Abstract
Background: Pre-eclampsia (PE) is a major pregnancy disorder complicating up to 8% of pregnancies. Increasing evidence indicates a sex-specific interplay between the mother, placenta and fetus. This may lead to different adaptive mechanisms during pregnancy.
Methods: We performed an individual participant data meta-analysis to determine associations of fetal sex and PE, with specific focus on gestational age at delivery in PE. This was done on 219 575 independent live-born singleton pregnancies, with a gestational age at birth between 22.0 and 43.0 weeks of gestation, from 11 studies participating in a worldwide consortium of international research groups focusing on pregnancy.
Results: Of the women, 9033 (4.1%) experienced PE in their pregnancy and 48.8% of the fetuses were female versus 51.2% male. No differences in the female/male distribution were observed with respect to term PE (delivered ≥ 37 weeks). Preterm PE (delivered < 37 weeks) was slightly more prevalent among pregnancies with a female fetus than in pregnancies with a male fetus [odds ratio (OR) 1.11, 95% confidence interval (CI) 1.02–1.21]. Very preterm PE (delivered < 34 weeks) was even more prevalent among pregnancies with a female fetus as compared with pregnancies with a male fetus (OR 1.36, 95% CI 1.17–1.59).
Conclusions: Sexual dimorphic differences in the occurrence of PE exist, with preterm PE being more prevalent among pregnancies with a female fetus as compared with pregnancies with a male fetus and with no differences with respect to term PE.
Keywords: Sexual dimorphism, pre-eclampsia, placenta, sex ratio, ALSPAC
Introduction
There are known large sex differences in disease incidence, presentation, diagnosis and outcome to treatment.1 During past years attention has focused on the female/male distribution during pregnancy and its interaction with maternal health. Apparently, maternal physiological functions are influenced in a fetal sex-specific manner during pregnancy.2 Pre-eclampsia (PE) is a major pregnancy disorder complicating up to 8% of pregnancies in some countries. PE is an important contributor to maternal and perinatal morbidity and mortality worldwide.3 Pre-eclamptic women as well as their children have an increased risk to develop cardiovascular disease and stroke later in life.4 A previous study indicated that fetal sex influenced gestational age at delivery in a Norwegian population from up to 50 years ago, with female fetuses predominating in pre-eclamptic pregnancies ending before 37 weeks.5 Gestational age has been suggested as an indicator of subsets of PE with a different pathophysiology and with different acute and long-range outcomes for both mother and baby. Therefore, in this study we sought to confirm and extend these earlier findings to very preterm pregnancies in a more diverse and contemporary pregnancy population. To assess sex-specific differences in gestational age at delivery in pre-eclamptic pregnancies, we conducted a meta-analysis of individual data from 219 575 pregnant women participating in 11 studies from several European, Oceanian and US centres.
Material and Methods
Inclusion criteria and participating cohorts
In 2011, the Global Pregnancy Collaboration (CoLab) was established to facilitate data and sample sharing between research groups studying PE and other pregnancy disorders [pre-empt.cfri.ca/Collaboration/global-pregnancy-Collaboration]. CoLab is a consortium of international research groups with data and biological samples from women before, during and in some cases long after pregnancy. Information on clinical data and samples is offered in a membership-wide shared database and available to CoLab members and to investigators sponsored by CoLab members.6 In 2012, we invited principal investigators of international research groups active in CoLab to participate in the current study. Studies participated if they included pregnant women with available information on the occurrence of PE. Information on gestational age at birth and fetal sex also had to be available. Only live-born singleton pregnancies with a gestational age at birth between 22.0 and 43.0 weeks of gestation were included. Both nulliparous and multiparous women could participate. Eleven studies agreed to participate, comprising 219 575 independent singleton pregnancies that met the inclusion criteria.7–16 The studies varied in sample size as well as study design, including both low- and high-risk pregnancies. Study-specific information with references to detailed information about each individual study is shown in Table 1. All studies were approved by the national, regional and local relevant research review boards. Regarding the ALSPAC study, ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the local research ethics committees. All participants provided written informed consent for use of their data. Anonymized data sets were stored on a single central secured data server with access for the main analysts only. MOOSE guidelines for reporting a meta-analysis were followed.
Table 1.
Characteristics of the participating studies
| Study | Country | Design | Setting | No. (= 219575) | Year inclusion | PE, n (% within study) |
|---|---|---|---|---|---|---|
| Avon Longitudinal Study of Parents and Children7 | UK | Prospective cohort study | Population based | 13444 | 1991–92 | 317 (2.4) |
| Danish National Birth Cohort (DNBC)8 | Denmark | Prospective cohort study | Population based | 83532 | 1996–2002 | 2040 (2.4) |
| Finnish Genetics of Pre-eclampsia Consortium9 | Finland | Case-control study | Hospital based | 1930 | 2008–12 | 1049 (54.4) |
| Generation R Study (GenR)10 | The Netherlands | Prospective cohort study | Population based | 8363 | 2002–06 | 198 (2.4) |
| Lund Database (Lund) | Sweden | Prospective cohort study | Hospital based | 545 | 1999-–014 | 239 (43.8) |
| The Norwegian Mother and Child Cohort (MoBa)11 | Norway | Prospective cohort study | Population based | 98436 | 1999–2009 | 3721 (3.8) |
| Oslo Pregnancy Biobank (OPB)12 | Norway | Case-control study | Hospital based | 472 | 2001–13 | 182 (38.6) |
| Pregnancy Exposures and Preeclampsia Prevention Study (PEPP)13 | USA | Prospective cohort study | Population based | 4274 | 1999–2007 | 597 (14.0) |
| Prediction and Prevention of Pre-eclampsia Project (PREDO)14 | Finland | Prospective cohort study | Hospital based | 1032 | 2005–09 | 92 (8.9) |
| The Screening for Pregnancy Endpoints (SCOPE)15 | New Zealand, Australia, UK and Ireland | Prospective cohort study | Hospital based | 5573 | 2004–11 | 275 (4.9) |
| Vitamin C and Vitamin E in Pregnant Women at Risk for Pre-Eclampsia trial (VIP)16 | UK | Randomized clinical trial | Hospital based | 1974 | 2003–05 | 323 (16.4) |
Studies are listed in alphabetical order.
No., number of participants with a live-born singleton pregnancy between 22–43 weeks of gestation and complete information on the occurrence of pre-eclampsia (PE) and fetal sex.
Pre-eclampsia
Information on PE per study was obtained per participating centre by using measurements, medical registries, hospital records and/or specific questionnaires. Gestational hypertension was defined as a blood pressure > 140 mmHg systolic or > 90 mmHg diastolic in a woman who was normotensive before 20 weeks’ gestation without concurrent new-onset proteinuria. In all studies participating in CoLab and in this study, PE is defined according to former International Society for the Study of Hypertension in Pregnancy criteria (de novo gestational hypertension with concurrent new-onset proteinuria [≥ 0.3 g protein in a 24-h specimen, correlating with ≥ 30 mg/dl (≥ 1 + reading on dipstick) in a random urine determination with no evidence of urinary tract infection].17 Superimposed PE was defined as chronic hypertension diagnosed before pregnancy or in the first 20 weeks of pregnancy, complicated by de novo proteinuria occurring after gestational week 20, in the absence of renal disease and urinary tract infection. As PE is a syndrome that does not necessarily present as de novo hypertension and proteinuria the same day, and as routine antenatal follow-up schedules differ between countries and pregnancies, the time of PE diagnosis is difficult to define precisely. Instead, gestational age at delivery was used as a proxy for the onset of disease. Women with a very early onset of PE (before gestational week 34) often present with combined intrauterine growth restriction (IUGR) or rapidly increasing maternal symptoms and rarely remain undelivered for many days or weeks. Women with term PE (from gestational week 37 + 0) are likely to be induced (provided vaginal delivery is feasible and clinically justified) and delivered shortly after diagnosis, complying with current international clinical PE guidelines. As gestational age at delivery was reliably registered in the centres that were included in this analysis, this was used as a proxy to distinguish between term, preterm and very preterm PE (i.e. delivery ≥ 37 + 0 weeks of gestation, < 37 weeks of gestation and < 34 weeks of gestation). This distinction between early and very early versus term ‘onset’ of PE is a commonly used categorization in PE studies.
Covariates
Information about maternal characteristics (maternal age, parity, body mass index and the presence of chronic hypertension) and birth characteristics (gestational age at birth, offspring birthweight and fetal sex) in each study was obtained per participating centre by using measurements, medical registries, hospital records and/or specific questionnaires.
Statistical analyses
Individual datasets were integrated into one central database. For the cleaning of the central database the following criteria were used: values had to be within three standard deviations at either side of the mean and/or values had to be clinically reasonable. Random-effects models as proposed by DerSimonian and Laird were used to take the potential between-study variation next to the within-study variation into account.18,19 In this model, the inverse of standard errors from the individual studies combined with the between-study variation were used as weights. Heterogeneity was assessed by the I2 index. The I2 index describes the proportion of total variation in the effect sizes that is due to heterogeneity between studies. To determine the influence of any particular cohort on overall results, we repeated each meta-analysis, leaving out one cohort at a time (leave-one-out methodology). The overall effects are presented as forest plots with the pooled odds ratios from the random-effects models with 95% confidence intervals (CI). Statistical analyses were performed with SAS 9.2 software (SAS Institute, Cary, NC) and Comprehensive Meta-Analysis 2.0 (Biostat, Englewood, USA).
Results
Subject characteristics
Study-specific information about maternal and birth characteristics is shown in Table 2. The overall distribution of female and male fetuses was 48.8% versus 51.2%. The overall prevalences of gestational hypertension and PE were 2.9% and 4.1% (n = 6150 and n = 9033), respectively. Of the pre-eclamptic women, 6.4% had superimposed PE (n = 575). Of the remaining 8458 de novo pre-eclamptic women, 15.4% were diagnosed with very preterm PE (<34 weeks of gestation, n = 1306).
Table 2.
Maternal and birth characteristics
| Total cohort | Alspac | DNBC | FINNPEC | GenR | Lund | |
|---|---|---|---|---|---|---|
| N = 219575 | n = 13444 | n = 83532 | n = 1930 | n = 8363 | n = 545 | |
| Maternal age, years (mean, SD) | 29.8 (4.7) | 28.0 (5.0) | 29.8 (4.4) | 29.9 (5.4) | 29.7 (5.3) | 30.0 (5.0) |
| Parity, % 0 | 50.4 | 45 | 50.6 | 66.1 | 55.0 | 68.5 |
| BMI, kg/m2 | 23.0 | 21.6 | 22.6 | 23.6 | 23.9 | NA |
| (median, 90% range) | (18.7–32.9) | (17.6–30.7) | (18.6–31.9) | (19.1–34.4) | (19.4–33.7) | NA |
| Chronic hypertension, % yes | 1.3 | 3.8 | 0.2 | 10.6 | 1.9 | 0.9 |
| Gestational age birth, weeks | 40.0 | 40.0 | 40.0 | 39.0 | 40.1 | 38.7 |
| (median, 90% range) | (36.0–42.0) | (36.0–42.0) | (37.0–42.0) | (31.0-–42.0) | (36.9–42.0) | (29.2–41.7) |
| Birthweight, grams (mean, SD) | 3547.5 (585.0) | 3408.7 (551.5) | 3574.3 (571.9) | 3096.3 (861.6) | 3411.9 (561.5) | 3156.1 (866.2) |
| Fetal sex, % female | 48.8 | 48.4 | 48.8 | 50.8 | 49.5 | 50.3 |
| MoBa | OPB | PEPP | PREDO | SCOPE | VIP | |
|---|---|---|---|---|---|---|
| n = 98436 | n = 472 | n = 4274 | n = 1032 | n = 5573 | n = 1974 | |
| Maternal age, years (mean, SD) | 30.2 (4.6) | 31.7 (4.9) | 26.3 (6.3) | 32.3 (5.8) | 28.7 (5.5) | 30.8 (5.9) |
| Parity, % 0 | 46.7 | 53.8 | 68.1 | 31.8 | 100 | 49.9 |
| BMI, kg/m2 | 23.1 | 23.9 | 24.1 | 25.5 | 24.2 | 31.2 |
| (median, 90% range) | (18.9–32.5) | (19.1–35.9) | (18.1–39.2) | (19.1–39.5) | (19.5–34.7) | (21.1–43.4) |
| Chronic hypertension, % yes | 0.5 | 0.2 | 2.5 | 18.4 | 2.7 | 41.0 |
| Gestational age birth, weeks | 40.0 | 38.4 | 39.0 | 39.9 | 40.1 | 39.0 |
| (median, 90% range) | (37.0–42.0) | (28.6–40.3) | (33.0–41.0) | (36.4–41.9) | (36.6–41.7) | (33.1–41.9) |
| Birthweight, grams (mean, SD) | 3600.3 (560.5) | 3129.5 (1015.8) | 3141.0 (728.3) | 3510.7 (597.6) | 3415.6 (555.4) | 3217.7 (761.2) |
| Fetal sex, % female | 48.7 | 50 | 49.2 | 46.6 | 49.2 | 49.1 |
NA, not available.
Pre-eclampsia and fetal sex
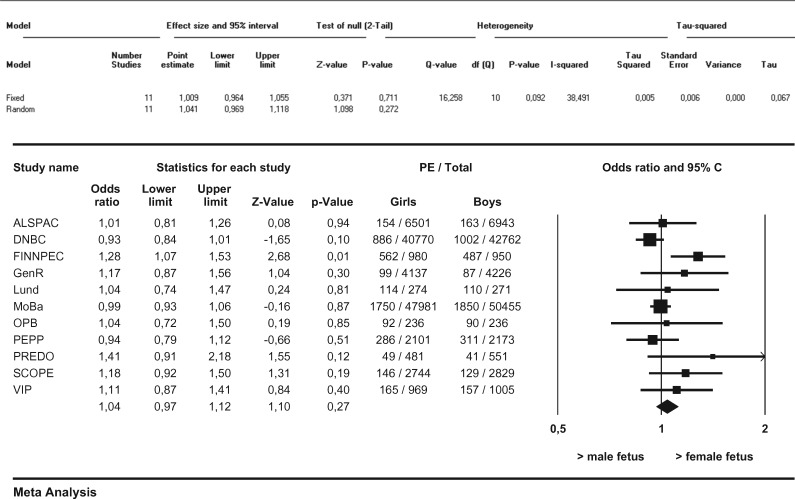
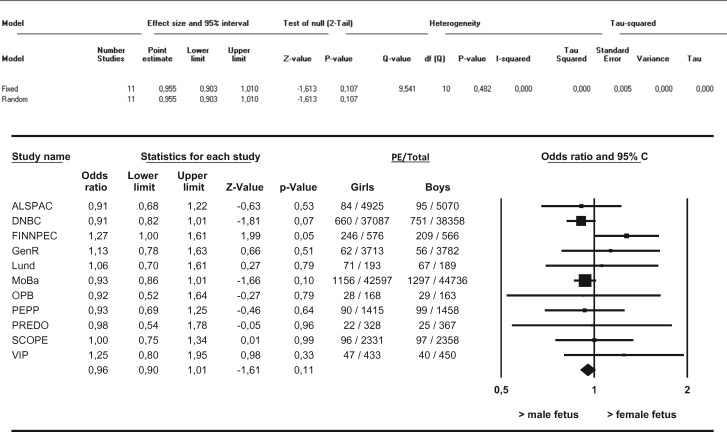
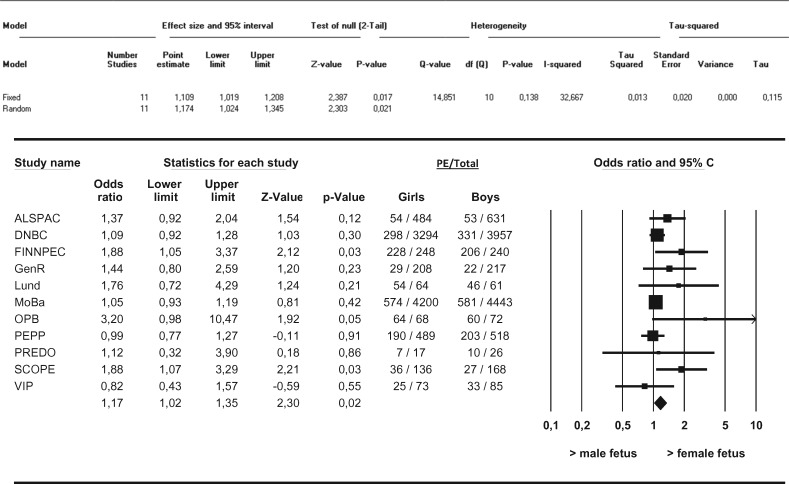
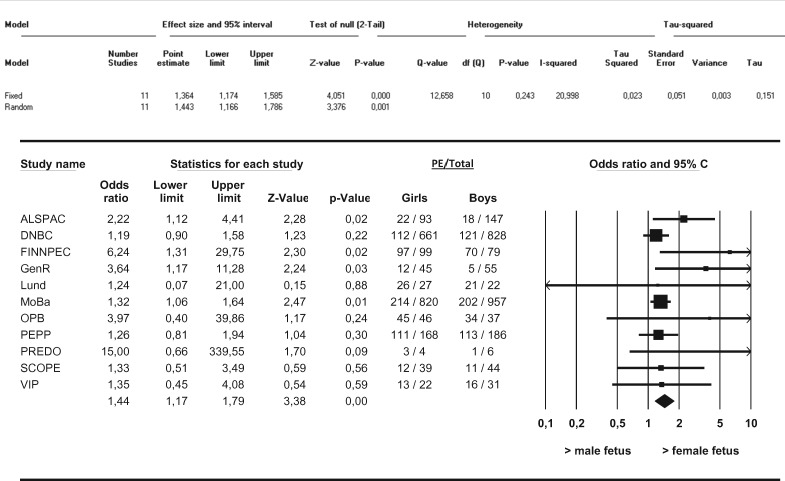
In this meta-analysis we observed no differences in the distribution of female versus male fetuses in the overall occurrence of PE (Figure 1). Furthermore, no differences in the distribution of female versus male fetuses with respect to de novo PE, superimposed PE or gestational hypertension were observed. We observed no differences in the female/male distribution with respect to term de novo PE (i.e. ≥ 37 weeks of gestation) (Figure 2). After stratification into preterm and very preterm de novo PE (i.e. < 37 weeks of gestation and < 34 weeks of gestation), differences in the distribution of female versus male fetuses in the occurrence of PE were observed. Female preterm PE was more prevalent than male preterm PE in pregnancies going beyond 22.0 weeks (OR 1.11, 95% CI 1.02–1.21, I2 = 32.7%) (Figure 3). These results did not change after applying the leave-one-out method nor did restriction of these analyses to nulliparous women change the results. Very preterm PE was even more prevalent among pregnancies with a female fetus as compared with pregnancies with a male fetus (OR 1.36, 95% CI 1.17–1.59, I2 = 21.0%) (Figure 4). Applying the leave-one-out method did not change the results nor did restriction of these analyses to nulliparous women (Supplementary Figures 1 and 2, available as Supplementary data at IJE online). Finally, no differences in the female/male distribution with respect to de novo PE between 34 and 37 weeks of gestation were observed. This suggests that the effects with respect to preterm PE are mainly determined by effects in the distribution of female versus male fetuses in very preterm PE (Supplementary Figure 3, available as Supplementary data at IJE online).
Figure 1.
Associations between fetal sex and overall PE between female and male pregnancies Results from random-effects models. Data reflect Odds ratios (95% Confidence Interval) in which female preeclampsia (PE) is compared to male PE.
Figure 2.
Associations between fetal sex and term de novo PE between female and male pregnancies Results from random-effects models. Data reflect Odds ratios (95% Confidence Interval) in which female term preeclampsia (PE) is compared to male term PE. Term PE was defined as gestational age ≥ 37+0 weeks at delivery.
Figure 3.
Associations between fetal sex and preterm de novo PE between female and male pregnancies Results from random-effects models. Data reflect Odds ratios (95% Confidence Interval) in which female preterm preeclampsia (PE) is compared to male preterm PE. Preterm PE was defined as gestational age < 37+0 weeks at delivery.
Figure 4.
Associations between fetal sex and very preterm de novo PE between female and male pregnancies Results from random-effects models. Data reflect Odds ratios (95% Confidence Interval) in which female very preterm preeclampsia (PE) is compared to male very preterm PE. Very preterm PE was defined as gestational age < 34+0 weeks at delivery.
Comment
Results from this large-scale meta-analysis of individual participants’ data show sexual dimorphic differences in the rates of PE subgroups, with preterm and very preterm PE being more prevalent among pregnancies with a female fetus as compared with pregnancies with a male fetus, and with no differences with respect to term PE. No differences in female/male distribution are observed in the overall risk of PE.
Comparison with earlier studies and interpretation of main findings
PE has a deleterious impact on maternal and fetal morbidity, mortality and future health. It is a heterogeneous disorder with a complex aetiology and pathogenesis. Progress in the understanding of the disorder would be assisted greatly if subtypes could be characterized.20 Despite increasing evidence that maternal physiological functions are influenced in a fetal sex-specific manner during pregnancy, in most studies that assess potential pathophysiological mechanisms of PE, fetal sex has not been taken into account.
Previously, a large Norwegian population-based data study suggested that the sex ratio in PE displays a pattern strongly dependent on length of gestation.5 They showed that female babies were more frequent in PE with preterm delivery, whereas PE with term delivery was dominated by male offspring. Interestingly, when only assessing normotensive pregnancies, opposite results were observed with a male predominance in preterm births.5 Our results on PE are in line with theirs, indicating that fetal sex influences gestational age at delivery in pre-eclamptic pregnancies. These results are further supported by a recent study by Broere-Brown et al.21 showing fetal sex-specific differences in maternal vascular adaptation to pregnancy. They observed sex-specific differences in Doppler measurements of the uterine artery and sex-specific differences in both systolic and diastolic blood pressure patterns throughout pregnancy. Interestingly, differential effects according to the presence or absence of the placental syndromes, encompassing PE, IUGR and preterm birth, were observed. In pregnancies complicated by the placental syndromes women pregnant with a female fetus showed a higher blood pressure compared with women with a male fetus at the beginning of pregnancy. In contrast, by the end of the second trimester a shift in the male blood pressure pattern and female blood pressure pattern was observed. This resulted in a higher blood pressure for women with a male fetus compared with women with a female fetus at the end of pregnancy.21
Gestational age has been suggested as an indicator of subsets of PE with a different pathophysiology and with different acute and long-range outcomes for both mother and baby. We hypothesize that perhaps we might be looking at a biological phenomenon in which the observed sex-specific differences reflect a functional placental difference and subsequent response by the mother between the sexes with differential PE phenotypes as a result.
So what underlies the sexual dimorphism in PE? According to the two-stage model, impaired placentation including dysfunctional remodelling of the utero-placental arteries has been considered as powerful predisposing step in the aetiology of PE. This has especially been suggested for the early-onset subtype of PE.3,22 The first decidua-associated remodelling step should be initiated around implantation. Exposures at this stage might influence the risk of PE. Previously, it was hypothesized by Vatten et al. that a sex-specific susceptibility to the process of embryonic implantation could partly explain sexual dimorphic differences in PE.5 The so-called ‘cross-over’ in the sex ratio of PE was interpreted as an indication for the existence of two separate pathogenetic entities. The first pathogenetic entity would be associated with IUGR. Unfortunately, we did not have information available on the occurrence of IUGR to test this. The other pathogenetic entity proposed was that late-onset disease originated from abnormal implantation. Male embryos would be more susceptible to suboptimal implantation or abnormal placental development.23 This might imply that those pregnancies with a male embryo that are susceptible to develop PE due to impaired placentation may already have miscarried in the first trimester. The male fetuses that survive the period of placentation will thereby represent a relatively healthy group of fetuses leading to a female-biased prevalence of PE. Orzsack et al.24 showed higher first-trimester male miscarriage rates.24 Furthermore, lower first-trimester human chorionic gonadotrophin hormone concentrations (hCG) have been described for pregnancies with a male fetus compared with pregnancies with a female fetus.25 Since progesterone levels are higher in male fetuses and exert an inhibitory effect on hCG production, this may result in a lower hCG production by the male placenta and thereby results in a differential endometrial receptivity.26 HCG is proposed to promote angiogenesis in the uterine vasculature and to block any immunological action by the mother on foreign invading placental cells.27 This might also be related to earlier reported observations on a positive correlation between hCG levels, hyperemesis gravidarum and early-onset PE and fetal sex. Hyperemesis gravidarum is associated with higher levels of hCG and with an increased risk of early-onset PE.28–30 The presence of a female fetus is associated with hyperemesis.
The second stage of the two-stage model is associated with an exaggerated endothelial activation and a generalized hyperinflammatory state.3,31,32 Episodes of placental hypoxia or reperfusion result in oxidative stress, subsequent apoptotic and necrotic disruption of syncytial architecture and release of various components from the intervillous space into the maternal circulation that stimulates the production of inflammatory cytokines.3,33,34 Broere-Brown et al.21 previously showed that the placental release of circulating angiogenic and fibrinolytic factors differs according to fetal sex.35 They observed higher S-Flt1, PAI-2 and PlGF blood concentrations in cases of female as compared with male placentas. In pregnancies complicated by PE, spontaneous preterm birth or IUGR, however, no fetal sex-specific differences were observed. From this they concluded that perhaps other mechanisms causing these complications dominated the fetal sex effect.35 Muralimanoharan et al.36 also presented evidence of sexual dimorphism in placentas from male fetuses compared with placentas from female foetuses, with higher levels of inflammatory, hypoxia and apoptotic molecules in males. This was observed in placental tissue of term pre-eclamptic pregnancies and is consistent with Vatten et al.5 In addition, they reported that in an obesogenic environment, primary trophoblasts derived from placentas of female fetuses have higher sensitivity to inflammatory stress compared with placentas of males. Interestingly, Minghetti et al.37 when studying preterm births, showed other results with higher umbilical cord blood levels of the oxidative stress biomarker 8-iso-PGF2α in male fetuses compared with female fetuses, using a natural twinning model.37 Isoprostanes are free radical-catalyzed prostaglandin-like products and considered as reliable markers of oxidative stress. In line with this, Yeganegi et al.38 and Challis et al.39 also demonstrated greater pro-inflammatory responses with a male fetus versus higher anti-inflammatory responses in pregnancies with a female fetus. They suggested that the male fetus exists in a relatively more ‘pro-inflammatory environment’ than the female fetus. This could account for the increased loss by miscarriage and spontaneous preterm birth with male fetuses. However, these latter three studies focused on preterm births in non-pre-eclamptic pregnancies and thereby are not completely pertinent to the distinct and multi-step entity of PE. We hypothesize that differences between pregnancies with male and female fetuses in the first (placental) but also second (systemic maternal) stage predispose to dimorphic differences in PE. Perhaps as previously suggested by Haig,40 PE is a disorder of failed interaction between two genetically different organisms. As PE is associated with long-term maternal health and in view of increasing interest in microchimerism (i.e. the long-term presence within an individual of a low level of cells derived from a different individual), the observed sexual dimorphic differences in the occurrence of PE might not be pertinent to pregnancy alone but also might have important long-term cardiovascular health implications for the mother.2,4
Strengths and limitations
We performed a large meta-analysis with individual data from 11 studies participating in the CoLab consortium. We did not rely on published data, which limits any potential publication bias. The large number of participants enabled us to assess small effects. We presented results from random-effects models which allow heterogeneity in the true effect estimates between different populations and take between-study variation into account. By applying the leave-one-out method, we were able to determine the influence of any particular cohort on overall results. In agreement with other studies, we used the dating of gestational age at delivery as a proxy for the onset of PE, and not the time of first diagnosis. In a small subset of women (n = 1716) however, we did have information available on actual gestational age at PE diagnosis. These data were highly correlated with gestational age at birth (r = 0.89, P < 0.001). We therefore think it is unlikely that non-differential misclassification affected our effect estimates greatly.
Finally, we chose to exclude stillbirths since some studies did only include live-born infants whereas in other studies the presence of stillbirths could have been under-sampled (due to participation bias or loss-to-follow-up bias). Some stillbirths might have occurred before PE has been recognized clinically, or fetal sex may not have been determined in some of the very early stillbirths. Vatten et al.5 showed an increased risk of perinatal death in pre-eclamptic pregnancies in case of male fetuses. We had information available on 660 stillbirths. Additional analyses, however, in this subgroup showed no differences in the female/male distribution.
Conclusion
In conclusion we found that there are fetal sex-specific differences in the occurrence of PE with a female dominance among preterm, but not term, pregnancies complicated by PE. Our results highlight the importance of fetal sex when studying placenta-mediated-diseases.
Supplementary Data
Supplementary data are available at IJE online.
Supplementary Material
Acknowledgements
Acknowledgements are due to: the Global Pregnancy Collaboration (CoLab); and the Avon Longitudinal Study of Parents and Children (ALSPAC) (we are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses); the Danish National Birth Cohort (DNBC); the Finnish Genetics of Pre-eclampsia Consortium (FINNPEC) (we thank the study participants as well as the members of the FINNPEC Board and the FINNPEC Study Group; the Generation R Study (GenR); the Lund Database (Lund); the Norwegian Mother and Child Cohort Study (MoBa) we are grateful to all the participating families in Norway who take part in this ongoing cohort study; the Oslo Pregnancy Biobank (OPB) (we are grateful for excellent biobank and study assistance from Lisa Øhra Levy, Research Centre for Obstetrics and Gynecology, Oslo University Hospital); the Pregnancy Exposures and Pre-eclampsia Prevention Study (PEPP); the Prediction and Prevention of Pre-eclampsia Project (PREDO); the Screening for Pregnancy Endpoints International Cohort Study (SCOPE); and the Vitamin C and Vitamin E in Pregnant Women at Risk for Pre-Eclampsia trial (VIP).
Funding
This work was supported by: the Global Pregnancy Collaboration (CoLab), part of the Pre-eclampsia-Eclampsia Monitoring, Prevention & Treatment initiative funded by the University of British Columbia, a grantee of the Bill & Melinda Gates Foundation; Avon Longitudinal Study of Parents and Children (ALSPAC) [the UK Medical Research Council, the Wellcome Trust (grant ref: 102215/2/13/2) and the University of Bristol provide core support for ALSPAC]; the Danish National Birth Cohort (DNBC) (the Danish National Research Foundation established the Danish Epidemiology Science Centre that initiated, created and funded the Danish National Birth Cohort with additional support from the Pharmacy Foundation, the Egmont Foundation, the March of Dimes Birth Defects Foundation, and the Augustinus Foundation); the Finnish Genetics of Pre-eclampsia Consortium (FINNPEC) (supported by Jane and Aatos Erkko Foundation, Päivikki and Sakari Sohlberg Foundation, Academy of Finland, Research Funds of the University of Helsinki, government special state subsidy for health sciences (EVO funding) at the Hospital District of Helsinki and Uusimaa, Novo Nordisk Foundation, Finnish Foundation for Pediatric Research, Emil Aaltonen Foundation, and Sigrid Jusélius Foundation); the Generation R Study (GenR) (funded by the Erasmus Medical Centre, Rotterdam; the Erasmus University Rotterdam and the Netherlands Organization for Health Research and Development). The researchers are independent from the funders. Additional support was available from the Netherlands Organization for Health Research and Development (VIDI) and the Dutch Asthma Foundation; Lund Database [supported by grants from the Swedish Research Council (Vetenskapsrådet) and with help from staff at Scania University Hospital, Sweden]; the Norwegian Mother and Child Cohort Study (MoBa) [supported by the Norwegian Ministry of Health and the Ministry of Education and Research, NIH/NIEHS (contract no N01-ES-75558), NIH/NINDS (grant no.1 UO1 NS 047537‐01 and grant no.2 UO1 NS 047537‐06A1)]; Oslo Pregnancy Biobank (OPB) (supported by grants from VIRUUS (Vitenskapsradet, Ulleval universitetssykehus) and the Woman and Child Division, Oslo University Hospital; Pregnancy Exposures and Pre-eclampsia Prevention Study (PEPP) (supported by National Institutes of Health grant P01-HD30367, National Centre for Research Resources Clinical and Translational Science Award grant 1 UL1 RR024153, and funding from Abbott Laboratories); Prediction and Prevention of Pre-eclampsia Project (PREDO) (supported by Academy of Finland, Clinical Graduate School in Pediatrics and Obstetrics/Gynecology, University of Helsinki, Finnish Medical Society Duodecim, Emil Aaltonen Foundation, Finnish Concordia Fund, Finnish Foundation For Pediatric Research, Finnish Medical Foundation, Signe and Ane Gyllenberg Foundation, Sigrid Juselius Foundation, Government Special Subsidy for Health Sciences at Helsinki and Uusimaa Hospital District, Jane and Aatos Erkko Foundation, Orion Foundation, Päivikki and Sakari Sohlberg Foundation, Yrjö Jahnsson Foundation); the Screening for Pregnancy Endpoints International Cohort Study (SCOPE) (maintained by MedSciNet AB); the Vitamin C and Vitamin E in Pregnant Women at Risk for Pre-Eclampsia trial (VIP) ([unded by the Wellcome Trust (registered charity number 210183) with additional support from Tommy’s the baby charity (registered charity number 1060508)].
Conflict of interest: The authors report no conflict of interest.
Key Message
This study highlights the importance of fetal sex during pregnancy showing fetal sex-specific differences in pre-eclampsia with a female dominance among preterm pre-eclamptic pregnancies.
References
- 1. Owens NJ. Sex and gender differences. In: Borgelt LM, O’Connell MB, Smith JA et al. (eds). Women’s Health Across the Lifespan. 2nd edn New York, NY: Elsevier, 2010. [Google Scholar]
- 2. Clifton VL, Stark MJ, Osei-Kumah A, Hodyl NA. Review:The feto-placental unit, pregnancy pathology and impact on long term maternal health. Placenta 2012;33(Suppl):S37_41. [DOI] [PubMed] [Google Scholar]
- 3. Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet 2010;376:631–44. [DOI] [PubMed] [Google Scholar]
- 4. Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129 290 births. Lancet 2001;357:2002–06. [DOI] [PubMed] [Google Scholar]
- 5. Vatten LJ, Skjaerven R. Offspring sex and pregnancy outcome by length of gestation. Early Hum Dev 2004;76:47–54. [DOI] [PubMed] [Google Scholar]
- 6. Myatt JP, Crompton RH, Thorpe SK. A new method for recording complex positional behaviours and habitat interactions in primates. Folia Primatol (Basel) 2011;82:13–24. [DOI] [PubMed] [Google Scholar]
- 7. Fraser A, Macdonald-Wallis C, Tilling K et al. . Cohort Profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol 2013;42:97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Olsen J, Melbye M, Olsen SF et al. . The Danish National Birth Cohort - its background, structure and aim. Scand J Public Health 2001;29:300–07. [DOI] [PubMed] [Google Scholar]
- 9. Lokki AI, Aalto-Viljakainen T, Meri S, Laivuori H. Genetic analysis of membrane cofactor protein (CD46) of the complement system in women with and without pre-eclamptic pregnancies. PLoS One 2015;10:e0117840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kruithof CJ, Kooijman MN, van Duijn CM et al. . The Generation R Study:Biobank update 2015. Eur J Epidemiol 2014;29:911–27. [DOI] [PubMed] [Google Scholar]
- 11. Magnus P, Irgens LM, Haug K et al. . Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol 2006;35:1146–50. [DOI] [PubMed] [Google Scholar]
- 12. Staff AC, Braekke K, Harsem NK, Lyberg T, Holthe MR. Circulating concentrations of sFlt1 (soluble FMS-like tyrosine kinase 1) in fetal and maternal serum during pre-eclampsia. Eur J Obstet Gynecol Reprod Biol 2005;122:33–39. [DOI] [PubMed] [Google Scholar]
- 13. Powers RW, Roberts JM, Plymire DA et al. . Low placental growth factor across pregnancy identifies a subset of women with preterm pre-eclampsia: type 1 versus type 2 pre-eclampsia? Hypertension 2012;60:239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Villa PM, Kajantie E, Raikkonen K et al. . Aspirin in the prevention of pre-eclampsia in high-risk women: a randomised placebo-controlled PREDO Trial and a meta-analysis of randomised trials. BJOG 2013;120:64–74. [DOI] [PubMed] [Google Scholar]
- 15. Kenny LC, Black MA, Poston L et al. . Early pregnancy prediction of pre-eclampsia in nulliparous women, combining clinical risk and biomarkers: the Screening for Pregnancy Endpoints (SCOPE) international cohort study. Hypertension 2014;64:644–52. [DOI] [PubMed] [Google Scholar]
- 16. Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH; Vitamins in Pre-eclampsia Trial C. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet 2006;367:1145–54. [DOI] [PubMed] [Google Scholar]
- 17. Group NHBPEPW. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 2000;183:S1–22. [PubMed] [Google Scholar]
- 18. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 19. van Houwelingen HC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med 2002;21:589–624. [DOI] [PubMed] [Google Scholar]
- 20. Dadelszen P, Magee LA, Roberts JM. Subclassification of pre-eclampsia. Hypertens Pregnancy 2003;22:143–48. [DOI] [PubMed] [Google Scholar]
- 21. Broere-Brown ZA, Schalekamp-Timmermans S, Hofman A, Jaddoe V, Steegers E. Fetal sex dependency of maternal vascular adaptation to pregnancy: a prospective population-based cohort study. BJOG 2016;123:1087–95. [DOI] [PubMed] [Google Scholar]
- 22. Redman CW, Sargent IL, Staff AC. IFPA Senior Award Lecture: making sense of pre-eclampsia ‐ two placental causes of pre-eclampsia? Placenta 2014;35(Suppl):S20–25. [DOI] [PubMed] [Google Scholar]
- 23. Murji A, Proctor LK, Paterson AD, Chitayat D, Weksberg R, Kingdom J. Male sex bias in placental dysfunction. Am J Med Genet A 2012;158A:779–83. [DOI] [PubMed] [Google Scholar]
- 24. Orzack SH, Stubblefield JW, Akmaev VR et al. . The human sex ratio from conception to birth. Proc Natl Acad Sci U S A 2015;112:E2102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Korevaar TI, Steegers EA, de Rijke YB et al. . Reference ranges and determinants of total hCG levels during pregnancy:the Generation R Study. Eur J Epidemiol 2015;30:1057–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hagemenas FC, Kittinger GW. The influence of fetal sex on the levels of plasma progesterone in the human fetus. J Clin Endocrinol Metab 1973;36:389–91. [DOI] [PubMed] [Google Scholar]
- 27. Cole LA. Biological functions of hCG and hCG-related molecules. Reprod Biol Endocrinol 2010;8:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bolin M, Akerud H, Cnattingius S, Stephansson O, Wikstrom AK. Hyperemesis gravidarum and risks of placental dysfunction disorders: a population-based cohort study. BJOG 2013;120: 541–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Verberg MF, Gillott DJ, Al-Fardan N, Grudzinskas JG. Hyperemesis gravidarum, a literature review. Hum Reprod Update 2005;11:527–39. [DOI] [PubMed] [Google Scholar]
- 30. Tan PC, Jacob R, Quek KF, Omar SZ. The fetal sex ratio and metabolic, biochemical, haematological and clinical indicators of severity of hyperemesis gravidarum. BJOG 2006;113: 733–37. [DOI] [PubMed] [Google Scholar]
- 31. Redman CW, Sacks GP, Sargent IL. Pre-eclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol 1999;180:499–506. [DOI] [PubMed] [Google Scholar]
- 32. Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Pre-eclampsia:an endothelial cell disorder. Am J Obstet Gynecol 1989;161:1200–04. [DOI] [PubMed] [Google Scholar]
- 33. Huppertz B. Placental origins of pre-eclampsia: challenging the current hypothesis. Hypertension 2008;51:970–75. [DOI] [PubMed] [Google Scholar]
- 34. Redman CWG, Sargent IL, Roberts JM. Immunology of Normal Pregnancy and Pre-eclampsia. Amsterdam: Academic Press, 2009. [Google Scholar]
- 35. Brown ZA, Schalekamp-Timmermans S, Tiemeier HW, Hofman A, Jaddoe VW, Steegers EA. Fetal sex-specific differences in human placentation: a prospective cohort study. Placenta 2014;35:359–64. [DOI] [PubMed] [Google Scholar]
- 36. Muralimanoharan S, Maloyan A, Myatt L. Evidence of sexual dimorphism in the placental function with severe pre-eclampsia. Placenta 2013;34:1183–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Minghetti L, Greco A, Zanardo V, Suppiej A. Early-life sex-dependent vulnerability to oxidative stress: the natural twining model. J Matern Fetal Neonatal Med 2013;26:259–62. [DOI] [PubMed] [Google Scholar]
- 38. Yeganegi M, Watson CS, Martins A et al. . Effect of Lactobacillus rhamnosus GR-1 supernatant and fetal sex on lipopolysaccharide-induced cytokine and prostaglandin-regulating enzymes in human placental trophoblast cells: implications for treatment of bacterial vaginosis and prevention of preterm labor. Am J Obstet Gynecol 2009;200:532 e1–8. [DOI] [PubMed] [Google Scholar]
- 39. Challis J, Newnham J, Petraglia F, Yeganegi M, Bocking A. Fetal sex and preterm birth. Placenta 2013;34:95–99. [DOI] [PubMed] [Google Scholar]
- 40. Haig D. Altercation of generations: genetic conflicts of pregnancy. Am J Reprod Immunol 1996;35:226–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.