Abstract
Pesticide exposure is linked to Parkinson’s disease, a neurodegenerative disorder marked by dopamine cell loss in the substantia nigra of the basal ganglia (BG) that often presents asymmetrically. We previously reported that pesticide-exposed agricultural workers (AW) have nigral diffusion tensor imaging (DTI) changes. The current study sought to confirm this finding, and explore its hemisphere and regional specificity within BG structures using an independent sample population. Pesticide exposure history, standard neurological exam, high-resolution magnetic resonance imaging (T1/T2-weighted and DTI), and [123I]ioflupane SPECT images (to quantify striatal dopamine transporters) were obtained from 20 AW with chronic pesticide exposure and 11 controls. Based on median cumulative days of pesticide exposure, AW were subdivided into high (AWHi, n = 10) and low (AWLo, n = 10) exposure groups. BG (nigra, putamen, caudate, and globus pallidus [GP]) fractional anisotropy (FA), mean diffusivity (MD), and striatal [123I]ioflupane binding in each hemisphere were quantified, and compared across exposure groups using analysis of variance. Left, but not right, nigral and GP FA were significantly lower in AW compared with controls (p’s < .029). None of the striatal (putamen and caudate) DTI or [123I]ioflupane binding measurements differed between AW and controls. Subgroup analyses indicated that significant left nigral and GP DTI changes were present only in the AWHi (p ≤ .037) but not the AWLo subgroup. AW, especially those with higher pesticide exposure history, demonstrate lateralized microstructural changes in the nigra and GP, whereas striatal areas appear relatively unaffected. Future studies should elucidate how environmental toxicants cause differential lateralized- and regionally specific brain vulnerability.
Keywords: substantia nigra, striatum, globus pallidus, MRI, DAT scan, pesticide exposure
Parkinson’s disease (PD) is marked primarily by loss of dopamine neurons in the substantia nigra (SN) of the basal ganglia (BG) and striatal dopamine deficiency (Dickson etal., 2009; Fearnley and Lees, 1991). The fact that identical twins frequently are discordant for PD suggests that environmental factors may be associated with the etiology of sporadic PD through mechanisms such as oxidative stress, neuroinflammation, and/or cytotoxicity (Tanner etal., 1999; Wirdefeldt etal., 2004). Recent epidemiologic data support the hypothesis that pesticide exposure is associated with increased risk of PD (McCormack etal. 2002; Pezzoli and Cereda, 2013; Tanner etal., 2011). In addition, a number of pesticides (eg, paraquat, rotenone, maneb) can cause direct dopaminergic toxicity in animal models (Desplats etal., 2012; Uversky, 2004).
The onset of PD motor symptoms is typically asymmetric (Riederer and Sian-Hulsmann, 2012) and more often present on the right side of the body (Uitti etal., 2005). Several studies have sought to identify a cause for this laterality. For example, asymmetrical changes in dopamine transporter levels have been reported in the striatum of PD patients (Siebyl etal., 1995), but the exact causes and mechanisms of the lateralized vulnerability to PD are unknown.
Recently, magnetic resonance imaging (MRI) has been used extensively to study PD-related pathological changes in human subjects (Chan etal., 2007; Du etal., 2012; Martin etal., 2008; Vaillancourt etal., 2009). In particular, diffusion tensor imaging (DTI), by measuring microstructural organization or integrity, has shown promise as a tool for assessing PD-related nigral changes probably due to dopamine cell loss (Chan etal., 2007; Du etal., 2011; Peran etal., 2010). In the MPTP PD mouse model, DTI changes are correlated with dopamine neuron loss in the SN (Boska etal., 2007). Furthermore, several human studies have demonstrated reduced FA values in the SN of early PD patients, consistent with the notion that DTI changes may be able to detect nigral microstructural changes and track their progression invivo (Du etal., 2012; Ofori etal., 2015; Vaillancourt etal., 2009).
We recently demonstrated decreased diffusion measures in asymptomatic agricultural workers (AW) exposed to pesticides (Du etal., 2014), providing the first direct evidence that chronic pesticide exposure may lead to nigral microstructural changes in humans that are qualitatively similar to PD. In this study, we first sought to confirm this finding, and then investigate whether changes occurred in a lateralized- and regionally specific manner within BG structures using MRI DTI (to reflect microstructural integrity). We hypothesized that: (1) other BG structures also may be susceptible to pesticide-related neurotoxicity; and (2) left side BG structures may be more vulnerable to pesticides because PD often presents with right side symptoms (Uitti etal., 2005). Second, we examined nigrostriatal integrity via [123I]ioflupane SPECT (single photon emission computed tomography) imaging (to visualize striatal dopamine transporters). Striatal dopaminergic terminal deficits are an essential feature of PD. We hypothesized that striatal dopaminergic transporter binding would be lower in AW with a history of pesticide exposure since our previous study (Du etal., 2014) showed that AW had similar, but less severe, nigral microstructural changes (Du etal., 2012; Ofori etal., 2015; Vaillancourt etal., 2009). Third, we investigated the relationships between BG imaging measurements and pesticide exposure. We hypothesized that: (1) subjects with longer durations of pesticide exposure will be more likely to have BG imaging changes; and (2) the BG imaging changes will be correlated with duration of pesticide exposure. Lastly, we explored the relationship between significant MRI DTI changes and dopamine transporter density.
MATERIALS AND METHODS
Subjects
A total of 20 AW and 11 controls were recruited from regional AW meetings in central Pennsylvania, USA, and from the community around the Penn State Hershey Medical Center (PSHMC). Those contacting us and expressing an interest in the study were screened for inclusion/exclusion criteria that included age 45-75 years, able/willing to provide consent, no significant memory impairment (Mini Mental Status Exam (MMSE) > 24), normal kidney or liver function, no significant medical and or neurological deficits, and no claustrophobia or other condition precluding an MRI. AW were defined as subjects who had applied pesticides at any point in their lifetime and controls as those who did not have any history of pesticide exposure. Seven subjects (1 control and 6 AW) participated in a previous study focused on pesticide exposure effects on MRI measures in the SN (Du etal., 2014). Subjects were all male and answered negatively for past diagnosis of neurological disorders. As part of the screening visit, detailed demographic information was obtained from all subjects. Since all subjects in the overall cohort were male, matching by gender was not an issue for the current study. Written informed consent was obtained from all subjects in accordance with the Declaration of Helsinki, and approved by the PHMC Internal Review Board/Human Subjects Protection Office.
Clinical Information
Height and weight were measured to calculate body mass index (BMI) for each subject. Handedness was determined by the dominant hand used during writing and eating, and all subjects were right-handed except for 1 control subject. A fasting blood sample was collected the morning of the study visit and assayed at the PSHMC clinical lab to rule out major medical issues (such as anemia, renal or liver dysfunctions). All subjects were examined and ascertained to be free of any obvious neurological and movement deficits using the UPDRS motor scores (UPDRS-III) with a threshold score of <15 indicating lack of parkinsonian motor signs as defined by a previous study (Lee etal., 2015). The UPDRS-III exams were performed by trained raters according to the Movement Disorder Society training modules. Subjects also completed sections I (a nonmotor-related functional questionnaire) and II (a motor-related functional questionnaire) of the UPDRS.
Exposure Assessment
Pesticide exposure was evaluated using a validated and detailed questionnaire from the Agriculture Health Study (AHS). The AHS is a large prospective study of the National Institute of Environmental Health Sciences that examines health effects among AW who have been exposed to pesticides (http://www.niehs.nih.gov). Using the AHS questionnaire, we evaluated frequency (ie, average annual number of days used) and duration (ie, number of exposed years) of pesticide exposures, including types of pesticide handled (ie, mixing, loading, and/or application), personal protective equipment use, and personal hygiene practices. Pesticides assessed were selected because of their frequency and overall use in the agricultural settings of central Pennsylvania, or because of human or animal data suggesting their possible adverse health effects. The pesticides to which subjects were exposed are listed in Supplementary Table 1.
Pesticide exposures in AW and control subjects were calculated based on the number of days of exposure. AW then were dichotomized into 2 groups based on the median: low exposure AW (AWLo) and high exposure AW (AWHi). The dichotomized exposure variable was the main exposure metric for all subjects.
Imaging Data Acquisition
Brain MRI data acquisition
All subjects were scanned with a 3.0 Tesla MRI system (Trio, Siemens Magnetom, Erlangen, Germany, 8-channel phased array head coil) with high-resolution T1-, T2-weighted, and DTI sequences. A 3D MPRAGE with TR = 1540 ms, TE = 2.3 ms, isotropic 1-mm voxels was utilized to acquire T1-weighted images. A fast-spin-echo sequence was used to obtain T2-weighted images with TR/TE = 2500/316, FOV = 256 × 256 mm, matrix = 256 × 256, slice thickness = 1 mm (with no gap), and slice number = 176. For DTI, acquisition parameters were as follows: TR/TE = 8300/82 ms, b value = 1000 s/mm2, diffusion gradient directions = 42 and 7 b = 0 scans, FOV = 256 × 256 mm, matrix = 128 × 128, slice thickness = 2 mm (with no gap), and slice number = 65.
Dopamine transporter data acquisition
[123I]Ioflupane (DaTscan, GE Healthcare, Ltd, Marlborough, Massachusetts) was used to image dopamine transporters. Subjects ingested Lugol’s solution diluted in water (equivalent to 100 mg of iodide) to protect the thyroid gland. An IV line was placed by an experienced nurse and 1 h after ingestion of the iodide solution, subjects were injected with 4 mCi of [123I]ioflupane. Images of the brain were acquired 4 h later using a SPECT camera (Siemens, Symbia Evo) with the recommended imaging parameters (Kupsch etal., 2012). The reconstructed images then were transferred to a secure research picture archiving and communication system for further analysis.
Data Analysis
Segmentation of BG structures on MRI
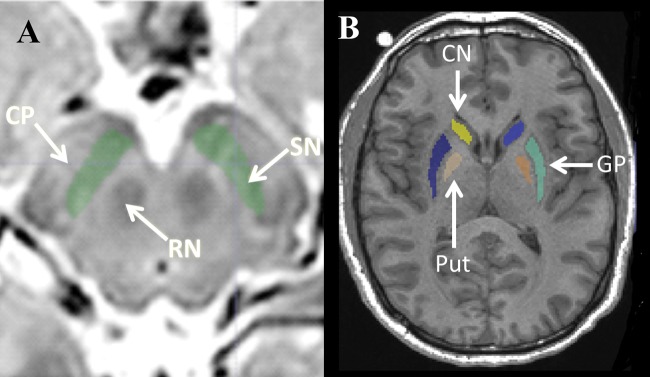
The SN was defined manually on T2-weighted images using ITK-SNAP (Yushkevich etal., 2006) by an investigator blinded to subject identification. This region of interest was defined as a hypointense band between the red nucleus (RN) and cerebral peduncle (CP) in axial sections (Du etal., 2011; Massey and Yousry, 2010; Vaillancourt etal., 2012), as illustrated in Figure 1A. The most superior extent of the SN segmentation was defined at 1 slice inferior to the axial section of the RN showing the largest radius. A total of 4–6 slices (from the rostral to caudal part of the SNpc) were used as the SN region of interest (ROI) (Yushkevich etal., 2006). The right and left SN were analyzed separately. Another set of SN ROIs also was obtained by a second rater (MG) using the same segmentation protocol, thus providing an estimate of the inter-rater reliability using intra-class correlation coefficients. Putamen (Put), caudate, and globus pallidus (GP) ROIs were defined on high-resolution T1-weighted images using multi-atlas automatic segmentation software (AutoSeg) for each subject (Gouttard etal., 2007; Figure 1B).
Figure 1.
Schematic of the segmentation of BG structures. A, Illustration of ROI definition of the SN. The arrows indicate the SN, RN, and CP. B, Illustration of the ROI definition of the caudate (CN), Put, and GP. Regions are indicated by arrows.
Estimation of DTI measures
DTI image quality control and tensor reconstruction was performed using DTIPrep (Neuro Image Research and Analysis Laboratory, University of North Carolina, Chapel Hill, North Carolina, USA) that first checks DWI images for appropriate quality by calculating the inter-slice and inter-image intra-class correlation, and then corrects for the distortions induced by eddy currents and head motion (Liu etal., 2010). Diffusion tensor images then were estimated via weighted least squares (Salvador etal., 2005). DTI is thought to reflect the diffusion of water molecules in tissues and thus is an indicator of the structural organization in a region. Two DTI values (fractional anisotropy [FA] and mean diffusivity [MD]) were calculated out of 3 diffusivity eigenvalues (λ1, λ2, λ3; Le Bihan etal., 2001). FA is a weighted average of pairwise differences of the 3 eigenvalues and may reflect the degree of diffusion anisotropy.
MD is an average of the 3 eigenvalues and provides information regarding the magnitude of the diffusion (Du etal., 2014; Le Bihan etal., 2001; Lee etal., 2016).
The segmented bilateral ROIs were mapped to FA maps by co-registering the T2-weighted images to the B0 images of DTI data using an affine registration pipeline implemented in 3D Slicer (www.slicer.org) (Rueckert etal., 1999). Mean FA and MD values then were obtained for further analysis.
Quantification of striatal DAT binding
To obtain striatal regions of interest, a common atlas was first generated from all acquired [123I]ioflupane (dopamine transporter [DAT]-binding density) images. The striatum was delineated manually on this common atlas, and divided into the anterior and posterior divisions to separate the caudate from the Put (Seibyl etal., 1995). Subject images first were aligned to a common atlas via linear affine registration (Avants etal., 2011). Mean signal intensity values were extracted from the delineated striatal ROIs on each subject image. Striatal binding was normalized for each image by subtracting the mean signal intensity region of an occipital ROI from the striatal intensity (Seibyl etal., 1995). All striatal measurements are reported according to hemisphere location, with each region separated into the anterior (caudate) and posterior (Put) areas. Hereafter, we refer to the results as DAT binding (ie, relative DAT density).
Inter-Rater Validation of DTI Measurements
There was moderate agreement between the 2 raters regarding the segmentation and resulting extraction of DTI measures in the SN. The inter-rater agreement using concordance correlation coefficients (CCCs) were 0.74 for left FA and 0.69 for right FA. The CCCs were 0.64 for left MD and 0.67 for right MD. The results presented in this article are based on the data from one rater, although an independent analysis of the ROIs from the second rater yielded similar results (data not shown).
Statistical Analysis
Demographic and clinical measures were compared between controls and AW as a whole using t-tests, whereas those among controls, low exposure (AWLo), and high exposure (AWHi) AW were conducted using 1-way analysis of variance (ANOVA). Basal ganglia DTI (FA and MD) and DAT-binding (anterior and posterior striatal regions) measures were compared between each hemisphere by paired t tests within controls, AW, or AW subgroups. Then, BG DTI (FA and MD) and DAT-binding (anterior and posterior striatal regions) measures were compared between controls and the agricultural group or subgroups by 1-way analysis of covariance (ANCOVA) with adjustment for age. We explored the associations between BG DTI measures and days of pesticide exposure using Spearman partial correlations while adjusting for age. Last, we explored the relationship between significant DTI measures and striatal DAT binding measurements adjusting for age. Due to the explorative nature, we did not control for multiple comparisons. Significance was defined as p < .05 and marginal significance as p ≥ .05 and <.10. All statistical analyses were performed using SAS 9.3 with 2-sided alphas (SAS Institute Inc., Cary, North Carolina, USA).
RESULTS
Demographic Data
There were no differences in age, BMI, University of Pittsburgh Smell Identification Test, MMSE, UPDRS-I, or UPDRS-II scores between controls and AW (Table 1). In contrast, AW tended to have higher UPDRS-III scores than controls (p = .062; Table 1).
Table 1.
Demographic and Clinical Characteristics of Study Subjects
| AWs |
Group comparisons |
|||||||
|---|---|---|---|---|---|---|---|---|
| Controls | AW | AWLo | AWHi | Controls vs. AW | Controls vs. AWLo | Controls vs. AWHi | AWLo vs. AWHi | |
| Number (n) | 11 | 20 | 10 | 10 | ||||
| Age | 59.1 ± 10.1 | 59.4 ± 7.4 | 60.6 ± 8.7 | 58.2 ± 5.9 | 0.923 | 0.688 | 0.812 | 0.534 |
| BMI | 27.2 ± 2.5 | 29.4 ± 3.8 | 29.2 ± 3.4 | 29.6 ± 4.2 | 0.102 | 0.206 | 0.130 | 0.798 |
| Days of exposurea | 0 | 638 (105–6825) | 245 (105–525) | 1750 (750–6825) | ||||
| MMSE | 29.8 ± 0.4 | 28.7 ± 1.3 | 28.2 ± 1.3 | 29.1 ± 1.0 | 0.783 | 0.754 | 0.876 | 0.876 |
| UPSIT | 31.5 ± 4.3 | 32.0 ± 5.0 | 29.7 ± 5.7 | 34.3 ± 3.0 | 0.790 | 0.376 | 0.173 | 0.030 |
| UPDRS | ||||||||
| UPDRS-I | 0.5 ± 0.7 | 0.9 ± 1.3 | 0.6 ± 0.8 | 1.1 ± 1.7 | 0.480 | 0.913 | 0.273 | 0.333 |
| UPDRS-II | 2.1 ± 2.5 | 2.8 ± 3.0 | 2.3 ± 3.0 | 3.3 ± 3.1 | 0.508 | 0.867 | 0.337 | 0.437 |
| UPDRS-III | 2.5 ± 2.3 | 4.8 ± 3.6 | 5.2 ± 3.5 | 4.4 ± 3.9 | 0.062 | 0.064 | 0.182 | 0.587 |
Data represent the mean ± SD unless otherwise indicated. Each measure was compared using either t tests (controls vs. AWs) or ANOVA (for the comparisons among controls, AWLo, and AWHi). BMI, Body mass index; MoCA, Montreal Cognitive Assessment; UPDRS, Unified Parkinson’s Disease Rating Scale; UPSIT, University of Pittsburgh Smell Identification Test.
Days of exposure for AWs and their subgroups are reported as median and range. The groups were not compared since controls had 0 days of exposure and thus statistics were not appropriate.
Exposure Assessment
Intrinsic to our study design, AW had more days of exposure compared with controls. The range of exposure duration, however, was extremely wide and a few subjects had extremely long exposure durations (see Supplementary Figures). The median cumulative days of pesticide exposure for all AW was 638 days (range = 105–6825). The median number of days for the AWLo subgroup was 245 days (range = 105-525), whereas that for the AWHi subgroup was 1750 days (range = 750–6825 days).
Diffusion Imaging Measures
Lateralized differences in DTI measurements
Within controls, the left FA values in the Put (p = .021) and GP (p = 0.039) were significantly higher than those on the right, and a similar but nonstatistically significant difference was observed for the SN (p = .058). The left MD in the SN (p = .030), Put (p = .0004), and GP (p < .0001) were significantly lower than on the right. There were no statistically significant hemisphere differences in the caudate.
Within AW overall, the left FA value was significantly higher in the Put (p = .002) and GP (p = .001) compared with the right side (a finding similar to that observed in controls), but there were no differences in the SN or caudate (p ≥ .19). Similar findings for MD values were seen in the overall AW group, with lower Put (p < .0001) and GP (p < .0001) MD measures in the left compared with the right but no difference in the SN or caudate.
Within the AW subgroups, left FA values in the Put (p = .002) and GP (p = .002) were significantly higher in AWLo but not AWHi (p ≥ .14) subjects. The left MD values were significantly lower in both AWLo (p ≤ .0003) and AWHi (p ≤ .018) subjects than those on the right, with a trend for the SN in AWLo subjects (p = .062).
Differences in DTI measurements between controls and AWs
When compared with controls, AW had lower left, but not right, SN and GP FA values after adjusting for age (p ≤ .029, Table 2). There were no significant differences in bilateral striatal (caudate or Put) DTI measurements (FA or MD) between AW and controls.
Table 2.
DTI Measures in Control and AWs
| BG regions | Hemi-sphere | Controls (Con) | AWs |
Group comparisons |
|||||
|---|---|---|---|---|---|---|---|---|---|
| AW | AWLo | AWHi | Con vs. AW | Con vs. AWLo | Con vs. AWHi | AWHi vs. AWLo | |||
| FA measures | |||||||||
| SN | Left | 0.503 ± 0.043 | 0.447 ± 0.071 | 0.463 ± 0.093 | 0.432 ± 0.040 | 0.026* | 0.151 | 0.017* | 0.310 |
| Right | 0.464 ± 0.059 | 0.445 ± 0.071 | 0.440 ± 0.073 | 0.450 ± 0.074 | 0.476 | 0.443 | 0.662 | 0.746 | |
| Caudate | Left | 0.223 ± 0.018 | 0.222 ± 0.027 | 0.225 ± 0.032 | 0.219 ± 0.023 | 0.863 | 0.934 | 0.709 | 0.658 |
| Right | 0.222 ± 0.039 | 0.218 ± 0.031 | 0.216 ± 0.038 | 0.219 ± 0.024 | 0.726 | 0.847 | 0.691 | 0.842 | |
| Put | Left | 0.322 ± 0.029 | 0.310 ± 0.030 | 0.315 ± 0.034 | 0.304 ± 0.026 | 0.280 | 0.577 | 0.202 | 0.477 |
| Right | 0.307 ± 0.027 | 0.294 ± 0.037 | 0.296 ± 0.036 | 0.291 ± 0.040 | 0.320 | 0.517 | 0.303 | 0.706 | |
| GP | Left | 0.401 ± 0.030 | 0.366 ± 0.044 | 0.371 ± 0.051 | 0.362 ± 0.038 | 0.029* | 0.101 | 0.037* | 0.635 |
| Right | 0.364 ± 0.049 | 0.337 ± 0.042 | 0.330 ± 0.046 | 0.344 ± 0.039 | 0.126 | 0.118 | 0.299 | 0.592 | |
| MD measures | |||||||||
| SN | Left | 8.19±1.48 x 10−4 | 8.55±1.21 x 10−4 | 8.33±1.26 x 10−4 | 8.77±1.18 x 10−4 | 0.439 | 0.710 | 0.350 | 0.581 |
| Right | 8.98±1.96 x 10−4 | 9.05±1.25 x 10−4 | 9.41±1.29 x 10−4 | 8.69±1.16 x 10−4 | 0.886 | 0.459 | 0.623 | 0.236 | |
| Caudate | Left | 1.049 ± 0.115 | 1.041 ± 0.212 | 1.092 ± 0.259 | 0.990 ± 0.149 | 0.906 | 0.630 | 0.496 | 0.262 |
| Right | 1.110 ± 0.183 | 1.040 ± 0.127 | 1.083 ±0.145 | 0.998 ± 0.094 | 0.187 | 0.562 | 0.093 | 0.273 | |
| Put | Left | 0.744 ± 0.020 | 0.751 ± 0.030 | 0.749 ± 0.026 | 0.752 ± 0.035 | 0.518 | 0.756 | 0.435 | 0.647 |
| Right | 0.788 ± 0.027 | 0.794 ± 0.043 | 0.799 ± 0.028 | 0.789 ± 0.056 | 0.638 | 0.593 | 0.795 | 0.788 | |
| GP | Left | 0.751 ± 0.035 | 0.789 ± 0.071 | 0.796 ± 0.091 | 0.783 ± 0.049 | 0.109 | 0.117 | 0.248 | 0.671 |
| Right | 0.815 ± 0.040 | 0.857 ± 0.078 | 0.872 ± 0.089 | 0.843 ± 0.068 | 0.106 | 0.077 | 0.319 | 0.430 | |
Data represent the mean ± SD. DTI measures were compared using ANCOVA with adjustment for age. p values are presented for the comparisons among controls, AW, and AW subgroups (Hi and Lo). Asterisks and bolded text represent significant comparisons.
The subgroup comparisons revealed that AWHi, but not AWLo, subjects showed significantly lower left nigral and GP (p ≤ .037) FA values compared with controls. Trend MD differences were observed between controls and AWHi subjects in the right caudate (p = .093) and between controls and AWLo in the right GP (p = .077; Table 2). The differences between the AWHi and AWLo groups were not statistically significant (p ≥ .24).
Striatal Dopaminergic Transporter (DAT Binding, [123I]-Ioflupane SPECT) Measures
There were no significant hemispheric differences in any striatal DAT binding measures in controls, AW, or the AW subgroups (p ≥ .36). Comparison of DAT-binding intensity among controls and AW subgroups (high- and low-exposure) also demonstrated no significant difference in striatal subregions (p ≥ .21; Table 3).
Table 3.
Dopamine Transporter Uptake Measures in Controls and AW
| AWs |
Group Comparisons (p values) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Controls | AW | AWLo | AWHi | Controls vs. AW | Controls vs. AWLo | Controls vs. AWHi | AWHivs. AWLo | |
| Anterior Striatum | ||||||||
| Left | 2.51 ± 0.51 | 2.40 ± 0.34 | 2.45 ± 0.34 | 2.34 ± 0.36 | 0.458 | 0.807 | 0.312 | 0.453 |
| Right | 2.44 ± 0.42 | 2.34 ± 0.32 | 2.40 ± 0.36 | 2.27 ± 0.27 | 0.445 | 0.953 | 0.213 | 0.248 |
| Posterior Striatum | ||||||||
| Left | 3.13 ± 0.62 | 3.17 ± 0.57 | 3.18 ± 0.68 | 3.16 ± 0.48 | 0.817 | 0.721 | 0.974 | 0.752 |
| Right | 3.11 ± 0.45 | 3.19 ± 0.53 | 3.19 ± 0.60 | 3.18 ± 0.49 | 0.650 | 0.612 | 0.796 | 0.807 |
Data represent the mean ± SD. DAT binding is expressed as a ratio of striatal/occipital cortex binding, and were compared using ANCOVA with adjustment for age. p values are presented for the comparisons among controls, AW, AW subgroups (Hi and Lo).
The Relationship of MRI DTI and Dopaminergic Transporter (DAT Binding) Measurements With Exposure Metrics
DTI measures (FA or MD) from the SN, Put, caudate, or GP did not correlate with days of exposure in the overall AW group (p ≥ .17). DAT-binding measurements also did not correlate with days of exposure (p ≥ .32; Supplementary Figure 2).
Since SN and GP FA measurements were significantly different between controls and AW, we graphed the relationships between FA measures and days of exposure using the raw data, and presented them in Supplementary Figure 1 for nigral FA and Supplementary Figure 2 for GP FA values.
The Relationship Between DTI and Striatal Dopaminergic Transporter Density
Since SN and GP DTI measures were significantly different between controls and AW, we explored their relationship to striatal DAT-binding measurements. Within controls, there were no significant correlations between any DTI measure and striatal dopaminergic transporter measurements (data not shown). Within AW, right SN FA values negatively correlated with DAT binding in the right posterior striatum (ρ = −0.489; p = .034; Table 4). In addition, FA values in the left GP correlated positively with DAT binding in the left posterior striatal region (ρ = 0.459, p = .048).
Table 4.
Relationships Between DTI FA and DAT Binding Values in AW
|
DAT Bindinga |
||||
|---|---|---|---|---|
| Anterior |
Posterior |
|||
| Left | Right | Left | Right | |
| Substantia nigra | ||||
| FA | ||||
| Left | −0.031 | 0.090 | 0.063 | −0.177 |
| 0.900 | 0.717 | 0.792 | 0.469 | |
| Right | −0.174 | 0.119 | −0.265 | −0.489* |
| 0.476 | 0.627 | 0.273 | 0.034 | |
| Globus Pallidus | ||||
| FA | ||||
| Left | 0.256 | 0.302 | 0.459* | 0.147 |
| 0.290 | 0.208 | 0.048 | 0.548 | |
| Right | 0.159 | 0.315 | −0.283 | 0.046 |
| 0.517 | 0.190 | 0.241 | 0.853 | |
Data represent the correlation coefficients (ρ, top number) and the p values (bottom number) of the Spearman partial correlation analyses with adjustment for age. Significant correlations are indicated by bolded text and asterisks.
DISCUSSION
The results from this study are consistent with prior cross-sectional findings of SN microstructural changes in AW (Du etal., 2014), but also revealed lower GP values in AW and lateralized (left) vulnerability of these BG structures to pesticide exposure. Conversely, our study did not reveal any DTI differences in striatal structures (caudate and Put). In addition, DAT binding was not decreased in AW as we hypothesized, suggesting that striatal regions are relatively resilient to pesticide neurotoxicity. The finding that nigral and pallidal FA measures are lower only in AW with high, but not low, exposures to pesticide hints at a potential exposure-MRI correlation. The direct correlation analysis, however, did not detect a significant association, which could be due to the small sample size. Collectively, these results suggest a lateralized and selective BG signature that can be captured by DTI may reflect damage due to occupational pesticide exposure among agricultural workers.
SN and GP Microstructural Changes as Markers of Pesticide Related Neurotoxicity
Lower SN FA values in AW observed previously (Du etal., 2014) and in the current study were in a similar to, but less severe, than those seen in PD patients (Du etal., 2012; Ofori etal., 2015; Prodoehl etal., 2009). The exact mechanism and implications of this DTI finding are unknown. Given the evidence from animal (Boska etal., 2007) and epidemiological (McCormack etal., 2002; Pezzoli and Cereda, 2013; Tanner etal., 2011) studies, one may hypothesize that this microstructural signature may reflect dopaminergic cell loss similar to that in PD. Yet no striatal field difference in DAT-binding argues against this hypothesis. There is a view that that PD may be caused by multiple “hits” (Hawkes etal., 2007; Sulzer, 2007), and the microstructural findings detected by DTI may mark one of these vulnerabilities. These nigral findings do not represent PD pathology per se, but rather a proximal event that may make exposed individuals more susceptible to a “next” hit that will cumulatively lead to PD.
We also discovered decreased GP FA values in AW, especially those with higher exposure levels. These data suggest that the GP, like the nigra, is vulnerable to pesticide exposure. The GP has higher energy demands, is a “sink” for divalent ions (eg, iron, manganese, copper, etc.), and displays FA alterations even at relatively low exposure levels (Lee etal., 2015). Yet GP FA alterations have not been described in PD patients or in our own dataset (unpublished). It is increasingly recognized, however, that REM behavior disorder is associated with the development of neurodegenerative disease, particularly α-synucleinopathies (eg, PD, multisystem atrophy, and Lewy body dementia; Boeve, 2010; Iranzo etal., 2006, 2013; Schenck etal., 1996). Radiotracer imaging in this prodromal population indicates increased metabolism in the GP (Holtbernd etal., 2014). Thus, it will be very interesting in the future to see if the GP FA differences observed in the current study may be related to GP metabolism. Our exploratory correlation analyses showed that lower left GP FA values may be associated with lower left Put (posterior striatal) DAT binding (Table 4). The traditional view suggests that the nigra projects to the striatum that then connects to the GP internus through the direct or indirect pathway (DeLong etal., 1984). Several recent studies, however, have reported that the GP, particularly the GP externus (GPe), projects to the dorsal striatum (reviewed by Hegeman etal., 2016). A systematic analysis of GPe inputs to the dorsal striatum currently is lacking, but it is possible that microstructural changes in the GP (reflected by lower FA values) could precipitate nigrostriatal dopaminergic terminal alterations that ultimately lead to PD vulnerability. Further studies are warranted to investigate both nigral and pallidal DTI modifications as biomarkers for pesticide-related toxicity.
Lateralized SN Change and Its Significance to the Asymmetrical Onset of PD Symptoms
The finding of lateralized vulnerability of the left SN and GP in AW is consistent with previous studies in PD indicating that the dominant hemisphere is more vulnerable to insult (Filippi etal., 1995). Previous studies also have suggested that handedness and hemisphere dominance may play a role in the development of PD symptoms (Barrett etal., 2011; Haaxma etal., 2010; Scherfler etal., 2012). Indeed, Uitti etal. (2005) demonstrated that right handed PD patients have a greater likelihood of having right- rather than left-side symptoms, although handedness explained relatively little of the variance (approximately 20%) associated with asymmetric onset in PD. Lower left SN FA values have been reported previously in PD patients (Prakash etal., 2012). Furthermore, Scherfler etal. (2012) measured dopamine transporter labeling in right-handed PD subjects and found a preponderance of lower left putaminal binding, asserting that the vulnerability of SN neurons may not be random. To our knowledge, this study is the first demonstration of lateralized vulnerability of the BG to environmental toxins.
The exact cause of the lateralized vulnerability, however, is unclear. In this study, left BG DTI measures were higher than right side values in control subjects. Whereas the higher SN FA values may symbolize better organization and efficiency of this hemisphere, it also may represent higher energy demands and its vulnerability to neurotoxins. Future studies regarding the timing of lateralized BG FA differences in the brain may be extremely useful in understanding neurodevelopment and may shed light on its contribution to the asymmetric onset of PD.
Relative Resilience of Striatal Dopaminergic Transporters and the Possibility of Contralateral Compensation
In our study, nigral and GP microstructural changes in AW occurred in the absence of DTI or DAT binding differences in any striatal region. These data suggest that striatal dopaminergic terminals are more resilient to pesticide exposure and microstructural alterations in the SN/GP occur prior to or without evidence of dopamine transporter loss. These results are contrary to our original hypothesis. An alternative hypothesis is that there are compensatory increases in dopamine transporters that occur in response to perturbation (Castro-Hernandez etal., 2015; Gordon etal., 1996; Kimmel etal., 2001; Lee etal., 2000; Meiergerd etal., 1993) as has been shown in animal models (Mamaligas etal., 2016) and humans (Berry etal., 2016; Rossi etal., 2017).
Interestingly, we observed significant associations between nigral DTI and DAT-binding measurements. Namely, right nigral FA measures correlated negatively with DAT binding in the right posterior striatum, the region known to be affected early in PD patients (Fearnley and Lees, 1991). Although nigral projections are primarily to the ipsilateral striatum, several studies have demonstrated contralateral projections in rodent models (Douglas etal., 1987; Veening etal., 1980), suggesting that approximately 3% of nigral projections are contralateral. Additional studies have shown electrophysiological and neurochemical changes in the striatum that result from manipulations of the contralateral nigra (Castellano and Rodriguez Diaz, 1991; Lawler etal., 1995; Nieoullon etal., 1979). Thus, it is possible that the compromise in the left SN may preferentially affect the left striatum such that it sends a “help” signal to the right nigrostriatal pathway that then assumes a predominant compensatory role for deficits in the left circuit. Consistent with this hypothesis, previous studies have suggested compensatory brain mechanisms may occur both in animal models exposed to a perturbation (Blanchard etal., 1996; Dravid etal., 1984) and in patients with neurological disorders (Lewis etal., 2011; Mufson etal., 2015).
Limitations of Our Studies and Future Directions
Despite the intriguing nature of the results, our study has several limitations. First, although the number of subjects is similar to that in many imaging studies, the sample size is relatively small. Second, previous research has indicated that FA values are affected by age and factors such as diet and socio-economic status (Salat etal., 2005; Vaillancourt etal., 2012; Wu etal., 2011). In our statistical analyses, we accounted for age, but not diet and socio-economic status. Third, there was a wide range of exposures in our AW cohort, with only a sparse sample with >1000 days of exposure that limited our attempt to correlate the exposure measurement with our imaging findings. We tried to mitigate this by separating the AWs into 2 groups based on the median days of exposure, but additional subjects are needed to fill in the sparse areas. Fourth, AWs are exposed to multiple pesticides, and there is no known biomarker (blood, urine, etc) to reflect past pesticide exposure. Thus, we were unable to determine the exact chemical(s) that may have contributed to the current findings.
Summary
This study demonstrated a lateralized microstructural signature in the BG that may be related to chronic pesticide exposure, with the left SN and GP being more vulnerable than the right, and striatal structures being more resistant. These occur without deficits in dopamine transporter density that is marked in PD. Our study also hinted that right nigrostriatal circuitry may compensate when pesticides insult the left pathway. These findings need to be replicated in larger cohorts and with longitudinal designs because they may be extremely important in understanding pesticide-related neurotoxicity and its link to PD-related pathoetiology.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
ACKNOWLEDGMENTS
We express gratitude to all of the participants who volunteered for this study and the study personnel who contributed to its completion. We also thank Mr John Bray and Ms Melissa Santos for their efforts in recruiting study participants and coordinating the study.
FUNDING
This work was supported in part by the National Institute of Neurological Disease and Stroke (NS060722 and NS082151 to X.H.), the National Institute of Environmental Health Sciences (ES022614 to B.C.J.), the Hershey Medical Center General Clinical Research Center (National Center for Research Resources, Grant UL1 RR033184 that is now at the National Center for Advancing Translational Sciences, Grant UL1 TR000127), and a GE HealthCare grant. All analyses, interpretations, and conclusions are those of the authors and not the research sponsors.
REFERENCES
- Avants B. B., Tustison N. J., Song G., Cook P. A., Klein A., Gee J. C. (2011). A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54, 2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett M. J., Wylie S. A., Harrison M. B., Wooten G. F. (2011). Handedness and motor symptom asymmetry in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 82, 1122–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry A. S., Shah V. D., Baker S. L., Vogel J. W., O’Neil J. P., Janabi M., Schwimmer H. D., Marks S. M., Jagust W. J. (2016). Aging affects dopaminergic neural mechanisms of cognitive flexibility. J. Neurosci. 36, 12559–12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard V., Anglade P., Dziewczapolski G., Savasta M., Agid Y., Raisman-Vozari R. (1996). Dopaminergic sprouting in the rat striatum after partial lesion of the substantia nigra. Brain Res. 709, 319–325. [DOI] [PubMed] [Google Scholar]
- Boeve B. F. (2010). REM sleep behavior disorder: Updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann. N. Y. Acad. Sci. 1184, 15–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boska M. D., Hasan K. M., Kibuule D., Banerjee R., McIntyre E., Nelson J. A., Hahn T., Gendelman H. E., Mosley R. L. (2007). Quantitative diffusion tensor imaging detects dopaminergic neuronal degeneration in a murine model of Parkinson’s disease. Neurobiol. Dis. 26, 590–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano M. A., Rodriguez Diaz M. (1991). Nigrostriatal dopaminergic cell activity is under control by substantia nigra of the contralateral brain side: Electrophysiological evidence. Brain Res. Bull. 27, 213–218. [DOI] [PubMed] [Google Scholar]
- Castro-Hernandez J., Afonso-Oramas D., Cruz-Muros I., Salas-Hernandez J., Barroso-Chinea P., Moratalla R., Millan M. J., Gonzalez-Hernandez T. (2015). Prolonged treatment with pramipexole promotes physical interaction of striatal dopamine D3 autoreceptors with dopamine transporters to reduce dopamine uptake. Neurobiol. Dis. 74, 325–335. [DOI] [PubMed] [Google Scholar]
- Chan L. L., Rumpel H., Yap K., Lee E., Loo H. V., Ho G. L., Fook-Chong S., Yuen Y., Tan E. K. (2007). Case control study of diffusion tensor imaging in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 78, 1383–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong M. R., Alexander G. E., Georgopoulos A. P., Crutcher M. D., Mitchell S. J., Richardson R. T. (1984). Role of basal ganglia in limb movements. Hum. Neurobiol. 2, 235–244. [PubMed] [Google Scholar]
- Desplats P., Patel P., Kosberg K., Mante M., Patrick C., Rockenstein E., Fujita M., Hashimoto M., Masliah E. (2012). Combined exposure to Maneb and Paraquat alters transcriptional regulation of neurogenesis-related genes in mice models of Parkinson’s disease. Mol. Neurodegener. 7, 49.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson D. W., Braak H., Duda J. E., Duyckaerts C., Gasser T., Halliday G. M., Hardy J., Leverenz J. B., Del T. K., Wszolek Z. K., et al. (2009). Neuropathological assessment of Parkinson’s disease: Refining the diagnostic criteria. Lancet Neurol. 8, 1150–1157. [DOI] [PubMed] [Google Scholar]
- Douglas R., Kellaway L., Mintz M., van Wageningen G. (1987). The crossed nigrostriatal projection decussates in the ventral tegmental decussation. Brain Res. 418, 111–121. [DOI] [PubMed] [Google Scholar]
- Dravid A., Jaton A. L., Enz A., Frei P. (1984). Spontaneous recovery from motor asymmetry in adult rats with 6-hydroxydopamine-induced partial lesions of the substantia nigra. Brain Res. 311, 361–365. [DOI] [PubMed] [Google Scholar]
- Du G., Lewis M. M., Sen S., Wang J., Shaffer M. L., Styner M., Yang Q. X., Huang X. (2012). Imaging nigral pathology and clinical progression in Parkinson’s disease. Mov. Disord. 27, 1636–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G., Lewis M. M., Sterling N. W., Kong L., Chen H., Mailman R. B., Huang X. (2014). Microstructural changes in the substantia nigra of asymptomatic agricultural workers. Neurotoxicol. Teratol. 41, 60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G., Lewis M. M., Styner M., Shaffer M. L., Sen S., Yang Q. X., Huang X. (2011). Combined R2* and diffusion tensor imaging changes in the substantia nigra in Parkinson’s disease. Mov. Disord. 26, 1627–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnley J. M., Lees A. J. (1991). Ageing and Parkinson’s disease: Substantia nigra regional selectivity. Brain. 114, 2283–2301. [DOI] [PubMed] [Google Scholar]
- Filippi M., Martino G., Mammi S., Campi A., Comi G., Grimaldi L. M. (1995). Does hemispheric dominance influence brain lesion distribution in multiple sclerosis? J Neurol. Neurosurg. Psychiatry 58, 748–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I., Weizman R., Rehavi M. (1996). Modulatory effect of agents active in the presynaptic dopaminergic system on the striatal dopamine transporter. Eur. J. Pharmacol. 298, 27–30. [DOI] [PubMed] [Google Scholar]
- Gouttard S., Styner M., Joshi S., Smith R. G., Hazlett H. C., Gerig G. (2007) Subcortical structure segmentation using probabilistic atlas priors. Medical Imaging. International Society for Optics and Photonics, pp. 65111-651221.
- Haaxma C. A., Helmich R. C., Borm G. F., Kappelle A. C., Horstink M. W., Bloem B. R. (2010). Side of symptom onset affects motor dysfunction in Parkinson’s disease. Neuroscience 170, 1282–1285. [DOI] [PubMed] [Google Scholar]
- Hawkes C. H., Del T. K., Braak H. (2007). Parkinson’s disease: A dual-hit hypothesis. Neuropathol. Appl. Neurobiol. 33, 599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman D. J., Hong E. S., Hernandez V. M., Chan C. S. (2016). The external globus pallidus: Progress and perspectives. Eur. J. Neurosci. 43, 1239–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtbernd F., Gagnon J. F., Postuma R. B., Ma Y., Tang C. C., Feigin A., Dhawan V., Vendette M., Soucy J. P., Eidelberg D., et al. (2014). Abnormal metabolic network activity in REM sleep behavior disorder. Neurology 82, 620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iranzo A., Molinuevo J. L., Santamaria J., Serradell M., Marti M. J., Valldeoriola F., Tolosa E. (2006). Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: A descriptive study. Lancet Neurol. 5, 572–577. [DOI] [PubMed] [Google Scholar]
- Iranzo A., Tolosa E., Gelpi E., Molinuevo J. L., Valldeoriola F., Serradell M., Sanchez-Valle R., Vilaseca I., Lomena F., Vilas D., et al. (2013). Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: An observational cohort study. Lancet Neurol. 12, 443–453. [DOI] [PubMed] [Google Scholar]
- Kimmel H. L., Joyce A. R., Carroll F. I., Kuhar M. J. (2001). Dopamine D1 and D2 receptors influence dopamine transporter synthesis and degradation in the rat. J Pharmacol. Exp. Ther. 298, 129–140. [PubMed] [Google Scholar]
- Kupsch A. R., Bajaj N., Weiland F., Tartaglione A., Klutmann S., Buitendyk M., Sherwin P., Tate A., Grachev I. D. (2012). Impact of DaTscan SPECT imaging on clinical management, diagnosis, confidence of diagnosis, quality of life, health resource use and safety in patients with clinically uncertain parkinsonian syndromes: A prospective 1-year follow-up of an open-label controlled study. J. Neurol. Neurosurg. Psychiatry 83, 620–628. [DOI] [PubMed] [Google Scholar]
- Lawler C. P., Gilmore J. H., Watts V. J., Walker Q. D., Southerland S. B., Cook L. L., Mathis C. A., Mailman R. B. (1995). Interhemispheric modulation of dopamine receptor interactions in unilateral 6-OHDA rodent model. Synapse 21, 299–311. [DOI] [PubMed] [Google Scholar]
- Le Bihan D., Mangin J. F., Poupon C., Clark C. A., Pappata S., Molko N., Chabriat H. (2001). Diffusion tensor imaging: Concepts and applications. J. Magn. Reson. Imaging 13, 534–546. [DOI] [PubMed] [Google Scholar]
- Lee C. S., Samii A., Sossi V., Ruth T. J., Schulzer M., Holden J. E., Wudel J., Pal P. K., de lF-F., Calne D. B., et al. (2000). In vivo positron emission tomographic evidence for compensatory changes in presynaptic dopaminergic nerve terminals in Parkinson’s disease. Ann. Neurol. 47, 493–503. [PubMed] [Google Scholar]
- Lee E. Y., Flynn M. R., Du G., Lewis M. M., Fry R., Herring A. H., Van Buren E., Van Buren S., Smeester L., Kong L., et al. (2015). T1 relaxation rate (R1) indicates nonlinear Mn accumulation in brain tissue of welders with low-level exposure. Toxicol. Sci. 146, 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. Y., Flynn M. R., Du G., Lewis M. M., Herring A. H., Van Buren E., Van Buren S., Kong L., Mailman R. B., Huang X. (2016). Highlight: Lower fractional anisotropy in the globus pallidus of asymptomatic welders, a marker for long-term welding exposure. Toxicol. Sci. 153, 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M. M., Du G., Sen S., Kawaguchi A., Truong Y., Lee S., Mailman R. B., Huang X. (2011). Differential involvement of striato- and cerebello-thalamo-cortical pathways in tremor- and akinetic/rigid-predominant Parkinson’s disease. Neuroscience 177, 230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Wang Y., Gerig G., Gouttard S., Tao R., Fletcher T., Styner M. (2010) Quality control of diffusion weighted images. Proceedings of SPIE–the International Society for Optical Engineering, Mar 11, 7628. [DOI] [PMC free article] [PubMed]
- Mamaligas A. A., Cai Y., Ford C. P. (2016). Nicotinic and opioid receptor regulation of striatal dopamine D2-receptor mediated transmission. Sci Rep 6, 37834.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W. R., Wieler M., Gee M. (2008). Midbrain iron content in early Parkinson disease: A potential biomarker of disease status. Neurology 70, 1411–1417. [DOI] [PubMed] [Google Scholar]
- Massey L. A., Yousry T. A. (2010). Anatomy of the substantia nigra and subthalamic nucleus on MR imaging. Neuroimaging Clin. N. Am. 20, 7–27. [DOI] [PubMed] [Google Scholar]
- McCormack A. L., Thiruchelvam M., Manning-Bog A. B., Thiffault C., Langston J. W., Cory-Slechta D. A., Di Monte D. A. (2002). Environmental risk factors and Parkinson’s disease: Selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol. Dis. 10, 119–127. [DOI] [PubMed] [Google Scholar]
- Meiergerd S. M., Patterson T. A., Schenk J. O. (1993). D2 receptors may modulate the function of the striatal transporter for dopamine: Kinetic evidence from studies invitro and invivo. J. Neurochem. 61, 764–767. [DOI] [PubMed] [Google Scholar]
- Mufson E. J., Mahady L., Waters D., Counts S. E., Perez S. E., DeKosky S. T., Ginsberg S. D., Ikonomovic M. D., Scheff S. W., Binder L. I. (2015). Hippocampal plasticity during the progression of Alzheimer’s disease. Neuroscience 309, 51–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieoullon A., Cheramy A., Leviel V., Glowinski J. (1979). Effects of the unilateral nigral application of dopaminergic drugs on the invivo release of dopamine in the two caudate nuclei of the cat. Eur. J. Pharmacol. 53, 289–296. [DOI] [PubMed] [Google Scholar]
- Ofori E., Pasternak O., Planetta P. J., Li H., Burciu R. G., Snyder A. F., Lai S., Okun M. S., Vaillancourt D. E. (2015) Longitudinal changes in free-water within the substantia nigra of Parkinson’s disease. Brain 138 (Pt8), 2322–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peran P., Cherubini A., Assogna F., Piras F., Quattrocchi C., Peppe A., Celsis P., Rascol O., Demonet J. F., Stefani A., et al. (2010). Magnetic resonance imaging markers of Parkinson’s disease nigrostriatal signature. Brain 133, 3423–3433. [DOI] [PubMed] [Google Scholar]
- Pezzoli G., Cereda E. (2013). Exposure to pesticides or solvents and risk of Parkinson disease. Neurology 80, 2035–2041. [DOI] [PubMed] [Google Scholar]
- Prakash B. D., Sitoh Y. Y., Tan L. C., Au W. L. (2012). Asymmetrical diffusion tensor imaging indices of the rostral substantia nigra in Parkinson’s disease. Parkinsonism Relat. Disord. 18, 1029–1033. [DOI] [PubMed] [Google Scholar]
- Prodoehl J., Corcos D. M., Vaillancourt D. E. (2009). Basal ganglia mechanisms underlying precision grip force control. Neurosci. Biobehav. Rev. 33, 900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer P., Sian-Hulsmann J. (2012). The significance of neuronal lateralisation in Parkinson’s disease. J. Neural Transm. 119, 953–962. [DOI] [PubMed] [Google Scholar]
- Rossi C., Genovesi D., Marzullo P., Giorgetti A., Filidei E., Corsini G. U., Bonuccelli U., Ceravolo R. (2017). Striatal dopamine transporter modulation after rotigotine: Results from a pilot single-photon emission computed tomography study in a group of early stage parkinson disease patients. Clin. Neuropharmacol. 401, 34–36. [DOI] [PubMed] [Google Scholar]
- Rueckert D., Sonoda L. I., Hayes C., Hill D. L., Leach M. O., Hawkes D. J. (1999). Nonrigid registration using free-form deformations: Application to breast MR images. Med. Imag. IEEE Trans. 18, 712–721. [DOI] [PubMed] [Google Scholar]
- Salat D. H., Tuch D. S., Greve D. N., van der Kouwe A. J., Hevelone N. D., Zaleta A. K., Rosen B. R., Fischl B., Corkin S., Rosas H. D., et al. (2005). Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol. Aging 26, 1215–1227. [DOI] [PubMed] [Google Scholar]
- Salvador R., Pena A., Menon D. K., Carpenter T. A., Pickard J. D., Bullmore E. T. (2005). Formal characterization and extension of the linearized diffusion tensor model. Hum. Brain Mapp. 24, 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck C. H., Bundlie S. R., Mahowald M. W. (1996). Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology 46, 388–393. [DOI] [PubMed] [Google Scholar]
- Scherfler C., Seppi K., Mair K. J., Donnemiller E., Virgolini I., Wenning G. K., Poewe W. (2012). Left hemispheric predominance of nigrostriatal dysfunction in Parkinson’s disease. Brain 135, 3348–3354. [DOI] [PubMed] [Google Scholar]
- Seibyl J. P., Marek K. L., Quinlan D., Sheff K., Zoghbi S., Zea-Ponce Y., Baldwin R. M., Fussell B., Smith E. O., Charney D. S. (1995). Decreased single-photon emission computed tomographic [123I]beta-CIT striatal uptake correlates with symptom severity in Parkinson’s disease. Ann. Neurol. 38, 589–598. [DOI] [PubMed] [Google Scholar]
- Sulzer D. (2007). Multiple hit hypotheses for dopamine neuron loss in Parkinson’s disease. Trends Neurosci. 30, 244–250. [DOI] [PubMed] [Google Scholar]
- Tanner C. M., Kamel F., Ross G. W., Hoppin J. A., Goldman S. M., Korell M., Marras C., Bhudhikanok G. S., Kasten M., Chade A. R., et al. (2011). Rotenone, paraquat, and Parkinson’s disease. Environ. Health Perspect. 119, 866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner C. M., Ottman R., Goldman S. M., Ellenberg J., Chan P., Mayeux R., Langston J. W. (1999). Parkinson disease in twins: An etiologic study. Jama 281, 341–346. [DOI] [PubMed] [Google Scholar]
- Uitti R. J., Baba Y., Whaley N. R., Wszolek Z. K., Putzke J. D. (2005). Parkinson disease: Handedness predicts asymmetry. Neurology 64, 1925–1930. [DOI] [PubMed] [Google Scholar]
- Uversky V. N. (2004). Neurotoxicant-induced animal models of Parkinson’s disease: Understanding the role of rotenone, maneb and paraquat in neurodegeneration. Cell Tissue Res. 318, 225–241. [DOI] [PubMed] [Google Scholar]
- Vaillancourt D. E., Spraker M. B., Prodoehl J., Abraham I., Corcos D. M., Zhou X. J., Comella C. L., Little D. M. (2009). High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology 72, 1378–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt D. E., Spraker M. B., Prodoehl J., Zhou X. J., Little D. M. (2012). Effects of aging on the ventral and dorsal substantia nigra using diffusion tensor imaging. Neurobiol. Aging 33, 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veening J. G., Cornelissen F. M., Lieven P. A. (1980). The topical organization of the afferents to the caudatoputamen of the rat. A horseradish peroxidase study. Neuroscience 5, 1253–1268. [DOI] [PubMed] [Google Scholar]
- Wirdefeldt K., Gatz M., Schalling M., Pedersen N. L. (2004). No evidence for heritability of Parkinson disease in Swedish twins. Neurology 63, 305–311. [DOI] [PubMed] [Google Scholar]
- Wu T., Wang L., Hallett M., Chen Y., Li K., Chan P. (2011). Effective connectivity of brain networks during self-initiated movement in Parkinson’s disease. Neuroimage 55, 204–215. [DOI] [PubMed] [Google Scholar]
- Yushkevich P. A., Piven J., Hazlett H. C., Smith R. G., Ho S., Gee J. C., Gerig G. (2006). User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 31, 1116–1128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.