Abstract
Retinoic acid (RA) plays pivotal roles in organogenesis, and both excessive and reduced amounts of RA cause developmental abnormalities. Reproductive organs are susceptible to teratogen toxigenicity, and the genital tubercle (GT) is one such representative organ. The physiological function of endogenous RA signaling and the mechanisms of RA-induced teratogenicity are poorly understood during the GT development. The objective of this study is to understand the developmental and teratogenic roles of RA during GT development by analyzing genetically modified mouse models. We found dynamic patterns of gene expression for the RA-synthesizing enzyme, Raldh2, and for the RA-catabolizing enzyme, Cyp26b1, during GT development. Rarb, an indicator gene for RA signaling, starts its expression in the prospective corpus cavernosum penis and in the urethral plate epithelium (UE), which plays central roles during GT development. Excessive RA signaling in Cyp26b1−/− mutants leads to abnormal extents of cell proliferation and differentiation during GT development, and also upregulates expression of growth factor signalings. They include Sonic hedgehog (Shh) signaling and Bone morphogenetic protein (Bmp) signaling, which are expressed in the UE and its bilateral mesenchyme. RA signaling positively regulatesShh and Bmp4 expression during GT development as testified also by the experiment of RA administration and analyses of loss–of-function of RA signaling mutants. Thus, RA signaling is involved in the developmental cascade necessary for UE formation and GT development.
Keywords: retinoic acid, genital tubercle, Sonic hedgehog (Shh), Bone morphogenetic protein (Bmp), urethral plate epithelium
INTRODUCTION
Retinoic acid (RA), an active derivative of vitamin A, exerts crucial functions in vertebrate development (Batourina et al., 2002; Niederreither and Dollé, 2008). Both excessive and reduced RA signaling cause developmental abnormalities (Niederreither and Dollé, 2008).
The supply of vitamin A in vivo is regulated by the blood stream. Additionally, RA is a diffusible molecule among tissues. However, it has been reported to function in a spatiotemporal manner (Duester, 2008). The regulation of RA activity depends on both the conversion of vitamin A to biological active RA by oxidation and by the catabolism of RA into an inactive form. Many enzymes are in fact involved in such processes. Among those enzymes, the RA-synthesizing enzyme, Raldh, and the RA-catabolizing enzyme, Cyp26, are expressed in spatiotemporal-specific patterns during embryonic development (Yashiro et al., 2004; Duester, 2008; Niederreither and Dollé, 2008). It has been suggested that the restricted expression of these two kinds of RA-metabolizing enzymes regulates region-specific RA signaling in the embryo (Duester, 2008).
The genital tubercle (GT) is a representative tissue for reproductive organ formation. Many researchers study GT development in the fields of developmental biology and pharmacology (Pask et al., 2005, 2008). The urethral plate epithelium (UE), an endoderm-derived tissue (Haraguchi et al., 2001; Seifert et al., 2008), develops at the ventral midline of the GT. GT possesses a unique developmental process that involves tissues derived from all three germ layers and the UE plays a central role in GT development (Haraguchi et al., 2000, 2001).
RA signaling has been suggested to function in organogenesis, including that of the brain, spinal cord, heart, eye, limb buds, and genitourinary tract (Duester, 2008; Mendelsohn et al., 1994). However, due to early lethality and severe disorganization of the entire caudal body of several RA signaling mutants (Abu-Abed et al., 2001; Niederreither and Dollé, 2008; Ribes et al., 2009), few studies have analyzed RA function during GT development. RA administration has been utilized as a good model system for teratology and toxicology studies. Because the GT is susceptible to the toxicity of certain external factors, many studies have assessed such effects on the GT (Jiang et al., 2007; Nakata et al., 2009). Exposure of mouse embryos to excessive amounts of RA at E8.5 to E9.5 induces agenesis of the UE on the ventral side of the GT (Ogino et al., 2001). Human genetic studies have also linked GT agenesis and defects in urethra formation to abnormal RA levels in patients with cytochrome P450 oxidoreductase deficiency (Fukami et al., 2010). It is essential, therefore, to investigate the mechanisms underlying the teratogenic effects of RA during GT development.
Many growth factor signaling pathways have been reported to be involved in GT embryonic development, including Sonic hedgehog (Shh) (Haraguchi et al., 2001; Seifert et al., 2009a) and Bone morphogenetic protein (Bmp) signaling pathways (Suzuki et al., 2003; Chiu et al., 2010). During organogenesis, RA signaling regulates Shh signaling during brain development, caudal body trunk formation, and prostate budding (Ribes et al., 2006; Vezina et al., 2008; Ribes et al., 2009). RA signaling regulates Bmp signaling during prostate development, the formation of craniofacial structures and early gastrulation (Zhu et al., 1999; Halilagic et al., 2007; Vezina et al., 2008). However, RA regulates Shh or Bmp signaling in different ways under aforementioned developmental contexts.
In this study, we investigated the developmental and teratogenic roles of RA during GT development. We suggest that Shh and Bmp4 are possible targets of RA signaling during GT development and RA-induced teratogenicity.
MATERIALS AND METHODS
Mice
The mutant alleles employed in this study were Cyp26b1−/− (Yashiro et al., 2004), RARaDNFlox (Rosselot et al., 2010), Gli1CreERT2 (Ahn and Joyner, 2004; Miyagawa et al., 2009), and Isl1MerCreMer (Laugwitz et al., 2005). All experimental procedures and protocols were approved by the committee on animal research at Kumamoto University and Wakayama Medical University. Noon on the day when a vaginal plug is detected is designated as E0.5.
The Tamoxifen (TM)-inducible Cre recombinase system removes the floxed sequence of the target genome (Feil et al., 1997). TM (Sigma Chemical Co., St. Louis, MO) was dissolved in sesame oil (Kanto Chemical, Tokyo, Japan) at a final concentration of 10 mg/ml and administered at 100 mg/kg body weight (intraperitoneally [i.p.]) to pregnant mice. Isl1MerCreMer/+:RARaDNFlox/− and Gli1CreERT2/+:RARaDNFlox/− males were mated with RARaDNFlox/Flox females, and only mutant embryos with homozygous RARaDNFlox/Flox alleles were analyzed. The Isl1MerCreMer/+:RARaDNFlox/Flox mutants were treated with TM at E9.5, and Gli1CreERT2/+:RARaDNFlox/Flox animals were treated at E10.5. Male embryos were used for all experiments unless otherwise indicated.
RA Treatment
Pregnant Crij:CD1 mice were given a single dose of All-trans-RA (100 mg/kg; Sigma-Aldrich) by gastric intubation via oral gavage at E14.5. The fetuses were harvested 2, 4, 6, and 8 hr after RA administration. RA was dissolved in DMSO. Control animals were given an equivalent volume of DMSO.
Section and Whole-Mount In Situ Hybridization for Gene Expression Analysis
Section in situ hybridization for gene expression was performed as described previously (Miyagawa et al., 2009). Each experimental set for section in situ hybridization was conducted strictly in the same condition between the control and the mutant or RA-treated embryos, including sample fixation, paraffin section preparation, and the in situ hybridization procedures. Three pairs of mice were analyzed for each genes on Cyp26b1−/− mutant analysis (Fig. 3); two pairs of mice were analyzed for each genes in RA administration analysis (Fig. 4) and RA loss-of-function mutants analysis (Fig. 5). Whole-mount in situ hybridization was performed with digoxigenin-labeled probes using standard procedure (Haraguchi et al., 2000). The following probes were used for in situ hybridization: Rarb (P. Chambon), Raldh2 (P. Chambon), Cyp26b1 (H. Hamada), Hoxd13 (P. Dolle), Bmp4 (C.M. Jones), Shh (C. Shukunami), Ptc1 (J. Motoyama), Meis2 (K. Backs), and Hoxa13 (H.S. Stadler).
Fig. 3.
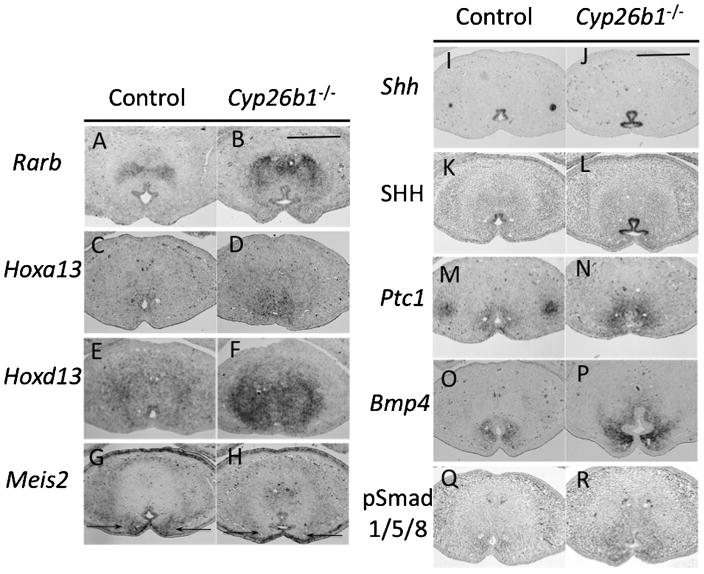
Altered expression of the homeobox genes, Shh and Bmp signaling factors in the GTs of Cyp26b1−/− mutants. A and B: Rarb expression is upregulated in the dorsal and bilateral mesenchyme of the UE as well as in the prospective corpus cavernosum penis in Cyp26b1−/− mutants. C and D: Expression level of Hoxa13 is upregulated in the mesenchyme around the urethra in the Cyp26b1−/− mutants compared with control. E and F: Expression level of Hoxd13 is increased in the mesenchyme of the GT. G and H:Meis2 expression level is decreased in the ventral bilateral mesenchyme of the UE (indicated by black arrows) in Cyp26b1−/− mutants. I–L: Shh expression level in the UE is increased in the mutant, both in mRNA (I and J) and in protein (K and L) levels. M and N: Patched1 (Ptc1) expression is upregulated in the bilateral mesenchyme of the UE in the mutant. O and P: Bmp4 expression is upregulated in the bilateral mesenchyme of the UE in the mutant. Q and R: pSmad1/5/8 expression is upregulated in the ventral region of mutant GTs. All images of the GT section are placed ventral side down. Scale bar, 400 μm. UE, urethral plate epithelium; GT, genital tubercle; Shh, Sonic hedgehog; Bmp, bone morphogenetic protein.
Fig. 4.
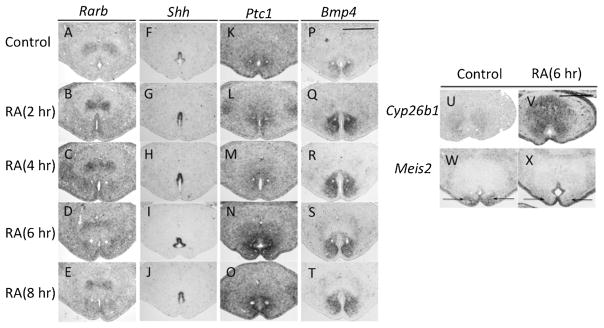
Prompt alterations of expression for Shh and Bmp4 induced by RA administration. A–E: Rarb expression level is upregulated 2 hr after RA administration (A and B). Such upregulated expression continued for 4 hr (C), and decreased 6 hr (D and E) after RA treatment. F–I: Shh expression level is increased 2 hr (F and G) after RA treatment, sustained at high levels for 6 hr (H and I), and slightly decreased 8 hr after RA treatment (J). K–O: Ptc1 expression is unaltered within 4 hr of RA treatment (K–M), then prominently increased 6 and 8 hr after RA treatment (N and O). P–T: Bmp4 expression is upregulated 2 hr after RA treatment, and sustained with increased level until 8 hr after RA treatment. U and V: Cyp26b1 expression is upregulated in the GT mesenchyme and ectoderm epithelium 6 hr after RA administration. W and X: Meis2 expression in the ventral bilateral mesenchyme of the UE (indicated by black arrows) is reduced 6 hr after RA treatment. All images are placed ventral side down. Scale bar, 400 μm. GT, genital tubercle; RA, retinoic acid; UE, urethral plate epithelium.
Fig. 5.
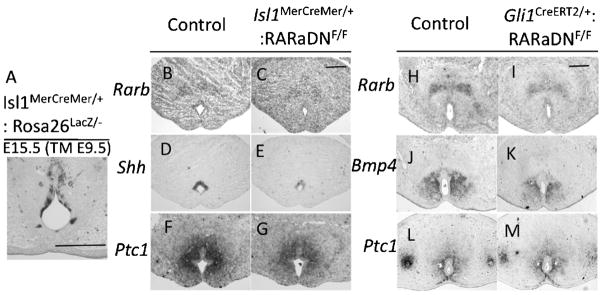
Impaired RA signaling reduces Shh and Bmp4 expression levels in the GT. A: The Isl1MerCreMer mouse line shows Tamoxifen (TM)-induced Cre expression in the UE. B and C: Rarb expression in the UE is reduced in the Isl1MerCreMer/+:RARaDNF/F mutant. D and E: Shh expression in the UE is reduced in the Isl1MerCreMer/+:RARaDNF/F mutant. F and G: Ptc1 expression in the bilateral mesenchyme of the UE is reduced in the Isl1MerCreMer/+:RARaDNF/F mutant. H and I: Rarb expression in the GT mesenchyme is reduced in the Gli1CreERT2/+:RARaDNF/F mutant. J and K: Bmp4 expression in bilateral mesenchyme of the UE is reduced in the Gli1CreERT2/+: RARaDNF/F mutant. L and M: Ptc1 expression in the bilateral mesenchyme of the UE is unaltered in the Gli1CreERT2/+: RARaDNF/F mutant. All images of the cross-section are placed ventral side down. Scale bar, 200 μm. GT, genital tubercle; RA, retinoic acid; UE, urethral plate epithelium; Bmp, bone morphogenetic protein.
Histological staining, BromodeoxyUridine Incorporation, and Immunohistochemical Analysis
Hematoxylin and Eosin staining and X-gal staining were performed by standard procedures as previously described (Haraguchi et al., 2007).
Pregnant females were injected (i.p.) with 100 mg of 5-BromodeoxyUridine (BrdU; Sigma)/kg body weight at E13.5 or E14.5. One hour after injection, embryos were collected and fixed.
Immunohistochemistry procedures were performed as previously described (Miyagawa et al., 2009). Deparaffinized sections were autoclaved at 121°C for 1 min in citrate buffer (pH 6.0) for antigen retrieval. The sections were incubated at a 1:100 dilution for anti-Laminin antibody (L9393; Sigma), a 1:500 dilution for anti-SHH antibody (clone H-160, SC9024; Santa Cruz Biotechnology), and a 1:300 dilution for anti-pSmad1/5/8 (♯9511s; Cell signaling). For SHH and pSmad1/5/8 immunohistochemistry, both Vectastain ABC (Vector Laboratories, Burlingame, CA) and TSA (NEL700001KT; PerkinElmer Inc., Waltham, MA) kits were employed to amplify the signals. For BrdU immunostaining, sections were additionally treated with 2.4 N HCl for 15 min to denature the DNA after antigen retrieval.
Statistical Analyses
Statistical analyses were performed using the Student’s t test followed by the F-test. p<0.05 is considered statistically significant.
RESULTS
Expression of Genes Involved in RA Signaling is Detected During GT Development
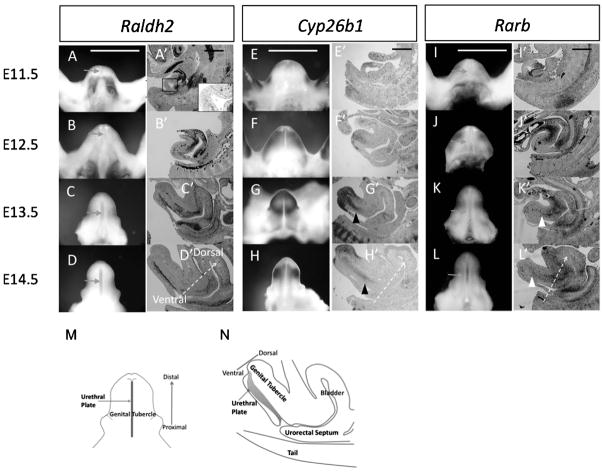
Retinaldehyde dehydrogenase 2 (Raldh2) is one of the key RA-synthesizing enzyme during embryonic development. The initiation of GT outgrowth begins at E10.5. Raldh2 is expressed in the caudal ventral region adjacent to the GT during initial outgrowth before E11.5 (Fig. 1A and A′). Expression of Raldh2is gradually shifted from the outside of the GT to the inner midline of the GT. Such expression is specifically restricted to the UE at E12.5 (Fig. 1B and B′). Formation of the UE is an essential morphogenetic process during GT development (Yamada et al., 2003). The UE plays essential roles that affect the development of its bilateral mesenchyme and, hence, the development of the GT (Haraguchi et al., 2000, 2001). Expression levels of Raldh2 in the UE gradually increase at E13.5 and E14.5 (Fig. 1C, C′, D, and D′).
Fig. 1.
RA signaling activities are detected during GT development. A–L are ventral views of GT; A′–L′ are midline sagittal sections of the caudal urogenital region. A–D and A′–D′: Raldh2 is expressed in the caudal ventral region adjacent to the GT at E10.5, but not in the urethral plate epithelium (UE, enlarged view in A′) (A and A′); its expression begins in the UE from E12.5 (B and B′), becoming stronger in the UE at E13.5 and E14.5 (C, C′, D and D′). Arrows in A–D indicate the UE. The areas in between the dashed lines in A′–D′ indicate the UE. E–H and E′–H′: Cyp26b1 is expressed in the mesenchyme adjacent to the distal ectodermal epithelium of the GT at E11.5 (E and E′), becomes broadly expressed in the mesenchyme at E12.5 (F and F′); and its expression becomes restricted to the mesenchyme dorsal of the UE in the proximal region and in the distal mesenchyme of the GT at E13.5 and E14.5 (G, G′, H, and H′). Black arrowheads indicate Cyp26b1 expression in the proximal GT. I–L and I′–L′: Rarb is expressed in the caudal region adjacent to the proximal GT at E11.5 and E12.5 (I, I′, J, and J′). Its expression begins in the UE and mesenchyme of the prospective corpus cavernosum penis in the proximal GT areas at E13.5 to E14.5 (K, K′, L, and L′). White arrowheads indicate Rarb expression in the prospective corpus cavernosum penis of the GT. Scale bar, 400 μm. Arrows with dashed stems in D′, H′, and L′ indicate the ventral–dorsal direction of the GT. M: schematic picture for ventral view of E14.5 GT. N: schematic picture for midline sagittal view of E14.5 GT. GT, genital tubercle; RA, retinoic acid.
Cytochrome P450 26b1(Cyp26b1), a RA-catabolizing enzyme, is initially expressed in the mesenchyme adjacent to the distal ectodermal epithelium of the GT at E11.5 (Fig. 1E and E′), and becomes broadly expressed in the GT mesenchyme at E12.5 (Fig. 1F and F′). Such expression remains in the distal portion of the GT. However, its proximal expression is gradually restricted to the mesenchyme on the dorsal side of the UE at E13.5 and E14.5 (Fig. 1G, G′, H, and H′).
Retinoic acid receptor beta (Rarb) is one of the RA downstream target genes (Sucov et al., 1990). Rarb is expressed in the caudal region adjacent to the proximal GT and the mesenchyme beneath the ectodermal epithelium in the lateral side of the GT at E11.5 and E12.5 (Fig. 1I, I′, J, and J′). Such expression gradually shifts to the UE and the prospective corpus cavernosum penis of the GT at E13.5 and E14.5 (Fig. 1K, K′, L, and L′).
Abnormal Cell Proliferation and Differentiation in the GTs of Cyp26b1−/− Mutants
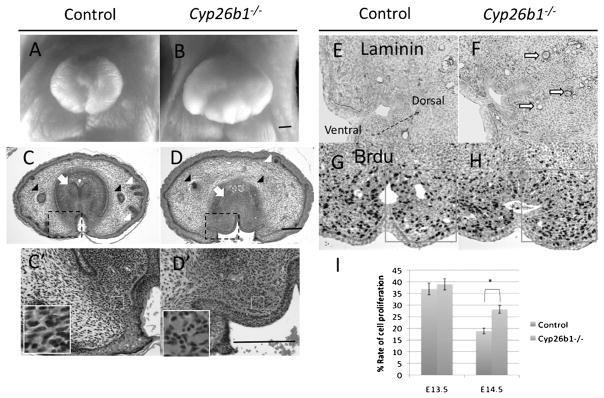
To understand the role of RA signaling during GT development, we analyzed the phenotypes of Cyp26b1−/− mutants during GT development. The mutants display hyperplastic GTs in both male and female embryos at E18.5 (Fig. 2A and B). The development of several GT accessory structures is impaired in Cyp26b1−/− mutants, including the hair follicles under the preputial genital skin and the preputial glands (Fig. 2C and D). During normal development, condensed mesenchyme in the proximal GT develops at E18.5 (Fig. 2C). Such condensed mesenchyme corresponds to the corpus cavernosum penis in adult mice (Murakami, 1987). Cyp26b1−/− mutants fail to develop such a prospective cavernous body (Fig. 2D). Ectopic blood vessels were observed surrounding the UE (Fig. 2C′ and D′). Epithelia isolating the glans and prepuce in the control GTs are absent in the mutants (Fig. 2C, C′, D, and D′).
Fig. 2.
Abnormal cell proliferation and differentiation in the GTs of Cyp26b1−/− mutants. A and B: Frontal view of an E18.5 female GT in a control embryo and Cyp26b1−/− mutant. The size of the GT in the mutant is enlarged compared with that of the control. C, C′, D, and D′: Transverse section of the proximal GT in an E18.5 female; C′ and D′ are enlarged views of the framed area (dashed black line) in C and D. The development of the preputial gland (black arrowhead) and hair follicles (white arrowheads) under the genital skin is impaired in the Cyp26b1−/− mutants; mesenchymal condensation of the prospective corpus cavernosum penis is abnormal in the mutants (white arrow); ectopic blood vessels (area indicated by white frame) develop in the bilateral and dorsal mesenchyme of the UE in the mutants (C′ and D′); epithelium (black arrow) between the glans and prepuce is absent in the mutant (C, C′, D, and D′). E and F: Blood vessels indicated by Laminin immunostaining developed ectopically (hollow arrow) in the dorsal and bilateral mesenchyme of the UE at E14.5 in the mutant. To show the images of the mesenchyme on the dorsal side of the UE, the picture is displayed with an angled orientation, indicated by arrows. G and H: BrdU immunostaining of an E14.5 GT section. I: Cell proliferation assay. Framed area in G and H was counted, and the ratio of BrdU-positive cells was compared between the control and the mutant at E13.5 (n = 6) and E14.5 (n = 6). The asterisk (*) indicates significant statistical differences. All images of the cross-section are placed ventral side down unless otherwise indicated. Arrow with dashed stem in E indicates the ventral–dorsal direction of the GT. Scale bar, 200 μm. GT, genital tubercle; UE, urethral plate epithelium; BrdU, 5-BromodeoxyUridine.
In order to analyze the cause and onset of late embryonic stage phenotypes, we analyzed the phenotypes of Cyp26b1−/− mutants at earlier embryonic stages. Laminin is an essential component of basal membrane for blood vessel (Laurie et al., 1984). Blood vessel structures, indicated by laminin immunostaining, developed ectopically in the dorsal and bilateral mesenchyme of the UE at E14.5 in the mutants (Fig. 2E and F). Regarding the hyperplastic phenotype of the GTs at E18.5, BrdU incorporation was performed to examine cell proliferation (Fig. 2G and H). The rate of cell proliferation in the bilateral mesenchyme of the UE is not different between the mutants and control littermates at E13.5, but significantly higher in the mutants at E14.5 (Fig. 2I). Cell death was also examined by Terminal deoxynucleotidyl transferase dUTP nick-end labeling (Tunel) staining. There is an increased rate of apoptosis in the distal glans of the GTs at E14.5. A thinner and sharpened shape of the distal glans of the GTs was observed at E16.5, but the proximal–distal lengths of the GTs are rather similar between the mutants and controls (data not shown).
All together, Cyp26b1−/− mutants display abnormal degrees of cell proliferation and differentiation processes during the embryonic development of the GT.
Altered Expression of Homeobox Genes and Growth Factors in the GTs of Cyp26b1−/− Mutants
Cyp26b1 is one of the most important enzymes that catabolizes RA during development. Inactivation of such enzyme causes excessive RA signaling in affected organs, such as the limbs (Yashiro et al., 2004). Consistently, there is ectopic RA signaling in the GTs of the mutants, indicated by upregulated Rarb expression in the dorsal and bilateral mesenchyme of the UE as well as in the mesenchyme of the prospective corpus cavernosum penis in the mutants (Fig. 3A and B).
The GT is one of the appendages from the trunk of the body, like the case of the limb. The developmental programs of the GT show some shared aspects with those of the limb (Dollé et al., 1991; Yamada et al., 2003). Both Hoxa13 and Hoxd13 show decreased expression in the limbs of Cyp26b1−/− mutants (Yashiro et al., 2004). Human patients possessing a Hoxa13mutation and the corresponding mouse mutants display appendicular phenotypes, including the limbs and the GTs (Kondo et al., 1997; Morgan et al., 2003; Tüzel et al., 2007). Thus, we investigated expression patterns of such homeobox genes during GT development. Hoxa13 is expressed in the UE and its surrounding mesenchyme. Hoxd13 is also expressed in the UE and in a broader part of the mesenchyme surrounding the UE compared with Hoxa13. Both Hoxa13 and Hoxd13 show upregulated expression in GT mesenchyme at E14.5 (Fig. 3C–F). Meis2 is another homeobox gene that shows distally expanded expression patterns in the limb mesenchyme of Cyp26b1−/− mutants (Yashiro et al., 2004). Its expression is detected in ectodermal epithelium and the ventral bilateral mesenchyme of the UE in the proximal GT at E14.5 in control embryos (Fig. 3G). Such mesenchymal expression of Meis2 in the corresponding region is prominently decreased in Cyp26b1−/− mutants (Fig. 3H).
Cyp26b1−/− mutants show multiple defects in both cell differentiation and proliferation in the GT. We further examined the possibility that certain growth factor activities may be altered under excessive RA signaling in the mutants. Shh signaling plays pivotal roles in both the initial outgrowth of the GT and its patterning (Haraguchi et al., 2001; Seifert et al., 2009a). Patched1 (Ptc1) is expressed downstream of Shh signaling, suggesting its activity. Ptc1 expression in the bilateral mesenchyme of the UE is slightly upregulated in Cyp26b1−/− mutants at E13.5 (data not shown). The upregulated expression of Ptc1 in the mutant becomes more prominent at E14.5 (Fig. 3M and N). Such enhanced Shh signaling is supported by an increased Shh gene expression at both the mRNA and protein levels (Fig. 3I–L). Bmp signaling plays important roles in the growth and differentiation of the GT (Suzuki et al., 2003; Wu et al., 2009). Bmp4, a Bmp signaling ligand, is normally expressed in the bilateral mesenchyme of the UE. In Cyp26b1−/− mutants, the expression of Bmp4 is upregulated in the bilateral mesenchyme at E14.5 (Fig. 3O and P). Phosphorylated Smad1/5/8, downstream of Bmp signaling transcriptional factors, are detected more prominently in the ventral region of mutant GTs at E14.5 (Fig. 3Q and R). Thus, expression of the Shh and Bmp signaling factors are increased in the Cyp26b1−/− mutants.
Prompt Alterations of Bmp4 and Shh Expression are Induced by RA Administration
Expression of several factors involved inShh and Bmp signaling are increased in the ventral GT region in Cyp26b1−/− mutants. To examine whether such changes in gene expression patterns are induced by excessive RA signaling and how early such alterations can be detected, we performed in vivo RA administration and examined the effects. We investigated the kinetics of gene expression changes at various times after RA administration. Expression levels of Rarb as well as of Shh and Bmp4 are increased 2 hr after RA administration (Fig. 4A, B, F, G, P, and Q). The increased expression level of Rarbpeaks 4 hr (Fig. 4A–E), Shh level peaks 6 hr (Fig. 4F–J), and Bmp4 level peaks 2 hr (Fig. 4P–T) after RA administration. Ptc1 expression in the bilateral mesenchyme of the UE is unaltered 4 hr after RA administration, but is upregulated 6 hr after RA administration (Fig. 4K–O). Cyp26b1 expression is upregulated prominently in the GT mesenchyme and ectodermal epithelium 6 hr after RA administration (Fig. 4U and V). Meis2 expression in the ventral bilateral mesenchyme of the UE is prominently reduced 6 hr after RA treatment (Fig. 4W and X). The consequences of gain-of-function experiments in RA signaling done by RA administration and achieved in Cyp26b1−/− mutants indicate similar effects of downstream gene expression.
Impaired RA Signaling Reduces Shh and Bmp4 Expression in the GT
Multiple phenotypes in the GTs of Cyp26b1−/− mutants indicate that excessive RA signaling leads to dysregulation of GT development. To examine whether the upregulated expression of Shh and Bmp4 in Cyp26b1−/− mutants is derived by consequences of abnormal RA signaling, loss-of-function mutants were examined to identify the physiological function of RA signaling. By employing the Creloxp recombination system, we conditionally activated the expression of the dominant-negative RA receptor (RARaDNFlox) (Rosselot et al., 2010) to obtain loss-of-function RA signaling phenotypes. The Isl1MerCreMer allele shows induced Cre recombinase expression in the UE (Fig. 5A). Expression level of Rarb in the UE decreases in Isl1MerCreMer: RARaDN mutants compared with controls (Fig. 5B and C). Shh expression in the UE is also reduced (Fig. 5D and E), consistent with the decreased expression level of Ptc1in the bilateral mesenchyme of the UE (Fig. 5F and G).
The Gli1CreERT2 allele has been utilized to modulate the mesenchymal expression of target genes in the GT (Miyagawa et al., 2009). By employing the Gli1CreERT2 driver strain, induction of mesenchymal expression of RARaDN was achieved. As a result, Rarb expression in the GT mesenchyme is reduced (Fig. 5H and I). Bmp4 expression in the bilateral mesenchyme of the UE is also reduced in contrast to Ptc1 expression, which is unaltered (Fig. 5J–M).
Altogether, experiments on both excessive and reduced RA signaling indicate its positive regulation on Shh and Bmp4expression in the GT.
DISCUSSION
RA signaling for organogenesis has been analyzed for decades. It is known to regulate various organogenesis processes. Administration of RA to pregnant animals induces multiple abnormalities, including abnormalities in GT development (Abu-Abed et al., 2001; Ogino et al., 2001; Lee et al., 2010). However, functional analysis of RA signaling has not been performed during GT development due to early lethality and severe caudal disorganization in RA signaling mutants, including Cyp26a1−/− and Raldh2−/− (Niederreither et al., 1999; Abu-Abed et al., 2001; Ribes et al., 2009). Comprehensive analysis of RA actions during GT development is therefore required.
In this study, we revealed dynamic changes in the expression patterns of genes in the RA signaling pathway from E12.5 to E14.5, after the initiation of GT outgrowth. Raldh2 switches its expression to the UE. Cyp26b1 shows gradually restricted expression in the mesenchyme on the dorsal-bilateral side of the UE. Following such expression pattern changes in RA-metabolizing enzymes, Rarb starts to be expressed in the UE. Regarding such emerging expression profiles of RA signaling factors in the UE and surrounding tissues, RA signaling might be involved in the formation of the UE, which regulates the development of the GT.
Abnormal RA Signaling Leads to Multiple Defects During GT Development in Cyp26b1−/− Mutants
The distal UE (DUE) was originally demonstrated to be essential for GT outgrowth (Haraguchi et al., 2000). Conditional knockout of several Fgf family genes suggested a redundancy of Fgf genes. Such observation raised a question about the significance of DUE for GT outgrowth (Lin et al., 2009; Miyagawa et al., 2009; Seifert et al., 2009b). Fgf8 is expressed in the DUE in control embryos and was suggested to be a marker for the DUE (Haraguchi et al., 2000, 2001; Seifert et al., 2009b). It is similarly expressed in Cyp26b1−/− mutants at E10.5, indicating that the initiation process of GT outgrowth is unaffected in the mutants (data not shown). Both the limb and GT are developing appendages from the body trunk. Changes in the endogenous distribution of RA following genetic ablation of Cyp26b1 result in truncated limb formation, accompanied by increased cell death and delayed chondrocyte maturation (Yashiro et al., 2004). Slightly increased cell apoptosis was also observed in Cyp26b1−/− mutants in the distal GT at E14.5, which might contribute to a thinner and sharpened shape of the distal glans of the GT observed at E16.5 (data not shown). However, the proximal–distal length of the GT in the mutants was not affected as observed at E16.5 (data not shown). Despite increased apoptosis in the distal glans, the proximal GT shows a prominent hyperplastic phenotype at E18.5. Such hyperplasia is consistent with the increased cell proliferation detected in the bilateral mesenchyme of the UE at E14.5. Cell proliferation was significantly reduced with conditional ablation of Shh expression in the UE (Seifert et al., 2010) (data not shown), suggesting that Shh may positively regulate cell proliferation. Increased expression level of Shh in the Cyp26b1−/− mutant might contribute to the hyperplastic phenotype of the GT.
There are defects in differentiation in various regions of the GT in the Cyp26b1−/− mutant, including the formation of ectopic blood vessels on the dorsal and lateral sides of the UE. Hoxa13 was shown to regulate vascularization in the development of the glans penis (Morgan et al., 2003; Shaut et al., 2007). Its expression is increased in the mesenchymal cells surrounding the UE of Cyp26b1−/− mutants, which might be involved in abnormal blood vessel formation in mutant GTs. Ablation of the cytochrome P450 oxidoreductase gene, which is an electron donor for all cytochrome P450 enzymes including Cyp26s, leads to defects in vasculogenesis (Otto et al., 2003). Such a phenotype is induced by the increased level of RA in the mutants (Ribes et al., 2007). Considering the ectopic blood vessel formation observed in the GT with excessive RA signaling in Cyp26b1−/− mutants, vasculogenesis might be a susceptible developmental process affected by excessive RA signaling.
Several differentiation defects other than ectopic blood vessel formation were observed in the GTs of Cyp26b1−/− mutants. Such abnormalities include impaired condensation of the prospective cavernosum penis, failure of the formation of hair follicles and preputial glands, and agenesis of the epithelia that separate the glans mesenchyme from the preputial tissues. In fact, expression of Bmp and Shh, as well as several homeobox genes, were altered in mutant GTs. Excessive RA signaling in Cyp26b1−/− mutants exerts teratogenic effects on the differentiation and growth processes of the GT.
Alterations in GT gene expression are quite different from those in the limbs of Cyp26b1−/− mutants. Expression of both Hoxa13and Hoxd13 is upregulated in the proximal GT, whereas their levels are reduced in the distal limbs of the mutants. Meis2 is another homeobox gene used as a proximal marker of limb development. Its expression is expanded to the distal limbs of the mutant, however, reduced in the ventral bilateral mesenchyme of the UE in mutant GTs. RA affects the expression of the same genes in different ways depending on the developmental context.
Shh and Bmp4 are Possible Target Genes of RA Signaling During GT Development
Excessive RA signaling in Cyp26b1−/− mutants upregulates Shh and Bmp signaling factors in mutant GTs. Administration of teratogenic doses of RA confirmed a prompt increase in Shh and Bmp4 expression in the GTs. Rarb expression is regulated by RA signaling via the binding of the heterodimeric RA receptor to RA (Sucov et al., 1990), and maternal administration of teratogenic doses of RA induces prominent upregulation of Rarb expression in E11.5 limbs within 3 hr, reaching a peak of expression after 6 hr (Harnish et al., 1992). Increased expression levels of Rarb are prominent 2 hr after RA treatment in the GT at E14.5, reaching a peak in the GT 4 hr after RA administration. Notably, expression levels of Shh and Bmp4 are also increased 2 hr after RA administration. The responding timing of these genes after RA treatment is comparable with that of Rarb, the direct target gene of RA signaling. This report is the first showing that Shh and Bmp genes can be immediate targets of RA signaling during GT formation. Thus, alterations in Shh and Bmp4 expression would be primary molecular events inducing growth and differentiation abnormalities of the GT in Cyp26b1−/− mutants.
Shh is expressed in the UE, and Bmp4 is expressed in the bilateral mesenchyme of the UE. Downregulation of Shh was achieved by conditionally inactivating RA signaling in the UE; similarly, downregulation of Bmp4 was achieved by conditionally inactivating RA signaling in the GT mesenchyme. The in situ downregulation of Shhand Bmp4 with tissue-specific inactivation of RA signaling suggests that RA signaling may regulate the expression of Shh and Bmp4 within the tissue compartments where they are expressed.
Expression of Bmp4 was shown to be positively regulated by Shh signaling during GT development (Haraguchi et al., 2001; Seifert et al., 2010). It is possible that upregulated Shh signaling promotes Bmp4 expression in Cyp26b1−/− mutants. However, increased Bmp4 expression was induced 2 hr after RA treatment when the mesenchymal expression of Ptc1 was unchanged, indicating that RA regulation of Bmp4 expression in the mesenchyme may not be mediated through Shh signaling in the GT. Bmp4 expression is reduced with conditional inactivation of RA signaling in the bilateral mesenchyme of the UE in Gli1CreERT2/+: RARaDNF/F mutants. In the corresponding areas of such mutants, expression level of Ptc1 is not different from that of control embryos. Hence, RA signaling presumably regulates Bmp4 expression independent of Shh signaling.
Teratogenic mechanisms induced by abnormal RA signaling are suggested by experiments adapting RA administration and the use of genetically modified mouse models in this study. Both experimental systems identified potential downstream target genes of Shh and Bmp4 during GT formation. It is known that insufficient maternal supplies of Vitamin A or dysregulation of RA in genetically deficient patients induce various developmental abnormalities, including those of the genitourinary system. This study offers important information on the downstream targets of RA signaling and may provide insights for further studies investigating RA-induced teratogenicity.
Supplementary Material
Acknowledgments
Grant sponsors: Ministry of Education, Culture, Sports, Science, and Technology, Japan; National Institutes of Health; Grant number: R01-ES016597-01A1.
We thank Drs. Alexandra L. Joyner, Pierre Chambon, Takashi Seki, John A. McLachlan, Yasuo Sakai, Chi-Chung Hui, and Carolina Rosselot for their great help. We also express our appreciation to Devangini Gandhi, Masayo Harada, Mylah D. Villacorte, Akiko Omori, Aki Murashima, Lerrie DG. Ipulan, Yuka Endo, and Shiho Miyaji for their assistance. This work was supported by Scientific Research on Innovative Areas Molecular Mechanisms for Establishment of Sex Differences (22132006) and the global COE Cell Fate Regulation Research and Education Unit from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, Grant-in-Aid for Young Scientists (B). This work was also supported by National Institutes of Health Grant R01-ES016597-01A1.
Footnotes
Additional Supporting Information maybe found in the online version of this article.
References
- Abu-Abed S, Dollé P, Metzger D, Beckett B, Chambon P, Petkovich M. The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes Dev. 2001;15:226–240. doi: 10.1101/gad.855001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S, Joyner AL. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell. 2004;118:505–516. doi: 10.1016/j.cell.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Batourina E, Choi C, Paragas N, Bello N, Hensle T, Costantini FD, Schuchardt A, Bacallao RL, Mendelsohn CL. Distal ureter morphogenesis depends on epithelial cell remodeling mediated by vitamin A and Ret. Nat Genet. 2002;32:109–115. doi: 10.1038/ng952. [DOI] [PubMed] [Google Scholar]
- Chiu HS, Szucsik JC, Georgas KM, Jones JL, Rumballe BA, Tang D, Grimmond SM, Lewis AG, Aronow BJ, Lessard JL, Little MH. Comparative gene expression analysis of genital tubercle development reveals a putative appendicular Wnt7 network for the epidermal differentiation. Dev Biol. 2010;344:1071–1087. doi: 10.1016/j.ydbio.2010.05.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollé P, Izpisúa-Belmonte JC, Brown JM, Tickle C, Duboule D. HOX-4 genes and the morphogenesis of mammalian genitalia. Genes Dev. 1991;5:1767–1767. doi: 10.1101/gad.5.10.1767. [DOI] [PubMed] [Google Scholar]
- Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997;237:752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- Fukami M, Nagai T, Mochizuki H, Muroya K, Yamada G, Takitani K, Ogata T. Anorectal and urinary anomalies and aberrant retinoic acid metabolism in cytochrome P450 oxidoreductase deficiency. Mol Genet Metab. 2010;100:269–273. doi: 10.1016/j.ymgme.2010.03.023. [DOI] [PubMed] [Google Scholar]
- Halilagic A, Ribes V, Ghyselinck NB, Zile MH, Dollé P, Studer M. Retinoids control anterior and dorsal properties in the developing forebrain. Dev Biol. 2007;303:362–375. doi: 10.1016/j.ydbio.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Haraguchi R, Suzuki K, Murakami R, Sakai M, Kamikawa M, Kengaku M, Sekine K, Kawano H, Kato S, Ueno N, Yamada G. Molecular analysis of external genitalia formation: the role of fibroblast growth factor (Fgf) genes during genital tubercle formation. Development. 2000;127:2471–2479. doi: 10.1242/dev.127.11.2471. [DOI] [PubMed] [Google Scholar]
- Haraguchi R, Mo R, Hui C, Motoyama J, Makino S, Shiroishi T, Gaffield W, Yamada G. Unique functions of Sonic hedgehog signaling during external genitalia development. Development. 2001;128:4241–4250. doi: 10.1242/dev.128.21.4241. [DOI] [PubMed] [Google Scholar]
- Haraguchi R, Motoyama J, Sasaki H, Satoh Y, Miyagawa S, Nakagata N, Moon A, Yamada G. Molecular analysis of coordinated bladder and urogenital organ formation by Hedgehog signaling. Development. 2007;134:525–533. doi: 10.1242/dev.02736. [DOI] [PubMed] [Google Scholar]
- Harnish DC, Jiang H, Soprano KJ, Kochhar DM, Soprano DR. Retinoic acid receptor beta 2 mRNA is elevated by retinoic acid in vivo in susceptible regions of mid-gestation mouse embryos. Dev Dyn. 1992;194:239–246. doi: 10.1002/aja.1001940309. [DOI] [PubMed] [Google Scholar]
- Jiang J, Ma L, Yuan L, Wang X, Zhang W. Study on developmental abnormalities in hypospadiac male rats induced by maternal exposure to di-n-butyl phthalate (DBP) Toxicology. 2007;232:286–293. doi: 10.1016/j.tox.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Kondo T, Zákány J, Innis JW, Duboule D. Of fingers, toes and penises. Nature. 1997;390:29. doi: 10.1038/36234. [DOI] [PubMed] [Google Scholar]
- Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie GW, Leblond CP, Inoue S, Martin GR, Chung A. Fine structure of the glomerular basement membrane and immunolocalization of five basement membrane components to the lamina densa (basal lamina) and its extensions in both glomeruli and tubules of the rat kidney. Am J Anat. 1984;169:463–481. doi: 10.1002/aja.1001690408. [DOI] [PubMed] [Google Scholar]
- Lee GS, Liao X, Shimizu H, Collins MD. Genetic and pathologic aspects of retinoic acid-induced limb malformations in the mouse. Birth Defects Res A Clin Mol Teratol. 2010;88:863–882. doi: 10.1002/bdra.20712. [DOI] [PubMed] [Google Scholar]
- Lin C, Yin Y, Veith GM, Fisher AV, Long F, Ma L. Temporal and spatial dissection of Shh signaling in genital tubercle development. Development. 2009;136:3959–3967. doi: 10.1242/dev.039768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn C, Lohnes D, Décimo D, Lufkin T, LeMeur M, Chambon P, Mark M. Function of the retinoic acid receptors (RARs) during development (II) Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development. 1994;120:2749–2771. doi: 10.1242/dev.120.10.2749. [DOI] [PubMed] [Google Scholar]
- Miyagawa S, Moon A, Haraguchi R, Inoue C, Harada M, Nakahara C, Suzuki K, Matsumaru D, Kaneko T, Matsuo I, Yang L, Taketo MM, Iguchi T, Evans SM, Yamada G. Dosage-dependent hedgehog signals integrated with Wnt/{beta}-catenin signaling regulate external genitalia formation as an appendicular program. Development. 2009;136:3969–3978. doi: 10.1242/dev.039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EA, Nguyen SB, Scott V, Stadler HS. Loss of Bmp7 and Fgf8 signaling in Hoxa13-mutant mice causes hypospadia. Development. 2003;130:3095–3109. doi: 10.1242/dev.00530. [DOI] [PubMed] [Google Scholar]
- Murakami R. Immunohistochemical and immunoblot analyses of collagens in the developing fibrocartilage in the glans penis of the rat. Acta Morphol Neerl Scand. 1987;25:279–288. [PubMed] [Google Scholar]
- Nakata M, Takada Y, Hishiki T, Saito T, Terui K, Sato Y, Koseki H, Yoshida H. Induction of Wnt5a-expressing mesenchymal cells adjacent to the cloacal plate is an essential process for its proximodistal elongation and subsequent anorectal development. Pediatr Res. 2009;66:149–154. doi: 10.1203/PDR.0b013e3181aa304a. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Dollé P. Retinoic acid in development: towards an integrated view. Nat Rev Genet. 2008;9:541–553. doi: 10.1038/nrg2340. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Subbarayan V, Dollé P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- Ogino Y, Suzuki K, Haraguchi R, Satoh Y, Dolle P, Yamada G. External genitalia formation: role of fibroblast growth factor, retinoic acid signaling, and distal urethral epithelium. Ann N Y Acad Sci. 2001;948:13–31. [PubMed] [Google Scholar]
- Otto DM, Henderson CJ, Carrie D, Davey M, Gundersen TE, Blomhoff R, Adams RH, Tickle C, Wolf CR. Identification of novel roles of the cytochrome p450 system in early embryogenesis: effects on vasculogenesis and retinoic acid homeostasis. Mol Cell Biol. 2003;23:6103–6116. doi: 10.1128/MCB.23.17.6103-6116.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pask AJ, Kanasaki H, Kaiser UB, Conn PM, Janovick JA, Stockton DW, Hess DL, Justice MJ, Behringer RR. A novel mouse model of hypogonadotrophic hypogonadism: N-ethyl-N-nitrosourea-induced gonadotropin-releasing hormone receptor gene mutation. Mol Endocrinol. 2005;19:972–981. doi: 10.1210/me.2004-0192. [DOI] [PubMed] [Google Scholar]
- Pask AJ, McInnes KJ, Webb DR, Short RV. Topical oestrogen keratinises the human foreskin and may help prevent HIV infection. PLoS One. 2008;3:e2308. doi: 10.1371/journal.pone.0002308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribes V, Wang Z, Dollé P, Niederreither K. Retinaldehyde dehydrogenase 2 (RALDH2)-mediated retinoic acid synthesis regulates early mouse embryonic forebrain development by controlling FGF and sonic hedgehog signaling. Development. 2006;133:351–361. doi: 10.1242/dev.02204. [DOI] [PubMed] [Google Scholar]
- Ribes V, Otto DM, Dickmann L, Schmidt K, Schuhbaur B, Henderson C, Blomhoff R, Wolf CR, Tickle C, Dollé P. Rescue of cytochrome P450 oxidoreductase (Por) mouse mutants reveals functions in vasculogenesis, brain and limb patterning linked to retinoic acid homeostasis. Dev Biol. 2007;303:66–81. doi: 10.1016/j.ydbio.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Ribes V, Le Roux I, Rhinn M, Schuhbaur B, Dollé P. Early mouse caudal development relies on crosstalk between retinoic acid, Shh and Fgf signalling pathways. Development. 2009;136:665–676. doi: 10.1242/dev.016204. [DOI] [PubMed] [Google Scholar]
- Rosselot C, Spraggon L, Chia I, Batourina E, Riccio P, Lu B, Niederreither K, Dolle P, Duester G, Chambon P, Costantini F, Gilbert T, Molotkov A, Mendelsohn C. Non-cell-autonomous retinoid signaling is crucial for renal development. Development. 2010;137:283–292. doi: 10.1242/dev.040287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Harfe BD, Cohn MJ. Cell lineage analysis demonstrates an endodermal origin of the distal urethra and perineum. Dev Biol. 2008;318:143–152. doi: 10.1016/j.ydbio.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Bouldin CM, Choi KS, Harfe BD, Cohn MJ. Multiphasic and tissue-specific roles of sonic hedgehog in cloacal septation and external genitalia development. Development. 2009a;136:3949–3957. doi: 10.1242/dev.042291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Yamaguchi T, Cohn MJ. Functional and phylogenetic analysis shows that Fgf8 is a marker of genital induction in mammals but is not required for external genital development. Development. 2009b;136:2643–2651. doi: 10.1242/dev.036830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Zheng Z, Ormerod BK, Cohn MJ. Sonic hedgehog controls growth of external genitalia by regulating cell cycle kinetics. Nat Commun. 2010;1:1–9. doi: 10.1038/ncomms1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaut CA, Saneyoshi C, Morgan EA, Knosp WM, Sexton DR, Stadler HS. HOXA13 directly regulates EphA6 and EphA7 expression in the genital tubercle vascular endothelia. Dev Dyn. 2007;236:951–960. doi: 10.1002/dvdy.21077. [DOI] [PubMed] [Google Scholar]
- Sucov HM, Murakami KK, Evans RM. Characterization of an autoregulated response element in the mouse retinoic acid receptor type beta gene. Proc Natl Acad Sci USA. 1990;87:5392–5396. doi: 10.1073/pnas.87.14.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Bachiller D, Chen YP, Kamikawa M, Ogi H, Haraguchi R, Ogino Y, Minami Y, Mishina Y, Ahn K, Crenshaw EB, Yamada G. Regulation of outgrowth and apoptosis for the terminal appendage: external genitalia development by concerted actions of BMP signaling [corrected] Development. 2003;130:6209–6220. doi: 10.1242/dev.00846. [DOI] [PubMed] [Google Scholar]
- Tüzel E, Samli H, Kuru I, Türkmen S, Demir Y, Maralcan G, Güler C. Association of hypospadias with hypoplastic synpolydactyly and role of HOXD13 gene mutations. Urology. 2007;70:161–164. doi: 10.1016/j.urology.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Vezina CM, Allgeier SH, Fritz WA, Moore RW, Strerath M, Bushman W, Peterson RE. Retinoic acid induces prostatic bud formation. Dev Dyn. 2008;237:1321–1333. doi: 10.1002/dvdy.21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Ferrara C, Shapiro E, Grishina I. Bmp7 expression and null phenotype in the urogenital system suggest a role in re-organization of the urethral epithelium. Gene Expr Patterns. 2009;9:224–230. doi: 10.1016/j.gep.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada G, Satoh Y, Baskin LS, Cunha GR. Cellular and molecular mechanisms of development of the external genitalia. Differentiation. 2003;71:445–460. doi: 10.1046/j.1432-0436.2003.7108001.x. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Zhao X, Uehara M, Yamashita K, Nishijima M, Nishino J, Saijoh Y, Sakai Y, Hamada H. Regulation of retinoic acid distribution is required for proximodistal patterning and outgrowth of the developing mouse limb. Dev Cell. 2004;6:411–422. doi: 10.1016/s1534-5807(04)00062-0. [DOI] [PubMed] [Google Scholar]
- Zhu CC, Yamada G, Blum M. Retinoic acid teratogenicity: the role of goosecoid and BMP-4. Cell Mol Biol (Noisy-le-grand) 1999;45:617–629. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.