Abstract
Objectives
In Haiti, routine screening for Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG), and Trichomonas vaginalis (TV) among pregnant women is not conducted, yet these sexually transmitted infections (STIs) are associated with adverse birth and newborn health outcomes. We aimed to assess the acceptability and feasibility of screening and the prevalence of STIs among pregnant women in Port-au-Prince, Haiti.
Methods
Pregnant women of at least 18 years of age attending Haitian Study Group for Kaposi’s sarcoma and Opportunistic Infections (GHESKIO) clinics in Port-au-Prince, Haiti provided self-collected vaginal swab specimens. Laboratory testing was done with Xpert® CT/NG and Xpert® TV.
Results
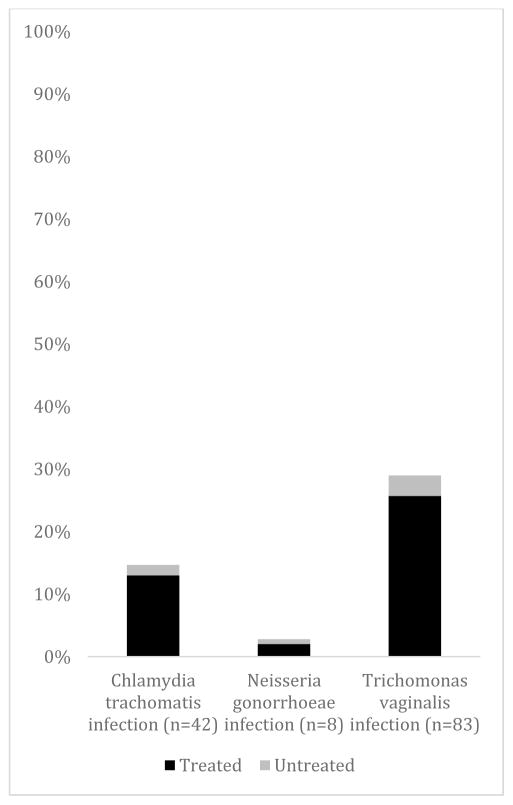
Of the 322 pregnant women who visited GHESKIO for their regularly scheduled appointments, 300 (93.2%) consented for CT, NG and TV testing. Of those, 107 women (35.7%) tested positive for at least one STI. There were 42 (14.7%) cases of CT, 8 (2.8%) NG, and 83 (29.0%) TV infections. Most infections were treated 122 of 133 (91.7%).
Conclusions
We found that it was highly acceptable and feasible to implement CT, NG, and TV screening amongst pregnant women in Port-au-Prince, Haiti. We found high prevalences of STIs among pregnant women, which suggests that STI screening in this population may be warranted.
Keywords: Sexually Transmitted Infections, Haiti, STI diagnostics, STI screening, Pregnancy
Introduction
Sexually transmitted infections (STI) cause a significant global health burden and cervico-vaginal infections are related to many adverse health outcomes. Though curable, sexually transmitted Trichomonas vaginalis (TV), Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) infections were responsible for over 350 million new infections worldwide in 2012 1. A medical history alone is insufficient for the accurate diagnosis of genitourinary tract infections 2. Many STIs can be asymptomatic and can only be diagnosed with screening tests 3. Therefore, although the current World Health Organization guidelines recommend syndromic management for STI diagnosis in low and middle income countries 4; syndromic management is unlikely to enhance STI control2. The early identification and subsequent treatment of STIs and other genital infections among index patients and their partners is paramount to achieving an effective reduction in the disease burden.
The immunologic response triggered by lower genital tract infections with CT and NG leads to significant inflammation of the cervico-endometrial tissue 5. Due to infection and the associated chronic inflammatory response, several important sequelae may result from those infections, including pelvic inflammatory disease, ectopic pregnancy, and infertility 6. TV has been associated with a more than a 2.7-fold increase in the risk of HIV acquisition 7,8, a 1.3-fold increase in preterm labor, and a 4.7-fold increase in pelvic inflammatory disease 9. STIs such as CT, NG and TV during pregnancy may be associated with increased rates of adverse pregnancy outcomes including neonatal and infant death, preterm birth, low birth weight, and spontaneous abortion 9–12.
Although routine screening and treatment for CT, NG and TV for pregnant women is done in several high-income countries like the United States, screening is not routinely done many settings around the world, including Haiti 13–16. Although few recent studies have documented the prevalence of CT, NG, and TV infections in Haiti, two reports estimated that 40% of women in rural areas who were receiving antenatal care and 47% of women in urban areas who were receiving antenatal care had at least one STI 17,18.
In Haiti, certain adverse birth outcomes remain high, including neonatal mortality, which was estimated at 25/1000 live births in 2012, and preterm birth was estimated at 14.1/100 live births in 2010 19,20. Improvements in STI diagnosis and treatment may play a role in reducing the high rates of those adverse pregnancy outcomes. The objective of this study was to assess the acceptability and feasibility of genital CT, NG, and TV infections screening and to estimate the prevalence of these infections in routine antenatal care in Port-au-Prince, Haiti.
Methods
All pregnant women over age 18 who visited the Haitian Study Group for Kaposi’s sarcoma and Opportunistic Infections (GHESKIO) antenatal clinic in Port-au-Prince, Haiti between October 26, 2015 and January 14, 2016 were offered screening for cervico-vaginal Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis infections. GHESKIO is a nonprofit organization that works in partnership with the Haitian Government to provide integrated primary care services, including HIV counseling, AIDS care, antenatal care, and management of tuberculosis and sexually transmitted infections. Participants that gave informed consent provided self-collected vaginal swab specimens after verbal instructions provided by GHESKIO health care employees. Patients were not offered any incentives for screening. Acceptability was measured by the percentage of patients who consented for participation of those offered testing.
Testing was conducted by study personnel using the FDA-Xpert® CT/NG and Xpert® TV assays (Cepheid, Sunnyvale, CA). The high sensitivity of nucleic acid amplification tests for Chlamydia trachomatis, Neisseria gonorrhoeae and Trichomonas vaginalis13 make them suitable as screening tests particularly because non-invasive specimens like urine and self-collected vaginal swabs can be used. Nucleic acid amplification tests do not require viable organisms and provide convenient specimen processing allowing use in diverse settings. Several studies have evaluated the utility and performance of self-collected specimens for CT and NG testing 21. The Xpert® CT/NG and TV assays have test performance equal to other FDA approved nucleic acid amplification tests in central clinical laboratories. Laboratory testing was done within 72 hours at the GHESKIO central laboratory.
The women returned to GHESKIO within 7 days from specimen collection to receive their test results and free treatment was provided for those who tested positive. Women who test positive for Chlamydia trachomatis, Neisseria gonorrhoeae or Trichomonas vaginalis were given 1 dose (1 gram) of azithromycin, ceftriaxone 250 mg injection and 1g of azithromycin, or 1 dose (2g) of metronidazole respectively. They were also counseled on how to prevent infection. Feasibility was measured by the percentage of infections that were treated. Women with positive tests were asked to return no earlier than 3 weeks after treatment for test-of-cure. Women were counseled to bring their partners or refer their partners with a patient referral card to GHESKIO for onsite treatment. Partner delivered therapy was provided to some women for treatment of CT and TV infections.
Data were collected electronically in secure databases and were analyzed using SAS v9.4 (Cary, NC, USA). Ethical approval was provided by Weill Cornell Medical College, New York General Institutional Review Board protocol number 15032016010. Ethical approval was also given by the GHESKIO Institutional Review Board.
Results
A total of 322 pregnant women presenting to GHESKIO were screened for participation over a 4-month study period. Of the 322 pregnant women who visited GHESKIO for their regularly scheduled appointments, 300 (93.2%) of them participated in the study and were tested for CT, NG and TV. Of those, 286 (95.3%) returned to receive their test results. There were 107 women (35.7%) who tested positive for at least one STI [Figure 1]. There were 42 women (14.0%) who tested positive for CT. Of the women who tested positive for CT, 39 (92.9%) of them were treated. For NG, 8 women (2.7%) tested positive, and 6 (75.0%) returned and received treatment. And lastly, for TV, 83 women (27.7%) tested positive. Of those 83 women, 77 (92.8%) received treatment. Of all of the participants, 4 were co-infected with all infections tested: NG, CT and TV. Additionally, of the women who tested positive initially, there were 13 partner referrals to GHESKIO that came for treatment.
Figure 1.
Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis prevalence and the proportion treated among pregnant women in antenatal care in Port-au-Prince, Haiti (N=300).
Among the 107 women who tested positive for at least 1 STI, 83 (77.6%) returned for a test-of-cure. 62 TV-infected women returned for the test-of-cure, of whom 28 (45.2%) tested positive for TV. Five NG infected women returned for test of cure, of whom 1 (20.0%) was positive. 32 CT infected women returned for test-of-cure, of whom 3 (9.4%) tested positive for CT. Those women were retreated.
Discussion
We provided screening for genital CT, NG, and TV infections for 300 pregnant women in routine antenatal care in Port-au-Prince, Haiti. We found that STI screening in antenatal care was acceptable with most, over 90%, of antenatal patients consenting to STI testing at their antenatal visit. Screening lead to identification of prevalent CT, NG and TV infections; and over a third of participants tested positive for at least one of the three STIs tested. We found high STI prevalence among this population with almost 15% testing positive for CT, an infection known to be associated with serious adverse outcomes in pregnancy 10,12. NG infections were identified in over 2% of participants. TV infection was the most prevalent STI identified in this study with over a quarter of participants testing positive. Infection of TV, a motile protozoan, is the most common, non-viral STI worldwide 1. Of those infections identified, a very high proportion, over 90%, were treated making screening during antenatal care an effective way to both identify and treat STIs in pregnancy. Some participants with STIs did not return for treatment even after follow up phone calls were made.
Test of cure visits were conducted approximately 3 weeks after treatment. Some participants continued to test positive even following adequate treatment. Most striking is that almost half of those treated for TV had a positive TV test of cure result. Molecular tests detect nucleic acid from organisms whether they are alive or not. However, that means that treated individuals may continue to test positive on a molecular test after treatment beyond the period of infectivity. In addition, there is emerging evidence of metronidazole resistant TV globally22 and could be another explanation for the high positive TV rates at the test of cure, however were unable to explore these possibilities in the present study. Further research is needed to determine time to organism clearance to inform appropriate timing of test of cure, however some evidence suggests that nucleic acid-based tests for NG infection will be negative 7 to 14 days after treatment 23 and over 85% of women will be negative for TV and CT nucleic acid 21 days after treatment 24. We were unable to determine if the high positivity rate for TV at test of cure was due to the Xpert® TV assay detecting nucleic acid from dead TV organism, treatment failure or reinfection.
Many nucleic acid amplification tests have been optimized for use with several specimen types including non-invasive specimens like urine and patient self-collected specimens (such as vaginal and rectal swabs) 21. An additional advantage of nucleic acid amplification tests is the advent of multiplex assays, such as the Xpert® CT/NG Assay used in this study, that simultaneously detect multiple targets to diagnose multiple pathogens. Those factors make nucleic acid amplification tests ideal for a range of settings.
This study was not without limitations. Participants were those visiting GHESKIO Centres, a non-governmental clinical setting in Port-au-Prince, Haiti and therefore may not be representative of participants in other settings. Additionally, the moderate sample size and short duration of this study did not provide enough power to look at the impact of testing on pregnancy outcomes or to provide highly precise estimates of prevalence. Despite those limitations, our intervention was able to identify prevalent CT, NG and TV infections that likely would have gone untreated in the absence of testing.
Screening for STIs in pregnancy provides an opportunity to improve health outcomes of women and infants 10,12. This study is comparable to similar pilot studies in Botswana and Peru that have demonstrated the acceptability and feasibility of clinic-based screening for STIs among pregnant women 25,26.
STI testing and treatment in antenatal care is acceptable, feasible and led to treatment of prevalence infections at GHESKIO Centers in Port-au-Prince, Haiti. Further, the high prevalence of STIs found in our sample provides support for efforts to increase screening and treatment in order to reduce the disease burden of STIs and subsequent adverse health outcomes among pregnant women and infants.
Synopsis.
We assessed the acceptability and feasibility of screening and the prevalence of STIs among 322 pregnant women in Port-au-Prince, Haiti.
Acknowledgments
Funding Statement: The testing supplies were donated by Cepheid Inc. (Sunnyvale, CA). CCB acknowledges funding from NIDA T32 DA023356.
The testing supplies were donated by Cepheid Inc. (Sunnyvale, CA). CCB acknowledges funding from NIDA T32 DA023356 and NIDA R01 DA037773-01A1.
Footnotes
Author Contributions: CCB provided oversight of the study, conducted the analysis, and wrote the manuscript. PM assisted with study implementation, data collection, data analysis. OO oversaw the study and laboratory-based processes. JWP, DB and JDK oversaw and conceived of the study. AW assisted with the data analysis and provided critical review. All co-authors provided review of the manuscript.
Conflict of Interest: None declared.
References
- 1.Newman L, Rowley J, Vander Hoorn S, et al. Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PloS one. 2015;10(12):e0143304. doi: 10.1371/journal.pone.0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garrett NJ, McGrath N, Mindel A. Advancing STI care in low/middle-income countries: has STI syndromic management reached its use-by date? Sexually transmitted infections. 2016 doi: 10.1136/sextrans-2016-052581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moss NJ, Ahrens K, Kent CK, et al. The decline in clinical sequelae of genital Chlamydia trachomatis infection supports current control strategies. The Journal of infectious diseases. 2006;193(9):1336–8. doi: 10.1086/503114. author reply 38–9. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Guidelines for the Management of Sexually Transmitted Infections. http://wwwwhoint/hiv/pub/sti/pub6/en/2004.
- 5.Reighard SD, Sweet RL, Vicetti Miguel C, et al. Endometrial leukocyte subpopulations associated with Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis genital tract infection. American journal of obstetrics and gynecology. 2011;205(4):324e1–7. doi: 10.1016/j.ajog.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmes KK. Sexually transmitted diseases. 4. New York: McGraw-Hill Medical; 2008. [Google Scholar]
- 7.Kissinger P, Adamski A. Trichomoniasis and HIV interactions: a review. Sexually transmitted infections. 2013;89(6):426–33. doi: 10.1136/sextrans-2012-051005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McClelland RS, Sangare L, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. The Journal of infectious diseases. 2007;195(5):698–702. doi: 10.1086/511278. [DOI] [PubMed] [Google Scholar]
- 9.Cotch MF, Pastorek JG, 2nd, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sexually transmitted diseases. 1997;24(6):353–60. doi: 10.1097/00007435-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Adachi K, Nielsen-Saines K, Klausner JD. Chlamydia trachomatis Infection in Pregnancy: The Global Challenge of Preventing Adverse Pregnancy and Infant Outcomes in Sub-Saharan Africa and Asia. Biomed Res Int. 2016;2016:9315757. doi: 10.1155/2016/9315757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva MJ, Florencio GL, Gabiatti JR, et al. Perinatal morbidity and mortality associated with chlamydial infection: a meta-analysis study. Braz J Infect Dis. 2011;15(6):533–9. doi: 10.1590/s1413-86702011000600006. [DOI] [PubMed] [Google Scholar]
- 12.Adachi K, Klausner JD, Xu J, et al. Chlamydia trachomatis and Neisseria gonorrhoeae in HIV-infected Pregnant Women and Adverse Infant Outcomes. Pediatr Infect Dis J. 2016 doi: 10.1097/INF.0000000000001199. [DOI] [PMC free article] [PubMed]
- 13.Centers for Disease C, Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae--2014. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control. 2014;63(RR-02):1–19. [PMC free article] [PubMed] [Google Scholar]
- 14.Chiduo M, Theilgaard ZP, Bakari V, et al. Prevalence of sexually transmitted infections among women attending antenatal clinics in Tanga, north eastern Tanzania. International journal of STD & AIDS. 2012;23(5):325–9. doi: 10.1258/ijsa.2011.011312. [DOI] [PubMed] [Google Scholar]
- 15.Mayaud P, Mabey D. Approaches to the control of sexually transmitted infections in developing countries: old problems and modern challenges. Sexually transmitted infections. 2004;80(3):174–82. doi: 10.1136/sti.2002.004101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. Global Strategy for the Prevention and Control of Sexually Transmitted Infections 2006–2015. Geneva: 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitzgerald DW, Behets FM, Lucet C, et al. Prevalence, burden, and control of syphilis in Haiti’s rural Artibonite region. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 1998;2(3):127–31. doi: 10.1016/s1201-9712(98)90113-8. [DOI] [PubMed] [Google Scholar]
- 18.Behets FM, Desormeaux J, Joseph D, et al. Control of sexually transmitted diseases in Haiti: results and implications of a baseline study among pregnant women living in Cite Soleil Shantytowns. The Journal of infectious diseases. 1995;172(3):764–71. doi: 10.1093/infdis/172.3.764. [DOI] [PubMed] [Google Scholar]
- 19.UNICEF. [accessed Accessed 17 May 2016];Statistics at a glance. http://www.unicef.org/infobycountry/haiti_statistics.html2013.
- 20.WHO. Born Too Soon: The Global Action Report on Preterm Birth. 2012. [Google Scholar]
- 21.Taylor D, Lunny C, Wong T, et al. Self-collected versus clinician-collected sampling for sexually transmitted infections: a systematic review and meta-analysis protocol. Systematic reviews. 2013;2:93. doi: 10.1186/2046-4053-2-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forbes GL, Drayton R, Forbes GD. A case of metronidazole-resistant Trichomonas vaginalis in pregnancy. International journal of STD & AIDS. 2016;27(10):906–8. doi: 10.1177/0956462415601295. [DOI] [PubMed] [Google Scholar]
- 23.Wind CM, Schim van der Loeff MF, Unemo M, et al. Test of Cure for Anogenital Gonorrhoea Using Modern RNA-Based and DNA-Based Nucleic Acid Amplification Tests: A Prospective Cohort Study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;62(11):1348–55. doi: 10.1093/cid/ciw141. [DOI] [PubMed] [Google Scholar]
- 24.Williams JA, Ofner S, Batteiger BE, et al. Duration of polymerase chain reaction-detectable DNA after treatment of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis infections in women. Sexually transmitted diseases. 2014;41(3):215–9. doi: 10.1097/OLQ.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 25.Wynn A, Ramogola-Masire D, Gaolebale P, et al. Acceptability and Feasibility of Sexually Transmitted Infection Testing and Treatment among Pregnant Women in Gaborone, Botswana, 2015. Biomed Res Int. 2016;2016:1251238. doi: 10.1155/2016/1251238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cabeza J, Garcia PJ, Segura E, et al. Feasibility of Chlamydia trachomatis screening and treatment in pregnant women in Lima, Peru: a prospective study in two large urban hospitals. Sexually transmitted infections. 2015;91(1):7–10. doi: 10.1136/sextrans-2014-051531. [DOI] [PMC free article] [PubMed] [Google Scholar]