Abstract
Background: Gastrin, which induces gastric acid secretion, and a structurally similar hormone, cholecystokinin (CCK)–a potent acid inhibitor, may each play a role in gastric cancer. However, few studies have investigated this hypothesis in humans. We therefore investigated whether serum gastrin or CCK concentrations at baseline were associated with the incidence of gastric non-cardia adenocarcinomas (GNCA), oesophagogastric junctional adenocarcinomas (EGJA) or gastric carcinoid tumours over 24 years of follow-up in a study nested within the all-male Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study of Finnish smokers.
Methods: Totals of 283 incident GNCA, 96 EGJA and 10 gastric carcinoid cases, and 778 matched controls, were included in our analysis. Gastrin and CCK were measured using specific radioimmunoassays. Odds ratios (OR) and 95% confidence intervals (95% CI) were estimated by multivariable logistic regression with adjustment for all known or suspected confounding factors, including Helicobacter pylori seropositivity.
Results: Those with high gastrin (Q4 vs Q1), had an increased risk of GNCA (fully adjusted OR: 1.92; 95% CI: 1.21, 3.05) and gastric carcinoids, though the small number of carcinoid cases meant the fully adjusted model was unstable (age-adjusted continuous model OR: 4.67; 95% CI: 2.67, 8.15). CCK was associated with risk of GNCA only for those in Q3 relative to Q1 (OR: 0.56; 95% CI: 0.33, 0.96), and no significant trend was observed.
Conclusions: Our data suggest that high serum concentrations of gastrin may be associated independently with an increased risk of gastric cancer; the role of CCK in cancer risk is less clear.
Keywords: Gastrin, cholecystokinin, gastric non-cardia adenocarcinoma, oesophagogastric junctional adenocarcinoma, gastric carcinoids
Introduction
Gastric cancer killed 723 000 people in 2012, making it the third leading cause of cancer death worldwide.1 Gastric non-cardia cancer (GNCA) usually develops against a background of persistent Helicobacter pylori (H. pylori) infection and associated chronic inflammation, which results in the destruction of gastric glands, a condition known as gastric atrophy. Gastric carcinoid tumours, which are relatively rare, are also thought to arise against a backdrop of gastric atrophy, although the role of H. pylori infection in these tumours is not known. Atrophy of the gastric glands almost certainly alters the gastric hormonal milieu; however, the role of this hormonal alteration in the aetiology of GNCA and gastric carcinoid tumours, as well as an adjacent third type of gastric cancer which occurs in the gastric junction (oesophagogastric junctional cancer, EGJA) is not known.
Gastrin is a hormone produced within the G cells of the pyloric glands of the stomach. Gastrin induces gastric acid secretion by binding to CCK2 receptors on enterochromaffin-like cells of the gastric corpus/fundus to cause the release of histamine, which in turn stimulates the gastric parietal cells to secrete H+ ions. In addition to inducing gastric acid secretion, gastrin is known to regulate processes including cellular proliferation, apoptosis, invasion and angiogenesis2 and, as such, has been hypothesized to have a role in the development of gastric cancer.3,4 InsGas mice, which over-express human gastrin, develop atrophy, intestinal metaplastic-type lesions, dysplasia and ultimately gastric adenocarcinoma within 20 months.4 Conversely, in studies of gastrin-deficient mice (G-/G-), parietal cell atrophy is observed and antral inflammation can develop and progress to antral atrophy, intestinal metaplasia and adenocarcinoma,5,6 suggesting significant roles for gastrin deregulation in gastric cancer.5 Furthermore, clinical and mechanistic studies link H. pylori infection to high gastrin concentrations. For example, incubation of canine G cells with H. pylori extracts and recombinant cytokines can stimulate the release of gastrin.7,8 Few studies, however, have evaluated the hypothesis prospectively in humans.
Cholecystokinin (CCK) is a structurally-related hormone which is produced within the gastrointestinal tract, in this case by the endocrine I cells of the small intestine. CCK may also contribute to gastric cancer. CCK is a potent inhibitor of gastric acid secretion; it combines with CCK1 receptors to stimulate somatostatin release, which controls gastrin release by the gastric G cells. CCK also controls the emptying of the gallbladder and the release of pancreatic enzymes, and may regulate cellular growth.9
As gastrin and CCK comprise a family of gastrointestinal hormones which share an identical carboxyl-terminal pentapeptide sequence, it has been historically difficult to measure CCK and avoid cross-reactivity with gastrin.10,11 Thus, there are few data from human studies examining the separate associations of gastrin and CCK with gastric or other cancers.12 Here, we took advantage of highly specific radioimmunoassays which detect all bioactive (i.e. α-amidated) forms of gastrin and CCK separately, to investigate the independent associations between circulating serum gastrin and CCK concentrations, and subsequent risk of GNCA, EGJA and gastric carcinoid tumours in a case-control study nested within the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study.
Methods
Participants in this study were drawn from the ATBC Study, a randomized, double-blind placebo-controlled, primary prevention trial designed to determine whether daily supplementation with alpha-tocopherol (50 mg/day), beta-carotene (20 mg/day) or both would reduce the incidence of lung cancer in male smokers.13–15 Between 1985 and 1988, 29 133 eligible Finnish male smokers (of at least five cigarettes daily), between 50 and 69 years old, were recruited to the ATBC Study. Supplementation ended in 1993; since that time, participants have been followed as a cohort. The ATBC Study was approved by the institutional review boards of both the National Institutes of Health in the USA and the National Public Health Institutes in Finland. All participants provided written informed consent.
Participants identified here as cases developed incident GNCA, EGJA or gastric carcinoid tumours between baseline and over 24 years of follow-up. Cancer cases were identified primarily via the Finnish Cancer Registry. Gastric cancer cases were defined according to the International Classification of Diseases, 9th Revision.16 GNCA cases included all adenocarcinomas coded as 151 or 151.1–151.9 (151.9: n = 144); EGJA cases included all adenocarcinomas coded as 151.0; and gastric carcinoid cancers were malignant carcinoid tumours coded as 151.4 or 151.8. Controls were alive and free of gastric cancer at the time of case diagnosis and were individually matched to cases on age at randomization (± 1 year), date of blood draw (± 30 days) and sample availability.
Data collection
Participants completed questionnaires at baseline regarding general risk factors, medical history and dietary intake. Dietary intake was assessed using a food frequency questionnaire which asked about usual food consumption over the previous year, including 276 common foods and mixed dishes, using a picture booklet to aid estimation of portion size.17,18 The weight and height of all participants was measured by trained study staff. Blood samples were collected from fasting participants at baseline (1985–88) and serum samples have been stored at -70°C since that time.
Laboratory analysis
Radioimmunoassays: gastrin and CCK
At the time of sample selection, 405 incident gastric adenocarcinomas had occurred in the ATBC Study: 283 cases of incident GNCA were selected for gastrin and CCK measurement, as were 96 cases of EGJA, 10 gastric carcinoid cases and 778 controls. A radioimmunoassay was used to measure gastrin; the gastrin antiserum (no. 2604) is directed against the C-terminus of gastrin-17 and binds all C-terminally amidated gastrins (−14, −17, −34, −52 and −71) in serum with equimolar potency, but is not cross-reactive with CCK.19,20 CCK was measured with a radioimmunoassay (RIA) in which antiserum (no. 92128) binds the bioactive forms of CCK (−8, −22, −33 and −58) with equal potency, without cross-reactivity with gastrin.11,21 Using 105 blinded quality control samples (10% of the samples within each batch for gastrin and CCK measurement) from a single serum pool from the ATBC Study, the coefficient of variation for these assays was calculated as 32% for gastrin and 23% for CCK.
A total of 13 samples from the control group failed gastrin RIA and 14 samples failed CCK RIA because of low sample volume. Because of limited serum volume, aliquots for gastrin measurements were made preferentially over CCK; 60 of the selected gastric cancer samples were dropped from gastrin measurement because of low serum volume and a further 65 were dropped from CCK measurement.
Helicobacter pylori multiplex serology assay
Helicobacter pylori (H. pylori) multiplex serology was performed as previously described and validated.22 Results are based on glutathione S-transferase (GST) capture immunosorbent assays combined with fluorescent bead technology23 and simultaneously quantify antibodies to multiple protein antigens. The panel included fifteen H. pylori proteins (UreA, Catalase, GroEL, NapA, CagA, CagM, Cagδ, HP0231, VacA, HpaA, Cad, HyuA, Omp, HcpC and HP0305) that were bacterially-expressed and affinity-purified recombinant GST tagged as per previous studies.24,25H. pylori positivity was defined as those seropositive for ≥ 4 antigens.22H. pylori serology was available for 92% cases and 48% of controls.
Serum pepsinogen I (PGI) was measured by enzyme-linked immunosorbent assay (ELISA) (BIOHIT, Finland). A low serum PGI level was defined as 56 µg/l or less. Using quality control samples (∼10%) from the ATBC Study, the coefficient of variation for this assay was 7%.
Statistical analysis
Statistical analyses were performed using STATA version 11.2 (Stata Corp., College Station, TX) and all P-values were two-sided. The distributions of baseline characteristics across GNCA, EGJA and gastric carcinoids, relative to controls, and associations between baseline characteristics and high vs normal serum gastrin and CCK were compared using Student’s t tests for continuous variables and Pearson’s χ2 or Fisher’s exact tests for categorical variables.
Odds ratios (OR) and 95% confidence intervals (95% CI) for associations between high concentrations of serum gastrin or CCK, as defined above, and risk of GNCA, EGJA and gastric carcinoids, were calculated using unconditional logistic regression models. Analyses included modelling gastrin and CCK as continuous data and by quartiles. Preliminary multivariate models of risk were adjusted for: age at randomization, total years of smoking, total cigarettes/day, alcohol (g/day), body mass index (BMI) (kg/m2), fruit intake (g/day), vegetable intake (g/day), education (post-primary school), H. pylori (+/-), ATBC treatment group assignment28 and blood draw date. Blood draw date was omitted from our final models because it has no significant effect on risk estimates. Estimates derived from conditional and unconditional logistic regression models were similar; as such, only the results of the unconditional models, which offered more precise risk estimates, are presented within this manuscript (conditional models can be found as Supplementary Tables, available as Supplementary data at IJE online). Missing data were included in the logistic regression models, using flag variables to indicate missing values and by setting the value of the missing data to 0.
Lag analyses were also performed: cases of GNCA and EGJA cases were divided into those occurring within 5 years (< 5 years) of serum collection/baseline, between 5 and 10 years (≥ 5 and ≤ 10 years) of baseline, or more than 10 years (> 10 years) after baseline; gastric carcinoids were omitted from this lag analysis due to small numbers.
Results
Baseline characteristics of the cases and control subjects are shown in Table 1. Incident GNCA cases were significantly less likely to have received post-primary school education and more likely to be seropositive for H. pylori than controls. Both GNCA and EGJA cases smoked more cigarettes per day than controls.
Table 1.
Descriptive characteristics of gastric non-cardia adenocarcinoma (GNCA), oesophagogastric junctional adenocarcinoma (EGJA) and gastric carcinoid cases and controls from the ATBC Study
| Variable | Controls | Gastric non-cardia adenocarcinoma | aP-value | Oesophagogastric junctional adenocarcinoma | aP-value | Gastric carcinoids | aP-value |
|---|---|---|---|---|---|---|---|
| Total N | 778 | 283 | 96 | 10 | |||
| Age at baseline, mean years (SD) | 58 (5) | 58 (5) | 0.78 | 59 (5) | 0.51 | 58 (5) | 0.76 |
| Years of smoking, mean (SD) | 36 (9) | 37 (10) | 0.35 | 37 (7) | 0.12 | 38 (6) | 0.39 |
| Cigarettes/day, mean (SD) | 19 (9) | 20 (9) | 0.02 | 21 (9) | 0.03 | 18(7) | 0.69 |
| Alcohol, g/day, mean (SD) | 15 (18) | 17 (21) | 0.16 | 15 (16) | 0.97 | 24 (32) | 0.13 |
| Education: post-primary school, N (%) | 137 (18) | 27 (9) | 0.001 | 18 (19) | 0.78 | 1 (10) | 1.00 |
| BMI (kg/m2), mean (SD) | 26 (4) | 26 (4) | 0.22 | 27 (4) | 0.36 | 29 (4) | 0.04 |
| Fruit, g/day, mean (SD) | 210 (180) | 195 (154) | 0.23 | 210 (164) | 0.99 | 139 (124) | 0.22 |
| Vegetables, g/day, mean (SD) | 290 (112) | 280 (104) | 0.20 | 289 (101) | 0.93 | 283 (124) | 0.85 |
| Helicobacter pylori-positive, N (%)b | 351 (93) | 257 (99) | < 0.001 | 82 (94) | 0.76 | 8 (80) | 0.10 |
| Low pepsinogen 1 (≤ 56 ug/l), N (%)c | 10 (3) | 34 (17) | < 0.001 | 6 (9) | 0.04 | 4 (80) | < 0.001 |
aP-values from Student’s t test, Pearson’s χ2 test or Fisher’s exact test, as appropriate.
bHelicobacter pylori multiplex positivity was available for 376/778 controls, 259/283 GNCA, 87/96 EGJA and 10/10 gastric carcinoid cases.
cPepsinogen 1 data were available for 309/778 controls, 202/283 GNCA, 67/96 EGJA and 5/10 gastric carcinoid cases.
Table 2 shows the relationship between baseline characteristics and quartiles of serum gastrin or CCK among controls. Serum gastrin, by quartile, was significantly associated with education (P = 0.01). Serum CCK, by quartile, was significantly associated with alcohol intake (g/day; P = 0.0001), vegetable intake (g/day; P = 0.04) and Helicobacter pylori seropositivity (P = 0.01).
Table 2.
Baseline characteristics by quartiles of serum gastrin and CCK concentration in the 778 ATBC Study controls
| Gastrin |
|||||
|---|---|---|---|---|---|
| Characteristics | Q1 (< 9.47 pmol/l) | Q2 (9.48–13.88 pmol/l) | Q3 (13.91–22.00 pmol/l) | Q4 (> 22.10 pmol/l) | aPtrend |
| Number of controls | 190 | 193 | 191 | 191 | |
| Age at baseline (years) | 58 | 58 | 58 | 59 | 0.23 |
| Mean years of smoking | 36 | 36 | 36 | 36 | 0.50 |
| Mean cigarettes/day | 19 | 18 | 20 | 19 | 0.96 |
| Alcohol: g/day | 15 | 15 | 16 | 14 | 0.35 |
| Education: post-elementary school % | 23 | 17 | 20 | 11 | 0.01 |
| Body mass index: kg/m2 | 26 | 27 | 26 | 26 | 0.82 |
| Fruit: g/day | 240 | 198 | 205 | 201 | 0.16 |
| Vegetables: g/day | 314 | 275 | 281 | 291 | 0.11 |
| Helicobacter pylori: % positive | 88 | 94 | 95 | 95 | 0.05 |
| Cholecystokinin |
|||||
|---|---|---|---|---|---|
| Characteristics | Q1 (< 0.47 pmol/l) | Q2 (0.48–1.00 pmol/l) | Q3 (1.01–1.46 pmol/l) | Q4 (> 1.47 pmol/l) | aPtrend |
| Number of controls | 189 | 193 | 191 | 191 | |
| Age at baseline | 58 | 58 | 59 | 59 | 0.05 |
| Mean years of smoking | 37 | 35 | 34 | 37 | 0.73 |
| Mean cigarettes/day | 19 | 20 | 18 | 19 | 0.71 |
| Alcohol: g/day | 21 | 15 | 13 | 11 | 0.0001 |
| Education: post-elementary school % | 17 | 18 | 22 | 14 | 0.71 |
| Body mass index: kg/m2 | 26 | 27 | 26 | 26 | 0.82 |
| Fruit: g/day | 177 | 233 | 231 | 202 | 0.16 |
| Vegetables: g/day | 272 | 298 | 299 | 290 | 0.04 |
| Helicobacter pylori: % positive | 99 | 95 | 88 | 91 | 0.01 |
aMantel-Haenszel trend test for categorical variables and Jonckheere-Terpstra test for trend for continuous variables.
Serum gastrin and CCK concentrations across gastric cancer case types are shown in Figure 1. The median and interquartile range (IQR) for serum gastrin and CCK were similar across controls, GNCA and EGJA. The interquartile range of gastrin and CCK concentrations in controls was 9.5–22.0 pmol/l and 0.5–1.5 pmol/l, respectively. Serum gastrin concentrations for those with carcinoid tumours (median: 153.0 pmol/l) were significantly higher than controls (median: 13.9 pmol/l), GNCA (median: 17.0 pmol/l) and EGJA (median: 13.0 pmol/l).
Figure 1.
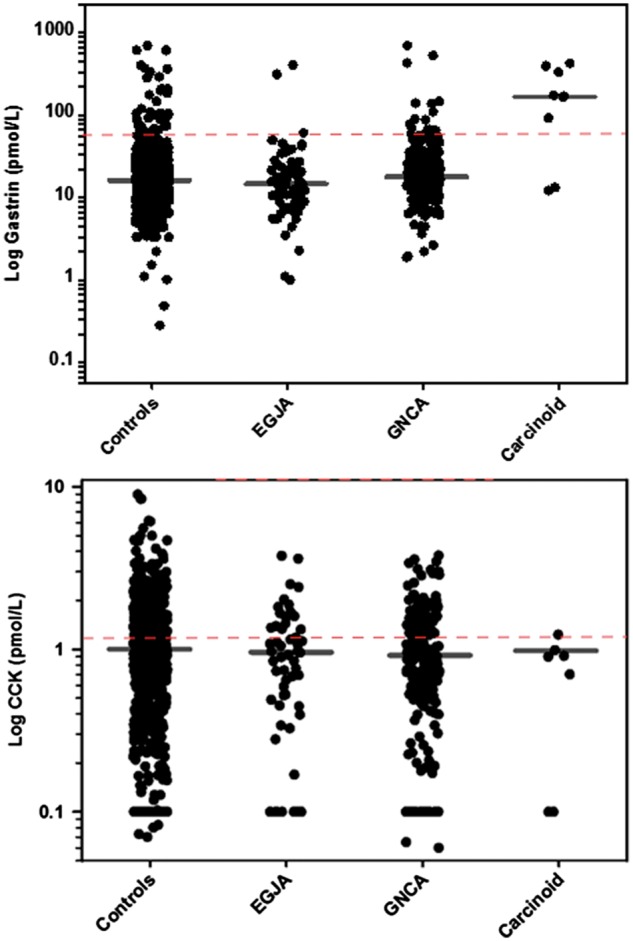
Scatterplots showing the distribution of serum gastrin and CCK concentrations by case type. Horizontal lines denote the median concentration in each group.
Higher gastrin concentrations were significantly associated with increased risk of gastric cancer. For every unit increase in gastrin, the OR for gastric cancer was 1.23 (adjusted model; 95% CI: 1.04, 1.45). Those with higher gastrin (Q4 vs Q1), had significantly increased risk of GNCA (fully adjusted OR: 1.92; 95% CI: 1.21, 3.05; n = 84 cases in Q4, Table 3a) and this trend was statistically significant (P = 0.002). The association between high gastrin and increased risk of GNCA become stronger on additional adjustment for CCK (Q4 vs Q1: OR: 2.56; 95% CI: 1.52, 4.30). In contrast, the OR for gastrin quartiles 2–4 for EGJA was generally below one, with the OR for quartile 2 reaching statistical significance (OR: 0.44; 95% CI: 0.22, 0.90; n = 14 cases). However this association was based on a small number of cases and was not significant following adjustment for CCK (OR: 0.46; 95% CI: 0.20, 1.04). Serum gastrin was also significantly associated with risk of gastric carcinoids, though the small number of carcinoid cases meant the fully adjusted model was unstable (age-adjusted continuous model OR: 4.67; 95% CI: 2.67, 8.15).
Table 3b.
Odds ratios (and 95% confidence intervals) for developing gastric cancer for those individuals by serum concentration of cholecystokinin (continuous and quartile models)
| Group | Cholecystokinin: continuous, log |
Cholecystokinin: quartiles |
Ptrend | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 |
Q3 |
Q4 |
|||||||||
| OR | 95% CI | P-value | OR | 95% CI | OR | 95% CI | OR | 95% CI | ||||
| Gastric cancer (n = 264) | ||||||||||||
| Age-adjusted | 0.80 | 0.70, 0.92 | 0.002 | REF | 1.09 | 0.75, 1.56 | 0.53 | 0.35, 0.82 | 0.72 | 0.48, 1.07 | 0.006 | |
| Adjusted | 0.85 | 0.73, 0.99 | 0.033 | REF | 1.13 | 0.74, 1.70 | 0.60 | 0.37, 0.96 | 0.79 | 0.50, 1.22 | 0.063 | |
| Adj. + gastrin | 0.84 | 0.72, 0.98 | 0.028 | REF | 1.11 | 0.73, 1.70 | 0.58 | 0.35, 0.93 | 0.77 | 0.49, 1.21 | 0.056 | |
| Gastric non-cardia adenocarcinoma (n = 200) | ||||||||||||
| Age-adjusted | 0.80 | 0.69, 0.93 | 0.004 | REF | 1.08 | 0.72, 1.62 | 0.48 | 0.30, 0.79 | 0.75 | 0.49, 1.16 | 0.020 | |
| Adjusted | 0.85 | 0.72, 1.01 | 0.054 | REF | 1.16 | 0.74, 1.82 | 0.56 | 0.33, 0.96 | 0.83 | 0.51, 1.35 | 0.126 | |
| Adj. + gastrin | 0.85 | 0.72, 1.01 | 0.054 | REF | 1.13 | 0.72, 1.80 | 0.53 | 0.31, 0.93 | 0.80 | 0.49, 1.31 | 0.096 | |
| Oesophagogastric junctional adenocarcinoma (n = 57) | ||||||||||||
| Ag-adjusted | 0.84 | 0.65, 1.09 | 0.185 | REF | 0.97 | 0.48, 1.96 | 0.72 | 0.34, 1.56 | 0.67 | 0.30, 1.47 | 0.182 | |
| Adjusted | 0.85 | 0.64, 1.13 | 0.266 | REF | 0.96 | 0.46, 2.01 | 0.77 | 0.34, 1.74 | 0.69 | 0.30, 1.60 | 0.331 | |
| Adj. + gastrin | 0.86 | 0.64, 1.13 | 0.285 | REF | 1.01 | 0.48, 2.15 | 0.75 | 0.32, 1.76 | 0.73 | 0.32, 1.70 | 0.373 | |
| Gastric carcinoid tumours (n = 3) | ||||||||||||
| Age adjusted | 0.66 | 0.34, 1.31 | 0.236 | REF | 0.57; 0.27, 1.22 | 0.127 | ||||||
Adjusted for: age at randomization, total years of smoking and total cigarettes/day, alcohol (g/day), BMI (kg/m2), fruit intake (g/day), vegetable intake (g/day), education (post-primary school), Helicobacter pylori (+/-) and ATBC treatment group assignment; additional adjustment as indicated for gastrin.
REF, reference value.
Table 3a.
Odds ratios (and 95% confidence intervals) for developing gastric cancer for those individuals by serum concentration of gastrin (continuous and quartile models)
| Group | Gastrin: continuous, log |
Gastrin: quartiles |
Ptrend | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 |
Q3 |
Q4 |
||||||||||
| OR | 95% CI | P-value | OR | 95% CI | OR | 95% CI | OR | 95% CI | |||||
| Gastric cancer (n = 329) | |||||||||||||
| Age-adjusted | 1.24 | 1.06, 1.44 | 0.016 | REF | 0.84 | 0.57, 1.24 | 1.03 | 0.71, 1.50 | 1.44 | 1.01, 2.06 | 0.022 | ||
| Adjusted | 1.23 | 1.04, 1.45 | 0.018 | REF | 0.81 | 0.52, 1.25 | 1.08 | 0.71, 1.65 | 1.54 | 1.02, 2.32 | 0.022 | ||
| Adj. + CCK | 1.31 | 1.09, 1.59 | 0.004 | REF | 0.91 | 0.56, 1.48 | 1.34 | 0.84, 2.14 | 1.91 | 1.22, 3.01 | 0.001 | ||
| Gastric non-cardia adenocarcinoma (n = 245) | |||||||||||||
| Age-adjusted | 1.29 | 1.08, 1.54 | 0.005 | REF | 1.03 | 0.66, 1.61 | 1.37 | 0.90, 2.10 | 1.79 | 1.19, 2.70 | 0.002 | ||
| Adjusted | 1.30 | 1.07, 1.59 | 0.009 | REF | 0.99 | 0.61, 1.62 | 1.43 | 0.89, 2.30 | 1.92 | 1.21, 3.05 | 0.002 | ||
| Adj. + CCK | 1.45 | 1.17, 1.79 | 0.001 | REF | 1.17 | 0.67, 2.05 | 1.77 | 1.04, 3.03 | 2.56 | 1.52, 4.30 | < 0.001 | ||
| Oesophagogastric junctional adenocarcinoma (n = 75) | |||||||||||||
| Age-adjusted | 0.82 | 0.61, 1.12 | 0.21 | REF | 0.47 | 0.24, 0.93 | 0.47 | 0.24, 0.92 | 0.63 | 0.34, 1.18 | 0.106 | ||
| Adjusted | 0.89 | 0.66, 1.20 | 0.45 | REF | 0.44 | 0.22, 0.90 | 0.51 | 0.25, 1.04 | 0.71 | 0.36, 1.39 | 0.106 | ||
| Adj. + CCK | 0.86 | 0.61, 1.21 | 0.38 | REF | 0.46 | 0.20, 1.04 | 0.71 | 0.33, 1.52 | 0.65 | 0.30, 1.44 | 0.415 | ||
| Gastric carcinoid tumours (n = 9) | |||||||||||||
| Age-adjusted | 4.67 | 2.67, 8.15 | < 0.001 | REF | 3.07; 1.25, 7.54 | 0.005 | |||||||
Adjusted for: age at randomization, total years of smoking and total cigarettes/day, alcohol (g/day), BMI (kg/m2), fruit intake (g/day), vegetable intake (g/day), education (post-primary school), Helicobacter pylori (+/-) and ATBC treatment group assignment; additional adjustment as indicated for CCK.
REF, reference value.
In continuous models, increasing concentration of CCK was statistically significantly associated with a decrease in risk of gastric cancer (adjusted model: OR: 0.85; 95% CI: 0.73, 0.99) and a similar trend was observed across quartiles of serum CCK, though this was not statistically significant (P = 0.063). No significant trend was noted for CCK concentrations and risk of GNCA, either in the continuous model (OR: 0.85; 95% CI: 0.72, 1.01) or the model using quartiles (Ptrend = 0.126). However, individuals in Q3 of CCK concentration had a significantly decreased risk of GNCA (OR: 0.56; 95% CI: 0.33, 0.96; n = 29 cases), though this association was based on a small number of cases and may be spurious. The small number of gastric carcinoids meant that we were unable to rule out an association between high CCK and risk of gastric carcinoid tumour, and we caution against any definitive interpretation of this finding (OR: 0.66; 95% CI: 0.34, 1.31). No change in risk estimates was noted when gastrin was added to the CCK risk models.
The temporal nature of the association between serum gastrin and CCK and risk of gastric cancer was explored in a series of lag analyses (Table 4; gastric carcinoids were excluded because of small numbers). Cases were divided into those occurring with 5 years of serum collection/baseline, between 5 and 10 years of baseline, and more than 10 years after baseline serum collection. For those with high serum gastrin, risk of GNCA was highest for those diagnosed more than 10 years after baseline blood draw compared with those with normal gastrin concentrations. In this group, each unit increase in gastrin (log-scale) was associated with a 55% increase in risk of GNCA (OR: 1.55; 95% CI: 1.24, 1.95). When EGJA cases were classified by time to diagnosis, no significant associations with gastrin were noted. CCK concentrations were significantly associated with risk of GNCA and EGJA for tumours developing within 5 years of blood draw. For these tumours, every unit increase in CCK was associated with a 45% decrease in risk of GNCA (OR: 0.54; 95% CI: 0.36, 0.81) and a 63% decrease in risk of EGJA (OR: 0.37; 95% CI: 0.14, 0.98). The association for GNCA was not consistent over time, in that no significant association was noted for tumours developing between 5 and 10 years of baseline; However, there was a significant 25% decrease in risk for tumours developing more than 10 years after blood draw (OR: 0.74; 95% CI: 0.62, 0.89). For EGJA cases in particular, we note relatively few cases in each stratum.
Table 4.
Odds ratios (and 95% confidence intervals) for developing gastric cancer, by time from baseline to cancer diagnosis, gastrin and CCK continuous (log-transformed)
| Gastrin: continuous |
Cholecystokinin: continuous |
|||||
|---|---|---|---|---|---|---|
| N cases | ORa | 95% CI | N cases | ORa | 95% CI | |
| Gastric cancera | ||||||
| < 5 years | 54 | 0.97 | 0.69, 1.35 | 24 | 0.49 | 0.34, 0.71 |
| 5–10 years | 108 | 1.19 | 0.94, 1.51 | 87 | 1.09 | 0.86, 1.38 |
| > 10 years | 166 | 1.41 | 1.15, 1.72 | 153 | 0.75 | 0.64, 0.89 |
| Gastric non-cardia adenocarcinomab | ||||||
| < 5 years | 42 | 0.77 | 0.52, 1.14 | 19 | 0.54 | 0.36, 0.81 |
| 5–10 years | 81 | 1.24 | 0.95, 1.63 | 64 | 1.08 | 0.82, 1.41 |
| > 10 years | 122 | 1.55 | 1.24, 1.95 | 117 | 0.74 | 0.62, 0.89 |
| Oesophagogastric junctional adenocarcinomab | ||||||
| < 5 years | 10 | 0.72 | 0.33, 1.59 | 4 | 0.37 | 0.14, 0.98 |
| 5–10 years | 24 | 0.73 | 0.43, 1.22 | 20 | 1.11 | 0.69, 1.77 |
| > 10 years | 41 | 0.91 | 0.60, 1.37 | 33 | 0.79 | 0.57, 1.11 |
1Adjusted for: age at randomization.
bGastric carcinoid tumours were not included in this analysis because of small numbers (so that GNCA and EGJA case numbers will not sum to gastric cancer numbers).
Discussion
In our prospective study, we observed that men with higher serum gastrin concentrations had a significantly increased risk of developing GNCA many years later. We also observed that higher gastrin levels were strongly associated with increased risk of gastric carcinoid tumours. No clear association was noted between concentrations of CCK and risk of GNCA, in that the trend was not significant. These associations appeared independent of several known risk factors for gastric cancer and were statistically significant even for those cancers diagnosed many years after blood draw.
Results from previous cross-sectional studies and case reports of gastrin and gastric carcinoid tumours are consistent with our findings.29,30 Carcinoid tumours are of enterochromaffin-like (ECL) cell origin and can arise anywhere in the digestive tract. It seems likely that the association we describe here between gastric carcinoid tumours and high levels of serum gastrin arises from early, pre-diagnostic, gastric carcinoid tumours producing high levels of gastrin. Because gastrin is trophic (tumour promoting), it is possible that these small gastric carcinoid tumours may be either produce gastrin, or be in proximity to cells which produce gastrin, which then feeds back in a positive loop to stimulate growth of the carcinoid tumour and further secretion of gastrin. The small numbers of carcinoid tumours in our study meant we could not explore the association; however, we note it here as an interesting observation. Likewise, we note that small numbers also limit any interpretation of our models relating to high CCK and risk of gastric carcinoid tumours.
We also observed a statistically significant positive association between high gastrin levels and GNCA, but no significant association with EGJA where, if anything, the association was inverse. These two upper gastrointestinal tumours are acknowledged to be difficult to distinguish, and yet a growing body of evidence suggests that they have distinct risk factors. Most notably, previous work by our group within this same population demonstrated that whereas H. pylori seropositivity is a strong risk factor for GNCA, it is inversely associated with EGJA.31 In contrast, serological atrophy has been shown to be a risk factor for both GNCA and EGJA in this population.26 We note with interest that Fossmark et al.,32 in a cross-sectional study, had observed that hypergastrinaemia is more frequently observed for gastric cancer arising in the gastric corpus.33
Although few previous prospective data on gastrin levels and gastric cancer are available, one previous prospective study did link elevated gastrin levels to increased risk of incident colorectal adenocarcinoma,34 finding an OR for high gastrin (defined as > 90 pg/ml) of 3.9 (95% CI: 1.5, 9.7). Gastrin is known to influence a number of carcinogenic processes: inducing expression and function of anti-apoptotic proteins and increasing proliferation of gastric mucosal cells through transcriptional activation of a number of genes.4 It is known to be pro-angiogenic, promoting tubule formation in human umbilical vascular endothelial cells (HUVEC) by stimulating heparin-binding epidermal growth factor expression/shedding which results in enhanced matrix metalloproteinase expression.35 Further, human gastric epithelial cells stimulated with gastrin express matrix metalloproteinase 9, and increased invasion of the gastric cancer cells through an artificial basement membrane is observed.36
As gastrin is produced within the stomach, high levels could alternatively simply reflect the altered milieu of gastric atrophy, rather than playing a specific aetiological role in carcinogenesis. Unfortunately, within the confines of the current study, it is not possible to distinguish between these scenarios. Furthermore, we found no correlation between levels of gastrin and the most widely used serological marker of gastric atrophy, pepsinogen I, with little change to our observed associations after pepsinogen I adjustment. Measuring gastrin and pepsinogen within an endoscopic surveillance setting, with additional histological data on atrophy, would help provide additional data on whether gastrin levels merely reflect altered gastric mucosa preceding gastric cancer.
Given the current widespread use of proton pump inhibitors (PPI) in the over-the-counter treatment of general indigestion, one might wonder about the role of proton pump inhibitor (PPI) usage in our study, as PPI treatment is known to cause high levels of gastrin.37–39 A particular strength of our study is that it began before widespread use of PPIs and so we can rule PPI usage out as an explanation of the hypergastrinaemia we observe. Future studies are needed to determine the role of PPIs in gastric cancer.40 High gastrin levels may also mediate previously observed associations between clinical conditions and gastric cancer. For example, the prevalence of pernicious anaemia, in which patients appear to have both higher gastrin levels and higher risk of gastric adenocarcinoma as well as gastric carcinoid tumours,41 is estimated to range between 2% and 5%42,43 in older populations. Zollinger-Ellison syndrome (ZES) is a rare syndrome which causes very high gastrin levels. The true incidence and prevalence of ZES is unknown in Finland, although the estimated annual incidence in the general population is one case per million people.44,45 It is therefore very unlikely that the associations we see result from a large number of ZES cases. We believe our findings are therefore likely applicable to the general population.
CCK has not been as intensively studied as gastrin, largely because accurate measurement of the hormone has only become possible recently.9,11 CCK is a potent inhibitor of gastric acid secretion, and it is also known to regulate pancreatic growth and enzyme secretion and contraction of the gallbladder. Interestingly, CCK also relaxes the proximal stomach and stimulates the pyloric sphincter, which inhibits gastric emptying and induces satiety.9 The physiological consequences of high concentrations of CCK have not been widely studied; exogenous administration of CCK has been shown to promote pancreatic cancer in animal models, though usually this is concomitant with the administration of a carcinogen or in the presence of pre-existing inflammation.46 We note with interest that a case report of a patient with a CCK-oma detailed symptoms of non-watery diarrhoea, severe weight loss, and gallbladder and peptic ulcer disease.47 As with gastrin, non-aetiological mechanisms for the associations we observed with CCK are also possible. It is interesting to note that adjustment for CCK in the risk models for gastrin appear to strengthen the association between gastrin and risk of GNCA, though the converse appears to have no effect. This may reflect the physiological cross-talk between gastrin and CCK, or chance.
Our results further underscore the importance of accurate measurement of gastrin and CCK. Many commercially available kits in use by diagnostic clinical laboratories are known to be suboptimal in that they measure only individual forms of gastrin.10 In contrast, our measurement included gastrin -14, -17, -34, -52 and -71 with equimolar potency, regardless of the degree of sulphation. Similarly, our CCK measurement included CCK-8, -22, -33 and -58. Further, our gastrin and CCK assays did not cross-react. Given the independent associations we report between high concentrations of gastrin and CCK and increased risk of gastric cancer, accurate and specific measurement of both total gastrin and CCK seems particularly important.
Our study has several significant strengths, including its large size and prospective design, which allowed us to look at risk of gastric cancer after many years of follow-up. The ATBC study was also carried out on serum collected before the introduction of PPIs, which allowed us to rule this out as a possible reason for elevated serum gastrin concentrations. We also had a wealth of information on potential confounders for our analyses, including: H. pylori infection status, pepsinogen 1, smoking and alcohol data, dietary data and body mass index, for example. Last, we used assays for both gastrin and CCK that are entirely specific to the individual circulating forms of the hormones, and importantly, can distinguish between these structurally similar peptides.
We also acknowledge notable limitations in that our study included only middle-aged male Finnish smokers and our results require replication in other populations. We measured gastrin and CCK at just a single point in time and we acknowledge the limited dynamic range for circulating concentrations of CCK. We included relatively few gastric carcinoid tumours, reflective of the relative rarity of these tumours, and so our statistical power was low. Also, we lacked H. pylori infection sero-status on one of our two matched controls per case, although the prevalence of H. pylori is very high in the ATBC, and restricting our analyses to cases and controls with H. pylori serology measurements showed a similar association. Last, the coefficient of variation for our gastrin and CCK assays was higher than we would have liked, though this suggests that the true associations may be even stronger than those reported here.
In conclusion, in this prospective study, we report that individuals with higher serum concentrations of gastrin were at significantly increased risk of developing gastric non-cardia adenocarcinomas and gastric carcinoid tumours. In contrast, higher circulating concentrations of CCK were associated with a lower risk of GNCA, though this association was limited to a single quartile and showed no significant trend. These intriguing results require replication in future studies to further elucidate the potential role of gastrin and CCK in gastric cancer.
Supplementary Data
Supplementary data are available at IJE online.
Funding
This research was supported in part by the Intramural Research Program of the NIH and the National Cancer Institute. Additionally, this research was supported by U.S. Public Health Service contracts N01-CN-45165, N01-RC-45035, N01-RC-37004 and HHSN261201000006C from the National Cancer Institute, Department of Health and Human Services.
Conflict of interest: None declared.
Key Messages
Gastrin has long been hypothesized to play a role in gastric carcinogenesis.
The role of cholecystokinin (CCK) in carcinogenesis has not been studied, largely due to difficulties in measuring CCK in serum.
We used highly specific radioimmunoassays to measure gastrin and CCK concentrations in serum, to determine whether these concentrations were associated with risk of gastric cancer many years later.
Participants with higher serum concentrations of gastrin at baseline had an increased risk of gastric non-cardia adenocarcinoma and gastric carcinoid tumours.
In contrast, no clear associations with serum CCK concentrations were observed.
Supplementary Material
References
- 1. Ferlay JSI, Ervik M, Dikshit R. et al. GLOBOCAN 2012 v1.0. Cancer Incidence and Mortality Worldwide. Lyon, France: International Agency for Research on Cancer, 2013. [Google Scholar]
- 2. Burkitt MD, Varro A, Pritchard DM. Importance of gastrin in the pathogenesis and treatment of gastric tumours. World J Gastroenterol 2009;15:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferrand A, Wang TC. Gastrin and cancer: a review. Cancer Lett 2006;238:15–29. [DOI] [PubMed] [Google Scholar]
- 4. Watson SA, Grabowska AM, El-Zaatari M, Takhar A. Gastrin - active participant or bystander in gastric carcinogenesis? Nat Rev Cancer 2006;6:936–46. [DOI] [PubMed] [Google Scholar]
- 5. Friis-Hansen L, Rieneck K, Nilsson HO, Wadstrom T, Rehfeld JF. Gastric inflammation, metaplasia, and tumour development in gastrin-deficient mice. Gastroenterology 2006;131:246–58. [DOI] [PubMed] [Google Scholar]
- 6. Zavros Y, Eaton KA, Kang W. et al. Chronic gastritis in the hypochlorhydric gastrin-deficient mouse progresses to adenocarcinoma. Oncogene 2005;24:2354–66. [DOI] [PubMed] [Google Scholar]
- 7. Beales I, Blaser MJ, Srinivasan S. et al. Effect of Helicobacter pylori products and recombinant cytokines on gastrin release from cultured canine G cells. Gastroenterology 1997;113:465–71. [DOI] [PubMed] [Google Scholar]
- 8. Suzuki T, Grand E, Bowman C. et al. TNF-alpha and interleukin 1 activate gastrin gene expression via MAPK- and PKC-dependent mechanisms. Am J Physiol Gastrointest Liver Physiol 2001;281:G1405–12. [DOI] [PubMed] [Google Scholar]
- 9. Rehfeld JF. Clinical endocrinology and metabolism. Cholecystokinin. Best Pract Res Clin Endocrinol Metab 2004;18:569–86. [DOI] [PubMed] [Google Scholar]
- 10. Rehfeld JF, Gingras MH, Bardram L, Hilsted L, Goetze JP, Poitras P. The Zollinger-Ellison syndrome and mismeasurement of gastrin. Gastroenterology 2011;140:1444–53. [DOI] [PubMed] [Google Scholar]
- 11. Rehfeld JF. Accurate measurement of cholecystokinin in plasma. Clin Chem 1998;44:991–1001. [PubMed] [Google Scholar]
- 12. Rehfeld JF, van Solinge WW. The tumour biology of gastrin and cholecystokinin. Adv Cancer Res 1994;63:295–347. [DOI] [PubMed] [Google Scholar]
- 13. The ATBC cancer Prevention Study Group. The alpha-tocopherol, beta-carotene lung cancer prevention study: Design, methods, participant characteristics, and compliance. Ann Epidemiol 1994;4:1–10. [DOI] [PubMed] [Google Scholar]
- 14. Albanes D, Heinonen OP, Taylor PR. et al. Alpha-tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of base-line characteristics and study compliance. J Natl Cancer Inst 1996;88:1560–70. [DOI] [PubMed] [Google Scholar]
- 15. The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. The ATBC Cancer Prevention Study Group. Ann Epidemiol 1994;4:1–10. [DOI] [PubMed] [Google Scholar]
- 16. World Health Organization. International Statistical Classification of Diseases and Related Health Problems. Geneva: WHO, 1978. [Google Scholar]
- 17. Pietinen P, Hartman AM, Haapa E. et al. Reproducibility and validity of dietary assessment instruments. II. A qualitative food frequency questionnaire. Am J Epidemiol 1988;128:667–76. [DOI] [PubMed] [Google Scholar]
- 18. Pietinen P, Hartman AM, Haapa E. et al. Reproducibility and validity of dietary assessment instruments. I. A self-administered food use questionnaire with a portion size picture booklet. Am J Epidemiol 1988;128:655–66. [DOI] [PubMed] [Google Scholar]
- 19. Rehfeld JF, Stadil F, Rubin B. Production and Evaluation of Antibodies for the Radioimmunoassay of Gastrin. Scand J Clin Lab Invest 1972;30:221–32. [DOI] [PubMed] [Google Scholar]
- 20. Rehfeld JF. Gastrins in serum. A review of gastrin radioimmunoanalysis and the discovery of gastrin heterogeneity in serum. Scand J Gastroenterol 1973;8:577–83. [PubMed] [Google Scholar]
- 21. Rehfeld JF, Sun G, Christensen T, Hillingso JG. The predominant cholecystokinin in human plasma and intestine is cholecystokinin-33. J Clin Endocrinol Metab 2001;86:251–58. [DOI] [PubMed] [Google Scholar]
- 22. Michel A, Waterboer T, Kist M, Pawlita M. Helicobacter pylori multiplex serology. Helicobacter 2009;14:525–35. [DOI] [PubMed] [Google Scholar]
- 23. Waterboer T, Sehr P, Michael KM. et al. Multiplex human papillomavirus serology based on in situ-purified glutathione s-transferase fusion proteins. Clin Chem 2005;51:1845–53. [DOI] [PubMed] [Google Scholar]
- 24. Gao L, Michel A, Weck MN, Arndt V, Pawlita M, Brenner H. Helicobacter pylori infection and gastric cancer risk: evaluation of 15 H.pylori proteins determined by novel multiplex serology. Cancer Res 2009;69:6164–70. [DOI] [PubMed] [Google Scholar]
- 25. Gao L, Weck MN, Michel A, Pawlita M, Brenner H. Association between chronic atrophic gastritis and serum antibodies to 15 Helicobacter pylori proteins measured by multiplex serology. Cancer Res 2009;69:2973–80. [DOI] [PubMed] [Google Scholar]
- 26. Murphy G, Kamangar F, Dawsey SM. et al. The relationship between serum ghrelin and the risk of gastric and esophagogastric junctional adenocarcinomas. J Natl Cancer Inst 2011;103:1123–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Varis K, Sipponen P, Laxen F. et al. Implications of serum pepsinogen I in early endoscopic diagnosis of gastric cancer and dysplasia. Helsinki Gastritis Study Group. Scand J Gastroenterol 2000;35:950–56. [DOI] [PubMed] [Google Scholar]
- 28. Malila N, Taylor PR, Virtanen MJ. et al. Effects of alpha-tocopherol and beta-carotene supplementation on gastric cancer incidence in male smokers (ATBC Study, Finland). Cancer Causes Control 2002;13:617–23. [DOI] [PubMed] [Google Scholar]
- 29. Thomas D, Tsolakis AV, Grozinsky-Glasberg S. et al. Long-term follow-up of a large series of patients with type 1 gastric carcinoid tumours: data from a multicenter study. Eur J Endocrinol 2013;168:185–93. [DOI] [PubMed] [Google Scholar]
- 30. Hung OY, Maithel SK, Willingham FF, Farris AB III, Kauh JS. Hypergastrinemia, type 1 gastric carcinoid tumors: diagnosis and management. J Clin Oncol 2011;29:e713–15. [DOI] [PubMed] [Google Scholar]
- 31. Kamangar F, Dawsey SM, Blaser MJ. et al. Opposing risks of gastric cardia and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity. J Natl Cancer Inst 2006;98:1445–52. [DOI] [PubMed] [Google Scholar]
- 32. Fossmark R, Sagatun L, Nordrum IS, Sandvik AK, Waldum HL. Hypergastrinemia is associated with adenocarcinomas in the gastric corpus and shorter patient survival. APMIS 2015;123:509–14. [DOI] [PubMed] [Google Scholar]
- 33. Waldum HL, Hauso O, Sordal OF, Fossmark R. Gastrin May Mediate the Carcinogenic Effect of Helicobacter pylori Infection of the Stomach. Dig Dis Sci 2015;60:1522–27. [DOI] [PubMed] [Google Scholar]
- 34. Thorburn CM, Friedman GD, Dickinson CJ, Vogelman JH, Orentreich N, Parsonnet J. Gastrin and colorectal cancer: a prospective study. Gastroenterology 1998;115:275–80. [DOI] [PubMed] [Google Scholar]
- 35. Clarke PA, Dickson JH, Harris JC, Grabowska A, Watson SA. Gastrin enhances the angiogenic potential of endothelial cells via modulation of heparin-binding epidermal-like growth factor. Cancer Res 2006;66:3504–12. [DOI] [PubMed] [Google Scholar]
- 36. Wroblewski LE, Pritchard DM, Carter S, Varro A. Gastrin-stimulated gastric epithelial cell invasion: the role and mechanism of increased matrix metalloproteinase 9 expression. Biochem J 2002;365:873–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Orlando LA, Lenard L, Orlando RC. Chronic hypergastrinemia: causes and consequences. Dig Dis Sci 2007;52:2482–89. [DOI] [PubMed] [Google Scholar]
- 38. Creutzfeldt W, Lamberts R. Is hypergastrinaemia dangerous to man? Scand J Gastroenterol Suppl 1991;180:179–91. [DOI] [PubMed] [Google Scholar]
- 39. Jensen RT. Consequences of long-term proton pump blockade: insights from studies of patients with gastrinomas. Basic Clin Pharmacol Toxicol 2006;98:4–19. [DOI] [PubMed] [Google Scholar]
- 40. Song H, Zhu J, Lu D. Long-term proton pump inhibitor (PPI) use and the development of gastric pre-malignant lesions. Cochrane Database Syst Rev 2014;12:CD010623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Murphy G, Dawsey SM, Engels EA. et al. Cancer Risk Following Pernicious Anemia in the US Elderly Population. Clin Gastroenterol Hepatol 2015;13:2282–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carmel R. Prevalence of undiagnosed pernicious anemia in the elderly. Arch Intern Med 1996;156:1097–100. [PubMed] [Google Scholar]
- 43. Lindenbaum J, Rosenberg IH, Wilson PW, Stabler SP, Allen RH. Prevalence of cobalamin deficiency in the Framingham elderly population. Am J Clin Nutr 1994;60:2–11. [DOI] [PubMed] [Google Scholar]
- 44. Tomassetti P, Salomone T, Migliori M, Campana D, Corinaldesi R. Optimal treatment of Zollinger-Ellison syndrome and related conditions in elderly patients. Drugs Aging 2003;20:1019–34. [DOI] [PubMed] [Google Scholar]
- 45. Jacobsen O, Bardram L, Rehfeld JF. The requirement for gastrin measurements. Scand J Clin Lab Invest 1986;46:423–26. [DOI] [PubMed] [Google Scholar]
- 46. Smith JP, Solomon TE. Cholecystokinin and pancreatic cancer: the chicken or the egg? Am J Physiol Gastrointest Liver Physiol 2014;306:G91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rehfeld JF, Federspiel B, Bardram L. A neuroendocrine tumour syndrome from cholecystokinin secretion. N Engl J Med 2013;368:1165–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


