Abstract
Aims
Alcohol is a risk factor for cardiac arrhythmias. Retrospective analyses suggest supraventricular arrhythmias consecutive to acute alcohol consumption, but prospective data are limited. We intended to prospectively associate acute alcohol consumption with cardiac arrhythmias.
Methods and results
At the 2015 Munich Octoberfest, we enrolled 3028 voluntary participants who received a smartphone-based ECG and breath alcohol concentration (BAC) measurements. ECGs were analysed for cardiac arrhythmias (sinus tachycardia, sinus arrhythmia, premature atrial/ventricular complexes, atrial fibrillation/flutter) and respiratory sinus arrhythmia. By multivariable adjusted logistic regression we associated BACs with cardiac arrhythmias. Similarly, we analysed 4131 participants of the community-based KORA S4 Study (Co-operative Health Research in the Region of Augsburg) and associated cardiac arrhythmias with chronic alcohol consumption. In our acute alcohol cohort (mean age 34.4 ± 13.3 years, 29% women), mean BAC was 0.85 ± 0.54 g/kg. Cardiac arrhythmias occurred in 30.5% (sinus tachycardia 25.9%; other arrhythmia subtypes 5.4%). Breath alcohol concentration was significantly associated with cardiac arrhythmias overall (odds ratio (OR) per 1-unit change 1.75, 95% confidence interval (CI) 1.50–2.05; P < 0.001) and sinus tachycardia in particular (OR 1.96, 95%CI 1.66–2.31; P < 0.001). Respiratory sinus arrhythmia measuring autonomic tone was significantly reduced under the influence of alcohol. In KORA S4, chronic alcohol consumption was associated with sinus tachycardia (OR 1.03, 95%CI 1.01–1.06; P = 0.006).
Conclusions
Acute alcohol consumption is associated with cardiac arrhythmias and sinus tachycardia in particular. This partly reflects autonomic imbalance as assessed by significantly reduced respiratory sinus arrhythmia. Such imbalance might lead to sympathetically triggered atrial fibrillation resembling the holiday heart syndrome.
ClinicalTrials.org accession number
Introduction
Acute and chronic alcohol intake result in desirable and undesirable health effects.1 Modest recreational alcohol consumption may lead to perceived wellbeing,2 beyond moderate intake confers the risk of adverse outcomes. Common symptoms of acute alcohol consumption are disorientation, disinhibition, nausea, and vomiting.3,4 Excessive acute abuse may lead to death.5 Chronic consumption has been ascribed cardio-protective effects.6 More commonly, chronic alcohol use dose-dependently is associated with detrimental effects including substance addiction and severe nutritional, hepatic, neurological and social consequences.7
From a cardiovascular perspective, acute excessive alcohol consumption has been associated with the so called ‘Holiday Heart Syndrome’.8 This syndrome affects individuals without a specific cardiac history resulting in both ventricular and supraventricular arrhythmias, predominantly atrial fibrillation.8,9 Small case series confirmed a relation between alcohol use and atrial fibrillation.10,11 Regarding chronic alcohol consumption, population-based studies reported a dose-response effect on the development of atrial fibrillation.12
Many open questions remain. All reports on effects of acute alcohol intake were derived from small retrospective analyses with relations to arrhythmias as secondary findings. In our study, we thus intended to conduct a large, sufficiently powered, observational, cross-sectional analysis of cardiac arrhythmia prevalence in participants with quantitatively measured acute alcohol intake. Investigating visitors of the 2015 Munich Octoberfest, we hypothesized that increased breath alcohol concentration (BAC) is associated with a higher burden of cardiac arrhythmias. To parallel acute with chronic alcohol use, we studied participants of the community-based KORA Study.
Methods
Study cohorts
For the analysis of BAC and arrhythmia prevalence, we designed an observational, cross-sectional cohort study and recruited voluntary visitors at the annual Octoberfest in Munich, Germany between September and October 2015 (acute alcohol cohort). Participants had to be ≥18 years of age and provide written informed consent to study inclusion. We screened and consented 3042 individuals. After exclusion of individuals with a BAC ≥3.00 g/kg (n = 4) and individuals with uninterpretable electrocardiogram (ECG) recordings (n = 8), the final study cohort comprised 3028 participants. Individuals with a BAC ≥ 3.00 g/kg are considered disabled due to intoxication according to German law, and must not be consented. The ethics committee at the Ludwig Maximilians University of Munich, Germany approved the study, which was registered at clinicaltrials.org (NCT02550340).
To assess chronic alcohol consumption on arrhythmia prevalence, we investigated participants of the Survey S4 of the community-based Co-operative Health Research in the region of Augsburg Study (KORA S4, chronic alcohol cohort).13 Study participants were identified through the registration office. Ten strata of equal size according to sex and age comprised 4261 individuals; after exclusion of those without (n = 95) or with uninterpretable (n = 17) ECGs, or without information on alcohol consumption (n = 18), the final cohort consisted of 4131 individuals. The ethics committee of the Bavarian Medical Association approved the study.
Electrocardiogram recordings
In the acute alcohol cohort, 30 s ECG recordings were obtained using the smart phone based AliveCor device (AliveCor, San Francisco, CA, USA). A two-electrode hardware extension wirelessly communicating with a software application was held with both hands by the participant, resembling a lead I ECG.
In KORA S4, digital 10 s 12-lead ECGs were obtained using the Hannover ECG System version 3.22-12 (HES, Corscience, Erlangen, Germany). ECGs were recorded after 10 min rest in supine position.
Electrocardiogram analysis was performed in parallel by two senior cardiologists blinded to BAC, chronic alcohol consumption levels, or clinical covariates. Discrepant findings were resolved by consent. Assessment of arrhythmias employed a standardized coding scheme. Arrhythmias were classified as sinus tachycardia (heart rate >100 b.p.m.), sinus arrhythmia, premature atrial complex, premature ventricular complex, and atrial fibrillation or flutter. We further assessed all ECGs for respiratory sinus arrhythmia as a measure of autonomic tone. For this, we modified the previously described respiratory sinus arrhythmia bedside test.14 Per 30 s ECG recording, we measured RR intervals with a scaled caliper, neglecting RR intervals before and after premature beats, and determined the shortest, longest, and mean RR interval of each participant. We considered respiratory sinus arrhythmia present, when the absolute difference of the shortest and longest RR interval was ≥20% of the mean RR interval duration.
Alcohol assessment
In the acute alcohol cohort, BAC was determined using a Dräger Alcotest 7510 handheld device (Drägerwerk AG, Lübeck, Germany). The device accounts for remaining oral alcohol. To further reduce influence of the latter, BAC was obtained at the end of participant recruitment, when individuals had not ingested alcohol for several minutes. Alcohol was measured in gram per kilogram (g/kg). In KORA S4, chronic alcohol consumption was assessed in gram alcohol per day (g/d), surveyed using the validated 7-day-recall method.15
Clinical covariates
In our acute alcohol cohort, assessment of clinical covariates was restricted by the study setting and the aim to maintain participant privacy. We were thus limited to survey self-reported age, sex, country of origin, history of heart disease, use of cardiovascular and antiarrhythmic drugs, and active smoking status.
In KORA S4, participants underwent a standardized interview and examination.13 We used information on age, sex, hypertension, smoking status, history of angina, myocardial infarction, diabetes mellitus, and stroke, as well as the use of cardiovascular and antiarrhythmic medication.
Statistical considerations
We express categorical variables as frequency (percentage). Continuous data are presented as mean ± standard deviation or median (25th;75th percentile) as appropriate. We applied logistic regression models to assess the relation of alcohol as predictor on the primary and secondary outcomes of arrhythmia prevalence, adjusted for age and sex or in addition for the remaining available covariates. Odds ratios are provided per unit (i.e. 1 g/kg) increase of BAC. Arrhythmia prevalence across quartiles of BAC was compared by χ2 tests for trend. Primary outcome was the occurrence of any arrhythmia including sinus arrhythmia, sinus tachycardia, premature atrial complex, premature ventricular complex, and atrial fibrillation or flutter. Secondary outcomes were arrhythmia subtype separately or combinations thereof. Another secondary outcome in our acute alcohol cohort was the presence of respiratory sinus arrhythmia. For sensitivity analyses to adjudicate the influence of alcohol consumption on the baseline prevalence of sinus tachycardia, we selected a subgroup of participants from our acute alcohol cohort with no exposure to acute alcohol consumption (BAC 0 g/kg). Similarly, in KORA S4 we selected participants without chronic alcohol use. In these sensitivity analyses, we determined the prevalence of cardiac arrhythmias as detailed above. Finally, we assessed interaction with sex by adding multiplicative interaction terms (sex*BAC) to our models.
For sample size calculations in the acute alcohol cohort, we assumed a 1% prevalence of arrhythmias in the general population assessed by a 10 s ECG. In our 30 s recordings, we assumed a 1.5% prevalence in those under no or low influence of alcohol (<0.5 g/kg). For the group under intermediate (≥0.5–<1.5 g/kg) and high (≥1.5 g/kg) influence, we assumed an odds of 2 and 4 for the prevalence of arrhythmias, respectively. For 85% power with a two-sided α-level set at 5%, we required 2754 participants. To account for a 10% drop-out rate, we aimed at 3029 enrolled individuals.
Results
During all 16 days of the 2015 Munich Octoberfest we included 3028 participants for analysis. Their mean age was 34.7 ± 13.3 years, 905 (29.9%) were women (Table 1). Reflecting the international attendance, individuals originated from 60 different countries, whereas the majority of 69% was from Germany.
Table 1.
Characteristics of the study cohorts
| Acute alcohol cohort |
Chronic alcohol cohort |
|||||
|---|---|---|---|---|---|---|
| Men | Women | Men | Women | |||
| n | 3028 | 2123 | 905 | 4131 | 2021 | 2110 |
| Sex (women), n(%) | 905 (29.9%) | - | - | 2110 (51.1%) | - | - |
| Age, years | 34.7 ± 13.3 | 35.5 ± 13.4 | 33.1 ± 12.8 | 49.1 ± 13.9 | 49.5 ± 14.0 | 48.7 ± 13.8 |
| History of | ||||||
| Heart disease, n(%) | 175 (5.8%) | 140 (6.6%) | 35 (3.9%) | - | - | - |
| Angina, n(%) | - | - | - | 249 (6.0%) | 116 (5.7%) | 133 (6.3%) |
| Myocardial infarction, n(%) | - | - | - | 82 (2.0%) | 71 (3.5%) | 11 (0.5%) |
| Diabetes mellitus, n(%) | - | - | - | 159 (3.8%) | 82 (4.1%) | 77 (3.6%) |
| Stroke, n(%) | - | - | - | 49 (1.2%) | 33 (1.6%) | 16 (0.8%) |
| Arrhythmias, n(%) | 80 (2.6%) | 57 (2.7%) | 23 (2.5%) | - | - | - |
| Medication use, n(%) | 185 (6.1%) | 149 (7.0%) | 36 (4.0%) | 859 (20.8%) | 409 (20.2%) | 450 (21.3%) |
| Current smoking, n(%) | 858 (28.3%) | 605 (28.5%) | 253 (28.0%) | 1066 (25.6%) | 616 (30.5%) | 450 (21.3%) |
The KORA S4 cohort consisted of 4131 individuals with a mean age of 49.1 ± 13.9 years and 2110 (51.1%) females (Table 1). Owing to the study composition, >99.5% were of German descent.
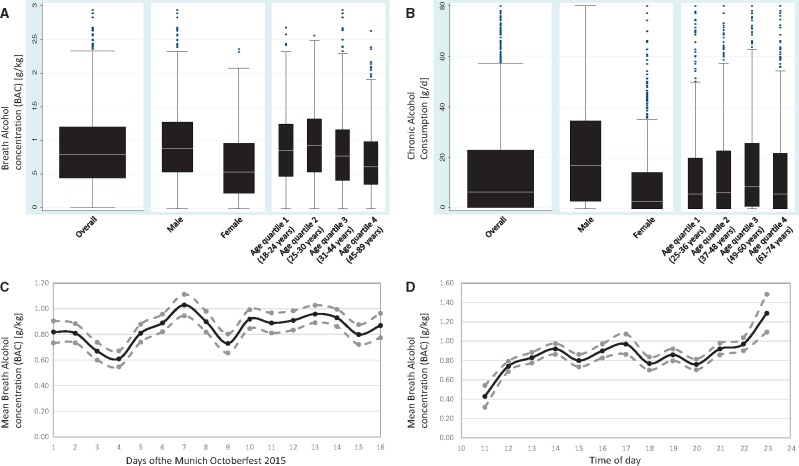
In the acute alcohol cohort, the mean BAC was 0.85 ± 0.54 g/kg (range 0–2.94 g/kg). Generally, men had a higher BAC compared to women. Across quartiles of age, the highest BAC was found in the 2nd quartile (25–30 years of age, Figure 1A). We noted limited day-by-day variation, tending to higher BAC measurements towards the weekends (Figure 1C). The circadian distribution confirmed a constant rise in BAC toward the closing hour of each day. A slight decline in the afternoon likely reflects a partial exchange of visitors due to seat reservation time slots (Figure 1D).
Figure 1.
Alcohol consumption. A. Distribution of breath alcohol concentration (BAC) across the acute alcohol cohort in g/kg. Results presented for the entire cohort, and stratified by sex and quartiles of age. B. Distribution of chronic alcohol consumption in KORA S4 in g/d. Results presented for the entire cohort, and stratified by sex and quartiles of age. Outliers truncated at 80 g/d. C. Day-by-day variability of mean BAC for each of the 16 days of the Octoberfest. D. Circadian variability of mean BAC across recruitment days at the Octoberfest.
The mean chronic consumption of alcohol in KORA S4 was 15.8 ± 21.4 g/d. On average, men consumed nearly three time as much alcohol as women. The highest chronic alcohol consumption was observed in the 3rd age quartile (37–48 years of age, Figure 1B).
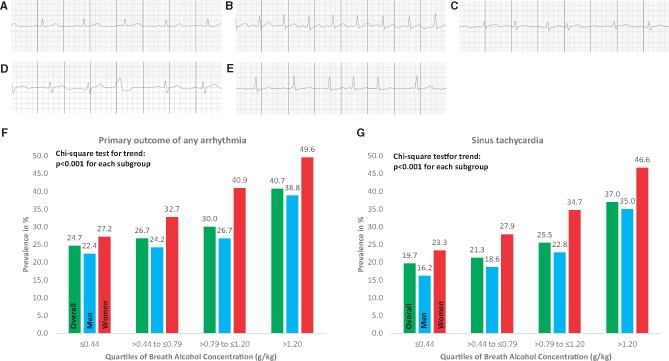
The quality of ECG recordings in our acute alcohol cohort was high, despite disadvantageous recording conditions. Only eight ECGs were uninterpretable. Examples of encountered arrhythmias are presented in Figure 2A–E. In the acute alcohol cohort, the primary outcome of any arrhythmia occurred in 30.5%. Thereby, sinus tachycardia was most common and affected 25.9% of participants. The combined prevalence of other arrhythmias was 5.4%, including sinus arrhythmia, premature atrial and ventricular complexes, and atrial fibrillation or flutter. The secondary outcome of respiratory sinus arrhythmia as a qualitative measure of autonomic tone was noted in 22.2% of participants (Table 2).
Figure 2.
Examples and Prevalence of Cardiac Arrhythmias. A–E. Representative ECG recordings obtained in our acute alcohol cohort. ECG recordings show sinus rhythm (A), sinus tachycardia (B), premature atrial complex (C), premature ventricular complex (D), atrial fibrillation (E). F–G. Clustered bars represent the prevalence of the primary outcome of any cardiac arrhythmia (F) and sinus tachycardia (G) in our acute alcohol cohort by quartiles of BAC. Within each cluster, bars represent the overall cohort (green), and sex-stratified results for men (blue) and women (red). Clusters compared by χ2 test for trend.
Table 2.
Prevalence of arrhythmias
| Acute alcohol cohort |
Chronic alcohol cohort |
||||||
|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | ||||
| 1 | Sinus arrhythmia | 51 (1.7%) | 40 (1.9%) | 11 (1.2%) | 9 (0.2%) | 4 (0.2%) | 5 (0.2%) |
| 2 | Sinus tachycardia | 785 (25.9%) | 514 (24.2%) | 271 (29.9%) | 17 (0.4%) | 9 (0.4%) | 8 (0.4%) |
| 3 | Premature atrial complexes | 39 (1.3%) | 30 (1.4%) | 9 (1.0%) | 26 (0.6%) | 11 (0.7%) | 11 (0.5%) |
| 4 | Premature ventricular complexes | 52 (1.7%) | 39 (1.8%) | 13 (1.4%) | 46 (1.1%) | 19 (0.9%) | 27 (1.3%) |
| 5 | Atrial fibrillation/flutter | 25 (0.8%) | 12 (0.6%) | 13 (1.4%) | 22 (0.5%) | 18 (0.9%) | 4 (0.2%) |
| Combination of 1, 2, 3, 4, 5 | 925 (30.5%) | 614 (28.9%) | 311 (34.4%) | 112 (2.7%) | 58 (2.9%) | 54 (2.6%) | |
| Combination of 1, 3, 4, 5 | 164 (5.4%) | 118 (5.6%) | 46 (5.1%) | 95 (2.3%) | 50 (2.2%) | 45 (2.1%) | |
| Combination of 3, 4, 5 | 113 (3.7%) | 78 (3.7%) | 35 (3.9%) | 87 (2.1%) | 47 (2.0%) | 40 (1.9%) | |
| Respiratory sinus arrhythmia | 673 (22.2%) | 509 (24.0%) | 163 (18.0%) | - | - | - | |
In comparison, arrhythmias occurred less frequently in KORA S4. Only 2.7% of the participants met the primary outcome, at which 0.4% had sinus tachycardia and 2.3% presented with sinus arrhythmia, premature atrial and ventricular complexes, and atrial fibrillation or flutter (Table 2).
In our sensitivity analysis of study participants with 0 g/kg BAC in the acute alcohol cohort, the primary outcome of any arrhythmia occurred in 23.9%, and sinus tachycardia occurred in 18.5%. In those without alcohol use in the chronic alcohol cohort, prevalence of any arrhythmia was 2.5% and of sinus tachycardia was 0.4%.
For our primary outcome (sinus tachycardia; sinus arrhythmia; premature atrial and ventricular complexes; atrial fibrillation or flutter), we identified a robust association with higher BAC in our acute alcohol cohort. Analysis by arrhythmia subtype revealed that this association was driven by sinus tachycardia, both after age and sex, and after multivariable adjustment. (Table 3) Across quartiles of BAC in the overall and sex-stratified cohort, we found increasing prevalences of any arrhythmia and sinus tachycardia, respectively (Figure 2F and G). In sex-stratified analyses, the effect of BAC on sinus tachycardia was similar in males and females, both after adjustment for age (males: OR 2.12 (95% CI 1.75–2.58), P < 0.001; females: OR 2.02 (95% CI 1.51–2.69), P < 0.001) and after multivariable adjustment (males: OR 2.00 (95% CI 1.64–2.44), P < 0.001; females: OR 1.89 (95% CI 1.40–2.55), P < 0.001). We also noted a significant inverse association of respiratory sinus arrhythmia with BAC (Table 3). Interaction analyses did not suggest interaction between sex and BAC.
Table 3.
Association of arrhythmia prevalence with alcohol consumption
| Acute alcohol cohort |
Chronic alcohol cohort |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Adjusted for | Multivariable | Adjusted for | Multivariable | ||||||
| Age and Sex |
Adjustment |
Age and Sex |
Adjustment |
||||||
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | ||
| 1 | Sinus arrhythmia | 0.74 (0.43–1.30) | 0.3 | 0.77 (0.44–1.35) | 0.36 | 0.97 (0.92–1.03) | 0.31 | 0.97 (0.91–1.02) | 0.25 |
| 2 | Sinus tachycardia | 2.08 (1.77–2.45) | <0.001 | 1.96 (1.66–2.31) | <0.001 | 1.02 (1.01–1.04) | 0.003 | 1.03 (1.01–1.05) | 0.006 |
| 3 | Premature atrial complexes | 0.88 (0.46–1.70) | 0.71 | 0.93 (0.48–1.81) | 0.84 | 0.99 (0.96–1.01) | 0.31 | 0.99 (0.96–1.01) | 0.35 |
| 4 | Premature ventricular complexes | 1.11 (0.64–1.92) | 0.71 | 1.07 (0.62–1.86) | 0.81 | 0.99 (0.97–1.01) | 0.43 | 0.99 (0.98–1.01) | 0.45 |
| 5 | Atrial fibrillation/flutter | 1.45 (0.67–3.13) | 0.35 | 1.39 (0.64–3.00) | 0.83 | 1.00 (0.98–1.02) | 0.80 | 1.00 (0.98–1.02) | 0.84 |
| Combination of 3, 4, 5 | 1.03 (0.70–1.51) | 0.87 | 1.03 (0.70–1.51) | 0.89 | 0.99 (0.98–1.00) | 0.21 | 0.99 (0.98–1.01) | 0.27 | |
| Combination of 1, 3, 4, 5 | 0.93 (0.68–1.27) | 0.64 | 0.93 (0.68–1.28) | 0.67 | 0.99 (0.98–1.00) | 0.15 | 0.99 (0.98–1.00) | 0.18 | |
| Combination of 1, 2, 3, 4, 5 | 1.87 (1.60–2.18) | <0.001 | 1.75 (1.50–2.05) | <0.001 | 1.00 (0.99–1.01) | 0.94 | 1.00 (0.99–1.01) | 0.90 | |
| Respiratory sinus arrhythmia | 0.52 (0.44–0.63) | <0.001 | 0.54 (0.45–0.65) | <0.001 | – | – | – | – | |
Odds ratios (OR) are presented per unit (i.e. 1 g/kg) increase of continuously measured breath alcohol concentration. Multivariable adjustment in the acute alcohol cohort included age, sex, history of heart disease, use of cardiovascular drugs, use of antiarrhythmic drugs, and active smoking status. Multivariable adjustment in the chronic alcohol cohort included age, sex, hypertension, smoking status, history of angina, myocardial infarction, diabetes mellitus, stroke, and use of cardiovascular and antiarrhythmic medication. Significant P-values are highlighted by bold print.
In KORA S4, we confirmed an association between sinus tachycardia and chronic alcohol consumption. Yet, the associated effect size was remarkably smaller than with acute alcohol consumption. No associations with chronic alcohol consumption were found for other arrhythmias or a combination of sinus tachycardia with other arrhythmia subtypes (Table 3).
Discussion
In our observational, cross-sectional acute alcohol cohort, we have recruited over 3000 participants with quantitatively measured acute alcohol consumption. Each participant received a 30 s ECG for arrhythmia analysis. Our main finding was a significant association of sinus tachycardia with acute alcohol intake, occurring in 25.9% of individuals. We also confirmed this association with chronic alcohol consumption in the community-based cohort KORA study. In the acute alcohol cohort, respiratory sinus arrhythmia as a marker of balanced autonomic tone was more common in those exposed to less alcohol.
The Octoberfest is a traditional public festival in the city of Munich, Germany that is celebrated annually since 1810. It has gained strong international recognition and is renowned for serving Munich-brewed beer. Numerous visitors attend the festival primarily for the purpose of beer consumption. In 2015, 5.9 million visitors frequented the Octoberfest and consumed 7.5 million liters of beer. We thus considered the setting most suitable for conducting our acute alcohol study. We anticipated that by recruiting participants during all 16 days of the festival, we would be able to enrol a sufficient number of individuals presenting with a continuously distributed range of BAC. With an average BAC of 0.85 g/kg (range: 0–2.94 g/kg) we clearly achieved this goal. The modest day-by-day and circadian variabilities of BAC support the robustness of our results. The lively atmosphere in a beer tent deviated from AliveCor manufacturer recommendations for ECG recording. Despite adverse recording conditions, with >99.5% interpretable readings the obtained ECG quality was very high.
We conservatively based sample size considerations on an estimated 1.5% prevalence of any arrhythmia under no acute influence of alcohol and at rest. Even without considering sinus tachycardia, we detected arrhythmias in 2.3% of our chronic and in 5.4% of our acute alcohol cohorts. We thus submit that we present sufficiently powered results. In our acute alcohol cohort, we found a strong and robust association of increased BAC with sinus tachycardia in particular. We were not able to associate increased BAC with other arrhythmia subtypes and specifically atrial fibrillation. This is despite prior reports that repeatedly suggested such a relation coining the term ‘holiday heart syndrome’.8 It is notable that so far studies have considered patients presenting with atrial fibrillation and have then retrospectively identified recent acute alcohol consumption as a presumed cause.8–10 Ours is the first large and prospective investigation to systematically analyse the immediate occurrence of arrhythmias under the influence of acute alcohol intake. We thus conclude that acute alcohol consumption leads to increased arrhythmia prevalence overall, with sinus tachycardia as the immediate main effect.
For chronic alcohol consumption, prior results are more equivocal. Based on large community-based studies, modest use does not appear to be a major risk factor for atrial fibrillation.16 Only long-term use in the Framingham Heart Study and beyond moderate use (>35 standard drinks per week) in the Copenhagen City Heart Study resulted in an increased risk for atrial fibrillation.12,17 Along these findings, in our own community-based analysis of low to moderate chronic alcohol consumption with less than 20 g/d on average, we did not find an association with an increased arrhythmia prevalence.
A main result of our study is the profound association of acute alcohol consumption with sinus tachycardia. As demonstrated in our sensitivity analysis, it is intuitive to assume that participants of a festival present with significantly higher rates of sinus tachycardia than under resting conditions. However, sinus tachycardia occurred almost 30% more often in Octoberfest participants under the influence of alcohol compared with those without alcohol consumption. Furthermore, the association of sinus tachycardia with higher BAC remained even after accounting for confounders. Although less pronounced, also in KORA S4 increased chronic alcohol consumption was associated with sinus tachycardia.
Whereas ours is the largest prospective analysis to describe the association of acute alcohol consumption and sinus tachycardia under real life conditions, the relation has previously been noted. Prior studies have reported an increase in heart rate following alcohol intake in experimental settings.18–21 Consistently, these studies have identified alcohol induced alterations of the autonomic nervous system to elevate heart rate. Thereby, both an increase in sympathetic activity18,21 and a decrease in vagal tone19,20 have been described. Respiratory sinus arrhythmia is a simple measure of cardiac vagal tone and autonomic balance.22 In our acute alcohol cohort, participants with increased BAC presented with a significantly reduced prevalence of respiratory sinus arrhythmia. This finding concurs with the interpretation that the observed alcohol induced increase in heart rate is the consequence of changes in autonomic balance. Autonomic imbalance is strongly predisposing to the development of atrial fibrillation.23,24 Alcohol induced autonomic imbalance could thus hypothetically be a link to the later development of atrial fibrillation. Possibly, the short duration of our ECG recordings immediately during acute alcohol exposition resulted in an underestimation of alcohol induced cardiac arrhythmias and atrial fibrillation in particular. These considerations ask for additional research including longer ECG recordings during follow-up of hours and days after acute alcohol consumption.
A number of considerations are warranted when interpreting our study. Given the public environment of our study, we were restricted in assessing personal questions and conducting physical examinations. Hence, several possible confounding factors remained unaddressed, including but not limited to the amount of alcohol consumption prior to BAC measurement, the participants’ common alcohol consumption behaviour, their use of recreational drugs, or their usual physical activity. A single 30 s ECG recording prevented the investigation of temporal relations between alcohol consumption and arrhythmia occurrence. Importantly, we cannot comment on the relevance of baseline heart rate prior to alcohol intake. We were thus only able to assess the prevalence but not the incidence of arrhythmias during follow-up. Future studies with longer ECG recordings will need to fill this gap. Respiratory sinus arrhythmia only partially reflects the influence of autonomic tone on heart rate variability. Additional research is warranted to investigate more elaborate measures of autonomic tone in relation to alcohol consumption. Due to our exclusion criteria, we were not able to study severely intoxicated individuals (BAC ≥3.00 g/kg). In KORA S4, chronic alcohol consumption was rather low compared with other community-based cohorts. We might thus have underestimated arrhythmia prevalence secondary to long-term alcohol use. Information on chronic alcohol consumption in our acute alcohol cohort was unavailable.
In conclusion, we have conducted a large prospective analysis of acute alcohol consumption on ECG-assessed cardiac arrhythmias. We thereby report good technical feasibility of ECG screening even under lively conditions at the Munich Octoberfest. Acute and—to a lesser extent—chronic alcohol consumption were associated with sinus tachycardia. Analysis of respiratory sinus arrhythmia as a measure of autonomic tone suggested that acute alcohol intake confers autonomic imbalance. Additional research is warranted to investigate if autonomic imbalance constitutes the link between sinus tachycardia and the occurrence of arrhythmias like atrial fibrillation, as implicated by reports of the so-called ‘Holiday Heart Syndrome’ (Figure3).
Figure 3.
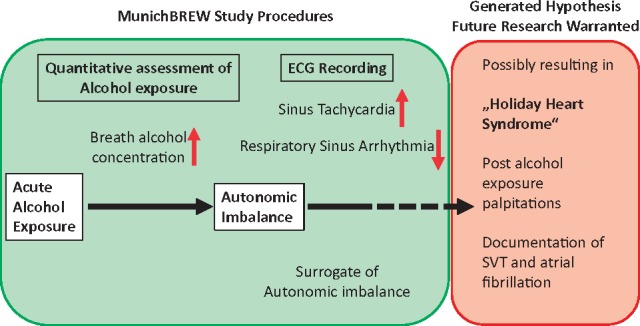
MunichBREW study conclusions. The figure summarizes the study procedures and results in the panel shaded in green. These findings influence the generated hypothesis on ‘Holiday Heart Syndrome’ pathophysiology illustrated in the panel shaded in red. Importantly, additional research is warranted to support this hypothesis.
Acknowledgements
We are grateful to Staatliches Hofbräuhaus in München (director Dr Michael Möller) for supporting the conduction of our research. This work is part of the doctoral theses of Rebecca Herbel and Cathrine Drobesch.
Funding
This study was funded by the Stiftung Biomedizinische Alkoholforschung, institutional funds of the Department of Medicine I, University Hospital Munich, and by the European Commission’s Horizon 2020 research and innovation programme [grant number 633196]: CATCH ME. The KORA study was initiated and financed by the Helmholtz Zentrum München, German Research Centre for Environmental Health, funded by the German Federal Ministry of Education and Research (BMBF) and by the State of Bavaria.
Conflict of interest: none declared.
References
- 1. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FD, Lochen ML, Lollgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WM; Authors/Task Force M. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gilman JM, Ramchandani VA, Davis MB, Bjork JM, Hommer DW.. Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci 2008;28:4583–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yost DA. Acute care for alcohol intoxication. Be prepared to consider clinical dilemmas. Postgrad Med 2002;112:14–16, 21–12, 25–16. [DOI] [PubMed] [Google Scholar]
- 4. Vonghia L, Leggio L, Ferrulli A, Bertini M, Gasbarrini G, Addolorato G, Alcoholism Treatment Study G.. Acute alcohol intoxication. Eur J Intern Med 2008;19:561–567. [DOI] [PubMed] [Google Scholar]
- 5. Kanny D, Brewer RD, Mesnick JB, Paulozzi LJ, Naimi TS, Lu H.. Vital signs: alcohol poisoning deaths - United States, 2010-2012. MMWR Morb Mortal Wkly Rep 2015;63:1238–1242. [PMC free article] [PubMed] [Google Scholar]
- 6. Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA.. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ 2011;342:d671.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O'Connor PG, Schottenfeld RS.. Patients with alcohol problems. N Engl J Med 1998;338:592–602. [DOI] [PubMed] [Google Scholar]
- 8. Ettinger PO, Wu CF, De La Cruz C Jr., Weisse AB, Ahmed SS, Regan TJ.. Arrhythmias and the “Holiday Heart”: alcohol-associated cardiac rhythm disorders. Am Heart J 1978;95:555–562. [DOI] [PubMed] [Google Scholar]
- 9. Koskinen P, Kupari M, Leinonen H, Luomanmaki K.. Alcohol and new onset atrial fibrillation: a case-control study of a current series. Br Heart J 1987;57:468–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krishnamoorthy S, Lip GY, Lane DA.. Alcohol and illicit drug use as precipitants of atrial fibrillation in young adults: a case series and literature review. Am J Med 2009;122:851–856. e853. [DOI] [PubMed] [Google Scholar]
- 11. Mandyam MC, Vedantham V, Scheinman MM, Tseng ZH, Badhwar N, Lee BK, Lee RJ, Gerstenfeld EP, Olgin JE, Marcus GM.. Alcohol and vagal tone as triggers for paroxysmal atrial fibrillation. Am J Cardiol 2012;110:364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Djousse L, Levy D, Benjamin EJ, Blease SJ, Russ A, Larson MG, Massaro JM, D'agostino RB, Wolf PA, Ellison RC.. Long-term alcohol consumption and the risk of atrial fibrillation in the Framingham Study. Am J Cardiol 2004;93:710–713. [DOI] [PubMed] [Google Scholar]
- 13. Holle R, Happich M, Lowel H, Wichmann HE, Group MKS.. KORA–a research platform for population based health research. Gesundheitswesen 2005;67(Suppl. 1):S19–S25. [DOI] [PubMed] [Google Scholar]
- 14. Katz A, Liberty IF, Porath A, Ovsyshcher I, Prystowsky EN.. A simple bedside test of 1-minute heart rate variability during deep breathing as a prognostic index after myocardial infarction. Am Heart J 1999;138:32–38. [DOI] [PubMed] [Google Scholar]
- 15. Schneider B, Baumert J, Schneider A, Marten-Mittag B, Meisinger C, Erazo N, Hammer GP, Ladwig KH.. The effect of risky alcohol use and smoking on suicide risk: findings from the German MONICA/KORA-Augsburg Cohort Study. Soc Psychiatry Psychiatr Epidemiol 2011;46:1127–1132. [DOI] [PubMed] [Google Scholar]
- 16. Mukamal KJ, Psaty BM, Rautaharju PM, Furberg CD, Kuller LH, Mittleman MA, Gottdiener JS, Siscovick DS.. Alcohol consumption and risk and prognosis of atrial fibrillation among older adults: the Cardiovascular Health Study. Am Heart J 2007;153:260–266. [DOI] [PubMed] [Google Scholar]
- 17. Mukamal KJ, Tolstrup JS, Friberg J, Jensen G, Gronbaek M.. Alcohol consumption and risk of atrial fibrillation in men and women: the Copenhagen City Heart Study. Circulation 2005;112:1736–1742. [DOI] [PubMed] [Google Scholar]
- 18. Iwase S, Matsukawa T, Ishihara S, Tanaka A, Tanabe K, Danbara A, Matsuo M, Sugiyama Y, Mano T.. Effect of oral ethanol intake on muscle sympathetic nerve activity and cardiovascular functions in humans. J Auton Nerv Syst 1995;54:206–214. [DOI] [PubMed] [Google Scholar]
- 19. Reed SF, Porges SW, Newlin DB.. Effect of alcohol on vagal regulation of cardiovascular function: contributions of the polyvagal theory to the psychophysiology of alcohol. Exp Clin Psychopharmacol 1999;7:484–492. [DOI] [PubMed] [Google Scholar]
- 20. Sagawa Y, Kondo H, Matsubuchi N, Takemura T, Kanayama H, Kaneko Y, Kanbayashi T, Hishikawa Y, Shimizu T.. Alcohol has a dose-related effect on parasympathetic nerve activity during sleep. Alcohol Clin Exp Res 2011;35:2093–2100. [DOI] [PubMed] [Google Scholar]
- 21. Spaak J, Merlocco AC, Soleas GJ, Tomlinson G, Morris BL, Picton P, Notarius CF, Chan CT, Floras JS.. Dose-related effects of red wine and alcohol on hemodynamics, sympathetic nerve activity, and arterial diameter. Am J Physiol Heart Circ Physiol 2008;294:H605–H612. [DOI] [PubMed] [Google Scholar]
- 22. Sinnecker D, Dommasch M, Steger A, Berkefeld A, Hoppmann P, Muller A, Gebhardt J, Barthel P, Hnatkova K, Huster KM, Laugwitz KL, Malik M, Schmidt G.. Expiration-triggered sinus arrhythmia predicts outcome in survivors of acute myocardial infarction. J Am Coll Cardiol 2016;67:2213–2220. [DOI] [PubMed] [Google Scholar]
- 23. de Vos CB, Nieuwlaat R, Crijns HJ, Camm AJ, LeHeuzey JY, Kirchhof CJ, Capucci A, Breithardt G, Vardas PE, Pisters R, Tieleman RG.. Autonomic trigger patterns and anti-arrhythmic treatment of paroxysmal atrial fibrillation: data from the Euro Heart Survey. Eur Heart J 2008;29:632–639. [DOI] [PubMed] [Google Scholar]
- 24. Park HW, Shen MJ, Lin SF, Fishbein MC, Chen LS, Chen PS.. Neural mechanisms of atrial fibrillation. Curr Opin Cardiol 2012;27:24–28. [DOI] [PMC free article] [PubMed] [Google Scholar]