Abstract
Zebrafish are a powerful model system to assess the molecular and cellular effects of exposure to toxic chemicals during embryonic development. To study the effects of environmental endocrine disruptors, embryos and larvae are commonly exposed to supraphysiologic concentrations of these compounds in the water, but their bioavailability in zebrafish is largely unknown. One hypothesis is that supraphysiologic concentrations of estrogens in the water are required to achieve physiologic levels in vivo; however, this has not been directly tested. To test this hypothesis, we developed an assay using radiolabeled estradiol ([3H]E2) to measure uptake from water at multiple concentrations and exposure durations in developing zebrafish from 0 to 5 days postfertilization (dpf). We found that [3H]E2 uptake increased with increasing concentration, duration, and developmental stage. Percent uptake from the total volume of treatment solution increased with increasing exposure duration and developmental stage, but remained constant with increasing concentration. We also found that the chorion, an acellular envelope surrounding embryos through 3 dpf, did not substantially affect [3H]E2 uptake. Finally, we found that at 1 dpf, E2 was preferentially taken up by the yolk at multiple exposure durations, while at 2 dpf E2 was preferentially taken up into the embryonic body. Our results support the hypothesis that exposing zebrafish embryos and larvae to supraphysiologic concentrations of estrogens is required to achieve physiologically relevant doses in vivo. The isotopic assay reported here will provide a foundation for determining the uptake of other compounds for teratogenicity, toxicology and drug discovery studies.
Keywords: endocrine disruptors, estrogens, embryo, environmental exposure
Exposure to environmental endocrine disruptors (EEDs), small molecules that mimic endogenous hormones, can cause deleterious effects in humans and aquatic animals (Diamanti-Kandarakis et al., 2009). The ubiquity of EEDs that influence estrogen receptor activity, such as the naturally occurring chemicals estradiol (E2) and genistein, pharmaceuticals such as ethinyl estradiol (EE2), and synthetic industrial compounds such as bisphenol A (BPA), is a particular cause for concern (Adeel et al., 2017; Lambert et al., 2015).
To study the effects of estrogen-like EEDs on development, zebrafish embryos are commonly used to screen for toxicity and to study the effects of estrogens on organ formation and function (Bouwmeester et al., 2016; Carroll et al., 2014; Gorelick and Halpern, 2011; Gorelick et al., 2014; Hao et al., 2013; Kinch et al., 2015; Namdaran et al., 2012; Padilla et al., 2012; Romano and Gorelick, 2014; Sun et al., 2010; Tal et al., 2016; Truong et al., 2014). Embryos are often exposed to micromolar concentrations of E2 in fish water, despite the fact that the EC50 of E2 for the zebrafish estrogen receptors alpha, beta 1 and beta 2 (ERα, ERβ1, ERβ2) is 77, 39, and 118 pM, respectively (Pinto et al., 2014). Such high concentrations in fish water are used under the assumption that only a small percentage of estrogens in the water are absorbed by the embryo; however, this assumption has not been tested directly.
E2 uptake is further complicated by the presence of the chorion, an acellular membrane surrounding embryos prior to hatching that may block chemical uptake (Bonsignorio et al., 1996; Rawson et al., 2000). Additionally, the lipid-rich yolk that provides embryos with nutrients prior to initiation of feeding (Kunz, 2004) may preferentially absorb small molecules and delay their reaching the embryonic body. How these structures affect E2 and estrogen-like EED uptake is not well understood.
Using radiolabeled estradiol ([3H]E2), we developed an assay to measure E2 uptake into zebrafish embryos during multiple stages of development. We found that less than 1% of E2 is absorbed following 1 h exposure, and <4% of E2 is absorbed following exposures 24 h or longer. Additionally, we found that the embryo chorion has little effect on E2 uptake, while the yolk absorbs a significant proportion of E2. Our results support the hypothesis that exposing zebrafish embryos and larvae to supraphysiologic concentrations of estrogens is required to achieve physiologically relevant doses in vivo.
MATERIALS AND METHODS
Zebrafish
Adult zebrafish were raised at 28.5 °C on a 14-h light, 10-h dark cycle in the UAB Zebrafish Research Facility in an Aquaneering recirculating water system (Aquaneering, Inc., San Diego, California). All zebrafish used for experiments were wildtype, AB strain (Westerfield, 2000). All procedures were approved by the UAB Institutional Animal Care and Use Committee.
Embryo collection
Adult zebrafish were allowed to spawn naturally in groups. Embryos were collected in intervals of 10 min to ensure precise developmental timing, placed in 60 cm2 Petri dishes at a density of no more than 100 per dish in E3B media (60× E3B: 17.2g NaCl, 0.76g KCl, 2.9g CaCl2-2H2O, 2.39g MgSO4 dissolved in 1 L Milli-Q water; diluted to 1× in 9 L Milli-Q water plus 100 μl 0.02% methylene blue), and then stored in an incubator at 28.5 °C on a 14-h light, 10-h dark cycle until treatment.
Embryo treatments
Embryos were treated in tritiated E2 ([6,7-3H(N)]-17β-E2, 1 mCi/ml, Perkin Elmer NET013250UC, Waltham, Massachusetts) or vehicle (0.1% ethanol) diluted to final concentration in E3B at the time of treatment.
Uptake assay
For uptake experiments using tritiated compounds, zebrafish between 6 and 96 hpf were exposed in 24-well plates to 2 ml of treatment solution in pools of 10 embryos or larvae per well. For experiments performed between 6 and 48 hpf, embryos were manually dechorionated unless otherwise specified. All treatments were diluted to specified concentrations in E3B embryo media, such that vehicle concentration never exceeded 0.25%. After the addition of treatment solution, 10 μl aliquots of treatment water were collected in duplicate from each well to measure initial radioactivity. During the exposure period, embryos were kept at 28.5 °C on a 14-h light, 10-h dark cycle. At the end of the exposure period, treatment water was aspirated from each well with a fine-tip pipette and fresh E3B was added. In embryos above 72 hpf at the time of measurement, 0.01 mg/ml tricaine was added to immobilize embryos for transfer. Embryos were next pipetted individually into a new well of a separate 24-well plate filled with fresh E3B using a 10 μl pipette tip with the narrow end cut with a razor blade to prevent damage to the embryo, and then immediately transferred with a new pipette tip into a 7 ml liquid scintillation vial (High Density Polyethylene MiniVial, Research Products International No. 125500, Mount Prospect, Illinois) containing 400 μl Biosol tissue solubilizer (National Diagnostics LS-310, Atlanta, Georgia). A single well was used for the individual wash step of each embryo, and washes were counted to ensure no radioactivity from the water was being transferred with the embryos into the scintillation vials. Vials were then heated in a water bath at 55 °C for 2–3 h and allowed to cool to room temperature prior to the addition of 4 ml Bioscint scintillation fluid (National Diagnostics EC-309, Atlanta, Georgia). Radioactivity of samples was measured on an LS 6500 Multi-purpose Scintillation Counter (Beckman, Indianapolis, Indiana). Picomole (pmol) absorption was quantified from the standard curve (Supplementary Figure 1). Percent uptake was determined by dividing the radioactivity (counts-per-minute [CPM]) detected in individual embryos by the radioactivity of the treatment solution prior to exposure. For experiments requiring yolk removal, at the end of the exposure period, the 24-well plate was placed on ice and 0.01 mg/ml tricaine was added to each well. In the individual wash step (see above), 10 μl pipette tips were used to separate the body of the embryo from the yolk by pipetting directly over the yolk 2–3 times until separation occurred, keeping the embryonic body intact. Background radioactivity from vehicle-treated embryos and their treatment solution was sufficiently low (<10 CPM) to be excluded from graphs and analysis of E2-treated groups.
Experimental design and data analysis
Experiments were performed on 10 embryos from a single clutch per treatment group or vehicle control group. Experiments were performed at least 3 times (n ≥ 3) using embryos from different clutches. Mean pmol uptake and mean percent uptake from each group were used for comparing treatment groups between experiments. A 2-tailed, unpaired Student’s t test was used when testing for statistical significance between 2 groups, and 1-way ANOVA with Tukey’s test for multiple comparisons was used when comparing more than 2 groups. Statistical significance was set at p ≤ .05. GraphPad Prism 7.0a software was used for all statistical analyses and for producing graphs.
RESULTS
Assay Development
We measured [3H]E2 levels in fish water and in zebrafish using a scintillation counter, detecting CPM [3H]E2. To convert CPM into picomoles [3H]E2 (pmol), we created a standard curve using increasing concentrations of [3H]E2 (0.01 nM to 1 μM in increments of 10×). 10 μl of each concentration of [3H]E2 in embryo water was measured in triplicate to obtain CPM values for each concentration. This experiment was repeated 2 more times (n = 3 experiments) to obtain a mean CPM value that was then used for curve-fitting analysis. The best fit was achieved by plotting log Y versus log X, where X = pmol [3H]E2 and Y = CPM (Suppleentary Figure 1; r2 = 0.9992, unweighted, best-fit linear regression line). The equation Y = 1.073*X + 4.459 was used in subsequent experiments to convert CPM to pmol [3H]E2. We determined the limit of detection of our assay to be 0.01 pmol, as this is the smallest measured amount that was reliably distinguished from background.
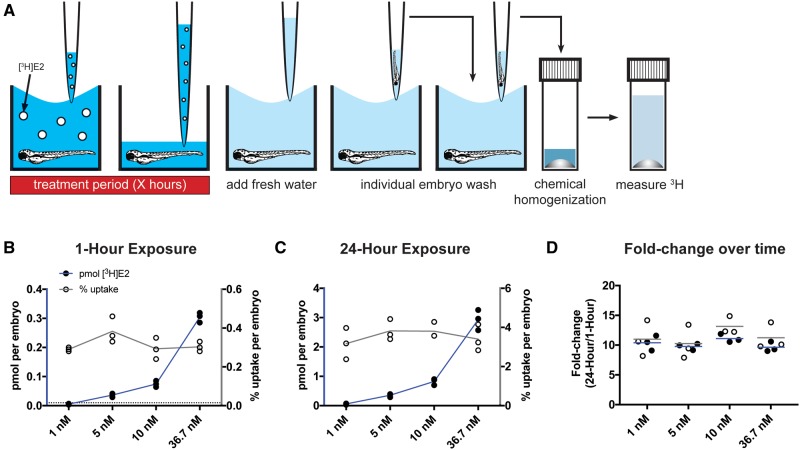
To maximize throughput, we initially attempted to measure E2 uptake using zebrafish embryos in 100–200 μl of treatment solution in 96-well plates. Results using this technique were highly variable within the same experiment, even when concentration, exposure duration, and developmental stage remained constant (coefficient of variability (CV) = 27–37%). We found that the radioactivity of treatment wells with no embryo present was also highly variable (data not shown), likely due to variable levels of evaporation between wells during treatment. To overcome intra-experimental variability due to evaporation, we exposed zebrafish in larger volumes. We combined embryos in pools of 25 per 60-mm diameter Petri dish in 7 mL treatment solution, or in pools of 10 embryos per well in 24-well plates in 2 ml treatment solution per well. With both methods, we were able to measure uptake in single embryos with lower variability between embryos than was demonstrated in the 96-well plate method at the same exposure conditions (CV = 12–23% with 60-mm dish method, CV = 17–29% with 24-well plate method). The variability among experimental means was also lower for the 24-well plate method (96-well: CV = 27%, 24-well: CV = 12%). As the 24-well plate method was more time and cost effective, we chose this method as the basis of our assay. A graphical method of the methods for our assay is shown in Figure 1A, details are described in Materials and Methods Section.
Figure 1.
E2 uptake is concentration- and duration-dependent, but percent uptake is not concentration-dependent. A, Graphic demonstration of the assay developed to measure [3H]E2 uptake. White circles represent molecules of [3H]E2. Embryos at 48 hpf were exposed to 4 different concentrations of [3H]E2 for 1 h (B) or 24 h (C). Radioactivity was measured using a scintillation counter and used to calculate pmol [3H]E2 per embryo (black circles) and percent [3H]E2 uptake per embryo (white circles). At both exposure durations, pmol [3H]E2 uptake increased in a concentration-dependent manner (blue lines connecting black circles), whereas percent [3H]E2 uptake remained constant (gray lines connecting white circles). Each circle represents the mean uptake from a single experiment (n = 3) assaying 10 embryos per experiment. Horizontal dotted line represents the limit of detection (0.01 pmol). D, Both pmol and percent [3H]E2 uptake increased approximately 10-fold following 24-h exposure compared with 1-h exposure. Horizontal lines represent the mean fold change in pmol (blue) or percent uptake (gray). Color image is available online, and text accurately reflects both black and white and color formats.
E2 Uptake Is Concentration- and Duration-Dependent
We first tested the effect of concentration on [3H]E2 uptake in 48 h postfertilization (hpf) dechorionated zebrafish embryos. Embryos were exposed to 4 different concentrations of [3H]E2, from 1 to 36.7 nM, for 1 h. We found that the amount of [3H]E2 absorbed per embryo increased 8-fold between 5 and 36.7 nM exposure (Figure 1B, Supplementary Figure 2A, Supplementary Table 1; 1 nM treatment = 0.0054 ± 0.0016 pmol (mean ± SD), 5 nM = 0.036 ± 0.0065 pmol, 10 nM = 0.075 ± 0.010 pmol, 36.7 nM = 0.30 ± 0.017 pmol). Note that at the 1 nM concentration, the measured pmol values were below the limit of detection (LOD) (0.01 pmol).
To determine how exposure duration influences E2 uptake, we exposed 48 hpf dechorionated embryos to the same 4 concentrations of [3H]E2 for 24 h, and compared absorption for each concentration following 1- and 24-h exposure. Following 24-h exposure, absorption was concentration-dependent, with an average 51-fold increase between 1 and 36.7 nM exposure (Figure1C; 1 nM treatment = 0.057 ± 0.022 pmol (mean ± SD), 5 nM = 0.35 ± 0.057 pmol, 10 nM = 0.83 ± 0.11 pmol, 36.7 nM = 2.93 ± 0.35 pmol). For a given concentration, absorption following 24- versus 1-h exposure increased by an average of 10.22- ± 0.96-fold (Figure 1D, Supplementary Figure 3), demonstrating that [3H]E2 uptake is dependent on duration of exposure.
Using these results, we also calculated the percent uptake of [3H]E2 per embryo. For a given concentration, percent uptake increased 10- to 13-fold between 1- and 24-h exposures (Figure 1D, Supplementary Figure 3). In contrast, percent uptake remained constant following the same exposure duration at different concentrations of [3H]E2 (Figs. 1B and C, Supplementary Figure 2B, Supplementary Table 2; 1-h treatment: 1 nM = 0.29% ± 0.01% (mean ± SD); 5 nM = 0.38% ± 0.068%, 10 nM = 0.29% ± 0.055%, 36.7 nM = 0.30% ± 0.025%; 24-h treatment: 1 nM = 3.17% ± 0.80%, 5 nM = 3.82% ± 0.54%, 10 nM = 3.81% ± 0.41%, 36.7 nM = 3.40% ± 0.68%). Thus, percent uptake of [3H]E2 is dependent on duration of exposure, but not on concentration.
For subsequent experiments testing variables other than concentration, the 5 nM concentration was chosen, as this was the lowest concentration we tested that was reliably above the LOD at both exposure durations. As percent uptake does not change with increasing exposure concentration, values observed with this concentration should be applicable to higher exposure concentrations.
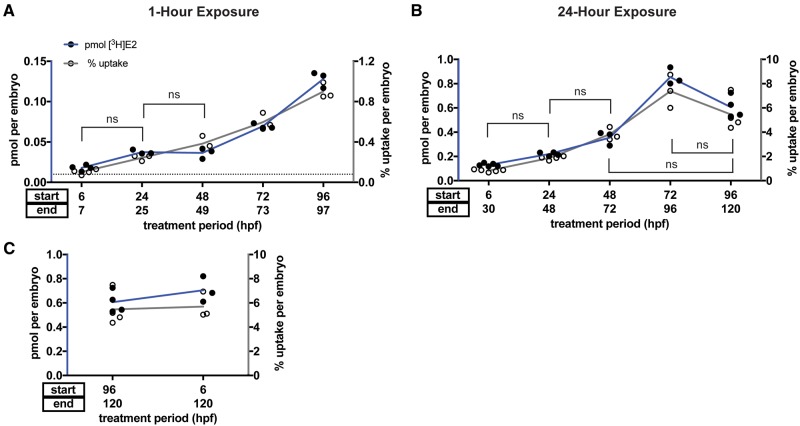
E2 Uptake Increases With Developmental Stage
Studies of the effects of endocrine disrupting compounds on development and organ function frequently require exposure of zebrafish embryos and larvae during multiple stages of development. To determine whether developmental stage influences E2 uptake, we exposed embryos beginning at five different developmental stages, between 6 and 96 hpf, to a single concentration of [3H]E2 (5 nM) for 1 h. Mean pmol [3H]E2 uptake increased 7-fold between 6 and 96 hpf (Figure 2A; 6 hpf treatment = 0.018 ± 0.004 pmol (mean ± SD), 24 hpf = 0.037 ± 0.003 pmol, 48 hpf = 0.036 ± 0.007 pmol, 72 hpf = 0.070 ± 0.003 pmol, 96 hpf = 0.13 ± 0.010 pmol). Mean pmol [3H]E2 uptake was not statistically significantly different when starting treatment at 24 versus 48 hpf (ANOVA with Tukey’s multiple comparisons test, p = .9994), but mean pmol uptake was significantly increased when comparing younger versus older developmental stages for all other groups (Supplementary Table 3).
Figure 2.
E2 uptake is age-dependent. Embryos were exposed to 5 nM [3H]E2 for 1 h (A) or 24 h (B) starting at five different developmental stages between 6 and 96 hpf. Lines denote percent uptake comparisons that are not statistically significant, all other comparisons are significant (p < .05). Radioactivity was measured using a scintillation counter and used to calculate pmol [3H]E2 per embryo (black circles) and percent [3H]E2 uptake per embryo (white circles). Both pmol (blue lines connecting black circles) and percent (gray lines connecting white circles) [3H]E2 uptake increased with age except for 24-h exposure at 96 hpf, where there was a partial reduction in uptake that could represent efflux. One-way ANOVA with Tukey’s multiple comparisons test shows significant increase in pmol uptake when comparing 1-h treatment (A) at 6 versus 24 hpf (p = .0064), 24 versus 72 hpf (p = .0002), 48 versus 72 hpf (p = .0001), and 72 versus 96 hpf (p < .0001); significant changes in percent uptake were found with 6 versus 48 hpf (p = .0005), 48 versus 72 hpf (p < .0001) and 72 versus 96 hpf (p = .0005). A significant increase in pmol was present when comparing 24-h treatments (B) at 6 versus 48 hpf (p = .0005), 24 versus 48 hpf (p = .0407) and 48 versus 72 hpf (p < .0001), while a significant decrease was found when comparing 72 versus 96 hpf (p = .0003); percent uptake was significantly increased when comparing 6 versus 48 hpf (p = .0023), 24 versus 72 hpf (p < .0001), and 48 versus 72 hpf (p = .0013). C, Static exposure to 5 nM [3H]E2 from 6 to 120 hpf (114 h total). Starting exposure at 6 versus 96 hpf has little effect on [3H]E2 uptake at 120 hpf (p = .8206). Data from the 96 hpf exposure group are the same as that shown in (B). Each circle represents the mean uptake from a single experiment (n = 3–5) assaying 10 embryos per experiment. Horizontal dotted line represents the limit of detection (0.01 pmol). Lines connect the mean for each group. Color image is available online, and text accurately reflects both black and white and color formats.
Similarly, percent uptake increased 9-fold between 6 and 96 hpf (Figure 2A; 6 hpf treatment = 0.10% ± 0.025% uptake (mean ± SD), 24 hpf = 0.24% ± 0.029% uptake, 48 hpf = 0.38% ± 0.068% uptake, 72 hpf = 0.60% ± 0.083% uptake, 96 hpf = 0.90% ± 0.078% uptake). Mean percent uptake was not statistically significant when starting treatment at 6 versus 24 hpf (ANOVA with Tukey’s test for multiple comparisons, p = .0620) or 24 versus 48 hpf (p = .0880), but was significantly increased when comparing younger versus older developmental stages for all other groups (Supplementary Table 3).
At exposure duration of 24 h, 72 hpf embryos absorbed the most [3H]E2 of all stages tested, with a 6.5-fold increase between 6 and 72 hpf (Figure 2B; 6 hpf treatment = 0.13 ± 0.013 pmol (mean ± SD), 24 hpf = 0.22 ± 0.015 pmol, 48 hpf = 0.35 ± 0.057 pmol, 72 hpf = 0.85 ± 0.071 pmol, 96 hpf = 0.61 ± 0.090 pmol). There was not a statistically significant difference in mean pmol uptake when starting treatment at 6 versus 24 hpf (ANOVA with Tukey’s multiple comparisons test, p = .1682 for pmol), but mean difference when comparing other developmental stage groups was statistically significant (Supplementary Table 4).
Mean percent uptake increased 9-fold between 6 and 72 hpf, (Figure 2B; 6 hpf treatment = 0.83% ± 0.10% uptake, 24 hpf = 1.86% ± 0.16% uptake, 48 hpf = 3.82% ± 0.54% uptake, 72 hpf = 7.38% ± 1.37% uptake, 96 hpf = 5.46% ± 1.39% uptake) with significantly different percent uptake between all groups except 6 versus 24 hpf (ANOVA with Tukey’s multiple comparisons test, p = .4151), 24 versus 48 hpf (p = .0627), 48 versus 96 hpf (p = .1450), and 72 versus 96 hpf embryos (p = .0694) (Supplementary Table 4). Together, these results suggest that older embryos absorb more E2 than younger embryos.
To monitor developmental toxicity, a frequently used assay calls for continuously exposing zebrafish to a compound from the first hours of development (0–8 hpf) until larvae are freely swimming and organogenesis is largely complete (96–120 hpf) (Gustafson et al., 2012; McCollum et al., 2011). We tested [3H]E2 uptake under these conditions by continuously exposing embryos to 5 nM [3H]E2 from 6 until 120 hpf. We found that percent uptake was 5.69% ± 1.08% per embryo (mean ± SD), compared with 5.46% ± 1.39% per embryo when exposing embryos from 96 to 120 hpf only (Figure 2C). This difference was not statistically significant (Student’s unpaired t test, p = .8206). Although this multi-day exposure technique is relevant for studies testing compound effect throughout multiple developmental stages, it should be noted that this technique does not lead to enhanced chemical uptake overall compared with treating embryos for a much shorter time period to the same developmental end-point (120 hpf).
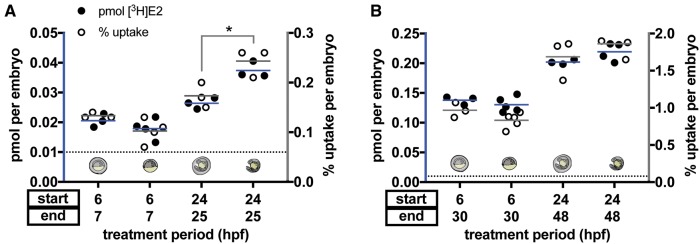
E2 Uptake Is Modestly Affected by Chorion Removal at 1-h, but Not 24-h, Exposures
Prior to hatching between 48 and 72 hpf, zebrafish embryos are enveloped in chorions that act as protective barriers during early development. The chorion is a 3-layered acellular envelope 1.5-2.5 μM wide, composed of multiple proteins with pores that exclude particles larger than 3–4 kDa (Bonsignorio et al., 1996; Pelka et al., 2017; Rawson et al., 2000). We hypothesized that E2 uptake would not be affected by chorion removal due to its small size, well under 3 kDa, and its lipophilicity as a steroid hormone. To test whether the chorion influences E2 uptake, we exposed embryos at 6 and 24 hpf, with chorions intact and following manual chorion removal, to 5 nM [3H]E2 for either 1 or 24 h. At 6 hpf, our results were consistent with the hypothesis that chorion removal does not affect E2 uptake. We found that pmol [3H]E2 uptake was not significantly changed in 6 hpf dechorionated embryos at either exposure duration compared with 6 hpf embryos with chorions intact (Figs. 3A and B; 1-h treatment: chorion = 0.021 ± 0.002 pmol, no chorion = 0.018 ± 0.004 pmol, p = .3076 Student’s unpaired t test; 24-h treatment: chorion = 0.14 ± 0.007, no chorion = 0.13 ± 0.013 pmol, p = .3863). In 24 hpf dechorionated embryos, pmol [3H]E2 uptake was increased approximately 1.4-fold following 1-h exposure compared with embryos with chorion intact (Figure 3A; chorion = 0.026 ± 0.002 pmol, no chorion = 0.037 ± 0.003 pmol, p = .0044 Student’s unpaired t test). Following 24-h exposure, there was no significant difference in uptake between dechorionated embryos and embryos with chorion intact (Figure 3B; chorion = 0.20 ± 0.004 pmol; no chorion = 0.22 ± 0.015 pmol, p = .1216).
Figure 3.
E2 uptake is modestly affected by chorion removal at 1-h, but not 24-h, exposures. Embryos at 6 or 24 hpf were exposed to 5 nM [3H]E2 for 1 h (A) or 24 h (B). Radioactivity was measured using a scintillation counter and used to calculate pmol [3H]E2 per embryo (black circles) and percent [3H]E2 uptake per embryo (white circles). Chorion-intact versus dechorionated embryos are indicated by images aligned with each treatment group. At 1-h exposure, both pmol [3H]E2 and percent uptake were not significantly decreased in 6 hpf dechorionated embryos (Student’s unpaired t-test, p = .31 for pmol, p = .096 for percent uptake), but were significantly increased in 24 hpf dechorionated embryos (*Student’s unpaired t-test, p = .0044 for pmol, p = .034 for percent uptake). At 24-h exposure, uptake was not statistically different when the chorion was present (Student’s unpaired t-test, 6 hpf: p = .39 for pmol, p = .12 for percent uptake; 24 hpf: p = .12 for pmol, p = .32 for percent uptake). Each circle represents the mean uptake from a single experiment (n = 3–5 per group) assaying 10 embryos per experiment. Lines represent the mean pmol (blue lines) or percent uptake (gray lines) of all experiments. Horizontal dotted line represents the limit of detection (0.01 pmol). Color image is available online, and text accurately reflects both black and white and color formats.
Correspondingly, percent uptake was not significantly changed in 6 hpf dechorionated embryos following 1- or 24-h exposure (Figs. 3A, and B; 1-h treatment: chorion = 0.13% ± 0.006% uptake, no chorion = 0.10 ± 0.025% uptake, p = .0958 Student’s unpaired t test; 24-h treatment: chorion = 0.967% ± 0.10% uptake, no chorion = 0.832% ± 0.10% uptake, p = .1156). Percent uptake in 24 hpf dechorionated embryos was also consistent with pmol uptake results, with uptake increased approximately 1.4-fold at 1-h exposure in dechorionated embryos versus embryos with chorion intact (Figure 3A; chorion = 0.17% ± 0.025% uptake, no chorion = 0.24% ± 0.029% uptake, p = .0340 Student’s unpaired t test). Following 24-h exposure, there was no significant difference in percent uptake between dechorionated embryos and embryos with chorion intact (Figure 3B; chorion = 1.69% ± 0.28% uptake; no chorion = 1.86% ± 0.16% uptake, p = .3249).
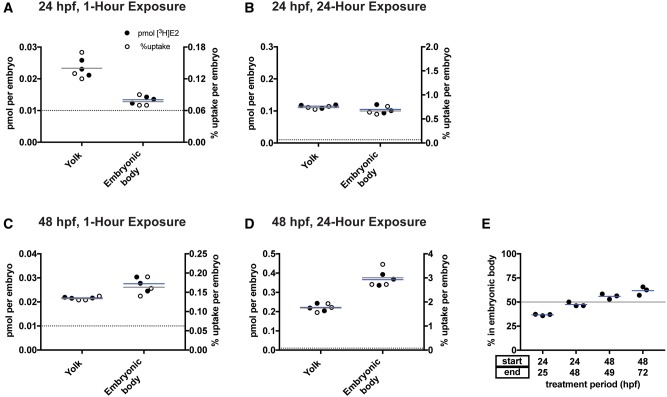
Developmental Stage and Exposure Duration Determine Whether E2 Is Preferentially Absorbed by the Yolk
Zebrafish embryos are lecithotrophic organisms that obtain nutrient supply and maternally deposited transcripts from their yolks throughout the first 4–5 days of development (Kunz, 2004). The lipid-rich yolk initially comprises a sizeable proportion of the embryonic mass. As the embryo matures, the ratio of yolk to embryo mass decreases until the yolk is fully absorbed by 7–10 days postfertilization (dpf).
We hypothesized that embryos at developmental stages with higher yolk content would absorb more E2 into the yolk versus into the embryonic body. To test this hypothesis, we exposed 24 and 48 hpf embryos to 5 nM [3H]E2 for 1–24 h and then separated the yolk from the embryonic body and assayed [3H]E2 levels in each (the 6 hpf stage was excluded from this analysis since the embryonic body is not sufficiently developed to make separation from the yolk possible). We found that when starting 5 nM [3H]E2 exposure at 24 hpf, E2 was preferentially absorbed by the yolk following 1-h exposure (Figure 4A; yolk = 0.023 ± 0.002 pmol, embryonic body = 0.013 ± 0.001 pmol), but was nearly equivalent in the yolk and embryonic body following 24-h exposure (Figure 4B; yolk = 0.12 ± 0.006 pmol, embryonic body = 0.11 ± 0.014 pmol). When starting treatment at 48 hpf, E2 was preferentially absorbed into the embryonic body at both exposure durations (Figs. 4C and D; 1-h: yolk = 0.022 ± 0.0002 pmol, embryonic body = 0.028 ± 0.0029 pmol; 24-h: yolk = 0.22 ± 0.020 pmol, embryonic body = 0.37 ± 0.028 pmol).
Figure 4.
Developmental stage and exposure duration determine whether E2 is preferentially absorbed by the yolk. Embryos at 24 (A, B) or 48 hpf (C, D) were exposed to 5 nM [3H]E2 for 1 h (A, C) or 24 h (B, D). Yolk was separated from embryonic body and radioactivity was measured using a scintillation counter and used to calculate pmol [3H]E2 per yolk and pmol [3H]E2 per embryonic body. E, Uptake into the embryonic body increases with increasing developmental stage. Circles represent percent [3H]E2 in the embryonic body versus [3H]E2 in the total embryo (embryonic body + yolk). Each circle represents the mean uptake from a single experiment (n = 3 per group) assaying 10 embryos per experiment. Horizontal blue lines are the mean of the 3 experiments. Horizontal gray line at y = 50 in (E) represents 50% absorption, indicating equal uptake into embryonic body and yolk. Color image is available online, and text accurately reflects both black and white and color formats.
To compare the percent of E2 taken up by the yolk versus embryonic body, we divided the pmol taken up per embryonic body by the pmol taken up by the entire embryo (yolk plus the embryonic body). In 24 hpf embryos, the majority of [3H]E2 was taken up by the yolk: the percent [3H]E2 taken up by the embryonic body was 36.7% ± 0.78% (mean ± SD) following 1 h exposure and 47.4% ± 2.2% following 24 h exposure (Figure 4E). In contrast, 48 hpf embryos absorbed the majority of [3H]E2 into the embryonic body: the percent [3H]E2 taken up by the embryonic body was 55.9% ± 2.8% following 1 h exposure and 61.8% ± 4.4% following 24 h exposure.
DISCUSSION
Comparison to Estrogen Uptake Assays in Other Aquatic Species
Our results support the hypothesis that supraphysiologic concentrations of E2 in zebrafish treatment water are required to obtain physiologic doses in vivo. Similar studies have measured the levels of exogenous estrogens and estrogen-like EEDs in multiple aquatic species (Bhandari et al., 2015; Miguel-Queralt and Hammond, 2008; Piferrer and Donaldson, 1994; Schwarz et al., 2017), but, to our knowledge, exogenous E2 uptake has not been previously measured in zebrafish embryos or larvae.
One study measured uptake of exogenous [3H]E2 in mussels (Mytilus spp.) by measuring tissue extracts after 48-h exposure and reported 18.7% uptake (Schwarz et al., 2017) versus the <4% uptake we found following 24-h exposure. This discrepancy could be explained by inherent differences in zebrafish and mussel absorption capacity, as mussels are much larger organisms and are not thought to produce endogenous E2. Further, a much lower concentration of [3H]E2 was used than in our study (4 pM vs 1–36.7 nM). Piferrer and Donaldson studied E2 uptake in coho salmon (Oncorhynchus kisutch) at different developmental stages using 97 pM [3H]E2 and found an uptake of 0.08%–0.33% in individual eggs after 96-h exposure, and a 0.13%–1.21% uptake in alevins (hatched eggs prior to feeding) (Piferrer and Donaldson, 1994). Though this is lower than the percent uptake we calculated in similarly staged zebrafish embryos at 24-h, this could be due to species differences. The increase in percent uptake observed at older developmental stages (alevins) is in agreement with our results. Another study measured [3H]BPA and [3H]EE2 uptake in fertilized medaka eggs (Oryzias latipes) at a single concentration for each chemical (44 nM for [3H]BPA and 0.17 nM [3H]EE2), reporting uptake of 0.125 pmol/mg/egg for [3H]BPA and 4.05 fmol/mg/egg for [3H]EE2 following 24-h exposure (Bhandari et al., 2015). Although [3H]BPA uptake appears similar to our [3H]E2 results, [3H]EE2 uptake was 30% of what we reported for [3H]E2 in zebrafish embryos. However, it is difficult to directly compare these results because the average mass of a medaka egg was not reported.
An uptake study in adult zebrafish exposed individual fish to radiolabeled steroids and measured aliquots of treatment water following exposure (Miguel-Queralt and Hammond, 2008). By measuring radioactivity in water containing 4 nM [3H]EE2 before and after zebrafish exposure, the authors reported 65% uptake of EE2 from treatment water after 1-h exposure. These results suggest that estrogen uptake is higher in adults compared with embryos and larvae, consistent with our findings that [3H]E2 uptake increases with developmental stage.
Comparison to HPLC, UPLC, and MS Steroid Measurement
Though chromatographic methods coupled to mass spectrometry (MS) could be utilized to assay levels of specific estrogens in zebrafish embryos (Alharthy et al., 2017), these methods require access to expensive and specialized equipment. A study of the uptake and clearance of small hydrophilic molecules like paracetamol in zebrafish embryos was developed using ultra performance liquid chromatography with MS (UPLC-MS) (Kantae et al., 2016); however, this approach is not easily translatable to E2 measurement due to the difficulty of E2 extraction from biological matrices and ion suppression effects that require chemical derivatization for MS analysis. Saili et al. (2012) measured BPA uptake in zebrafish embryos using high performance liquid chromatography with MS (HPLC-MS), finding an uptake of 0.02 μg/kg following 1 nM exposure for 50 h starting at 8 hpf. However, due to the high limit of detection of this assay (267 vs 1 nM for our assay), uptake was not directly measured. Rather, uptake following nM exposure levels was calculated by extrapolating uptake from embryos exposed to higher concentrations of BPA (1, 10, and 100 μM). Because BPA is a nonsteroidal estrogen, it may be absorbed and excreted differently than E2, and extraction protocols for BPA and E2 are not interchangeable due to their distinct chemical structures.
Additionally, separate extraction protocols would be needed to measure levels of E2 metabolites, such as sulfate and glucuronide conjugates and catechol estrogens, using HPLC-MS. Enzymes involved in E2 metabolism—sulfotransferase 1, UDP-glucuronosyltransferase 1, and catecholmethyltransferase—are maternally transferred to embryos and expressed prior to 5 dpf (Alazizi et al., 2011; Christen and Fent, 2014; Yasuda et al., 2005), so these metabolites should be considered when measuring E2 uptake and metabolism.
An alternative to chromatographic methods for measuring E2 uptake is the use of radiolabeled compounds. Though some radioactive isomers such as 32P and 35S are avoided due to their short half-lives and user-associated risk, the tritium isomer (3H) has a half-life of more than 12 years and a limited risk profile as a low energy beta emitter. 14C is another radioactive beta emitter with a half-life of 5730 years that is safe for human use. E2 and other endogenous steroids (including estrone, estriol, testosterone, androstenedione, progesterone, cholesterol, and cortisol) and estrogen-like environmental endocrine disrupting compounds (including BPA, EE2, polychlorinated biphenyl ethers, and phthalates) are commercially available as 3H and/or 14C isomers. Some compounds not commercially available may be custom synthesized commercially by PerkinElmer and American Radio Chemicals, Inc. Liquid scintillation counters used to measure radioactivity are inexpensive and user-friendly compared with chromatography and spectrometry equipment.
We therefore used tritiated E2 ([3H]E2) to develop an efficient assay with the ability to quantify uptake in the pmol range. Our assay can measure whole embryos individually, eliminating the need for chemical extraction protocols and pooled embryo measurements required by chromatographic spectrometry methods. As compound isolation is not required by our method, the resulting values include E2 and, potentially, its conjugates and polar metabolites that contain an 3H. The treatment water can also be easily measured, providing the ability to calculate percent uptake for each embryo. The minimal sample preparation makes this method adaptable to any compound soluble in embryo water and available as a radioisotope.
Variables That Affect E2 Uptake
We found that increasing E2 concentration, duration, or developmental stage increased absolute uptake from treatment water. In contrast, percent uptake was the same regardless of concentration. The increase in uptake we observed with increasing developmental stage could be due to increased embryonic mass at higher developmental stages, or the increased production of steroid-binding proteins such as albumin and sex-hormone binding globulin. Since these proteins bind steroids in the blood, they decrease the levels of free E2, thus increasing the capacity of an organism to absorb more E2 from their environment (Chrousos, 2015; Miguel-Queralt and Hammond, 2008; Miguel-Queralt et al., 2004).
We also found that the chorion had minimal effect on E2 uptake, though we did see a modest increase in E2 uptake at 1-h exposure in 24 hpf embryos with chorion removal. This could be due to increased E2 metabolism and the subsequent excretion of E2 conjugates that are unable to pass through the chorion, becoming trapped in the perivitelline space between the chorion and the embryo. Changes in absorption between chorionated and dechorionated embryos are likely not biologically relevant, however, suggesting that dechorionation is not necessary prior to E2 treatment. These results are in contrast to a prior study, which found that chorion removal increased chemical uptake (Panzica-Kelly et al., 2015). This discrepancy could be explained by the chemical properties of the compounds used in the Panzica-Kelly study. Several of the compounds tested were hydrophilic and nonsteroidal—such as sulfasalazine, fluconazole, and penicillamine—and may be blocked from diffusing through the chorion. In contrast, the biophysical properties of E2 suggest that it can passively diffuse through the chorion, as it does through plasma membranes. Our results suggest that chorion removal is not required when treating embryos with E2, and that higher concentrations of E2 are not required when treating embryos with their chorions intact. Similarly, chorion removal should not significantly affect E2 uptake. It is reasonable to assume that this holds true for structurally similar steroids, such as testosterone and progesterone, although this should be tested in the future.
Last, we found that a major proportion of E2 was absorbed into the yolk of the embryo when treatment was started before 3 dpf. This result was likely due to the lipid-rich nature of the yolk and its significant contribution to embryonic mass in early development. Future studies using E2 for embryo treatment should take this into consideration. Our results suggest that prior to 48 hpf, the majority of exogenous E2 is absorbed by the yolk and presumably not active in the embryonic body. Using results for percent uptake at multiple concentrations, durations, and developmental stages, we reviewed previously published studies testing the effects of E2 in developing zebrafish embryos and, based on the concentration of E2 in the fish water, we estimated the amount of E2 absorbed by the embryos and larvae (Table 1). For example, 1.8 nM E2 in fish water was used to visualize estrogen receptor activity in transgenic 5xERE:GFP zebrafish embryos (Gorelick and Halpern, 2011), though the biological relevance of this concentration was questioned. Our results suggest, however, that this concentration in the water corresponds to 70 pM in the embryo, assuming 3.8% uptake. This is consistent with the EC50 of E2 for ERα, 77 pM; ERβ1, 39 pM; and ERβ2, 118 pM (Pinto et al., 2014).
Table 1.
Estimated Concentration of E2 Taken Up Into Zebrafish Based on Published Studies Reporting Concentration of E2 in Fish Water
| E2 Concentration in Fish Water | Exposure Duration | Developmental Stage at Exposure Start | Estimated Percent Uptake | Estimated E2 in Zebrafish | References |
|---|---|---|---|---|---|
| 1.8 nM | 21 h | 3 hpf | 0.8% | 14 pM | (Kinch et al., 2016) |
| 1.835 nM | 3 days | 6–8 hpf | 3.8% | 70 pM | (Gorelick and Halpern, 2011) |
| 1 μM | 24 h | 96 hpf | 5.5% | 55 nM | (Hoffman et al., 2016) |
| 1 μM | 4 days | 3 hpf | 5.7% | 57 nM | (Hao et al., 2013) |
| 10 μM | 24 h | 12 hpf | 1.0% | 100 nM | (Carroll et al., 2014) |
Percent uptake of E2 was calculated based on percent [3H]E2 uptake found for a similar exposure duration and developmental stage. Estimated concentration in zebrafish was calculated by multiplying E2 concentration in fish water by estimated percent uptake for each exposure duration and developmental stage.
Assay Limitations
Two principle limitations of this assay are the inability to distinguish between absorbed E2 and its metabolites, and the need for isotopic chemicals. The isotopic E2 assay cannot distinguish between uptake of E2 and conversion of E2 to a metabolite (eg, catechol estrogens). Although knowing the total E2 uptake, regardless of how the absorbed E2 is metabolized, is useful in translating exposure concentration to a physiologic dose in vivo, identification of E2 metabolites and their concentrations would be advantageous. E2 and its metabolites may have different affinities for estrogen receptors (Zhu et al., 2006), therefore distinguishing between E2 metabolites is important for understanding how E2 exerts a particular effect. Future studies optimizing HPLC-MS methods to detect E2 and related molecules from zebrafish embryos will be necessary. Second, tritiated chemicals are significantly more expensive than their nonisotopic counterparts. There may be some compounds that are chemically unstable in isotopic form. The use of isotopic chemicals also limits the range of treatment concentrations available, as there are typically only 1 or 2 concentrations of these chemicals produced. This limitation may be avoided by adding nonlabeled compounds and extrapolating uptake from the percentage of total compound that is radiolabeled, though this method is less precise. Note that MS assays also require the use of internal standards, typically stable isotopes using 2H or 13C, making the limitations of cost of isotopic chemicals also applicable to HPLC-MS assays.
CONCLUSIONS AND FUTURE DIRECTIONS
The [3H]E2 assay provides an efficient, reliable, and sensitive method for determining E2 uptake into zebrafish embryos and larvae. This approach can be applied to a wide range of isotopic compounds and exposure parameters, using zebrafish and other aquatic species. For example, future studies could optimize our assay for use in 96-well plates to compare uptake between treatment vessels. Future studies could also utilize this assay to screen for drugs and/or genes that influence compound uptake. For example, to determine mechanisms of EE2 efflux, one could screen for compounds that reduce or promote isotopic EE2 uptake. Results could provide insight into how animals evade toxicity and reveal previously unappreciated small molecules that modulate steroid transporter activity. Our results provide a foundation for future studies utilizing fish embryos as a screening tool for developmental toxicity.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
ACKNOWLEDGMENTS
We thank Charles N. Falany for use of materials and equipment for liquid scintillation counting, Zachary Tibbs for advice on experimental design and Susan Farmer and the UAB zebrafish facility staff for zebrafish husbandry and maintenance.
FUNDING
This work was supported by National Institute of Environmental Health Sciences (R01ES026337) and National Institute of General Medical Sciences (T32GM008361). These data were presented in part at the Aquatic Animal Models of Human Disease Conference at the University of Alabama at Birmingham, Birmingham, AL on January 11, 2017.
REFERENCES
- Adeel M., Song X., Wang Y., Francis D., Yang Y. (2017). Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 99, 107–119. [DOI] [PubMed] [Google Scholar]
- Alazizi A., Liu M.-Y., Williams F. E., Kurogi K., Sakakibara Y., Suiko M., Liu M.-C. (2011). Identification, Characterization, and Ontogenic Study of a Catechol O-methyltransferase from Zebrafish. Aquat. Toxicol. (Amsterdam, Netherlands) 102, 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alharthy K. M., Albaqami F. F., Thornton C., Corrales J., Willett K. L. (2017). Mechanistic evaluation of benzo[a]pyrene’s developmental toxicities mediated by reduced Cyp19a1b activity. Toxicol. Sci. 155, 135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari R. K., vom Saal F. S., Tillitt D. E. (2015). Transgenerational effects from early developmental exposures to bisphenol A or 17α-ethinylestradiol in medaka, Oryzias latipes. Sci. Rep. 5, 9303.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsignorio D., Perego L., Del Giacco L., Cotelli F. (1996). Structure and macromolecular composition of the zebrafish egg chorion. Zygote 4, 101–108. [DOI] [PubMed] [Google Scholar]
- Bouwmeester M. C., Ruiter S., Lommelaars T., Sippel J., Hodemaekers H. M., van den Brandhof E.-J., Pennings J. L. A., Kamstra J. H., Jelinek J., Issa J.-P. J., et al. (2016). Zebrafish embryos as a screen for DNA methylation modifications after compound exposure. Toxicol. Appl. Pharmacol. 291, 84–96. [DOI] [PubMed] [Google Scholar]
- Carroll K. J., Esain V., Garnaas M. K., Cortes M., Dovey M. C., Nissim S., Frechette G. M., Liu S. Y., Kwan W., Cutting C. C., et al. (2014). Estrogen defines the dorsal-ventral limit of VEGF regulation to specify the location of the hemogenic endothelial niche. Dev. Cell 29, 437–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen V., Fent K. (2014). Tissue-, sex- and development-specific transcription profiles of eight UDP-glucuronosyltransferase genes in zebrafish (Danio rerio) and their regulation by activator of aryl hydrocarbon receptor. Aquat. Toxicol. 150, 93–102. [DOI] [PubMed] [Google Scholar]
- Chrousos G. P. (2015). The gonadal hormones & inhibitors In Basic and Clinical Pharmacology, 13th ed. (Katzung B. G., Trevor A. J., Eds.). McGraw-Hill Medical, New York, NY. [Google Scholar]
- Diamanti-Kandarakis E., Bourguignon J. P., Giudice L. C., Hauser R., Prins G. S., Soto A. M., Zoeller R. T., Gore A. C. (2009). Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr. Rev. 30, 293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick D. A., Halpern M. E. (2011). Visualization of estrogen receptor transcriptional activation in zebrafish. Endocrinology 152, 2690–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick D. A., Iwanowicz L. R., Hung A. L., Blazer V. S., Halpern M. E. (2014). Transgenic zebrafish reveal tissue-specific differences in estrogen signaling in response to environmental water samples. Environ. Health Perspect. 122, 356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson A. L., Stedman D. B., Ball J., Hillegass J. M., Flood A., Zhang C. X., Panzica-Kelly J., Cao J., Coburn A., Enright B. P., Tornesi M. B., et al. (2012). Inter-laboratory assessment of a harmonized zebrafish developmental toxicity assay — Progress report on phase I. Reprod Toxicol 33, 155–164. [DOI] [PubMed] [Google Scholar]
- Hao R., Bondesson M., Singh A. V., Riu A., McCollum C. W., Knudsen T. B., Gorelick D. A., Gustafsson J.-Ã. (2013). Identification of estrogen target genes during zebrafish embryonic development through transcriptomic analysis. PLoS One, 8, e79020.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman E. J., Turner K. J., Fernandez J. M., Cifuentes D., Ghosh M., Ijaz S., Jain R. A., Kubo F., Bill B. R., Baier H., et al. (2016). Estrogens suppress a behavioral phenotype in zebrafish mutants of the autism risk gene, CNTNAP2. Neuron 89, 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantae V., Krekels E. H. J., Ordas A., González O., van Wijk R. C., Harms A. C., Racz P. I., van der Graaf P. H., Spaink H. P., Hankemeier T. (2016). Pharmacokinetic modeling of paracetamol uptake and clearance in zebrafish larvae: Expanding the allometric scale in vertebrates with five orders of magnitude. Zebrafish 13, 504–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinch C. D., Ibhazehiebo K., Jeong J. H., Habibi H. R., Kurrasch D. M. (2015). Low-dose exposure to bisphenol A and replacement bisphenol S induces precocious hypothalamic neurogenesis in embryonic zebrafish. Proc. Natl. Acad. Sci. U.S.A. 112, 1475–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinch C. D., Kurrasch D. M., Habibi H. R. (2016). Adverse morphological development in embryonic zebrafish exposed to environmental concentrations of contaminants individually and in mixture. Aquat. Toxicol. 175, 286–298. [DOI] [PubMed] [Google Scholar]
- Kunz Y. W. (2004). Yolk (vitellus) In Developmental Biology of Teleost Fishes, pp. 23–40. Springer, Netherlands, Dordrecht. (doi: 10.1007/978-1-4020-2997-4_3). [Google Scholar]
- Lambert M. R., Giller G. S., Barber L. B., Fitzgerald K. C., Skelly D. K. (2015). Suburbanization, estrogen contamination, and sex ratio in wild amphibian populations. Proc. Natl. Acad. Sci. U.S.A. 112, 11881–11886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum C. W., Ducharme N. A., Bondesson M., Gustafsson J. A. (2011). Developmental toxicity screening in zebrafish. Birth Defects Res. C Embryo Today 93, 67–114. [DOI] [PubMed] [Google Scholar]
- Miguel-Queralt S., Hammond G. L. (2008). Sex Hormone-binding globulin in fish gills is a portal for sex steroids breached by xenobiotics. Endocrinology 149, 4269–4275. [DOI] [PubMed] [Google Scholar]
- Miguel-Queralt S., Knowlton M., Avvakumov G. V., Al-Nouno R., Kelly G. M., Hammond G. L. (2004). Molecular and functional characterization of sex hormone binding globulin in zebrafish. Endocrinology 145, 5221–5230. [DOI] [PubMed] [Google Scholar]
- Namdaran P., Reinhart K. E., Owens K. N., Raible D. W., Rubel E. W. (2012). Identification of modulators of hair cell regeneration in the zebrafish lateral line. J. Neurosci. 32, 3516–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla S., Corum D., Padnos B., Hunter D. L., Beam A., Houck K. A., Sipes N., Kleinstreuer N., Knudsen T., Dix D. J., et al. (2012). Zebrafish developmental screening of the ToxCast Phase I chemical library. Reprod. Toxicol. 33, 174–187. [DOI] [PubMed] [Google Scholar]
- Panzica-Kelly J. M., Zhang C. X., Augustine-Rauch K. A. (2015). Optimization and performance assessment of the chorion-off [dechorinated] zebrafish developmental toxicity assay. Toxicol. Sci. 146, 127–134. [DOI] [PubMed] [Google Scholar]
- Pelka K. E., Henn K., Keck A., Sapel B., Braunbeck T. (2017). Size does matter – Determination of the critical molecular size for the uptake of chemicals across the chorion of zebrafish (Danio rerio) embryos. Aquat. Toxicol. 185, 1–10. [DOI] [PubMed] [Google Scholar]
- Piferrer F., Donaldson E. M. (1994). Uptake and clearance of exogenous estradiol-17β and testosterone during the early development of coho salmon (Oncorhynchus kisutch), including eggs, alevins and fry. Fish Physiol. Biochem. 13, 219–232. journal article). [DOI] [PubMed] [Google Scholar]
- Pinto C., Grimaldi M., Boulahtouf A., Pakdel F., Brion F., Ait-Aissa S., Cavailles V., Bourguet W., Gustafsson J. A., Bondesson M., et al. (2014). Selectivity of natural, synthetic and environmental estrogens for zebrafish estrogen receptors. Toxicol. Appl. Pharmacol. 280, 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson D. M., Zhang T., Kalicharan D., Jongebloed W. L. (2000). Field emission scanning electron microscopy and transmission electron microscopy studies of the chorion, plasma membrane and syncytial layers of the gastrula-stage embryo of the zebrafish Brachydanio rerio: a consideration of the structural and functional relationships with respect to cryoprotectant penetration. Aqua. Res. 31, 325–336. [Google Scholar]
- Romano S. N., Gorelick D. A. (2014). Semi-automated imaging of tissue-specific fluorescence in zebrafish embryos. J. Vis. Exp. 87, 51533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saili K. S., Corvi M. M., Weber D. N., Patel A. U., Das S. R., Przybyla J., Anderson K. A., Tanguay R. L. (2012). Neurodevelopmental low-dose bisphenol A exposure leads to early life-stage hyperactivity and learning deficits in adult zebrafish. Toxicology 291, 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz T. I., Katsiadaki I., Maskrey B. H., Scott A. P. (2017). Mussels (Mytilus spp.) display an ability for rapid and high capacity uptake of the vertebrate steroid, estradiol-17β from water. J. Steroid Biochem. Mol. Biol. 165(Part B), 407–420. [DOI] [PubMed] [Google Scholar]
- Sun L., Wen L., Shao X., Qian H., Jin Y., Liu W., Fu Z. (2010). Screening of chemicals with anti-estrogenic activity using in vitro and in vivo vitellogenin induction responses in zebrafish (Danio rerio). Chemosphere 78, 793–799. [DOI] [PubMed] [Google Scholar]
- Tal T., Kilty C., Smith A., LaLone C., Kennedy B., Tennant A., McCollum C. W., Bondesson M., Knudsen T., Padilla S., et al. (2016). Screening for angiogenic inhibitors in zebrafish to evaluate a predictive model for developmental vascular toxicity. Reprod. Toxicol. doi: 10.1016/j.reprotox.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong L., Reif D. M., St Mary L., Geier M. C., Truong H. D., Tanguay R. L. (2014). Multidimensional in vivo hazard assessment using zebrafish. Toxicol. Sci. 137, 212–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. (2000). The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio Rerio), 4th ed.University of Oregon Press, Eugene, OR. [Google Scholar]
- Yasuda S., Liu C.-C., Takahashi S., Suiko M., Chen L., Snow R., Liu M.-C. (2005). Identification of a novel estrogen-sulfating cytosolic SULT from zebrafish: Molecular cloning, expression, characterization, and ontogeny study. Biochem. Biophys. Res. Commun. 330, 219–225. [DOI] [PubMed] [Google Scholar]
- Zhu B. T., Han G.-Z., Shim J.-Y., Wen Y., Jiang X.-R. (2006). Quantitative structure-activity relationship of various endogenous estrogen metabolites for human estrogen receptor α and β subtypes: Insights into the structural determinants favoring a differential subtype binding. Endocrinology 147, 4132–4150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.