaflets (B, n = 16), and ∼30% of N1KO mice have bicuspid aortic valves (C, n = 5). Haematoxylin-and-eosin-stained (HE) sections of aortic valves shows that N1KO mice (E and F) have thickened leaflets compared with the controls (D). Movat's staining shows increased presence of glycosaminoglycan (blue) and collagen (yellow) at the base of BAV of N1KO mice (I) compared with tricuspid aortic valves (TAV) of control (G) or N1KO mice (H). Sirius red staining shows increased staining for fibrotic cells within aortic valves of N1KO mice (K and L) compared with the controls (J). Bar = 100 µm (D–F) or 20 µm (G–L).
Notch1-Rbpj signalling in valvar endothelial cells controls post-endocardial-to-mesenchymal transformation arterial valve development
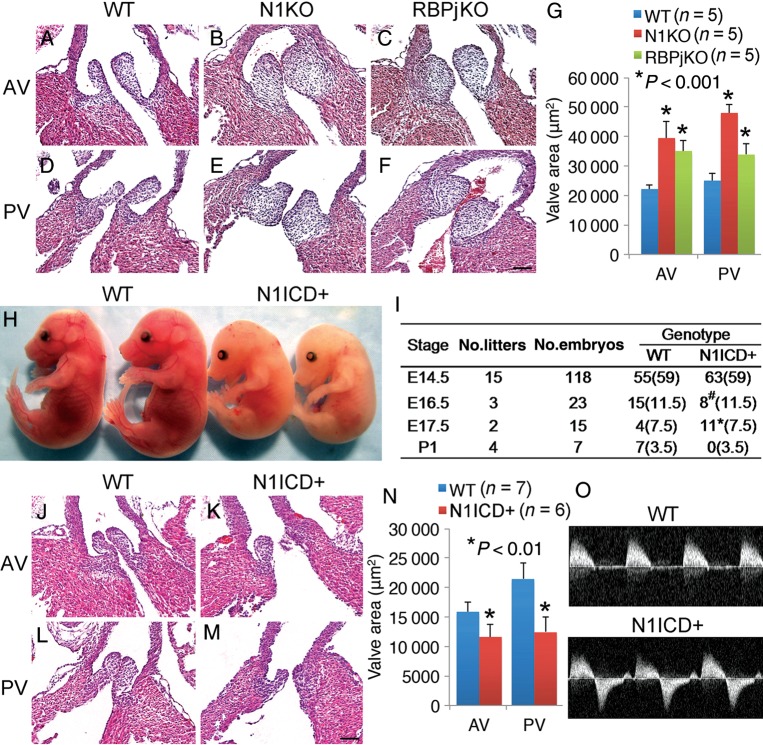
To understand how Notch1 signalling in VEC regulates post-EMT development of arterial valves, we examined the valves of VEC Notch1 (N1KO) or Rbpj deletion (RbpjKO) embryos between E11.5 and E16.5 and found that both had hypertrophic arterial valves (Figure 3A–G). Bicuspid aortic valves were present in E16.5 N1KO embryos (Supplementary material online, Figure S5), which resulted from excessive fusion between the two major outflow tract cushions beginning at E12.5 (Supplementary material online, Figure S6). All aortic sinuses of N1KO embryos were underdeveloped (Supplementary material online, Figure S7) and had high take-off of coronary arteries (Supplementary material online, Figure S8). In contrast to the arterial valves, the mitral and tricuspid valves appeared normal in N1KO mice (Supplementary material online, Figures S9 and S10).
Figure 3.
Notch1-Rbpj signalling in valvar endothelial cells is essential for development of arterial valves. HE sections show that E16.5 N1KO (B and E) and RbpjKO embryos (C and F) have enlarged arterial valves compared with controls (A, D, and G). (H) Gross view shows that E16.5 N1ICD+ embryos are underdeveloped compared with controls. (I) Table summarizes the embryonic lethal phenotype of N1ICD+ embryos. Numbers outside parentheses represent for observed embryos and numbers inside are expected. #, Runted embryos; *, dead embryos. HE sections show that E15.5 N1ICD+ embryos (K and M) have hypoplastic arterial valves compared with controls (J, L, and N). (O) Doppler analysis shows regurgitation across the aortic valves of E15.5 N1ICD+ embryos. AV, aortic valve; PV, pulmonary valve. Bar = 50 µm.
We then conducted ‘gain-of-function’ experiments and found that all the embryos with overexpression of the Notch1 intracellular domain in VEC (N1ICD+, hereafter) were runted by E16.5 and died before birth (Figure 3H and I). Histology revealed underdeveloped arterial valves in E15.5 N1ICD+ embryos (Figure 3J–N), and echocardiography showed regurgitation through defective aortic valves (Figure 3O). Valvar defects due to overexpression of N1ICD were restricted to the arterial valves (Supplementary material online, Figure S11). We also noted sub-aortic ventricular septum defects in 71% of N1ICD+, 62.5% of N1KO, and 60% of RbpjKO embryos (Supplementary material online, Table S3).
Since Jag1 is a main Notch1 ligand in VEC crucial for EMT and endocardial cushion formation,8 we therefore deleted Jag1 in VEC after EMT and found unexpectedly that the deletion did not affect development of arterial valves (Supplementary material online, Figure S12). To determine whether Notch signalling in VIC contributes to valvar development, we deleted Rbpj or overexpressed N1ICD in VIC using the VIC-specific PostnCre mice11 and found neither condition affected formation and homeostasis of arterial valves (Supplementary material online, Figures S13 and S14).
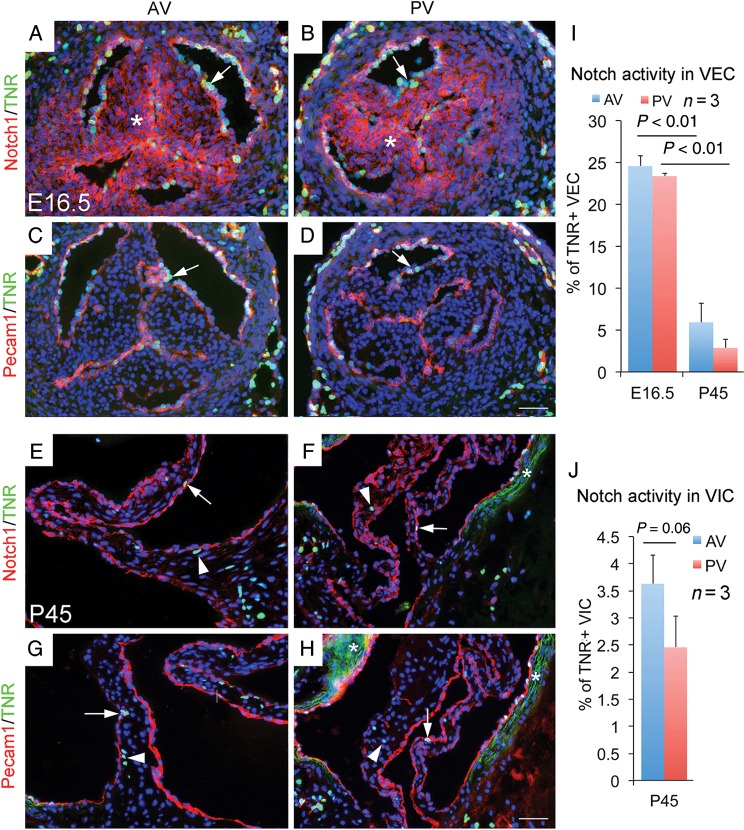
Notch1-Rbpj signalling is predominantly activated in the valvar endothelial cells of the developing valves
To better understand Notch1 expression vs. activity in VEC and VIC for valvar development and homeostasis, we examined the canonical Notch1 signalling in embryonic and adult valves by quantifying simultaneously the membrane Notch1 protein expression and nuclear Notch activity in transgenic Notch reporter (TNR) mice.12 The results showed that although both VEC and VIC of embryonic arterial valves expressed membrane Notch1 protein, nuclear Notch activity was only present in VEC (Figure 4A–D, I, and J). In adult valves, while membrane Notch1 protein was persistent in VEC, but greatly diminished in VIC, Notch activity was rare in both tissues (Figure 4E–H, I, and J). The findings are consistent with the observations that inactivation of Notch signalling in VEC, but not VIC, results in congenital anomalies of arterial valves.
Figure 4.
Notch activity in embryonic and adult arterial valves revealed by transgenic Notch reporter mice. (A and B) Simultaneous view of the canonical Notch activity (green) and membrane Notch1 protein expression (red) in E16.5 arterial valves shows membrane Notch1 proteins in valvar endothelial cells (arrow) and valvar interstitial cells (asterisk), but Notch activity is only present on the surface of valves (arrow). (C and D) Co-staining of Pecam1 (red) and transgenic Notch reporter (green) confirms valvar endothelial cells-specific Notch activation in embryonic valves (arrow). (E–H) Immunostaining shows that membrane Notch1 proteins are predominantly present in valvar endothelial cells (arrow) of arterial valves at postnatal (P) 45 day, and active Notch signalling is seen in a small set of valvar endothelial cells and valvar interstitial cells (arrowhead). Asterisks in E and H indicate background of auto-fluorescence associated with elastic fibres. (I) and (J) show quantitative values. Bar = 50 µm.
Notch1 signalling in valvar endothelial cells regulates proliferation and apoptosis of valvar interstitial cells
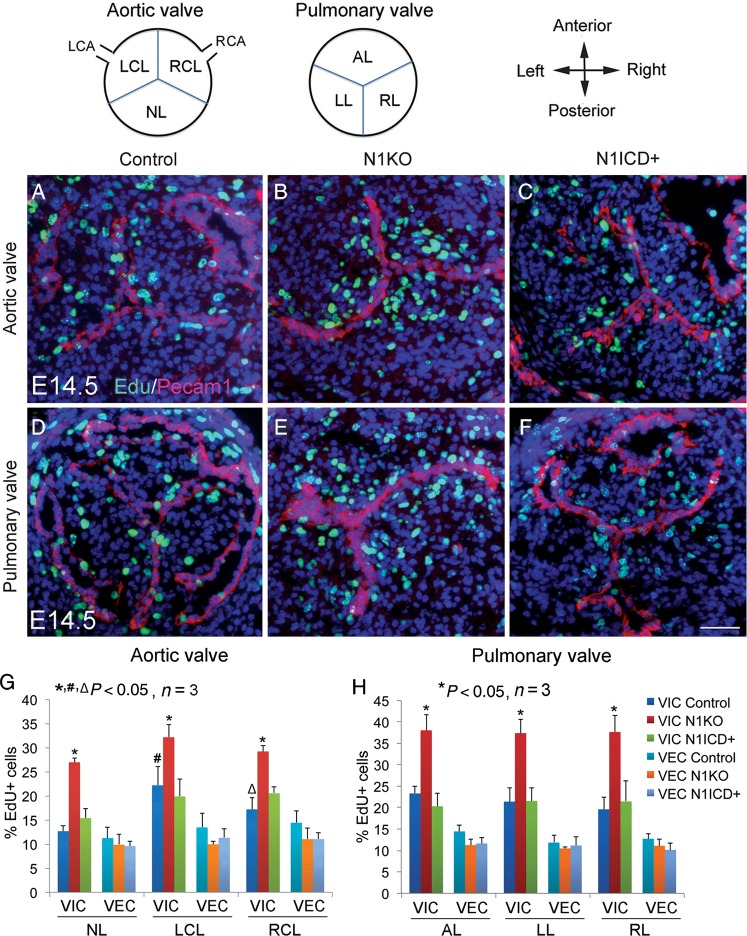
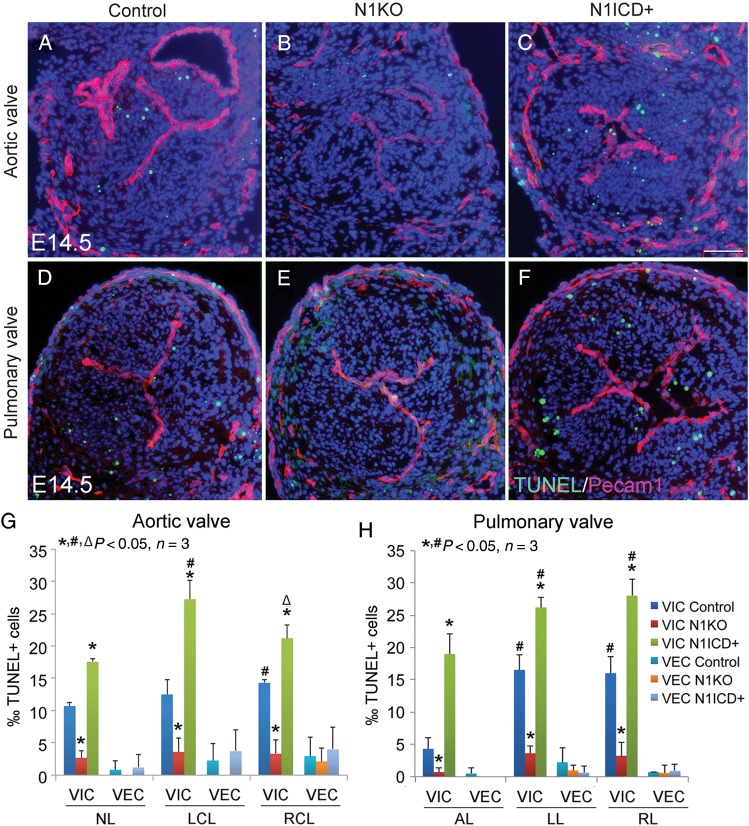
We next examined cell proliferation using 5-ethynyl-2'-deoxyuridine (EdU) labelling and found significantly increased proliferation of VIC, but not VEC, in the leaflets of arterial valves of E12.5 and E14.5N1KO embryos, compared with the control or N1ICD+ embryos at both stages (Figure 5; Supplementary material online, Figure S15). Interestingly, at E14.5, the two coronary leaflets had a relative high level of proliferative VIC compared with the non-adjacent aortic leaflet (Figure 5G). We then examined apoptosis at the same stages using terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay and found significant reduced apoptosis of VIC in all the leaflets of arterial valves of N1KO embryos, whereas N1ICD+ embryos had increased apoptosis of VIC at both stages (Figure 6; Supplementary material online, Figure S16). Notably, there was a relatively high level of apoptotic VIC in the left and right cushions at E12.5 (Supplementary material online, Figure S16), which gives rise to the two coronary leaflets. On the other hand, we found no significant changes in the proliferation and apoptosis of VEC at both stages under the condition of either loss or gain of Notch1 activity. We also examined the mitral and tricuspid valves and found no significant changes in proliferation and apoptosis of either VEC or VIC in N1KO mice (Supplementary material online, Figure S17, data not shown). Together, the results suggest that formation of two coronary leaflets is more vulnerable to deregulated proliferation and apoptosis of VIC, which might lead to the excessive fusion of two leaflets and subsequent BAV.
Figure 5.
Notch1 in valvar endothelial cells negatively regulates proliferation of valvar interstitial cells during development of arterial valves. Schematic diagram shows transverse view of arterial valves. LCA/RCA, left/right coronary artery; NL, non-adjacent leaflet; LCL/RCL, left/right coronary leaflet; AL/LL/RL, anterior/left/right leaflet. EdU labelling shows proliferating cells (green) in arterial valves of E14.5 control (A and D), N1KO (B and E), and N1ICD+ embryos (C and F). Pecam1 staining marks valvar endothelial cells (red). Bar = 50 µm. (G) and (H) show quantitative results. The data are presented as the ratio of EdU+ cells/total valvar endothelial cells or valvar interstitial cells. Statistical comparison was performed using one-way analysis of variance followed by Tukey's test. A P-value of <0.05 was considered significant. Asterisk indicates comparison with the control in each individual leaflet, hash indicates comparison with the non-adjacent leaflet in each genotype, and open triangle indicates comparison with the left coronary leaflet in each genotype.
Figure 6.
Notch1 in valvar endothelial cells promotes apoptosis of valvar interstitial cells during development of arterial valves. TUNEL assay shows apoptotic cells (green) in arterial valves of E14.5 control (A and D), N1KO (B and E), and N1ICD+ embryos (C and F). Pecam1 staining marks valvar endothelial cells (red). Bar = 50 µm. (G) and (H) show quantitative results. The data are presented as the ratio of TUNEL+ cells/total valvar endothelial cells or valvar interstitial cells. Statistical comparison was performed using one-way analysis of variance followed by Tukey's test. A P-value of <0.05 was considered significant. Asterisk indicates comparison with the control in each individual valve leaflet, hash indicates comparison with the non-adjacent leaflet (G) or anterior leaflet (H) in each genotype, and open triangle indicates comparison with the left coronary leaflet (G) in each genotype.
Notch1-Rbpj signalling regulates Tnfα for apoptosis of valvar interstitial cells and development of arterial valves
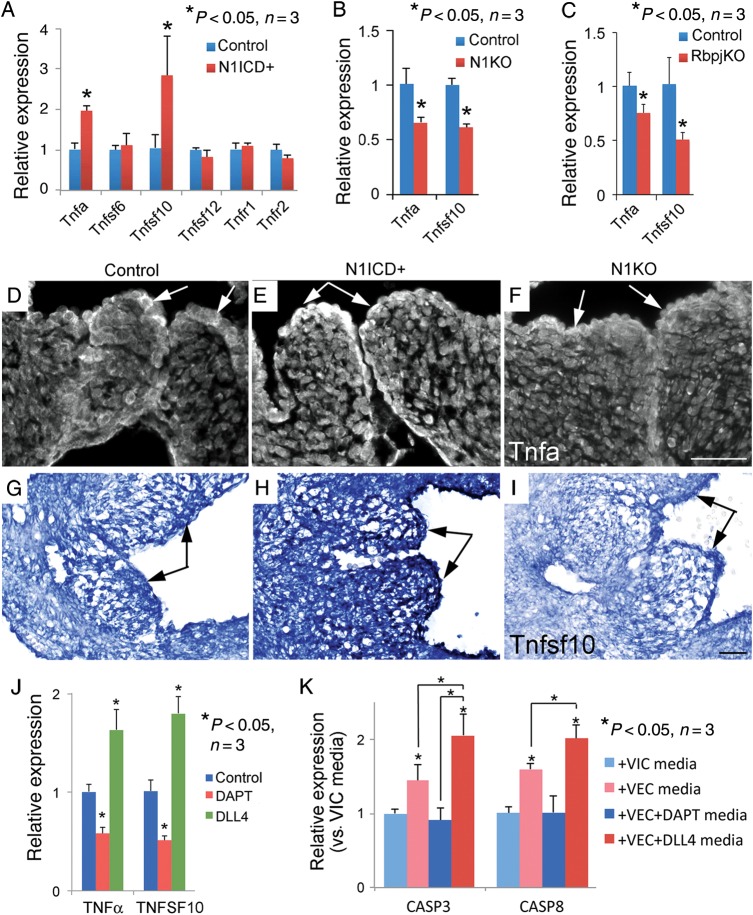
We then examined the expression of genes involved in proliferation (Supplementary material online, Figure S18), extracellular matrix (ECM) deposition (Supplementary material online, Figure S19), and apoptosis (Figure 7) in the arterial valves of E14.5 hearts. Notably, expression of apoptotic genes, Tnfα and Tnfsf10, in the post-EMT valves was dependent on the Notch1-Rbpj signalling in VEC (Figure 7A–I). To test if Notch1 regulated expression of Tnfα and Tnfsf10 in VEC, we treated cultures of pig aortic VEC with Notch inhibitor N-[(3,5-Difluorophenyl)acetyl]-L-alanyl-2-phenyl]glycine-1,1-dimethylethyl ester or activator DLL4 and found Notch inhibition significantly suppressed, whereas the activation augmented the expression of Tnfα and Tnfsf10 (Figure 7J). Moreover, conditioned media from Notch-activated VEC-promoted expression of apoptotic genes CASP3 and CASP8 by pig aortic VIC (Figure 7K).
Figure 7.
Notch1-Rbpj signalling regulates Tnf gene expression in the developing arterial valves. (A–C) Quantitative polymerase chain reaction analysis shows that expression of Tnf and Tnfsf10 in arterial valves of E14.5 embryos is dependent on Notch1 activity. (D–F) Immunostaining shows that expression of Tnfα proteins in valvar endothelial cells (arrows) of aortic valves is regulated by Notch1 activity. (G–I) In situ hybridization shows that expression of Tnfsf10 transcripts in the developing aortic valves (arrows) is dependent on Notch1 activity. Bar = 50 µm. (J) Quantitative polymerase chain reaction analysis shows expression of Tnfα and Tnfsf10 in cultured pig aortic valvar endothelial cells is regulated by Notch signalling. (K) Quantitative polymerase chain reaction analysis shows that conditioned media of valvar endothelial cells promotes expression of CASP3 and CASP8 by valvar interstitial cells, and the effect is dependent on Notch activation.
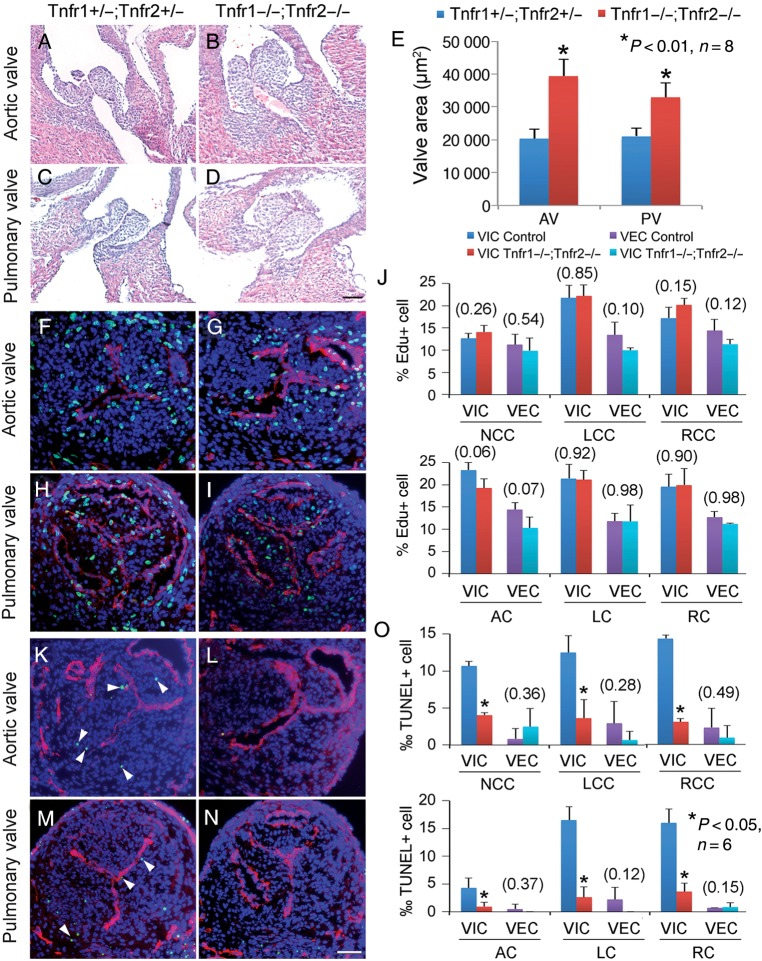
To determine whether Tnfα signalling is necessary for development of arterial valves, we deleted both Tnfr1 and Tnfr2 receptors in mice (Tnfr1−/−;Tnfr2−/−) to inactivate the entire Tnfα signalling. These mice developed hypertrophic arterial valves by E16.5 (Figure 8A–E). While the deletion did not affect valve cell proliferation (Figure 8F–J), it significantly repressed apoptosis of VIC (Figure 8K–O). Interestingly, although adult Tnfr1−/−;Tnfr2−/− mice had increased collagen deposition in the aortic valves, they did not develop valvar calcification (Supplementary material online, Figure S20) and had normal cardiac functions (Supplementary material online, Table S4). The observations demonstrate that Tnfα-regulated apoptosis is necessary for development of arterial valves and suggest that it is also involved in postnatal valvar calcification.
Figure 8.
Tnf signalling is required for apoptosis of valvar interstitial cells and development of arterial valves. (A–E) HE sections show that E16.5 Tnfr1−/−;Tnfr2−/− embryos have significantly thickened leaflets of arterial valves (B and D) compared with Tnfr1+/−;Tnfr2+/− littermates (A and C). Bar = 20 µm. EdU assay shows no difference in cell proliferation in arterial valves between E14.5 Tnfr1+/−;Tnfr2+/− (F, H, and J) and Tnfr1−/−;Tnfr2−/− embryos (G, I, and J). TUNEL assay shows significantly decreased apoptosis of valvar interstitial cells (arrowheads) in all the arterial leaflets of E14.5 Tnfr1−/−;Tnfr2−/− embryos (L, N, and O) compared with controls (K, M, and O). Pecam1 staining (red) labels valvar endothelial cells. Numbers in parentheses indicate P-valves. Bar = 50 µm.
Discussion
Despite its increasing prevalence and clinical severity, little is known about the developmental origins of congenital anomalies of arterial valves. This is partly due to inadequate animal models that target post-EMT development of valves. In this study, we generated new mouse models of congenital anomalies of arterial valves in which Notch signalling was altered in post-EMT valves and provided multiple lines of evidence showing collectively that the Notch-Tnf signalling between VEC and VIC regulates proliferation and apoptosis of VIC to form the leaflets of arterial valves.
Apoptosis of VIC in the developing arterial valves has been speculated to shape valvar leaflets.13 We have also observed that apoptosis occurs after EMT in a subpopulation of VIC located at the base of valves or underneath VEC of growing leaflets. Perhaps the most striking finding is the identification of pro-apoptotic Tnfα signalling as a necessary morphogenic factor for development of valvar leaflets. Apoptosis and proliferation are two necessary morphogenic forces for valvar development.14–17 Our results show that apoptosis and proliferation are both prominent in the two coronary leaflets compared with the non-adjacent one, suggesting that apoptosis of VIC is counteracted by proliferation to compensate the excessive loss of cells during development of valvar leaflets. In N1KO mice, there are increased proliferation and reduced apoptosis of VIC in the two major outflow tract cushions when they develop into arterial valve leaflets, and the imbalanced proliferation and apoptosis of VIC likely contribute to the excessive fusion of the cushions, resulting in abnormal formation of interleaflet triangles and BAV. In addition, the deregulated proliferation and apoptosis may contribute to the underdeveloped aortic sinuses and high take-off of coronary arteries. Collectively, these developmental defects in the aortic root resemble those anatomic abnormalities seen in the patients with the congenital malformation and stenosis of the arterial valves, arising from the excessive fusion of the major outflow tract cushions.18–20
Bicuspid aortic valves in N1KO mice are the result of fusion of the left and right coronary leaflets, which is seen in >70% human BAV.21,22 It is different from the BAV observed in other mouse genetic models,23 such as endothelial Gata5 null mice,24 which is the result of fusion of the right and non-adjacent leaflets. The different subtypes of BAV in different genetic deletions in the mouse models support that human BAV subtypes have distinct genetic aetiologies, which contribute to different clinical outcomes.25
In addition to VEC and VIC, other cellular sources such as haematopoietic stem cells may account for post-EMT development of valves and homeostasis,26 which we have not analysed in our models. Development of endocardial cushions into valvar leaflets also involves ECM.27,28 In our study, the thickening aortic valves of N1KO mice have increased deposition of glycoaminoglycans and collagens and decreased the expression of MMP9, a major collagenase, suggesting that valvar stenosis is complicated with dysplasia features, as seen in congenital malformed valves in the Alagille syndrome, caused by mutations in the NOTCH gene JAGGED1.29,30 Surprisingly, knockout of Mmp9 does not result in any valve phenotype (Supplementary material online, Figure S19), indicating that its role in valvar formation is compensated by other Mmps. Interestingly, heterozygous Notch1 null mice on hypercholesterolemic diet develop valvar aortic stenosis,31 supporting that Notch1 is required for adult valvar homeostasis.
It remains unclear from our study why VEC inactivation of Notch1 signalling results in defects in arterial but not atrioventricular valves. It is known that germline deletion of Nfatc1 causes the absence of arterial valves, with minimal effect on the atrioventricular valves,4,32–34 which suggests different intrinsic susceptibilities between the arterial and atrioventricular valves to a deleterious genetic mutation due to different origins of VIC and cellular interactions.4,35 Extrinsic factors such as haemodynamic stress may also superimpose and interact with intrinsic factors to cause distinct phenotypes.36
Our study reveals that the VEC-specific Notch1 signalling regulates post-EMT development of arterial valves through promoting expression of Tnfα in VEC, which then interacts with its receptors Tnfr1 and Tnfr2 on VIC and induces apoptosis of VIC to shape endocardial cushions into mature leaflets (Supplementary material online, Figure S21). To our knowledge, this is the first in vivo example demonstrating that Notch1-Tnfα signalling plays a critical role in heart development, and the VEC Notch1 signalling is required for postnatal aortic homeostasis. This conclusion has clinical implications, as mutations in the NOTCH-TNF pathway may contribute to the developmental pathogenesis of congenital anomalies and stenosis of arterial valves.
Supplementary material
Supplementary material is available at European Heart Journal online.
Authors’ contributions
Y.Z., D.Z., B.Z.: performed statistical analysis. K.E.Y., J.T.B., B.Z.: handled funding and supervision. Y.W., B.W., E.F., W.L., P.L., C.M.A., K.M., M.C., D.Y., D.X.: acquired the data. Y.W., B.W., B.Z.: conceived and designed the research. Y.W., B.Z.: drafted the manuscript. K.E.Y., J.T.B.: made critical revision of the manuscript for key intellectual content. M.R., V.T., S.J.C.: the names of the authors who did anything else on the manuscript other than what we have listed.
Funding
This work was supported by the National Institutes of Health (R01HL078881, R21HL104441, and R01HL111770 to B.Z.; R01HL110328 and R21HL118672 to J.T.B.; and R01HL094319 to K.E.Y.), the National Science Foundation (CBET-0955172 to J.T.B.), and the American Heart Association Postdoctoral Fellowship (AHA-POST16970056 to Y.W.).
Conflict of interest: none declared.
Supplementary Material
Acknowledgements
The Collagen type II (CIIC1) antibody, developed by Rikard Holmdahl and Kristofer Rubin, was obtained from the Developmental Studies Hybridoma Bank, created by NICHD of NIH and maintained at Department of Biology, University of Iowa, Iowa City, IA, USA.
References
- 1. Hinton RB, Yutzey KE. Heart valve structure and function in development and disease. Ann Rev Physiol 2011;73:29–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eisenberg LM, Markwald RR. Molecular regulation of atrioventricular valvuloseptal morphogenesis. Circ Res 1995;77:1–6. [DOI] [PubMed] [Google Scholar]
- 3. Wu B, Baldwin HS, Zhou B. Nfatc1 directs the endocardial progenitor cells to make heart valve primordium. Trends Cardiovasc Med 2013;23:294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu B, Wang Y, Lui W, Langworthy M, Tompkins KL, Hatzopoulos AK, Baldwin HS, Zhou B. Nfatc1 coordinates valve endocardial cell lineage development required for heart valve formation. Circ Res 2011;109:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Armstrong EJ, Bischoff J. Heart valve development: endothelial cell signaling and differentiation. Circ Res 2004;95:459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 2009;137:216–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in NOTCH1 cause aortic valve disease. Nature 2005;437:270–274. [DOI] [PubMed] [Google Scholar]
- 8. Wang Y, Wu B, Chamberlain AA, Lui W, Koirala P, Susztak K, Klein D, Taylor V, Zhou B. Endocardial to myocardial notch-wnt-bmp axis regulates early heart valve development. PLoS ONE 2013;8:e60244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grego-Bessa J, Luna-Zurita L, del Monte G, Bolos V, Melgar P, Arandilla A, Garratt AN, Zang H, Mukouyama YS, Chen H, Shou W, Ballestar E, Esteller M, Rojas A, Perez-Pomares JM, de la Pompa JL. Notch signaling is essential for ventricular chamber development. Dev Cell 2007;12:415–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Limbourg FP, Takeshita K, Radtke F, Bronson RT, Chin MT, Liao JK. Essential role of endothelial Notch1 in angiogenesis. Circulation 2005;111:1826–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lindsley A, Snider P, Zhou H, Rogers R, Wang J, Olaopa M, Kruzynska-Frejtag A, Koushik SV, Lilly B, Burch JB, Firulli AB, Conway SJ. Identification and characterization of a novel Schwann and outflow tract endocardial cushion lineage-restricted periostin enhancer. Dev Biol 2007;307:340–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nowotschin S, Xenopoulos P, Schrode N, Hadjantonakis AK. A bright single-cell resolution live imaging reporter of Notch signaling in the mouse. BMC Dev Biol 2013;13:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hurle JM, Colvee E, Blanco AM. Development of mouse semilunar valves. Anat Embryol 1980;160:83–91. [DOI] [PubMed] [Google Scholar]
- 14. Thompson RP, Sumida H, Abercrombie V, Satow Y, Fitzharris TP, Okamoto N. Morphogenesis of human cardiac outflow. Anat Record 1985;213:578–586, 538–9. [DOI] [PubMed] [Google Scholar]
- 15. Sugi Y, Ito N, Szebenyi G, Myers K, Fallon JF, Mikawa T, Markwald RR. Fibroblast growth factor (FGF)-4 can induce proliferation of cardiac cushion mesenchymal cells during early valve leaflet formation. Dev Biol 2003;258:252–263. [DOI] [PubMed] [Google Scholar]
- 16. Cheng G, Wessels A, Gourdie RG, Thompson RP. Spatiotemporal and tissue specific distribution of apoptosis in the developing chick heart. Dev Dyn 2002;223:119–133. [DOI] [PubMed] [Google Scholar]
- 17. Lin CJ, Lin CY, Chen CH, Zhou B, Chang CP. Partitioning the heart: mechanisms of cardiac septation and valve development. Development 2012;139:3277–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anderson RH. Clinical anatomy of the aortic root. Heart 2000;84:670–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anderson RH, Webb S, Brown NA, Lamers W, Moorman A. Development of the heart: (3) formation of the ventricular outflow tracts, arterial valves, and intrapericardial arterial trunks. Heart 2003;89:1110–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Loukas M, Bilinsky E, Bilinsky S, Blaak C, Tubbs RS, Anderson RH. The anatomy of the aortic root. Clin Anat 2014;27:748–756. [DOI] [PubMed] [Google Scholar]
- 21. Fernandez B, Duran AC, Fernandez-Gallego T, Fernandez MC, Such M, Arque JM, Sans-Coma V. Bicuspid aortic valves with different spatial orientations of the leaflets are distinct etiological entities. J Am Coll Cardiol 2009;54:2312–2318. [DOI] [PubMed] [Google Scholar]
- 22. Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007;133:1226–1233. [DOI] [PubMed] [Google Scholar]
- 23. Prakash SK, Bosse Y, Muehlschlegel JD, Michelena HI, Limongelli G, Della Corte A, Pluchinotta FR, Russo MG, Evangelista A, Benson DW, Body SC, Milewicz DM. A roadmap to investigate the genetic basis of bicuspid aortic valve and its complications: insights from the International BAVCon (Bicuspid Aortic Valve Consortium). J Am Coll Cardiol 2014;64:832–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Laforest B, Andelfinger G, Nemer M. Loss of Gata5 in mice leads to bicuspid aortic valve. J Clin Invest 2011;121:2876–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O, Grabenwoger M, Haverich A, Iung B, Manolis AJ, Meijboom F, Nienaber CA, Roffi M, Rousseau H, Sechtem U, Sirnes PA, Allmen RS, Vrints CJ, Guidelines ESCCfP. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873–2926. [DOI] [PubMed] [Google Scholar]
- 26. Visconti RP, Ebihara Y, LaRue AC, Fleming PA, McQuinn TC, Masuya M, Minamiguchi H, Markwald RR, Ogawa M, Drake CJ. An in vivo analysis of hematopoietic stem cell potential: hematopoietic origin of cardiac valve interstitial cells. Circ Res 2006;98:690–696. [DOI] [PubMed] [Google Scholar]
- 27. Norris RA, Moreno-Rodriguez RA, Sugi Y, Hoffman S, Amos J, Hart MM, Potts JD, Goodwin RL, Markwald RR. Periostin regulates atrioventricular valve maturation. Dev Biol 2008;316:200–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Snider P, Hinton RB, Moreno-Rodriguez RA, Wang J, Rogers R, Lindsley A, Li F, Ingram DA, Menick D, Field L, Firulli AB, Molkentin JD, Markwald R, Conway SJ. Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ Res 2008;102:752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li L, Krantz ID, Deng Y, Genin A, Banta AB, Collins CC, Qi M, Trask BJ, Kuo WL, Cochran J, Costa T, Pierpont ME, Rand EB, Piccoli DA, Hood L, Spinner NB. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet 1997;16:243–251. [DOI] [PubMed] [Google Scholar]
- 30. Oda T, Elkahloun AG, Pike BL, Okajima K, Krantz ID, Genin A, Piccoli DA, Meltzer PS, Spinner NB, Collins FS, Chandrasekharappa SC. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet 1997;16:235–242. [DOI] [PubMed] [Google Scholar]
- 31. Nus M, MacGrogan D, Martinez-Poveda B, Benito Y, Casanova JC, Fernandez-Aviles F, Bermejo J, de la Pompa JL. Diet-induced aortic valve disease in mice haploinsufficient for the Notch pathway effector RBPJK/CSL. Arteriosclerosis Thromb Vasc Biol 2011;31:1580–1588. [DOI] [PubMed] [Google Scholar]
- 32. de la Pompa JL, Timmerman LA, Takimoto H, Yoshida H, Elia AJ, Samper E, Potter J, Wakeham A, Marengere L, Langille BL, Crabtree GR, Mak TW. Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum. Nature 1998;392:182–186. [DOI] [PubMed] [Google Scholar]
- 33. Ranger AM, Grusby MJ, Hodge MR, Gravallese EM, de la Brousse FC, Hoey T, Mickanin C, Baldwin HS, Glimcher LH. The transcription factor NF-ATc is essential for cardiac valve formation. Nature 1998;392:186–190. [DOI] [PubMed] [Google Scholar]
- 34. Zhou B, Wu B, Tompkins KL, Boyer KL, Grindley JC, Baldwin HS. Characterization of Nfatc1 regulation identifies an enhancer required for gene expression that is specific to pro-valve endocardial cells in the developing heart. Development 2005;132:1137–1146. [DOI] [PubMed] [Google Scholar]
- 35. Jain R, Engleka KA, Rentschler SL, Manderfield LJ, Li L, Yuan L, Epstein JA. Cardiac neural crest orchestrates remodeling and functional maturation of mouse semilunar valves. J Clin Invest 2011;121:422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Butcher JT, McQuinn TC, Sedmera D, Turner D, Markwald RR. Transitions in early embryonic atrioventricular valvar function correspond with changes in cushion biomechanics that are predictable by tissue composition. Circ Res 2007;100:1503–1511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.