Abstract
Background: Questions remain about the strength and shape of the dose-response relationship between fruit and vegetable intake and risk of cardiovascular disease, cancer and mortality, and the effects of specific types of fruit and vegetables. We conducted a systematic review and meta-analysis to clarify these associations.
Methods: PubMed and Embase were searched up to 29 September 2016. Prospective studies of fruit and vegetable intake and cardiovascular disease, total cancer and all-cause mortality were included. Summary relative risks (RRs) were calculated using a random effects model, and the mortality burden globally was estimated; 95 studies (142 publications) were included.
Results: For fruits and vegetables combined, the summary RR per 200 g/day was 0.92 [95% confidence interval (CI): 0.90–0.94, I2 = 0%, n = 15] for coronary heart disease, 0.84 (95% CI: 0.76–0.92, I2 = 73%, n = 10) for stroke, 0.92 (95% CI: 0.90–0.95, I2 = 31%, n = 13) for cardiovascular disease, 0.97 (95% CI: 0.95–0.99, I2 = 49%, n = 12) for total cancer and 0.90 (95% CI: 0.87–0.93, I2 = 83%, n = 15) for all-cause mortality. Similar associations were observed for fruits and vegetables separately. Reductions in risk were observed up to 800 g/day for all outcomes except cancer (600 g/day). Inverse associations were observed between the intake of apples and pears, citrus fruits, green leafy vegetables, cruciferous vegetables, and salads and cardiovascular disease and all-cause mortality, and between the intake of green-yellow vegetables and cruciferous vegetables and total cancer risk. An estimated 5.6 and 7.8 million premature deaths worldwide in 2013 may be attributable to a fruit and vegetable intake below 500 and 800 g/day, respectively, if the observed associations are causal.
Conclusions: Fruit and vegetable intakes were associated with reduced risk of cardiovascular disease, cancer and all-cause mortality. These results support public health recommendations to increase fruit and vegetable intake for the prevention of cardiovascular disease, cancer, and premature mortality.
Keywords: Fruit and vegetables, diet, nutrition, cardiovascular disease, cancer, all-cause mortality, cohort, global assessment
Key Messages
Although a high fruit and vegetable intake has been recommended for prevention of cardiovascular disease and some cancers, questions remain with regard to the amounts and types of fruits and vegetables that are most strongly associated with a reduced risk of cardiovascular disease, total cancer or all-cause mortality and with regard to the burden of disease and mortality that may be attributed to a low fruit and vegetable intake.
In this meta-analysis of 95 studies (142 publications), reductions in risk of cardiovascular disease and all-cause mortality were observed up to an intake of 800 g/day of fruit and vegetables combined, whereas for total cancer no further reductions in risk were observed above 600 g/day.
Inverse associations were observed between intake of apples/pears, citrus fruits, green leafy vegetables/salads and cruciferous vegetables and cardiovascular disease and mortality, and between green-yellow vegetables and cruciferous vegetables and total cancer risk.
An estimated 5.6 and 7.8 million premature deaths worldwide in 2013 may be attributable to a fruit and vegetable intake below 500 and 800 g/day, respectively, if the observed associations are causal.
Introduction
A high intake of fruit and vegetables is one of the cornerstones of a healthy diet and has been recommended to the general public to reduce the risk of cardiovascular diseases and cancer, which are the two most common causes of premature death worldwide and which accounted collectively for 25.5 million deaths in 2013.1 These recommendations have to a large degree been based on findings from epidemiological studies which have shown inverse associations between high fruit and vegetable intake and risk of certain cancers,2 coronary heart disease3and stroke.4 However, the question of what is the optimal level of fruit and vegetable intake to reduce the risk of chronic diseases and premature death is still unanswered. This is reflected by the fact that recommendations for dietary intake vary globally. For example, current recommendations for fruit and vegetable intake range from at least 400 g/day by the World Cancer Research Fund, the WHO, and in England, to 500 g/day in Sweden, to 600 g/day in Denmark, 650–750 g/day in Norway, and 640–800 g/day in the USA (Table 5.2, page 60 in the report).5
Data regarding fruit and vegetable intake and cancer risk are less clear-cut today2 than a decade or two ago.6 A modest association between fruit and vegetable intake or specific subtypes of fruits and vegetables and total cancer risk cannot yet be excluded,7 but the available studies have been inconsistent.7–21 Some studies reported inverse associations,7,8,11–13,17, 21 whereas other studies found no clear association;9,10,14–16,18–20 however, some of these may have had statistical power too low to detect a modest association.9,10,14 Cohort studies have been more consistent in finding an inverse association between fruit and vegetable intake and risk of coronary heart disease and stroke11,22,23 than for cancer, and this has also been shown in meta-analyses3,4 as well as in several additional studies that have been published since these meta-analyses.16,19,23–29 In addition, several10,18,27,30–33 but not all34–40 cohort studies have found inverse associations between fruit and vegetable intake and all-cause mortality but again, some of these studies may have had statistical power too low to detect an association.34–36,38
However, the question of what is an optimal intake of fruit and vegetables remains unclear because the shape of the dose-response relationship between fruit and vegetable intake and incidence or mortality from cardiovascular disease and total cancer as well as the association with all-cause mortality has not been well defined. Although a recent meta-analysis found a reduced risk of all-cause mortality and cardiovascular disease mortality, but not cancer mortality, with greater fruit and vegetable intake,41 the review missed or excluded a large number of publications on all-cause mortality11,18,30,33–38,42–52 and included only studies of cardiovascular disease mortality and cancer mortality, not of disease incidence. Further, at least 16 additional cohort studies (17 publications) have since been published.29,53–68 Thus questions remain with regard to the strength and shape of the dose-response relationship between fruit and vegetable intake and chronic disease risk and mortality, and whether fruit and vegetables also reduce the risk of incident cardiovascular disease or cancer. In addition, it is not clear whether specific types of fruits and vegetables are particularly beneficial with regard to reducing chronic disease risk and mortality, since previous reviews have not analysed fruit and vegetable subtypes.3,4,41
We conducted a systematic review and meta-analysis of published prospective studies relating fruit and vegetable consumption to risk of incidence or mortality from coronary heart disease, stroke, total cardiovascular disease, and total cancer, and to all-cause mortality, and we specifically aimed to clarify the strength and shape of the dose-response relationship for these associations and whether specific types of fruit and vegetables were associated with risk. Last, we calculated the attributable fractions of all-cause and cause-specific mortality globally and by region under the assumption that the observed associations are causal.
Methods
Search strategy and inclusion criteria
We searched the PubMed and Embase databases from their inception (1966 and 1947, respectively) up to 19 July 2016, and the search was later updated to 29 September 2016. Details of the search terms used for the PubMed search are shown in Supplementary Table 1 (available at IJE online) and a similar search was conducted in Embase. Prospective studies of fruit and vegetable intake and risk of incidence or mortality from coronary heart disease (total coronary heart disease or major coronary event, nonfatal myocardial infarction (MI), any MI, fatal MI, incident ischaemic heart disease, fatal ischaemic heart disease, acute coronary syndrome), stroke (total stroke, ischaemic, haemorrhagic, intracerebral and subarachnoidal haemorrhage), total cardiovascular disease (coronary heart disease and stroke combined), and total cancer and all-cause mortality were included if they reported adjusted estimates of the relative risk (RR) (including odds ratios and hazard ratios) and 95% confidence intervals (CIs); and for the dose-response analyses, a quantitative measure of the intake for at least three categories of fruit and vegetable intake had to be available. The excluded studies are listed in Supplementary Table 2, available at IJE online.
Data extraction
Results and study characteristics were extracted into tables and included: name of first author, publication year, country or region, the name of the study, follow-up period, sample size and number of cases or deaths, type of outcome, gender, age, type of fruit and vegetables, amount or frequency of intake, RRs and 95% CIs and variables adjusted for in the analysis. We followed standard criteria for reporting meta-analyses.69 The data extraction was conducted by D.A., and was checked for accuracy by L.T.F. and N.K.
Statistical methods
We calculated summary relative risks (RRs) of incidence or mortality from coronary heart disease, stroke, total cardiovascular disease, and total cancer, and of all-cause mortality for the highest vs the lowest level and per 200 g/day of fruits, vegetables and total fruit and vegetable intake using the random-effects model by DerSimonian and Laird70 which takes into account both within- and between-study variation (heterogeneity). For specific types of fruits and vegetables we calculated summary RRs using 100 g/day as the increment. The average of the natural logarithm of the RRs was estimated and the RR from each study was weighted by the method of DerSimonian and Laird.70 The primary analysis of coronary heart disease, stroke, cardiovascular disease and total cancer included studies that reported on both incidence and mortality from these outcomes, but subgroup analyses were conducted separately for incidence and mortality. For studies that provided results stratified by gender, smoking status, or other subgroups, but not overall, the relative risks were pooled using a fixed-effects model before inclusion in the meta-analysis. One exception is a study which published separately on White37 and Black30 subjects in two different publications and which had different durations of follow-up in the two publications, and in this case both results were included without combining the subgroups. For two studies we recalculated the confidence intervals from 99% to 95%.43,60
We conducted linear dose-response analyses using the method by Greenland and Longnecker71 to calculate RRs and 95% CIs from the natural logarithm of the risk estimates across categories of intake. For each category of fruit and vegetable intake we used the mean or median if reported, and the midpoint of the upper and lower bound was estimated for the remaining studies. When extreme categories were open-ended we used the width of the adjacent interval to calculate an upper or lower cut-off value. Consistent with previous meta-analyses, we used 80 g as a serving size for fruit and vegetable intake.72,73 For specific fruit and vegetable types we used serving sizes as provided in a pooled analysis of cohort studies,74 but for some subtypes of fruits and vegetables which were not reported on in this publication we used 80 g as a serving size as well. We contacted the authors of six studies16,56,61,64,75,76 for information regarding the quantities of consumption for subtypes of fruits and vegetables or for more details of data which were only briefly described in the text, and all replied and provided supplementary information.16,56,61,64,75,76
A potential nonlinear dose-response relationship between fruit and vegetable intake and cardiovascular disease, cancer and mortality risks was assessed using restricted cubic splines with three knots at 10%, 50% and 90% percentiles of the distribution, which was combined using multivariate meta-analysis.77,78 We conducted a sensitivity analysis using fractional polynomial models for the nonlinear analysis as well,79 and we determined the best-fitting second-order fractional polynomial regression model, which was defined as the one with the lowest deviance.
Heterogeneity between studies was evaluated using Q and I2 statistics.80 To explore potential heterogeneity we conducted subgroup analyses by study characteristics. Small-study effects such as publication bias were assessed using Egger’s test81 and by inspection of the funnel plots. When Egger’s test indicated bias, we tested whether this affected the results by excluding studies with a low number of cases or by excluding obvious outlying studies based on inspection of the funnel plots. We also conducted sensitivity analyses excluding each study at a time from each analysis to clarify if the results were robust. Study quality was assessed using the Newcastle-Ottawa scale which awards 0–9 stars based on the selection, comparability and outcome assessment.82 We considered studies with scores of 0–3, 4–6 and 7–9 to represent low, medium and high quality studies, respectively. Stata version 13.0 software (StataCorp, TX, USA) was used for the analyses.
Attributable fractions
We calculated the fraction of deaths attributable worldwide due to low fruit and vegetable intake, assuming a causal relationship, using the relative risks from the nonlinear dose-response analysis. The prevalence of low fruit and vegetable intake was calculated based on data from the World Health Survey which provided estimates of fruit and vegetable intake from 26 national population-based surveys covering 14 geographical regions.83,84 We used data on mortality from the Global Burden of Disease Study 2013.1 Because all the epidemiological studies included in this meta-analysis have been conducted in mainly adult populations, we excluded the number of deaths occurring before 15 years age as well as the intake levels for subjects < 15 years. The preventable proportion of deaths and cause-specific deaths attributable to a low fruit and vegetable intake was calculated using the formula proposed by Miettinen.85 Further information about these calculations is provided in the Supplementary Methods, available at IJE online.
Results
A total of 142 publications from 95 unique cohort studies were included in the analyses7–40,42–68,75,76,86–164 (Figure 1; Supplementary Tables 3–7, available at IJE online); 44 studies were from Europe, 26 were from the USA, 20 from Asia and five from Australia. Five publications reported results from two studies that were combined.15,54,124,134,140 Throughout the text the total number of studies and publications are reported, but the number included in each high vs low analysis and dose-response analysis may differ slightly because some studies only reported dichotomous results or results on a continuous scale. The number of studies, cases, participants and the references for the studies included in each high vs low and dose-response analysis are provided in Table 1. The number of cases or deaths ranged between 17 742 and 43 336 for coronary heart disease, 10 560 and 46 951 for stroke, 20 329 and 81 807 for cardiovascular disease, 52 872 and 112 370 for total cancer and 71 160 and 94 235 for all-cause mortality (Table 1). The number of participants in each analysis ranged from 226 910 to 2 123 415 (any outcome) (Table 1). Supplementary Tables 3–7 show a summary of the study characteristics of the included studies. Figure 1 shows a flowchart of the study selection process. Figures 2–6 show the results for the dose-response analyses, and Supplementary Figures 1–31 (available at IJE online) shows the high vs low analyses for all outcomes and the high vs low, linear and nonlinear dose-response analyses for ischaemic and haemorrhagic stroke. Supplementary Tables 8-18 shows the results from the nonlinear dose-response analyses for all outcomes. Results for subtypes of fruit and vegetables are shown in Tables 2–6 (also see Supplementary Tables 19–27 and Supplementary Figures 32–242).
Figure 1.
Flow-chart of study selection.
Table 1.
Summary relative risks for high vs low and dose-response analyses and risk of coronary heart disease, stroke, cardiovascular disease, total cancer and mortality
| Food group | Comparison | n | Cases | Participants | RR (95% CI) | I2 | pheterogeneity | Egger | References |
|---|---|---|---|---|---|---|---|---|---|
| Coronary heart disease | |||||||||
| Fruit and vegetables | High vs low | 16 | 18516 | 792197 | 0.87 (0.83–0.91) | 0 | 0.52 | 0.21 | 23,29,31,32,56,58,64,86,88,90,91,93,94,98 |
| Per 200 g/d | 15 | 17742 | 775132 | 0.92 (0.90–0.94) | 0 | 0.96 | 0.12 | 23,29,31,32,56,58,64,86,88,90,91,94,132 | |
| Fruit | High vs low | 25 | 40229 | 1568460 | 0.86 (0.82–0.91) | 23.2 | 0.15 | 0.52 | 9–11,22,23,27,29,36,55,56,58,60,63,64,86,88,91,94,95,98–100,104 |
| Per 200 g/d | 24 | 43336 | 1555553 | 0.90 (0.86–0.94) | 43.7 | 0.01 | 0.04 | 9,10,22,23,27,29,36,56,58,60,62–64,86,88,91,92,94,95,99,104,132 | |
| Vegetables | High vs low | 22 | 34754 | 2123415 | 0.87 (0.84–0.90) | 1.9 | 0.43 | 0.03 | 9,10,23,27,29,34,55,56,58,64,86,88,91,94,95,98,100,101,103,104 |
| Per 200 g/d | 20 | 20853 | 1047071 | 0.84 (0.79–0.90) | 60.6 | < 0.0001 | 0.001 | 9,22,23,27,29,56,58,62,64,86,88,91,94,95,100,103,104,132 | |
| Stroke | |||||||||
| Fruit and vegetables | High vs low | 8 | 10560 | 226910 | 0.79 (0.71–0.88) | 37.6 | 0.13 | 0.80 | 31,56,58,64,109,118,120,122 |
| Per 200 g/d | 10 | 11644 | 303338 | 0.84 (0.76–0.92) | 73.3 | < 0.0001 | 0.08 | 31,56,58,64,108–110,118,120,122 | |
| Fruit | High vs low | 17 | 46951 | 960337 | 0.82 (0.77–0.87) | 36.3 | 0.07 | 0.60 | 11,25–27,55,56,58,60,63,64,109,112–117 |
| Per 200 g/d | 16 | 46203 | 964142 | 0.82 (0.74–0.90) | 72.9 | < 0.0001 | 0.62 | 25–27,56,58,60,62–64,109,112–114,116,117,126 | |
| Vegetables | High vs low | 13 | 14519 | 427124 | 0.87 (0.81–0.95) | 38.2 | 0.08 | 0.82 | 25–27,55,56,58,64,109,112,113,115–117 |
| Per 200 g/d | 13 | 14973 | 441670 | 0.87 (0.79–0.96) | 63.4 | 0.001 | 0.15 | 25–27,56,58,62,64,109,112,113,116,117,126 | |
| Cardiovascular disease | |||||||||
| Fruit and vegetables | High vs low | 16 | 27842 | 963240 | 0.84 (0.79–0.90) | 53.5 | 0.006 | 0.005 | 15,16,18,31,39,42,53,56,58,64,75,86–88,98,125 |
| Per 200 g/d | 13 | 20329 | 877925 | 0.92 (0.90–0.95) | 31.3 | 0.13 | 0.05 | 15,16,18,31,39,53,56,58,64,75,86–88 | |
| Fruit | High vs low | 21 | 81807 | 1605227 | 0.87 (0.82–0.92) | 70.9 | < 0.0001 | 0.99 | 11,16,19,24,27,53–56,58,60,63,64,75,76,88,98,124,125,127 |
| Per 200 g/d | 17 | 72648 | 1492617 | 0.87 (0.82–0.92) | 79.1 | < 0.0001 | 0.41 | 15,16,19,24,27,53,56,58,60–64,75,88,127 | |
| Vegetables | High vs low | 18 | 32049 | 1112174 | 0.89 (0.85–0.94) | 43.2 | 0.03 | 0.05 | 16,19,24,27,53–56,58,64,75,76,88,98,124,125,127 |
| Per 200 g/d | 14 | 23857 | 1009038 | 0.90 (0.87–0.93) | 11.5 | 0.33 | 0.53 | 15,16,19,24,27,53,56,58,62,64,75,88,127 | |
| Total cancer | |||||||||
| Fruit and vegetables | High vs low | 13 | 54123 | 904300 | 0.93 (0.87–0.98) | 41.2 | 0.06 | 0.97 | 7,8,13,15,16,18,20,42,53,56,59,87,128 |
| Per 200 g/d | 12 | 52872 | 902065 | 0.97 (0.95–0.99) | 48.7 | 0.03 | 0.18 | 7,8,13,15,16,18,20,53,56,59,87,128 | |
| Fruit | High vs low | 21 | 105401 | 1569168 | 0.92 (0.88–0.96) | 49.3 | 0.006 | 0.16 | 7–11,13,14,16,17,19–21,53,54,56,57,59,65,114,128 |
| Per 200 g/d | 20 | 112370 | 1648240 | 0.96 (0.94–0.99) | 52.1 | 0.004 | 0.05 | 7–10,13,15–17,19–21,53,56,57,59,61,65,114,128 | |
| Vegetables | High vs low | 17 | 101118 | 1505948 | 0.95 (0.90–0.99) | 52.6 | 0.006 | 0.25 | 7–9,13,16,17,19–21,53,54,56,57,59,65,128 |
| Per 200 g/d | 17 | 108855 | 1597722 | 0.96 (0.93–0.99) | 55.2 | 0.003 | 0.47 | 7–9,13,15–17,19–21,53,56,57,59,65,128 | |
| All-cause mortality | |||||||||
| Fruit and vegetables | High vs low | 22 | 87574 | 1035556 | 0.82 (0.79–0.86) | 62.3 | <0.0001 | 0.001 | 18,29,31,32,39,42–44,47–50,53,56,58,67,68,75,87,98,134,161 |
| Per 200 g/d | 15 | 71160 | 959083 | 0.90 (0.87–0.93) | 82.5 | <0.0001 | <0.0001 | 18,29,31,32,39,49,53,56,58,67,68,75,87,132,134 | |
| Fruit | High vs low | 30 | 93473 | >1144194 | 0.87 (0.84–0.90) | 60.2 | <0.0001 | 0.01 | 9–11,19,27,29,30,36,37,43,46,47,51–58,61,66,68,75,98,135–137,139 |
| Per 200 g/d | 27 | 94235 | >1104255 | 0.85 (0.80–0.91) | 89.5 | <0.0001 | 0.10 | 9,10,19,27,29,30,35–37,40,43,45,52,53,56–58,61,62,66,68,75,131–133,139 | |
| Vegetables | High vs low | 24 | 82904 | 1082960 | 0.87 (0.82–0.92) | 78.6 | <0.0001 | 0.02 | 9,19,27,29,34,38,46,47,51–58,66,68,75,98,136,137,139 |
| Per 200 g/d | 22 | 86264 | 1050795 | 0.87 (0.82–0.92) | 82.3 | <0.0001 | 0.02 | 9,19,27,29,33,35,40,45,52,53,56–58,62,66,68,75,131–133,139 | |
Figure 2.
Fruits, vegetables and coronary heart disease, linear and nonlinear dose-response..
Table 2.
Fruit and vegetable subtypes and coronary heart disease
| High vs low analysis |
Dose-response analysis |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fruit, vegetable subtype | n | RR (95% CI) | I2 | Ph | References | n | Increment | RR (95% CI) | I2 | Ph | References |
| Apples, pears | 10 | 0.85 (0.79–0.93) | 1.9 | 0.42 | 34,56,60,64,89,94,142,145,163 | 8 | Per 100 g/d | 0.99 (0.89–1.09) | 33.9 | 0.16 | 56,60,64,89,94,142,145 |
| Bananas | 3 | 0.90 (0.67–1.20) | 47.2 | 0.15 | 56,94 | 3 | Per 100 g/d | 0.81 (0.26–2.58) | 69.4 | 0.04 | 56,94 |
| Berries | 6 | 0.94 (0.81–1.08) | 51.6 | 0.07 | 34,56,60,89,102,163 | 4 | Per 100 g/d | 1.13 (0.90–1.43) | 14.0 | 0.32 | 56,60,89,102 |
| Citrus fruits | 14 | 0.91 (0.86–0.96) | 0 | 0.69 | 9,56,60,64,89,91,94,97,142,143,146,147 | 15 | Per 100 g/d | 0.95 (0.89–1.01) | 19.1 | 0.24 | 9,56,60,62,64,89,91,94,97,142,143,146,147 |
| Dried fruits | 2 | 0.93 (0.78–1.11) | 0 | 0.51 | 11,60 | 1 | Per 100 g/d | 0.55 (0.19–1.52) | - | - | 60 |
| Fruit juices | 2 | 0.79 (0.63–0.98) | 0 | 0.56 | 60,143 | 3 | Per 100 g/d | 0.93 (0.80–1.08) | 79.6 | 0.007 | 60,92,143 |
| Grapes | 4 | 0.91 (0.76–1.10) | 43.5 | 0.15 | 56,60,142,145 | 4 | Per 100 g/d | 0.87 (0.56–1.37) | 71.0 | 0.02 | 56,60,142,145 |
| Strawberries | 2 | 1.17 (0.71–1.91) | 76.5 | 0.04 | 142,144 | 1 | Per 100 g/d | 4.66 (1.14–19.03) | - | - | 144 |
| Watermelon | 2 | 0.87 (0.64–1.18) | 0 | 0.36 | 94 | 2 | Per 100 g/d | 0.91 (0.72–1.15) | 46.2 | 0.17 | 94 |
| Allium vegetables | 3 | 0.98 (0.79–1.20) | 0 | 0.40 | 89,94 | 3 | Per 100 g/d | 0.99 (0.34–2.86) | 4.4 | 0.35 | 89,94 |
| Cruciferous vegetables | 7 | 1.01 (0.90–1.13) | 30.4 | 0.20 | 56,64,91,94,147 | 8 | Per 100 g/d | 0.99 (0.89–1.09) | 0 | 0.59 | 56,62,64,91,94,147 |
| Green leafy vegetables | 10 | 0.83 (0.75–0.91) | 31.6 | 0.16 | 10,36,56,64,89,91,143,147 | 9 | Per 100 g/d | 0.72 (0.64–0.82) | 0.4 | 0.43 | 36,56,62,64,89,91,143,147 |
| Onions | 3 | 0.75 (0.56–1.02) | 61.9 | 0.07 | 34,105,145 | 2 | Per 100 g/d | 0.60 (0.15–2.42) | 76.5 | 0.04 | 105,145 |
| Potatoes | 5 | 0.92 (0.79–1.07) | 64.0 | 0.03 | 100,140,143,164 | 6 | Per 100 g/d | 0.99 (0.93–1.05) | 48.8 | 0.08 | 92,106,140,143,164 |
| Tomatoes | 5 | 0.90 (0.80–1.00) | 0 | 0.64 | 56,141,143,145,147 | 6 | Per 100 g/d | 0.94 (0.89–0.98) | 0 | 0.48 | 56,141,143,145,147,148 |
| Beta-carotene rich F&V | 3 | 0.83 (0.75–0.92) | 20.4 | 0.29 | 64,91 | 3 | Per 100 g/d | 0.77 (0.65–0.91) | 27.3 | 0.25 | 64,91 |
| Lutein rich F&V | 3 | 0.97 (0.89–1.05) | 31.2 | 0.23 | 64,91 | 3 | Per 100 g/d | 0.80 (0.60–1.07) | 9.5 | 0.33 | 64,91 |
| Lycopene rich F&V | 3 | 1.01 (0.93–1.09) | 0 | 0.39 | 64,91 | 3 | Per 100 g/d | 1.00 (0.91–1.10) | 0 | 0.77 | 64,91 |
| Vitamin C rich F&V | 3 | 0.86 (0.78–0.95) | 19.4 | 0.29 | 64,91 | 3 | Per 100 g/d | 0.91 (0.85–0.99) | 15.2 | 0.31 | 64,91 |
| Raw F&V | 2 | 0.89 (0.61–1.30) | 67.1 | 0.08 | 28,96 | 1 | Per 100 g/d | 0.88 (0.77–1.01) | - | - | 28 |
Some publications reported results from more than one study and for this reason the number of risk estimates and publications is not always the same.
F&V, fruit and vegetables.
Ph = P-value for heterogeneity.
Coronary heart disease
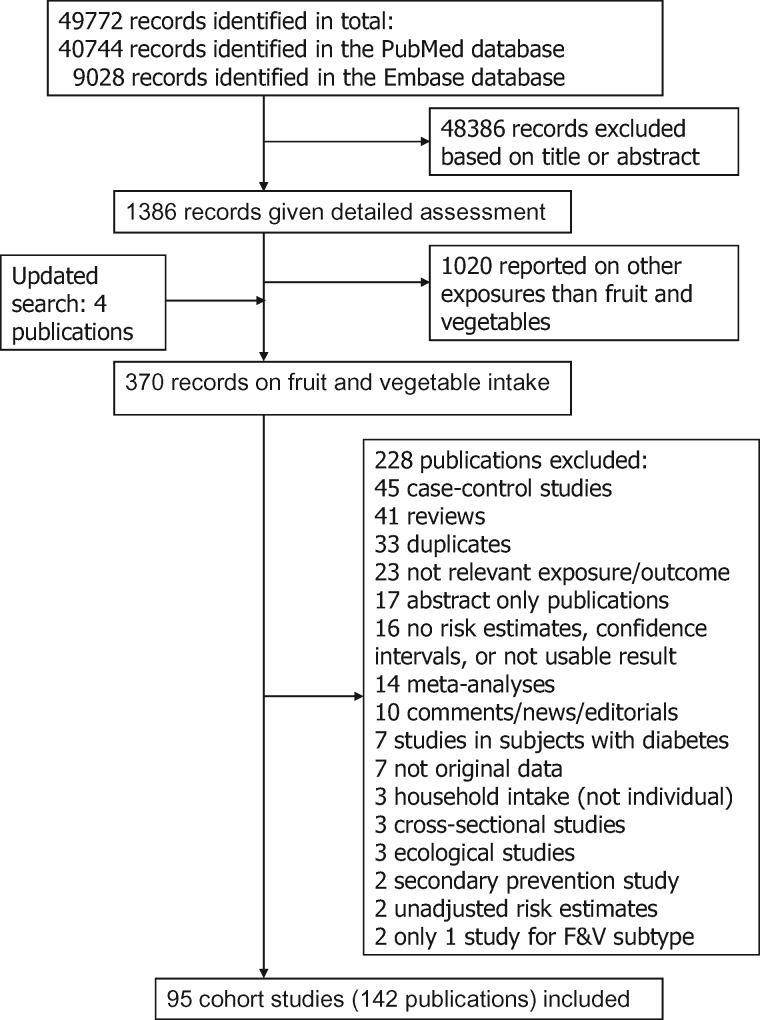
Seventeen studies (15 publications),23,29,31,32,56,58,64,86,88,90,91,93,94,98,132 26 studies (26 publications),9–11,22,23,27,29,36,55,56,58,60,62–64,86,88,91,92,94,95,98–100,104,132 and 23 studies (23 publications)9,10,22,23,27,29,34,55,56,58,62,64,86,88,91,94,95,98,100,101,103,104,132 were included in the analyses of fruit and vegetables combined, fruits alone and vegetables alone and coronary heart disease, respectively. The summary RR per 200 g/day was 0.92 (95% CI: 0.90–0.94, I2 = 0%) for fruits and vegetables (Figure 2a, b, Table 1; Supplementary Figure 1), 0.90 (95% CI: 0.86–0.94, I2 = 44%) for fruits (Figure 2c, d, Table 1; Supplementary Figure 2), and 0.84 (95% CI: 0.79–0.90, I2 = 61%) for vegetables (Figure 2e, 2f, Table 1; Supplementary Figure 3). There was no evidence of a nonlinear association for fruits and vegetables, Pnonlinearity = 0.30, and there was a 24% reduction in the relative risk at an intake of 800 g/day (Figure 2b; Supplementary Table 8). Nonlinear associations were observed for fruits, Pnonlinearity < 0.0001 (Figure 2d, Supplementary Table 9), and vegetables, Pnonlinearity < 0.0001 (Figure 2f, Supplementary Table 9), with most of the reductions in risk observed at the lower levels of intake, and there was a 21% reduction in relative risk up to 750–800 g/day for fruits and a 30% reduction in the relative risk up to 550–600 g/day for vegetables.
Of specific types of fruit and vegetables9,10,11,28,34,36,56,60,62,64,89,91,92,94,96,97,100,102,105,106,140–148,163,164 apples/pears, citrus fruits, fruit juices, green leafy vegetables, beta-carotene-rich fruits and vegetables and vitamin C-rich fruits and vegetables showed inverse associations with coronary heart disease in the high vs low analysis, and in addition tomatoes were inversely associated with coronary heart disease in the dose-response analysis (Table 2; Supplementary Tables 19–20, Supplementary Figures 32–76).
Stroke
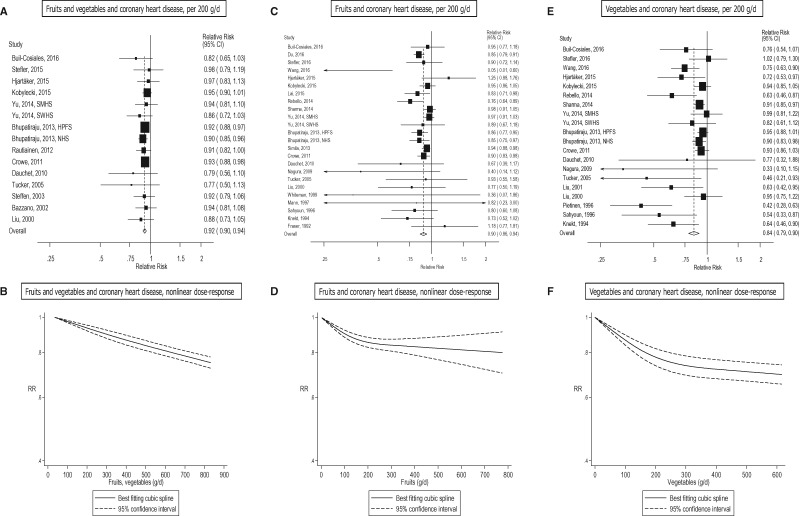
Ten studies (10 publications),31,56,58,64,108–110,118,120,122 19 studies (19 publications),11,25–27,55,56,58,60,62–64,109,112–117,126 and 14 studies (15 publications) 25–27,55,56,58,62,64,109,112,113,115–117,126 were included in the analysis of fruit and vegetables, fruits, and vegetables, and total stroke risk, respectively. The summary RR per 200 g/day was 0.84 (95% CI: 0.76–0.92, I2 = 73%) for fruits and vegetables (Figure 3a, b, Table 1; Supplementary Figure 4), 0.82 (95% CI: 0.74–0.90, I2 = 73%) for fruits (Figure 3c, d, Table 1; Supplementary Figure 5), and 0.87 (95% CI: 0.79–0.96, I2 = 63%) for vegetables (Figure 3e, f, Table 1; Supplementary Figure 6). There was evidence of a nonlinear association between fruit and vegetables, fruits, and vegetables, and total stroke, Pnonlinearity < 0.0001 (Figure 3b; Supplementary Table 10), Pnonlinearity < 0.0001 (Figure 3d; Supplementary Table 11), Pnonlinearity < 0.0001 (Figure 3f; Supplementary Table 11), with stronger reductions in risk at lower levels of intake. There was a 33% reduction in the relative risk at intakes of 800 g/day of fruits and vegetables, 20% reduction in the relative risk at 200–350 g/day of fruits and 28% reduction in the relative risk at 500 g/day of vegetables, and there was little evidence of further reductions in risk at higher intakes.
Figure 3.
Fruits and vegetables and stroke, linear and nonlinear dose-response..
Eight studies (seven publications),25,32,108–111,120 11 studies (10 publications),25–27,109,112–114,116,117,126 and nine studies (eight publications)25–27,109–111,113,116 were included in the analyses of fruits and vegetables combined, fruits, and vegetables, and ischaemic stroke, respectively. The summary RR per 200 g/day was 0.92 (95% CI: 0.87–0.97, I2 = 9%) for fruits and vegetables (Supplementary Figures 7–9, Supplementary Tables 12, 29, available at IJE online), 0.78 (95% CI: 0.69–0.89, I2 = 58%) for fruits (Supplementary Figures 10–12, Supplementary Tables 12, 29) and 0.86 (95% CI: 0.76–0.97, I2 = 55%) for vegetables (Supplementary Figures 13–15, Supplementary Tables 12, 29). Three studies (two publications, two risk estimates)108,109, eight studies (seven publications, seven risk estimates)19,25–27,63,109,113 and six studies (five publications, five risk estimates)19,25,27,63,113 were included in the analyses of fruits and vegetables combined, fruits, and vegetables, and haemorrhagic stroke, respectively. The summary RR per 200 g/day was 0.88 (95% CI: 0.78–0.99, I2 = 0%) for fruits and vegetables combined (Supplementary Figure 16, Supplementary Table 29), 0.66 (95% CI: 0.50–0.86, I2 = 57%) for fruits (Supplementary Figures 17–19, Supplementary Tables 13, 29), and 0.76 (95% CI: 0.55–1.06, I2 = 42%) for vegetables (Supplementary Figures 20–22, Supplementary Tables 13, 29).
Of specific types of fruit and vegetables,11,26,56,60,62,64,109,112,119,141–144,146,148–150,164 high intakes of apples/pears, citrus fruits, fruit juice, green leafy vegetables and pickled vegetables were inversely associated with total stroke risk, whereas intake of grapes was also inversely associated with total stroke in the dose-response analysis (Table 3, Supplementary Tables 21–22, Supplementary Figures 77–109). For ischaemic stroke26,109–111,121,146,150,162,164 there was evidence that intake of citrus fruits, citrus fruit juices, green leafy vegetables, and vitamin C-rich fruits and vegetables were inversely associated with risk, but none of the associations with haemorrhagic stroke26,109,146,150,164 were significant (Table 3; Supplementary Table 23, Supplementary Figures 110–140).
Table 3.
Fruit and vegetable subtypes and total stroke, ischaemic stroke and haemorrhagic stroke
| Total stroke |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| High vs low analysis |
Dose-response analysis |
||||||||||
| Fruit, vegetable subtype | n | RR (95% CI) | I2 | Ph | References | n | Increment | RR (95% CI) | I2 | Ph | References |
| Apples, pears | 6 | 0.88 (0.81–0.96) | 0 | 0.66 | 56,60,64,109,142,149 | 5 | Per 100 g/d | 0.94 (0.84–1.05) | 39.7 | 0.16 | 56,60,64,109,142 |
| Berries | 5 | 0.98 (0.86–1.12) | 51.4 | 0.08 | 26,56,60,109,150 | 5 | Per 100 g/d | 1.07 (0.79–1.45) | 39.0 | 0.16 | 26,56,60,109,150 |
| Citrus fruits | 8 | 0.74 (0.65–0.84) | 53.6 | 0.04 | 26,56,60,64,109,112,143,146 | 9 | Per 100 g/d | 0.78 (0.69–0.90) | 63.6 | 0.005 | 26,56,60,62,64,109,112,143,146 |
| Citrus fruit juice | 2 | 0.90 (0.74–1.10) | 0 | 0.55 | 60,142 | 2 | Per 100 g/d | 0.89 (0.72–1.10) | 0 | 0.61 | 60,142 |
| Dried fruits | 2 | 0.92 (0.74–1.15) | 0 | 0.98 | 11,60 | 1 | Per 100 g/d | 0.75 (0.32–1.81) | - | - | 60 |
| Fruit juice | 2 | 0.67 (0.60–0.76) | 0 | 0.98 | 60,143 | 2 | Per 100 g/d | 0.72 (0.63–0.83) | 0 | 0.69 | 60,143 |
| Grapes | 2 | 0.72 (0.47–1.10) | 35.8 | 0.21 | 56,60 | 2 | Per 100 g/d | 0.57 (0.34–0.97) | 0 | 0.66 | 56,60 |
| Allium vegetables | 2 | 0.89 (0.80–1.00) | 0 | 0.89 | 109,149 | 1 | Per 100 g/d | 0.89 (0.76–1.04) | - | - | 109 |
| Cruciferous vegetables | 4 | 0.97 (0.78–1.20) | 56.8 | 0.07 | 26,56,64,109 | 5 | Per 100 g/d | 1.04 (0.80–1.36) | 30.6 | 0.22 | 26,56,62,64,109 |
| Green leafy vegetables | 4 | 0.88 (0.81–0.95) | 0 | 0.60 | 56,64,109,143 | 5 | Per 100 g/d | 0.73 (0.57–0.94) | 35.9 | 0.18 | 56,62,64,109,143 |
| Pickled vegetables | 2 | 0.80 (0.73–0.88) | 0 | 0.93 | 119,143 | 2 | Per 100 g/d | 0.57 (0.43–0.74) | 0 | 0.70 | 119,143 |
| Potatoes | 4 | 0.94 (0.87–1.01) | 0 | 0.43 | 26,143,164 | 4 | Per 100 g/d | 0.98 (0.94–1.02) | 0 | 0.41 | 26,143,164 |
| Root vegetables | 2 | 1.01 (0.89–1.14) | 0 | 0.38 | 26,109 | 2 | Per 100 g/d | 0.96 (0.78–1.18) | 0 | 0.54 | 26,109 |
| Tomatoes | 3 | 0.95 (0.68–1.31) | 61.0 | 0.08 | 56,141,143 | 4 | Per 100 g/d | 1.01 (0.96–1.06) | 0 | 0.72 | 56,141,143,148 |
| Ischaemic stroke | |||||||||||
| Berries | 3 | 0.95 (0.75–1.21) | 74.7 | 0.02 | 26,109,150 | 3 | Per 100 g/d | 1.02 (0.61–1.72) | 70.3 | 0.03 | 26,109,150 |
| Citrus fruits | 7 | 0.78 (0.66–0.92) | 66.6 | 0.006 | 26,109–111,121,146,162 | 7 | Per 100 g/d | 0.87 (0.79–0.95) | 52.5 | 0.05 | 26,109–111,146,162 |
| Citrus fruit juices | 2 | 0.65 (0.51–0.84) | 0 | 0.47 | 110 | 2 | Per 100 g/d | 0.87 (0.80–0.96) | 0 | 0.74 | 110 |
| Allium vegetables | 2 | 0.90 (0.78–1.03) | 0 | 0.78 | 109,111 | 2 | Per 100 g/d | 0.93 (0.77–1.11) | 0 | 0.81 | 109,111 |
| Cruciferous vegetables | 5 | 0.82 (0.66–1.01) | 66.5 | 0.02 | 26,109–111 | 5 | Per 100 g/d | 0.66 (0.41–1.07) | 71.7 | 0.007 | 26,109–111 |
| Green leafy vegetables | 4 | 0.88 (0.78–0.99) | 0 | 0.45 | 109–111 | 4 | Per 100 g/d | 0.74 (0.62–0.89) | 0 | 0.78 | 109–111 |
| Potatoes | 5 | 0.97 (0.87–1.08) | 3.5 | 0.39 | 26,110,164 | 5 | Per 100 g/d | 1.00 (0.95–1.05) | 0 | 0.60 | 26,110,164 |
| Root vegetables | 3 | 0.93 (0.73–1.18) | 57.3 | 0.10 | 26,109,111 | 3 | Per 100 g/d | 0.91 (0.64–1.30) | 58.9 | 0.09 | 26,109,111 |
| Vitamin C-rich F&V | 2 | 0.80 (0.69–0.92) | 0 | 0.88 | 110 | 2 | Per 100 g/d | 0.92 (0.86–0.98) | 0 | 0.71 | 110 |
| Haemorrhagic stroke | |||||||||||
| Berries | 3 | 1.15 (0.89–1.49) | 0 | 0.60 | 26,109,150 | 3 | Per 100 g/d | 1.66 (0.91–3.03) | 0 | 0.66 | 26,109,150 |
| Citrus fruits | 3 | 0.74 (0.55–1.01) | 28.2 | 0.25 | 26,109,146 | 3 | Per 100 g/d | 0.79 (0.59–1.06) | 32.2 | 0.23 | 26,109,146 |
| Cruciferous vegetables | 2 | 0.83 (0.33–2.12) | 84.1 | 0.01 | 26,109 | 2 | Per 100 g/d | 0.27 (0.01–12.54) | 77.0 | 0.04 | 26,109 |
| Potatoes | 3 | 1.06 (0.83–1.36) | 0 | 0.90 | 26,164 | 3 | Per 100 g/d | 1.03 (0.91–1.16) | 0 | 0.77 | 26,164 |
| Root vegetables | 2 | 1.05 (0.76–1.44) | 0 | 0.92 | 26,109 | 2 | Per 100 g/d | 1.16 (0.66–2.02) | 0 | 0.85 | 26,109 |
F&V, fruit and vegetables.
Ph = P-value for heterogeneity.
Cardiovascular disease
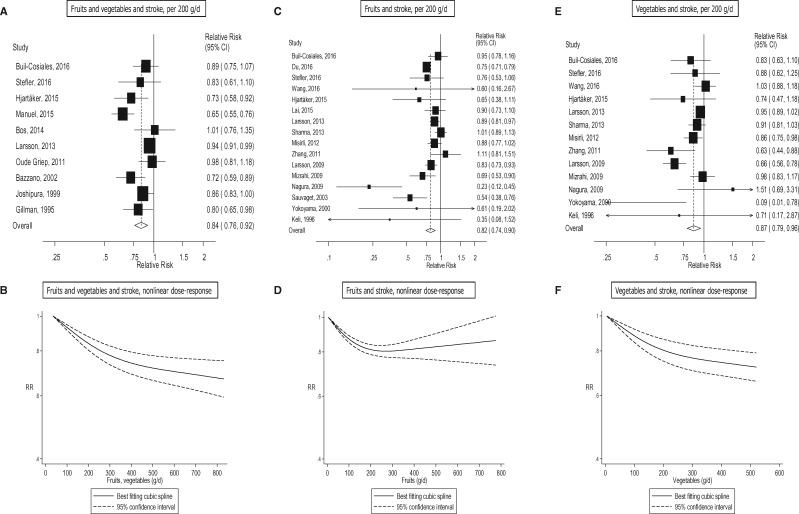
Seventeen studies (16 publications),15,16,18,31,39,42,53,56,58,64,75,86–88,98,125 25 studies (23 publications)11,15,16,19,24,27,53–56,58,60–64,75,76,88,98,124,125,127 and 22 studies (19 publications)15,16,19,24,27,53–56,58,62,64,75,76,88,98,124,125,127 were included in the analysis of fruit and vegetables, fruits, and vegetables, and cardiovascular disease, respectively. The summary RR was 0.92 (95% CI: 0.90–0.95, I2 = 31%) for fruits and vegetables (Figure 4a, b, Table 1; Supplementary Figure 23), 0.87 (95% CI: 0.82–0.92, I2 = 79%) for fruits (Figure 4c, d, Table 1; Supplementary Figure 24), and 0.90 (95% CI: 0.87–0.93, I2 = 12%) for vegetables (Figure 4e, 4f, Table 1, Supplementary Figure 25). There was evidence of nonlinearity, Pnonlinearity < 0.0001, for fruits and vegetables (Figure 4b; Supplementary Table 14), and fruits, Pnonlinearity < 0.0001, (Figure 4d; Supplementary Table 15), and for vegetables, Pnonlinearity = 0.04 (Figure 4f; Supplementary Table 15), with steeper inverse associations at lower levels of intake, although for vegetables the association was approximately linear. There were 28%, 27% and 28% reductions in relative risk at intakes of 800 g/day for fruits and vegetables and fruits, and 600 g/day of vegetables, respectively.
Figure 4.
Fruits and vegetables and cardiovascular disease, linear and nonlinear dose-response..
Of specific types of fruits and vegetables11,15,16,19,56,60–62,64,75,87,123,124,129,141,142,144,146,148,151,152,154,159,164 there was evidence that high vs low intake of apples/pears, citrus fruits, carrots and noncruciferous vegetables were inversely associated, and tinned fruits were positively associated with cardiovascular disease risk, and in the nonlinear dose-response analysis there was evidence that cruciferous vegetables, green leafy vegetables, and tomatoes were inversely associated with risk, although few studies were included in these analyses (Table 4; Supplementary Tables 24 and 25, Supplementary Figures 141–178).
Table 4.
Fruit and vegetable subtypes and cardiovascular disease
| High vs low analysis |
Dose-response analysis |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fruit, vegetable subtype | n | RR (95% CI) | I2 | Ph | References | n | Increment | RR (95% CI) | I2 | Ph | References |
| Apples, pears | 7 | 0.86 (0.80–0.93) | 0 | 0.74 | 56,60,61,64,129,142,152 | 7 | Per 100 g/d | 0.92 (0.82–1.03) | 46.9 | 0.08 | 56,60,61,64,129,142,152 |
| Berries | 2 | 1.02 (0.94–1.11) | 0 | 0.44 | 56,60 | 2 | Per 100 g/d | 1.13 (0.88–1.46) | 0 | 0.68 | 56,60 |
| Citrus fruits | 6 | 0.78 (0.66–0.92) | 72.3 | 0.003 | 16,56,60,64,124,146 | 8 | Per 100 g/d | 0.92 (0.84–1.00) | 65.8 | 0.005 | 15,16,56,60–62,64,146 |
| Citrus fruit juice | 2 | 0.88 (0.53–1.46) | 48.3 | 0.16 | 60,124 | 2 | Per 100 g/d | 0.98 (0.95–1.02) | 6.9 | 0.30 | 15,60 |
| Dried fruits | 3 | 0.94 (0.83–1.06) | 0 | 0.80 | 11,60,151 | 1 | Per 100 g/d | 0.66 (0.33–1.26) | - | - | 60 |
| Fruit juice | 1 | 0.67 (0.41–1.10) | - | - | 60 | 2 | Per 100 g/d | 0.99 (0.93–1.06) | 0 | 0.58 | 60,154 |
| Grapes | 3 | 0.86 (0.70–1.05) | 62.3 | 0.07 | 56,60,142 | 3 | Per 100 g/d | 0.83 (0.48–1.45) | 66.7 | 0.05 | 56,60,142 |
| Strawberries | 3 | 1.02 (0.79–1.32) | 57.2 | 0.10 | 142,144,151 | 1 | Per 100 g/d | 1.06 (0.95–1.17) | - | - | 144 |
| Tinned fruits | 3 | 1.23 (1.06–1.43) | 0 | 0.51 | 159 | 4 | Per 100 g/d | 1.30 (0.81–2.08) | 66.0 | 0.03 | 154,159 |
| Broccoli | 3 | 0.87 (0.67–1.13) | 25.0 | 0.26 | 142,144,151 | 2 | Per 100 g/d | 0.75 (0.49–1.14) | 0 | 0.57 | 142,144 |
| Carrots | 2 | 0.81 (0.70–0.93) | 0 | 0.73 | 56,123 | 1 | Per 100 g/d | 0.97 (0.72–1.30) | - | - | 56 |
| Cruciferous vegetables | 8 | 0.88 (0.73–1.05) | 77.5 | < 0.0001 | 16,19,56,64,87,124,151 | 9 | Per 100 g/d | 0.89 (0.77–1.02) | 65.1 | 0.003 | 15,16,19,56,62,64,87,154 |
| Green leafy vegetables | 5 | 0.84 (0.71–0.99) | 74.0 | 0.004 | 16,56,64,124,151 | 5 | Per 100 g/d | 0.83 (0.65–1.08) | 66.7 | 0.02 | 15,16,56,64 |
| Noncruciferous vegetables | 2 | 0.76 (0.59–0.97) | 50.1 | 0.16 | 19 | 2 | Per 100 g/d | 0.91 (0.82–1.01) | 74.5 | 0.05 | 19 |
| Potatoes | 3 | 1.01 (0.91–1.13) | 60.0 | 0.08 | 124,164 | 4 | Per 100 g/d | 1.01 (0.97–1.04) | 13.4 | 0.33 | 15,154,164 |
| Raw vegetables | 2 | 0.83 (0.66–1.05) | 88.0 | 0.004 | 11,75 | 1 | Per 100 g/d | 0.86 (0.81–0.90) | - | - | 75 |
| Tomatoes | 4 | 0.94 (0.86–1.02) | 0.4 | 0.39 | 56,141,142,151 | 4 | Per 100 g/d | 0.92 (0.80–1.07) | 52.6 | 0.10 | 56,141,142,148 |
| Beta-carotene rich F&V | 2 | 0.96 (0.89–1.03) | 0 | 0.77 | 64,124 | 2 | Per 100 g/d | 0.94 (0.89–0.99) | 0 | 0.55 | 64,124 |
| Vitamin C rich F&V | 1 | 0.91 (0.44–1.90) | - | - | 64 | 2 | Per 100 g/d | 0.95 (0.92–0.98) | 0 | 0.88 | 15, 64 |
F&V, fruit and vegetables.
Ph = P-value for heterogeneity.
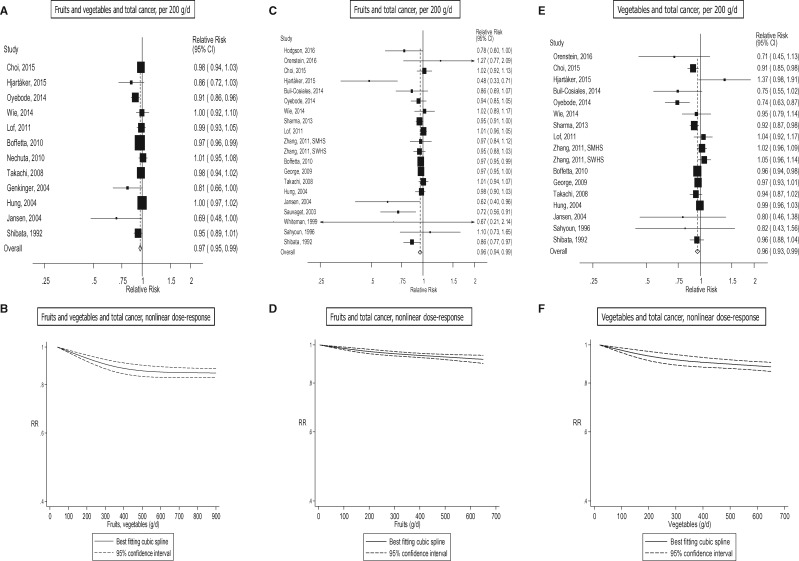
Total cancer
Fourteen studies (13 publications),7,8,13,15,16,18,20,42,53,56,59,87,128 25 studies (22 publications),7–11,13–17,19–21,53,54,56,57,59,61,65,114,128 and 19 studies (17 publications),7–9,13,15–17,19–21,53,54,56,57,59,65,128 were included in the analysis of fruit and vegetables, fruits, and vegetables, and total cancer, respectively. The summary RR was 0.97 (95% CI: 0.95–0.99, I2 = 49%) for fruits and vegetables combined (Figure 5a, b, Table 1; Supplementary Figure 26), 0.96 (95% CI: 0.94–0.99, I2 = 52%) for fruits (Figure 5c, d, Table 1; Supplementary Figure 27) and 0.96 (95% CI: 0.93–0.99, I2 = 55%) for vegetables (Figure 5e, f, Table 1; Supplementary Figure 28). There was evidence of nonlinearity for fruits and vegetables, Pnonlinearity = 0.02 (Figure 5b; Supplementary Table 16), fruits, Pnonlinearity = 0.02 (Figure 5d; Supplementary Table 17), and vegetables, Pnonlinearity = 0.03 (Figure 5f; Supplementary Table 17), with most of the reductions in risk at lower levels of intake. There were 14%, 8% and 12% reductions in the relative risk for intakes of 550–600 g/day for fruits and vegetables, fruits, and vegetables, respectively, but there was little evidence of further reductions in risk with higher intakes.
Figure 5.
Fruits and vegetables and total cancer, linear and nonlinear dose-response..
Of specific types of fruits and vegetables8–12,14–16,19,54,56,87,105,130,143,153–160 there were significant inverse associations between cruciferous vegetables and green-yellow vegetables and total cancer risk (Table 5; Supplementary Table 26, Supplementary Figures 179–209).
Table 5.
Fruit and vegetable subtypes and total cancer
| High vs low analysis |
Dose-response analysis |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fruit, vegetable subtype | n | RR (95% CI) | I2 | Ph | References | n | Increment | RR (95% CI) | I2 | Ph | References |
| Citrus fruits | 5 | 0.97 (0.90–1.04) | 61.0 | 0.04 | 9,16,143,155,156 | 6 | Per 100 g/d | 0.99 (0.95–1.04) | 70.3 | 0.005 | 15,16,143,156 |
| Dried fruits | 2 | 0.89 (0.61–1.30) | 32.7 | 0.22 | 11,153 | 0 | Per 100 g/d | - | - | - | - |
| Tinned fruits | 3 | 0.90 (0.77–1.05) | 0 | 0.99 | 159 | 4 | Per 100 g/d | 0.82 (0.66–1.01) | 0 | 0.96 | 154,159 |
| Fruit juice | 1 | 0.99 (0.92–1.07) | - | - | 143 | 2 | Per 100 g/d | 0.99 (0.95–1.02) | 0 | 0.84 | 143,154 |
| Broccoli | 2 | 1.03 (0.87–1.22) | 0 | 0.46 | 130,153 | 1 | Per 100 g/d | 1.22 (0.86–1.72) | - | - | 130 |
| Cruciferous vegetables | 5 | 0.84 (0.72–0.97) | 65.5 | 0.02 | 16,19,56,87 | 6 | Per 100 g/d | 0.91 (0.82–1.02) | 67.7 | 0.008 | 15,16,19,56,87,154 |
| Green vegetables | 3 | 0.96 (0.90–1.02) | 0 | 0.43 | 8,10,143 | 2 | Per 100 g/d | 0.91 (0.80–1.03) | 0 | 0.74 | 8,143 |
| Green yellow vegetables | 5 | 0.88 (0.77–1.00) | 17.1 | 0.31 | 9,12,14,153,157 | 4 | Per 100 g/d | 0.89 (0.83–0.96) | 8.0 | 0.35 | 9,12,153,157 |
| Mushrooms | 1 | 1.07 (0.99–1.16) | - | - | 143 | 2 | Per 100 g/d | 1.20 (1.01–1.44) | 0 | 0.80 | 143,154 |
| Noncruciferous vegetables | 2 | 1.05 (0.90–1.23) | 16.8 | 0.27 | 19 | 2 | Per 100 g/d | 1.01 (0.97–1.06) | 0 | 0.41 | 19 |
| Onions | 2 | 0.91 (0.81–1.02) | 0 | 0.34 | 105,130 | 2 | Per 100 g/d | 0.75 (0.54–1.04) | 0 | 0.52 | 105,130 |
| Pickled vegetbles | 2 | 0.96 (0.90–1.02) | 0 | 0.99 | 14,143 | 1 | Per 100 g/d | 0.92 (0.78–1.10) | - | - | 143 |
| Potatoes | 2 | 1.02 (0.86–1.22) | 31.0 | 0.23 | 14,143 | 3 | Per 100 g/d | 0.99 (0.95–1.02) | 0 | 0.77 | 15,143,154 |
| Salads | 4 | 0.98 (0.89–1.07) | 0 | 0.88 | 11,54,56,153 | 1 | Per 100 g/d | 0.88 (0.49–1.59) | - | - | 56 |
| Tomatoes | 3 | 0.86 (0.67–1.10) | 70.1 | 0.04 | 56,143,153 | 2 | Per 100 g/d | 0.94 (0.83–1.07) | 0 | 0.84 | 56,143 |
| Yellow vegetables | 3 | 0.97 (0.92–1.03) | 0 | 0.51 | 8,16,143 | 3 | Per 100 g/d | 0.97 (0.89–1.06) | 0 | 0.47 | 8,16,143 |
| Juice | 1 | 0.98 (0.81–1.19) | - | - | 160 | 2 | Per 100 g/d | 1.00 (0.94–1.07) | 0 | 0.54 | 158,160 |
Ph = P-value for heterogeneity.
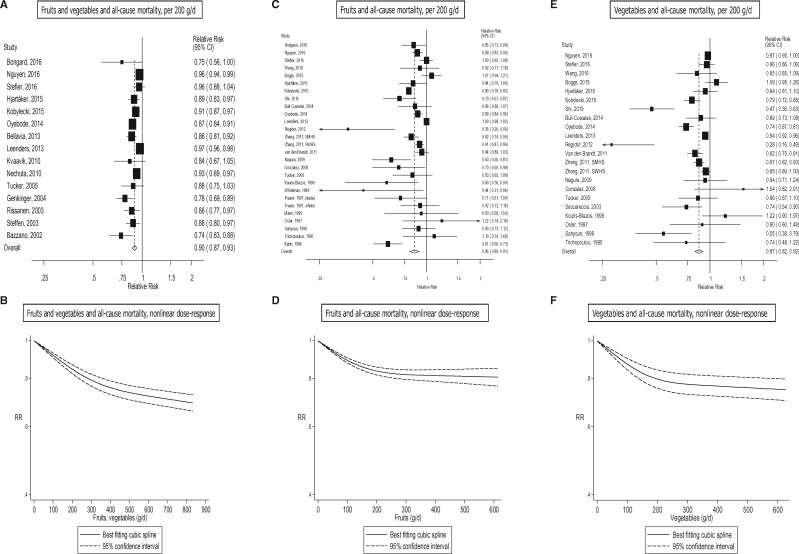
All-cause mortality
In all, 24 studies (23 publications),18,29,31,32,39,42–44,47–50,53,56,58,67,68,75,87,98,132,134,161 37 studies (36 publications),9–11,19,27,29,30,35–37,40,43,45–47,51–58,61,62,66,68,75,98,131–133,135–137,139 and 33 studies (31 publications)9,19,27,29,33–35,38,40,45–47,51–58,62,66,68,75,98,131–133,136,137,139 were included in the analysis of fruits and vegetables, fruits, and vegetables, and all-cause mortality, respectively. The summary RR was 0.90 (95% CI: 0.87–0.93, I2 = 83%) for fruits and vegetables (Figure 6a, b, Table 1; Supplementary Figure 29), 0.85 (95% CI: 0.80–0.91, I2 = 90%) for fruits (Figure 6c, d, Table 1; Supplementary Figure 30), and 0.87 (95% CI: 0.82–0.92, I2 = 82%) for vegetables (Figure 6e, f, Table 1; Supplementary Figure 31). There was evidence of nonlinearity for fruits and vegetables, Pnonlinearity < 0.0001 (Figure 6b; Supplementary Table 18), fruits, Pnonlinearity < 0.0001 (Figure 6d; Supplementary Table 18), and vegetables, Pnonlinearity < 0.0001 (Figure 6f; Supplementary Table 18), respectively, with stronger reductions in risk at lower levels of intake. There were 31%, 19% and 25% reductions in the relative risk with intakes of 800 g/day for fruits and vegetables combined, and at 600 g/day for fruits, and for vegetables, respectively.
Figure 6.
Fruits, vegetables and all-cause mortality, linear and nonlinear dose-response..
Of specific types of fruits and vegetables,9,11,19,30,34,37,38,43,44,53,54,56,61,62,67,68,75,87,105,133,138,143,159 there was evidence that high vs low intake of apples/pears, berries, citrus fruits, fruit juice, cooked vegetables, cruciferous vegetables, potatoes and green leafy vegetables/salads were inversely associated with all-cause mortality and tinned fruits were positively associated with all-cause mortality; whereas in the dose-response analysis fruit juice, cruciferous vegetables and green leafy vegetables/salads were significantly associated with reduced risk and tinned fruits were associated with increased risk (Table 6; Supplementary Table 27, Supplementary Figures 210–242).
Table 6.
Fruit and vegetable subtypes and all-cause mortality
|
High vs low analysis |
Dose-response analysis |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fruit, vegetable subtype | n | RR (95% CI) | I2 | Ph | References | n | Increment | RR (95% CI) | I2 | Ph | References |
| Apples, pears | 5 | 0.80 (0.70–0.91) | 84.9 | < 0.0001 | 34,56,61,75,138 | 3 | Per 100 g/d | 0.80 (0.64–1.01) | 95.3 | < 0.0001 | 56,61,75 |
| Bananas | 2 | 0.91 (0.80–1.03) | 18.8 | 0.27 | 56,61 | 2 | Per 100 g/d | 0.95 (0.80–1.14) | 70.5 | 0.07 | 56,61 |
| Berries | 3 | 0.92 (0.88–0.97) | 8.0 | 0.34 | 34,56,75 | 2 | Per 100 g/d | 0.85 (0.70–1.03) | 0 | 0.61 | 56,75 |
| Citrus fruits | 6 | 0.90 (0.86–0.94) | 12.3 | 0.34 | 9,38,56,61,75,143 | 7 | Per 100 g/d | 0.94 (0.88–1.00) | 49.9 | 0.06 | 9,38,56,61,62,75,143 |
| Fruit juice | 1 | 0.87 (0.83–0.91) | - | - | 143 | 2 | Per 100 g/d | 0.88 (0.84–0.92) | 0 | 0.71 | 53,143 |
| Tinned fruits | 3 | 1.13 (1.04–1.23) | 0 | 0.72 | 159 | 4 | Per 100 g/d | 1.14 (1.07–1.21) | 0 | 0.71 | 53,159 |
| Cruciferous vegetables | 6 | 0.88 (0.80–0.97) | 78.9 | < 0.0001 | 19,56,75,87,138 | 6 | Per 100 g/d | 0.90 (0.85–0.95) | 35.2 | 0.17 | 19,56,62,75,87 |
| Mushrooms | 2 | 0.93 (0.84–1.01) | 79.3 | 0.03 | 75,143 | 2 | Per 100 g/d | 0.74 (0.46–1.20) | 77.7 | 0.03 | 75,143 |
| Noncruciferous vegetables | 2 | 0.85 (0.67–1.07) | 82.7 | 0.02 | 19 | 2 | Per 100 g/d | 0.95 (0.89–1.02) | 83.1 | 0.02 | 19 |
| Onions, allium vegetables | 3 | 0.85 (0.69–1.06) | 84.0 | 0.002 | 34,75,105 | 2 | Per 100 g/d | 0.76 (0.40–1.46) | 50.3 | 0.16 | 75,105 |
| Potatoes | 4 | 0.78 (0.74–0.83) | 2.8 | 0.38 | 43,44,67,75 | 4 | Per 100 g/d | 0.91 (0.81–1.03) | 59.0 | 0.06 | 43,67,75,133 |
| Root vegetables | 2 | 0.93 (0.77–1.13) | 89.2 | 0.002 | 75,138 | 1 | Per 100 g/d | 0.76 (0.66–0.88) | - | - | 75 |
| Green leafy vegetables, salads | 7 | 0.92 (0.86–0.97) | 39.5 | 0.13 | 11,30,37,43,54,56,75 | 7 | Per 100 g/d | 0.78 (0.71–0.86) | 11.1 | 0.34 | 11,30,43,53,56,62,75 |
| Cooked vegetables | 5 | 0.87 (0.80–0.94) | 66.4 | 0.02 | 30,38,43,68,75 | 4 | Per 100 g/d | 0.89 (0.80–0.99) | 94.0 | < 0.0001 | 30,38,43,68,75 |
| Raw vegetables | 2 | 0.88 (0.79–0.98) | 74.4 | 0.05 | 68,75 | 2 | Per 100 g/d | 0.91 (0.80–1.02) | 90.8 | 0.001 | 68,75 |
Ph = P-value for heterogeneity.
Publication bias, subgroup and sensitivity analyses
There was evidence of publication bias in some of the analyses for coronary heart disease, stroke, cardiovascular disease and all-cause mortality (Table 1; Supplementary Figures 243–248, available at IJE online). However, excluding studies with < 150 or < 200 cases or outlying studies attenuated Egger’s test in several of the analyses, but did not materially affect the strength of the associations. In the analyses of vegetables and all-cause mortality, exclusion of two outlying studies explained the asymmetry in the funnel plots but none of these exclusions materially altered the summary estimates. The results persisted in sensitivity analyses excluding one study at a time from each analysis (Supplementary Figures 249–263, available at IJE online). We also repeated the nonlinear dose-response analyses using fractional polynomial models and in general found similar risk estimates compared with the restricted cubic spline models, although the confidence intervals were wider and there was more indication of nonlinearity (results not shown).
In subgroup analyses stratified by duration of follow-up, outcome type (incidence vs mortality), outcome subtype (MI vs. total CHD, or ischemic vs hemorrhagic), sex, geographical location, number of cases, study quality and adjustment for confounding factors, the findings persisted across most subgroups and there was little evidence of heterogeneity between most subgroups (Supplementary Tables 28–32, available at IJE online). The study quality was in general high as the vast majority of studies were in the group with 7–9 stars (Supplementary Tables 28–32). The mean (median) study quality scores of the studies included in the dose-response analysis were 8.0 (8.0), 7.5 (8.0), 7.7 (8.0) for fruits and vegetables, fruits, and vegetables and coronary heart disease, respectively. The respective means (medians) were 7.9 (8.0), 7.7 (8.0), and 7.7 (8.0) for stroke, 7.8 (8.0), 7.7 (8.0), 7.8 (8.0) for cardiovascular disease, 7.9 (8.0), 8.0 (8.0), and 8.1 (8.0) for total cancer, and 7.7 (8.0), 7.1 (7.0), and 7.5 (8.0) for all-cause mortality. There was suggestion of heterogeneity when studies of vegetables and coronary heart disease were stratified by adjustment for physical activity, with weaker (but still significant) associations among studies with such adjustment, Pheterogeneity = 0.003, compared to studies without such adjustment. In the analysis of fruits and vegetables and cardiovascular disease there was a weaker association among studies with adjustment for body mass index (BMI) than among studies without such adustment, Pheterogeneity = 0.03, and in the analysis of vegetables and cardiovascular disease there was a stronger association among studies with mortality as the outcome compared with studies with incidence as the outcome, Pheterogeneity = 0.03 (Supplementary Table 30). In the analysis of fruits and total cancer and all-cause mortality there was heterogeneity (Pheterogeneity = 0.03 and Pheterogeneity = 0.02, respectively) by adjustment for energy intake, with weaker (but still significant) associations in studies with such adjustment compared to studies without such adjustment (Supplementary Tables 31, 32). In the analysis of fruits and vegetables and all-cause mortality there was a weaker association among the studies that adjusted for red and processed meat intake compared to studies with such adjustment, Pheterogeneity < 0.0001, and in the analysis of vegetables and all-cause mortality there was a stronger association among the studies without adjustment for smoking than among studies with such adjustment, Pheterogeneity = 0.003 (Supplementary Table 32).
Estimation of the fraction of deaths preventable by increasing fruit and vegetable intake
Under the assumption that the observed associations are causal we estimated that the number of premature deaths attributable to a fruit and vegetable intake below 800 g/day in 2013 was 1 340 000 for coronary heart disease, 2 680 000 for stroke, 2 270 000 for cardiovascular disease, 660 000 for cancer and 7 800 000 for all-cause mortality (Table 7). We repeated these calculations using 500 g/day as a reference category and arrived at 710 000 coronary heart disease deaths, 1 470 000 stroke deaths, 1 260 000 cardiovascular disease deaths, 560 000 cancer deaths and 5 400 000 all-cause deaths (Table 7) (The combined number of deaths potentially preventable from coronary heart disease and stroke was larger than for cardiovascular disease probably because the studies included in each analysis were not the same and because there was a stronger association for stroke mortality than for coronary heart disease and cardiovascular disease mortality). The number of deaths attributable to a low fruit and vegetable intake for each country is provided in Supplementary Table 33. In a sensitivity analysis, we only counted the cause-specific deaths [from coronary heart disease, stroke and total cancer] for sub-Saharan Africa, and arrived at 5 240 000 and 7 630 000 premature deaths globally for an intake of 500 and 800 g/day, respectively.
Table 7.
Attributable fractions and numbers of deaths from coronary heart disease, stroke, cardiovascular disease, cancer and all-causes by region due to a fruit and vegetable intake below 500 g/day or 800 g/day (based on studies reporting on coronary heart disease, stroke, cardiovascular disease and cancer mortality only and excluding studies on incidence)
| Coronary heart disease |
Stroke |
Cardiovascular disease |
Total cancer |
All-cause mortality |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 500 g/d |
800 g/d |
500 g/d |
800 g/d |
500 g/d |
800 g/d |
500 g/d |
800 g/d |
500 g/d |
800 g/d |
|||||||||||
| Region | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n |
| America A | 9.1 | 55739 | 16.9 | 103482 | 23.2 | 44723 | 41.2 | 79448 | 8.8 | 70703 | 15.1 | 124284 | 7.3 | 53914 | 8.5 | 62586 | 11.9 | 360444 | 16.9 | 510322 |
| America B | 12.5 | 54117 | 20.7 | 89771 | 31.1 | 90528 | 49.7 | 144756 | 11.7 | 85227 | 18.8 | 135863 | 10.1 | 51598 | 11.4 | 58345 | 16.9 | 487772 | 22.0 | 637414 |
| America D | 8.5 | 4176 | 16.5 | 8081 | 21.6 | 7190 | 39.9 | 13247 | 8.3 | 6833 | 14.6 | 12436 | 7.4 | 4433 | 8.1 | 5196 | 11.0 | 43683 | 16.1 | 63877 |
| Europe A | 4.5 | 37864 | 10.2 | 85867 | 11.4 | 49792 | 24.2 | 105828 | 4.6 | 58739 | 8.9 | 121756 | 3.6 | 43103 | 4.4 | 52348 | 5.6 | 240217 | 9.2 | 397116 |
| Europe B | 8.4 | 35195 | 15.6 | 65281 | 21.6 | 66257 | 38.3 | 117708 | 8.1 | 58946 | 14.0 | 103551 | 6.8 | 23213 | 7.9 | 26921 | 11.1 | 193826 | 15.7 | 273948 |
| Europe C | 12.3 | 149042 | 21.5 | 260970 | 30.9 | 171813 | 51.9 | 288916 | 11.7 | 206530 | 19.3 | 345109 | 9.9 | 47412 | 11.3 | 54399 | 16.2 | 492439 | 22.0 | 669572 |
| South East Asia B | 12.2 | 33451 | 21.9 | 60025 | 30.6 | 111368 | 52.6 | 191272 | 11.7 | 74412 | 19.5 | 126895 | 9.8 | 24715 | 11.4 | 28590 | 15.9 | 324696 | 22.0 | 450661 |
| South East Asia D | 11.4 | 158497 | 20.9 | 291326 | 28.5 | 288454 | 50.1 | 506323 | 10.9 | 263073 | 18.6 | 457867 | 9.2 | 86619 | 10.7 | 100739 | 14.7 | 1537085 | 20.8 | 2171656 |
| Eastern Mediterranean B | 7.8 | 9949 | 15.5 | 19680 | 19.9 | 14075 | 37.4 | 26446 | 7.7 | 15197 | 13.7 | 28171 | 6.3 | 5005 | 7.5 | 5899 | 10.1 | 57236 | 15.0 | 85085 |
| Eastern Mediterranean D | 6.8 | 26452 | 13.9 | 54293 | 21.6 | 51646 | 33.4 | 100319 | 6.7 | 46384 | 12.3 | 88655 | 5.5 | 14127 | 6.5 | 16785 | 8.6 | 194416 | 13.2 | 297235 |
| Africa D | 7.0 | 8532 | 14.2 | 17381 | 24.2 | 46683 | 34.2 | 65992 | 6.9 | 21762 | 12.6 | 41334 | 7.4 | 11432 | 6.7 | 10283 | 8.9 | 122406 | 13.5 | 152326 |
| Africa E | 10.3 | 14537 | 19.5 | 27664 | 25.7 | 57088 | 46.6 | 103399 | 10.0 | 36223 | 17.3 | 64917 | 8.3 | 17767 | 9.7 | 20827 | 13.1 | 175970 | 19.0 | 253150 |
| Western Pacific A | 5.9 | 10780 | 12.3 | 22575 | 15.0 | 30799 | 29.6 | 60958 | 5.9 | 22802 | 10.9 | 44328 | 4.7 | 22851 | 5.7 | 27257 | 7.5 | 116071 | 11.6 | 180053 |
| Western Pacific B | 7.9 | 112025 | 16.9 | 238931 | 19.6 | 437848 | 39.3 | 877746 | 8.0 | 291165 | 14.9 | 571986 | 6.4 | 155867 | 7.7 | 187748 | 9.8 | 1035701 | 15.5 | 1648878 |
| Globally | 9.3 | 710356 | 17.7 | 1345327 | 22.9 | 1468264 | 41.8 | 2682358 | 7.7 | 1257996 | 13.8 | 2267152 | 6.9 | 562056 | 8.1 | 657923 | 11.3 | 5381962 | 16.3 | 7791293 |
A list of the country-specific attributable fractions and numbers of deaths attributable to a low fruit and vegetable intake is found in Supplementary Table 33
Discussion
There was a 8–16% reduction in the RR of coronary heart disease, 13–18% reduction in the RR of stroke, 8–13% reduction in the RR of cardiovascular disease, 3–4% reduction in the RR of total cancer and 10–15% reduction in the RR of all-cause mortality for each 200 g/day increment in intake of fruit, vegetables, and fruit and vegetables combined. In the nonlinear models, there were 16%, 28%, 22%, 13% and 27% reductions in the RR of coronary heart disease, stroke, cardiovascular disease, total cancer and all-cause mortality, respectively, for an intake of 500 g of fruits and vegetables per day vs 0–40 g/day, whereas an intake of 800 g/day was associated with 24%, 33%, 28%, 14% and 31% reductions in the RR, respectively. Globally an estimated 710 000 coronary heart disease deaths, 1.47 million stroke deaths, 560 000 cancer deaths and 5.4 million premature deaths were attributable to a fruit and vegetable intake below 500 g/day in 2013, and this increased to 1.34 million coronary heart disease deaths, 2.68 million stroke deaths, 660 000 cancer deaths and 7.8 million deaths from all causes when using 800 g/day as the optimal intake. Alternatively, using total cardiovascular disease instead of coronary heart disease and stroke mortality, an estimated 1.25 and 2.26 million cardiovascular disease deaths were attributable to a fruit and vegetable intake below 500 and 800 g/day, respectively.
There was evidence of nonlinearity in all analyses of fruits and vegetables combined, apart from one, and in most of the analyses the reduction in risk was steeper at the lower than at the higher range of fruit and vegetable intake. For fruits and vegetables combined the lowest risk was observed at an intake of 550–600 g/day (7–7.5 servings/day) for total cancer, with little evidence of further reductions in risk with higher intakes, whereas for coronary heart disease, stroke, cardiovascular disease and all-cause mortality the lowest risk was observed at 800 g/day (10 servings/day), which was at the high end of the range of intake across studies. Fruit and vegetable intake was only weakly associated with overall cancer risk, particularly cancer incidence, which is consistent with the change in the assessment of the evidence for several individual cancers as well;2 however, specific fruits and vegetables may be more strongly related to specific cancers. We found that several individual types of fruits and vegetables were inversely associated with coronary heart disease, stroke or cardiovascular disease (apples/pears, citrus fruits, cruciferous vegetables, green leafy vegetables, tomatoes and beta-carotene-rich and vitamin C-rich fruit and vegetables), total cancer (cruciferous vegetables and green-yellow vegetables) and all-cause mortality (apples/pears, berries, citrus fruits, cooked vegetables, cruciferous vegetables, potatoes, and green leafy vegetables/salads). In contrast, intake of tinned fruits was associated with increased risk of cardiovascular disease and all-cause mortality. However, because of the low number of studies on fruit and vegetable subtypes, the potential for selective reporting and publication of subtypes that are significantly associated with risk, as well as confounding from other types of fruits and vegetables, further studies are needed.
Our meta-analysis is in general consistent with previous meta-analyses of fruit and vegetable intake and coronary heart disease,3 stroke4 and mortality,41 but includes a much larger number of studies, more detailed dose-response, subgroup and sensitivity analyses, results for subtypes of fruit and vegetables and estimations of the burden of mortality due to a low fruit and vegetable intake as well. In comparison with the most recent meta-analysis on mortality which included less than half the studies in the present analysis, we found a slightly stronger association between fruit and vegetable intake and all-cause mortality with reductions in risk observed up to 800 g/day (or ten servings per day), although the reduction in risk was steepest up to 400 g/day, whereas the previous meta-analysis found no further benefit above five servings per day (400 g/day).41 In addition, we found significant inverse associations with overall cancer risk, which is consistent with data for some individual cancer sites,2,72,73,165–167 but in contrast to the previous meta-analysis which may have had limited power to detect a weak association.41 An interesting finding in the present meta-analysis is that fruit and vegetable intake was as strongly related to overall mortality as it was related to cardiovascular disease, but only modestly associated with cancer. One explanation may be that the studies included in each analysis do not fully overlap. In addition, it is possible that fruit and vegetable intake may be strongly associated with reduced incidence or mortality from other causes including respiratory, infectious, digestive, and inflammatory diseases,168–175 and recently in the EPIC- study inverse associations were also observed for all other causes of death for vegetables, and unknown causes of death for fruits.169 However, the available evidence for all these outcomes is very limited. Finally, fruit and vegetable intake may reduce the severity of disease and progression to death. Further studies are needed to clarify the association between fruit and vegetable intake and specific causes of death other than cardiovascular disease and cancer.
Fruit and vegetables contain of a myriad of nutrients and phytochemicals, including fibre, vitamin C, carotenoids, antioxidants, potassium, flavonoids and other unidentified compounds which are likely to act synergistically through several biological mechanisms to reduce risk of chronic diseases and premature mortality.176 Dietary fibre and fruit and vegetable intakes have been shown to reduce cholesterol levels, blood pressure, inflammation and platelet aggregation, and to improve vascular and immune function,177–180 and recent meta-analyses showed inverse associations between fibre intake and cardiovascular disease.181,182 Antioxidants in fruit and vegetables may neutralize reactive oxygen species and reduce DNA damage,178 glucosinolates in cruciferous vegetables induce detoxifying enzymes183 and intake of fruits, vegetables and fibre may modulate steroid hormone concentrations and hormone metabolism178 and may have a beneficial effect on gut microbiota.177 In addition, fruit and vegetable intake has been inversely associated with risk of developing overweight or obesity and with weight gain184–186 although data are not entirely consistent;187 however, the associations observed in these analyses appear to be independent of adiposity. A high fruit and vegetable intake may also reduce chronic disease risk indirectly, by displacement of unhealthy foods high in saturated fat, transfat, glycaemic load and sodium; however, most of the associations persisted in subgroups of studies that adjusted for dietary fat or meat intake.
Our analysis has several limitations. Combining studies from different populations increases the sample size and statistical power, but also results in heterogeneity because of differences in the characteristics of the study populations. Heterogeneity was low in the analyses of coronary heart disease, moderate to high for stroke, cardiovascular disease and total cancer and high for all-cause mortality. The heterogeneity appeared to be driven by differences in the size of the association more than by variation in the presence or absence of an association, as most of the studies found inverse associations. Some heterogeneity is expected as the studies varied by the age groups included, duration of follow-up, geographical location, sample sizes, detail of the dietary assessment method, and factors adjusted for in the analyses. There are also likely to be large differences between populations in the types, amounts and preparation methods of fruits and vegetables consumed, as well as differences in the stability of the intakes over time and differences in the incidence of specific cancers and specific causes of death that contribute to total cancer and all-cause mortality. However, when we conducted subgroup analyses to investigate sources of the heterogeneity, we found in general little evidence of heterogeneity between most subgroups. In the few subgroup analyses where the test for heterogeneity was significant there were often relatively few studies in one of the subgroups, and chance can not be excluded as an explanation.
Fruit and vegetable intake is often associated with other lifestyle factors such as lower prevalence of smoking, less overweight and obesity, higher physical activity and lower intakes of alcohol and red and processed meat, which could have confounded the observed associations. Many studies adjusted for these and other confounding factors, and we found little evidence that the results varied substantially whether or not adjustment for most of these confounders was done. It is possible that persons with a high fruit and vegetable intake may be more likely to undergo screening or have better access to or compliance with treatment, and this could lead to an improved survival and bias the results for mortality. There was little heterogeneity when studies were stratified by whether the outcome was incidence or mortality of cardiovascular disease or total cancer, although in a few analyses risk estimates were slightly stronger for mortality; however, power may have been low to detect a difference because of a moderate number of studies in each subgroup. The possibility of residual confounding by imprecisely measured, unknown or unmeasured confounders cannot be entirely excluded.
Measurement error in the assessment of fruit and vegetable intake and changes in fruit and vegetable intake during follow-up may have influenced the results, but to date only the EPIC study23,75 and the China Kadoorie Cohort Study63 have assessed the impact of measurement errors and regression dilution bias due to changes in intake during follow-up on these outcomes. The HR for all-cause mortality was 0.97 (95% CI: 0.96–0.98) per 200 g/day of fruit and vegetable intake without correction for measurement error compared with 0.94 (95% CI: 0.91–0.96) with correction for measurement error,75 and the corresponding HRs for ischaemic heart disease mortality (per 80 g/day) were 0.97 (95% CI: 0.95–0.99) and 0.95 (95% CI: 0.91–0.99),23 respectively, in the EPIC study. In the China Kadoorie Cohort Study the HR of cardiovascular death with one daily portion of fresh fruit intake was 0.77 (95% CI: 0.72–0.83) before and 0.63 (95% CI: 0.56–0.72) after correction for regression dilution. This suggests that both measurement error and regression dilution bias may have attenuated the observed risk estimates. We can also not entirely exclude the possibility that the weaker dose-response curve at higher compared with lower intakes could partly be due to measurement errors in the assessment of fruit and vegetable intake. Although not all studies had the same range of fruit and vegetable intake, there was a wide range of fruit and vegetable intake across most studies and thus the nonlinearity is not likely to be due to single studies with a more extreme range than others. Another limitation of the current analysis is that there were no prospective cohort data from some regions of the world including Africa, West Asia, South and Latin America, and we cannot exclude the possibility that the associations may differ by region or ethnicity. However, we did not find significant heterogeneity in the association between fruits and vegetables and the outcomes considered by region for the geographical locations for which data were available (North America, Europe, Australia and Asia). Two studies on fruit and vegetable intake and all-cause mortality in African Americans showed mixed results, with one reporting an inverse association,30 and a second study reporting no clear association.139 An analysis from the Latin American countries in the INTERHEART study suggested a similar association between fruit and vegetable intake and coronary heart disease188 as in the overall study.189
Last, the appropriateness of the estimates of the number of deaths attributable to a fruit and vegetable intake below 500 or 800 g/day is dependent on the validity of several assumptions that were made including that of: (i) a causal relationship between fruit and vegetable intake and these outcomes; (ii) lack of confounding; and (iii) that the results can be generalized across populations. The observed associations appear to meet several of Bradford-Hill’s criteria for causation including consistency of findings in different people and regions (by sex and geographical region), temporality, some evidence of a biological gradient, plausibility, coherence between epidemiological and laboratory findings and experimental evidence (on intermediate risk markers).190 However, although the strength of the association is moderate across the range of fruit and vegetable intake with a 31% reduction in the relative risk of all-cause mortality comparing an intake of 800 g/day with no intake, weak or moderate associations should not be dismissed as non-causal.190 In addition, the criteria of specificity is less important because many risk factors are causes of multiple diseases, and this is likely to be the case for a low fruit and vegetable intake. As already mentioned, the association between fruit and vegetable intake and cardiovascular disease, cancer and all-cause mortality persisted in many subgroup analyses when stratified by adjustment for confounding factors. Thus although we cannot entirely rule out the possibility that residual confounding could partly explain the associations, it seems less likely that it could entirely explain the observed associations. Confounding might exaggerate the observed associations but measurement errors would most likely tend to attenuate the observed associations. Because the distributions of causes of death differ substantially in sub-Saharan Africa compared with other regions, the results from existing cohort studies on all-cause mortality may not be generalizable to this region. The calculations for this region were done conservatively and we also conducted sensitivity analyses only counting the cause-specific deaths (coronary heart disease, stroke and cancer) from sub-Saharan Africa, but this did not substantially alter the number of deaths globally that were attributable to an inadequate fruit and vegetable intake (which was reduced from 5.4 to 5.2 million for an intake below 500 g/day and from 7.8 to 7.6 million for an intake below 800 g/d). Nevertheless, we cannot exclude the possibility that these estimates might change when additional data from other geographical locations and on specific causes of death become available.
As a meta-analysis of published literature, the analysis may have been affected by small-study effects such as publication bias. There was indication of small-study bias in several of the analyses, and we may therefore have slightly overestimated some of the associations. However, we found that exclusion of studies with a smaller number cases or deaths or exclusion of outlying studies attenuated the tests for publication bias, but in most cases did not substantially alter the summary estimates.
Strengths of this analysis include the wide search terms used, the large number of studies included from various geographical locations and the large number of cases and deaths (up to 43 000 coronary heart disease cases, 47 000 stroke cases, 81 000 cardiovascular disease cases, 112 000 cancer cases and >71 000–94 000 deaths among up to 2.1 million participants) which provided increased statistical power to detect significant associations, the robustness of the findings in comprehensive subgroup and sensitivity analyses, the high study quality of the included studies and the detailed dose-response analyses which allowed us to clarify the strength and shape of the dose-response relationship between fruit and vegetable intake and these outcomes. In addition, similar associations were observed when analyses were stratified by geographical regions which have different underlying patterns of diet and other confounding factors, suggesting that unknown confounders are not likely to entirely explain the associations observed. Our meta-analysis provides further support for public health recommendations, interventions, and policies to promote a high fruit and vegetable intake to reduce the risk of cardiovascular disease, cancer and premature mortality. Improving the availability and affordability of fruits and vegetables, particularly in low- and middle-income countries, might be important for increasing fruit and vegetable intake globally.191 Any further studies should try to further define the dose-response relationship at more extreme levels of intake and report more detailed results for subtypes of fruits and vegetables in relation to these outcomes. Further studies in other geographical locations and of other less common causes of death, and incorporating biomarkers of fruit and vegetable intake, are also urgently needed. This will allow us to conduct updated and more refined estimations of the mortality burden due to an inadequate fruit and vegetable intake worldwide in the future.
In conclusion, we found an inverse association between intake of fruits, vegetables, and fruit and vegetables combined, and the risk of coronary heart disease, stroke, cardiovascular disease, total cancer and all-cause mortality. In most of the analyses the reductions in risk were steeper at the lower range of intake. For total cancer the lowest risk was observed at an intake of 600 g/day (7.5 servings/day), whereas for coronary heart disease, stroke, cardiovascular disease and all-cause mortality the lowest risk was observed at 800 g/day (10 servings/day), a level of intake that is double the five servings per day (400 g/day) currently recommended by the World Cancer Research Fund, the WHO, and in England.5 In 2013, an estimated 1 340 000 coronary heart disease deaths, 2 680 000 stroke deaths, 660 000 cancer deaths and 7.8 million premature deaths were attributable to a fruit and vegetable intake below 800 g/day globally. A change in the diet towards a higher intake of fruit and vegetables and other plant foods could also have other important health168–175,192,193 as well as environmental benefits.194 Our meta-analysis provides further support for public health recommendations and interventions to increase fruit and vegetable intake for prevention of cardiovascular disease, cancer and premature mortality.
Supplementary Data
Supplementary data are available at IJE online.
Funding
This project was funded by Olav og Gerd Meidel Raagholt’s Stiftelse for Medisinsk Forskning, the Liaison Committee between the Central Norway Regional Health Authority (RHA) and the Norwegian University of Science and Technology (NTNU), and the Imperial College National Institute of Health Research (NIHR) Biomedical Research Centre (BRC). The funding sources had no role in the design or conduct of the study, collection, management, analysis or interpretation of the data; preparation, review or approval of the manuscript; or decision to submit the manuscript for publication.
Supplementary Material
Acknowledgements
We thank: Max Leenders (PhD, Department of Gastroenterology and Hepatology, University Medical Centre Utrecht, Utrecht, The Netherlands) for providing supplementary data from the EPIC study; Anette Hjartåker (Professor, Department of Nutrition, University of Oslo, Oslo, Norway) and Markus Knudsen (PhD, Department of Nutrition, University of Oslo, Oslo, Norway) for providing supplementary data from the Migrant Study; Hannah Gardener (PhD, Department of Neurology, Miller School of Medicine, University of Miami, Miami, FL, USA) for providing supplementary information from the Northern Manhattan Study; Jonathan Hodgson (Professor, School of Medicine and Pharmacology, University of Western Australia, Perth, WA, Australia) for providing supplementary information from the Calcium Intake Fracture Outcome Study; and Estefania Toledo (PhD, the PREDIMED Research Network, Madrid, Spain) and Pilar Buil-Cosiales (PhD, the PREDIMED Research Network, Madrid, Spain) for providing supplementary information from the PREDIMED study.
Author Contributions
D.A. had full access to all of the data and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: D.A., E.G., P.B., S.T. Acquisition, analysis or interpretation of data: D.A., E.G., P.B., N.K., L.T.F., T.N., D.C.G., E.R., L.J.V., S.T. Drafting of manuscript: D.A. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: D.A., D.C.G.. Obtained funding: D.A., E.G., P.B., E.R., L.J.V., S.T. Study supervision: S.T.
Conflict of interest: All the authors declare no conflict of interest.
References
- 1. GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385:117–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Cancer Research Fund/American Insitute for Cancer Research. Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective. Washington, DC: AICR, 2007. [Google Scholar]
- 3. He FJ, Nowson CA, Lucas M, MacGregor GA. Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: meta-analysis of cohort studies. J Hum Hypertens 2007;21:717–28. [DOI] [PubMed] [Google Scholar]
- 4. He FJ, Nowson CA, MacGregor GA. Fruit and vegetable consumption and stroke: meta-analysis of cohort studies. Lancet 2006;367:320–26. [DOI] [PubMed] [Google Scholar]
- 5. Nasjonalt råd for Ernæring. Kostråd for å fremme folkehelsen og forebygge kroniske sykdommer. Metodologi og vitenskapelig kunnskapsgrunnlag. Nasjonalt råd for Ernæring. 2011. (In English: National Nutrition Council. Dietary recommendations to improve public health and prevent chronic diseases. Methodology and scientific basis. National Nutrition Council 2011). https://helsedirektoratet.no/publikasjoner/kostrad-for-a-fremme-folkehelsen-og-forebygge-kroniske-sykdommer-metodologi-og-vitenskapelig-kunnskapsgrunnlag (8 December 2016, date last accessed). [Google Scholar]
- 6. World Cancer Research Fund / American Institute of Cancer Research. Food, Nutrition And The Prevention Of Cancer: A Global Perspective. London: WCRF/AICR, 1997. [Google Scholar]
- 7. Boffetta P, Couto E, Wichmann J. et al. Fruit and vegetable intake and overall cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC). J Natl Cancer Inst 2010;102:529–37. [DOI] [PubMed] [Google Scholar]
- 8. Shibata A, Paganini-Hill A, Ross RK, Henderson BE. Intake of vegetables, fruits, beta-carotene, vitamin C and vitamin supplements and cancer incidence among the elderly: a prospective study. Br J Cancer 1992;66:673–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sahyoun NR, Jacques PF, Russell RM. Carotenoids, vitamins C and E, and mortality in an elderly population. Am J Epidemiol 1996;144:501–11. [DOI] [PubMed] [Google Scholar]
- 10. Whiteman D, Muir J, Jones L, Murphy M, Key T. Dietary questions as determinants of mortality: the OXCHECK experience. Public Health Nutr 1999;2:477–87. [DOI] [PubMed] [Google Scholar]
- 11. Appleby PN, Key TJ, Burr ML, Thorogood M. Mortality and fresh fruit consumption. IARC Sci Pub 2002;156:131–33. [PubMed] [Google Scholar]
- 12. Sauvaget C, Nagano J, Hayashi M, Spencer E, Shimizu Y, Allen N. Vegetables and fruit intake and cancer mortality in the Hiroshima/Nagasaki Life Span Study. Br J Cancer 2003;88:689–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jansen MC, Bueno-de-Mesquita HB, Feskens EJ, Streppel MT, Kok FJ, Kromhout D. Quantity and variety of fruit and vegetable consumption and cancer risk. Nutr Cancer 2004;48:142–48. [DOI] [PubMed] [Google Scholar]
- 14. Khan MM, Goto R, Kobayashi K. et al. Dietary habits and cancer mortality among middle aged and older Japanese living in Hokkaido, Japan by cancer site and sex. Asian Pac J Cancer Prev 2004;5:58–65. [PubMed] [Google Scholar]
- 15. Hung HC, Joshipura KJ, Jiang R. et al. Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst 2004;96:1577–84. [DOI] [PubMed] [Google Scholar]
- 16. Takachi R, Inoue M, Ishihara J. et al. Fruit and vegetable intake and risk of total cancer and cardiovascular disease: Japan Public Health Centre-Based Prospective Study. Am J Epidemiol 2008;167:59–70. [DOI] [PubMed] [Google Scholar]
- 17. George SM, Park Y, Leitzmann MF. et al. Fruit and vegetable intake and risk of cancer: a prospective cohort study. Am J Clin Nutr 2009;89:347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nechuta SJ, Shu XO, Li HL. et al. Combined impact of lifestyle-related factors on total and cause-specific mortality among Chinese women: prospective cohort study. PLoS Med 2010;7:e1000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang X, Shu XO, Xiang YB. et al. Cruciferous vegetable consumption is associated with a reduced risk of total and cardiovascular disease mortality. Am J Clin Nutr 2011;94:240–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lof M, Sandin S, Lagiou P, Trichopoulos D, Adami HO, Weiderpass E. Fruit and vegetable intake and risk of cancer in the Swedish women’s lifestyle and health cohort. Cancer Causes Control 2011;22:283–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sharma S, Vik S, Pakseresht M, Shen L, Kolonel LN. Diet impacts mortality from cancer: results from the multiethnic cohort study. Cancer Causes Control 2013;24:685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Knekt P, Reunanen A, Jarvinen R, Seppanen R, Heliovaara M, Aromaa A. Antioxidant vitamin intake and coronary mortality in a longitudinal population study. Am J Epidemiol 1994;139:1180–89. [DOI] [PubMed] [Google Scholar]
- 23. Crowe FL, Roddam AW, Key TJ. et al. Fruit and vegetable intake and mortality from ischaemic heart disease: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Heart study. Eur Heart J 2011;32:1235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakamura K, Nagata C, Oba S, Takatsuka N, Shimizu H. Fruit and vegetable intake and mortality from cardiovascular disease are inversely associated in Japanese women but not in men. J Nutr 2008;138:1129–34. [DOI] [PubMed] [Google Scholar]
- 25. Larsson SC, Mannisto S, Virtanen MJ, Kontto J, Albanes D, Virtamo J. Dietary fibre and fibre-rich food intake in relation to risk of stroke in male smokers. Eur J Clin Nutr 2009;63:1016–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mizrahi A, Knekt P, Montonen J, Laaksonen MA, Heliovaara M, Jarvinen R. Plant foods and the risk of cerebrovascular diseases: a potential protection of fruit consumption. Br J Nutr 2009;102:1075–83. [DOI] [PubMed] [Google Scholar]
- 27. Nagura J, Iso H, Watanabe Y. et al. Fruit, vegetable and bean intake and mortality from cardiovascular disease among Japanese men and women: the JACC Study. Br J Nutr 2009;102:285–92. [DOI] [PubMed] [Google Scholar]
- 28. Oude Griep LM, Geleijnse JM, Kromhout D, Ocke MC, Verschuren WM. Raw and processed fruit and vegetable consumption and 10-year coronary heart disease incidence in a population-based cohort study in the Netherlands. PLoS One 2010;5:e13609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kobylecki CJ, Afzal S, Davey SG, Nordestgaard BG. Genetically high plasma vitamin C, intake of fruit and vegetables, and risk of ischemic heart disease and all-cause mortality: a Mendelian randomization study. Am J Clin Nutr 2015;101:1135–43. [DOI] [PubMed] [Google Scholar]
- 30. Fraser GE, Sumbureru D, Pribis P, Neil RL, Frankson MA. Association among health habits, risk factors, and all-cause mortality in a black California population. Epidemiology 1997;8:168–74. [DOI] [PubMed] [Google Scholar]
- 31. Bazzano LA, He J, Ogden LG. et al. Fruit and vegetable intake and risk of cardiovascular disease in US adults: the first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am J Clin Nutr 2002;76:93–99. [DOI] [PubMed] [Google Scholar]
- 32. Steffen LM, Jacobs DR Jr, Stevens J, Shahar E, Carithers T, Folsom AR. Associations of whole-grain, refined-grain, and fruit and vegetable consumption with risks of all-cause mortality and incident coronary artery disease and ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr 2003;78:383–90. [DOI] [PubMed] [Google Scholar]
- 33. Seccareccia F, Alberti-Fidanza A, Fidanza F. et al. Vegetable intake and long-term survival among middle-aged men in Italy. Ann Epidemiol 2003;13:424–30. [DOI] [PubMed] [Google Scholar]
- 34. Knekt P, Jarvinen R, Reunanen A, Maatela J. Flavonoid intake and coronary mortality in Finland: a cohort study. BMJ 1996;312:478–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Osler M, Schroll M. Diet and mortality in a cohort of elderly people in a North European community. Int J Epidemiol 1997;26:155–59. [DOI] [PubMed] [Google Scholar]
- 36. Mann JI, Appleby PN, Key TJ, Thorogood M. Dietary determinants of ischaemic heart disease in health conscious individuals. Heart 1997;78:450–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fraser GE, Shavlik DJ. Risk factors for all-cause and coronary heart disease mortality in the oldest-old. The Adventist Health Study. Arch Intern Med 1997;157:2249–58. [PubMed] [Google Scholar]
- 38. Fortes C, Forastiere F, Farchi S, Rapiti E, Pastori G, Perucci CA. Diet and overall survival in a cohort of very elderly people. Epidemiology 2000;11:440–45. [DOI] [PubMed] [Google Scholar]
- 39. Rissanen TH, Voutilainen S, Virtanen JK. et al. Low intake of fruits, berries and vegetables is associated with excess mortality in men: the Kuopio Ischaemic Heart Disease Risk Factor (KIHD) Study. J Nutr 2003;133:199–204. [DOI] [PubMed] [Google Scholar]
- 40. van den Brandt PA. The impact of a Mediterranean diet and healthy lifestyle on premature mortality in men and women. Am J Clin Nutr 2011;93:913–20. [DOI] [PubMed] [Google Scholar]
- 41. Wang X, Ouyang Y, Liu J. et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014;349:g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Elwood P, Galante J, Pickering J. et al. Healthy lifestyles reduce the incidence of chronic diseases and dementia: evidence from the Caerphilly Cohort Study. PLoS One 2013;8:e81877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kahn HA, Phillips RL, Snowdon DA, Choi W. Association between reported diet and all-cause mortality. Twenty-one-year follow-up on 27,530 adult Seventh-Day Adventists. Am J Epidemiol 1984;119:775–87. [DOI] [PubMed] [Google Scholar]
- 44. Rotevatn S, Akslen LA, Bjelke E. Lifestyle and mortality among Norwegian men. Prev Med 1989;18:433–43. [DOI] [PubMed] [Google Scholar]
- 45. Trichopoulou A, Kouris-Blazos A, Wahlqvist ML. et al. Diet and overall survival in elderly people. BMJ 1995;311:1457–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hays JC, Keller HH, Ostbye T. The effects of nutrition-related factors on four-year mortality among a biracial sample of community-dwelling elders in the North Carolina Piedmont. J Nutr Elder 2005;25:41–67. [DOI] [PubMed] [Google Scholar]
- 47. Knoops KT, Groot de LC, Fidanza F, Alberti-Fidanza A, Kromhout D, van Staveren WA. Comparison of three different dietary scores in relation to 10-year mortality in elderly European subjects: the HALE project. Eur J Clin Nutr 2006;60:746–55. [DOI] [PubMed] [Google Scholar]
- 48. Bazelmans C, De Henauw S, Matthys C. et al. Healthy food and nutrient index and all cause mortality. Eur J Epidemiol 2006;21:145–52. [DOI] [PubMed] [Google Scholar]
- 49. Kvaavik E, Batty GD, Ursin G, Huxley R, Gale CR. Influence of individual and combined health behaviors on total and cause-specific mortality in men and women: the United Kingdom health and lifestyle survey. Arch Intern Med 2010;170:711–18. [DOI] [PubMed] [Google Scholar]
- 50. Matheson EM, King DE, Everett CJ. Healthy lifestyle habits and mortality in overweight and obese individuals. J Am Board Fam Med 2012;25:9–15. [DOI] [PubMed] [Google Scholar]
- 51. Martinez-Gonzalez MA, Guillen-Grima F, De Irala J. et al. The Mediterranean diet is associated with a reduction in premature mortality among middle-aged adults. J Nutr 2012;142:1672–78. [DOI] [PubMed] [Google Scholar]
- 52. Regidor E, Franch J, Segui M, Serrano R, Rodriguez-Artalejo F, Artola S. Traditional risk factors alone could not explain the excess mortality in patients with diabetes: a national cohort study of older Spanish adults. Diabetes Care 2012;35:2503–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Oyebode O, Gordon-Dseagu V, Walker A, Mindell JS. Fruit and vegetable consumption and all-cause, cancer and CVD mortality: analysis of Health Survey for England data. J Epidemiol Community Health 2014;68:856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vormund K, Braun J, Rohrmann S, Bopp M, Ballmer P, Faeh D. Mediterranean diet and mortality in Switzerland: an alpine paradox?. Eur J Nutr 2015;54:139–48. [DOI] [PubMed] [Google Scholar]
- 55. Tognon G, Lissner L, Saebye D, Walker KZ, Heitmann BL. The Mediterranean diet in relation to mortality and CVD: a Danish cohort study. Br J Nutr 2014;111:151–59. [DOI] [PubMed] [Google Scholar]
- 56. Hjartaker A, Knudsen MD, Tretli S, Weiderpass E. Consumption of berries, fruits and vegetables and mortality among 10,000 Norwegian men followed for four decades. Eur J Nutr 2015;54:599–608. [DOI] [PubMed] [Google Scholar]
- 57. Buil-Cosiales P, Zazpe I, Toledo E. et al. Fibre intake and all-cause mortality in the Prevencion con Dieta Mediterranea (PREDIMED) study. Am J Clin Nutr 2014;100:1498–507. [DOI] [PubMed] [Google Scholar]
- 58. Stefler D, Pikhart H, Kubinova R. et al. Fruit and vegetable consumption and mortality in Eastern Europe: Longitudinal results from the Health, Alcohol and Psychosocial Factors in Eastern Europe study. Eur J Prev Cardiol 2016;23:493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Choi Y, Lee JE, Bae JM. et al. Vegetable intake, but not fruit intake, was associated with a reduction in the risk of cancer incidence and mortality in middle-aged Korean men. J Nutr 2015;145:1249–55. [DOI] [PubMed] [Google Scholar]
- 60. Lai HT, Threapleton DE, Day AJ, Williamson G, Cade JE, Burley VJ. Fruit intake and cardiovascular disease mortality in the UK Women’s Cohort Study. Eur J Epidemiol 2015;30:1035–48. [DOI] [PubMed] [Google Scholar]
- 61. Hodgson JM, Prince RL, Woodman RJ. et al. Apple intake is inversely associated with all-cause and disease-specific mortality in elderly women. Br J Nutr 2016;115:860–67. [DOI] [PubMed] [Google Scholar]
- 62. Wang JB, Fan JH, Dawsey SM. et al. Dietary components and risk of total, cancer and cardiovascular disease mortality in the Linxian Nutrition Intervention Trials cohort in China. Sci Rep 2016;6:22619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Du H, Li L, Bennett D. et al. Fresh Fruit consumption and major cardiovascular disease in China. N Engl J Med 2016;374:1332–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Buil-Cosiales P, Toledo E, Salas-Salvado J. et al. Association between dietary fibre intake and fruit, vegetable or whole-grain consumption and the risk of CVD: results from the PREvencion con DIeta MEDiterranea (PREDIMED) trial. Br J Nutr 2016;116:534–46. [DOI] [PubMed] [Google Scholar]
- 65. Orenstein L, Chetrit A, Dankner R. Healthy lifestyle pattern is protective against 30-yr cancer incidence in men and women: a cohort study. Nutr Cancer 2016;68:410–19. [DOI] [PubMed] [Google Scholar]
- 66. Shi Z, Zhang T, Byles J, Martin S, Avery JC, Taylor AW. Food habits, lifestyle factors and mortality among oldest old Chinese: The Chinese Longitudinal Healthy Longevity Survey (CLHLS). Nutrients 2015;7:7562–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bongard V, Arveiler D, Dallongeville J. et al. Food groups associated with a reduced risk of 15-year all-cause death. Eur J Clin Nutr 2016;70:715–22. [DOI] [PubMed] [Google Scholar]
- 68. Nguyen B, Bauman A, Gale J, Banks E, Kritharides L, Ding D. Fruit and vegetable consumption and all-cause mortality: evidence from a large Australian cohort study. Int J Behav Nutr Phys Act 2016;13:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 71. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301–09. [DOI] [PubMed] [Google Scholar]
- 72. Riboli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am J Clin Nutr 2003;78(Suppl 3):559–69S. [DOI] [PubMed] [Google Scholar]
- 73. Aune D, Lau R, Chan DS. et al. Nonlinear reduction in risk for colorectal cancer by fruit and vegetable intake based on meta-analysis of prospective studies. Gastroenterology 2011;141:106–18. [DOI] [PubMed] [Google Scholar]
- 74. Lee JE, Mannisto S, Spiegelman D. et al. Intakes of fruit, vegetables, and carotenoids and renal cell cancer risk: a pooled analysis of 13 prospective studies. Cancer Epidemiol Biomarkers Prev 2009;18:1730–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Leenders M, Sluijs I, Ros MM. et al. Fruit and vegetable consumption and mortality: European Prospective Investigation Into Cancer and Nutrition. Am J Epidemiol 2013;178:590–602. [DOI] [PubMed] [Google Scholar]
- 76. Gardener H, Wright CB, Gu Y. et al. Mediterranean-style diet and risk of ischemic stroke, myocardial infarction, and vascular death: the Northern Manhattan Study. Am J Clin Nutr 2011;94:1458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jackson D, White IR, Thompson SG. Extending DerSimonian and Laird’s methodology to perform multivariate random effects meta-analyses. Stat Med 2010;29:1282–97. [DOI] [PubMed] [Google Scholar]
- 78. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 2012;175:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bagnardi V, Zambon A, Quatto P, Corrao G. Flexible meta-regression functions for modeling aggregate dose-response data, with an application to alcohol and mortality. Am J Epidemiol 2004;159:1077–86. [DOI] [PubMed] [Google Scholar]
- 80. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 81. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wells G, Shea B, O’Connell D. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Department of Epidemiology and Community Medicine, University of Ottawa http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (8 December 2016, date last accessed). [Google Scholar]
- 83. Pomerleau J, Lock K, McKee M, Altmann DR. The challenge of measuring global fruit and vegetable intake. J Nutr 2004;134:1175–80. [DOI] [PubMed] [Google Scholar]
- 84. Lock K, Pomerleau J, Causer L, Altmann DR, McKee M. The global burden of disease attributable to low consumption of fruit and vegetables: implications for the global strategy on diet. Bull World Health Organ 2005;83:100–08. [PMC free article] [PubMed] [Google Scholar]
- 85. Miettinen OS. Proportion of disease caused or prevented by a given exposure, trait or intervention. Am J Epidemiol 1974;99:325–32. [DOI] [PubMed] [Google Scholar]
- 86. Liu S, Manson JE, Lee IM. et al. Fruit and vegetable intake and risk of cardiovascular disease: the Women’s Health Study. Am J Clin Nutr 2000;72:922–28. [DOI] [PubMed] [Google Scholar]
- 87. Genkinger JM, Platz EA, Hoffman SC, Comstock GW, Helzlsouer KJ. Fruit, vegetable, and antioxidant intake and all-cause, cancer, and cardiovascular disease mortality in a community-dwelling population in Washington County, Maryland. Am J Epidemiol 2004;160:1223–33. [DOI] [PubMed] [Google Scholar]
- 88. Dauchet L, Montaye M, Ruidavets JB. et al. Association between the frequency of fruit and vegetable consumption and cardiovascular disease in male smokers and non-smokers. Eur J Clin Nutr 2010;64:578–86. [DOI] [PubMed] [Google Scholar]
- 89. Oude Griep LM, Monique Verschuren WM, Kromhout D, Ocke MC, Geleijnse JM. Colours of fruit and vegetables and 10-year incidence of CHD. Br J Nutr 2011;106:1562–69. [DOI] [PubMed] [Google Scholar]
- 90. Rautiainen S, Levitan EB, Orsini N. et al. Total antioxidant capacity from diet and risk of myocardial infarction: a prospective cohort of women. Am J Med 2012;125:974–80. [DOI] [PubMed] [Google Scholar]
- 91. Bhupathiraju SN, Wedick NM, Pan A. et al. Quantity and variety in fruit and vegetable intake and risk of coronary heart disease. Am J Clin Nutr 2013;98:1514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Simila ME, Kontto JP, Mannisto S, Valsta LM, Virtamo J. Glycaemic index, carbohydrate substitution for fat and risk of CHD in men. Br J Nutr 2013;110:1704–11. [DOI] [PubMed] [Google Scholar]
- 93. Gunnell AS, Einarsdottir K, Galvao DA. et al. Lifestyle factors, medication use and risk for ischaemic heart disease hospitalisation: a longitudinal population-based study. PLoS One 2013;8:e77833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yu D, Zhang X, Gao YT. et al. Fruit and vegetable intake and risk of CHD: results from prospective cohort studies of Chinese adults in Shanghai. Br J Nutr 2014;111:353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sharma S, Vik S, Kolonel LN. Fruit and vegetable consumption, ethnicity and risk of fatal ischemic heart disease. J Nutr Health Aging 2014;18:573–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Eriksen A, Tillin T, O’Connor L. et al. The impact of health behaviours on incident cardiovascular disease in Europeans and South Asians – a prospective analysis in the UK SABRE study. PLoS One 2015;10:e0117364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dauchet L, Ferrieres J, Arveiler D. et al. Frequency of fruit and vegetable consumption and coronary heart disease in France and Northern Ireland: the PRIME study. Br J Nutr 2004;92:963–72. [DOI] [PubMed] [Google Scholar]
- 98. Atkins JL, Whincup PH, Morris RW, Lennon LT, Papacosta O, Wannamethee SG. High diet quality is associated with a lower risk of cardiovascular disease and all-cause mortality in older men. J Nutr 2014;144:673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Fraser GE, Sabate J, Beeson WL, Strahan TM. A possible protective effect of nut consumption on risk of coronary heart disease. The Adventist Health Study. Arch Intern Med 1992;152:1416–24. [PubMed] [Google Scholar]
- 100. Pietinen P, Rimm EB, Korhonen P. et al. Intake of dietary fibre and risk of coronary heart disease in a cohort of Finnish men. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Circulation 1996;9:2720–27. [DOI] [PubMed] [Google Scholar]
- 101. Watkins ML, Erickson JD, Thun MJ, Mulinare J, Heath CW Jr. Multivitamin use and mortality in a large prospective study. Am J Epidemiol 2000;152:149–62. [DOI] [PubMed] [Google Scholar]
- 102. Hirvonen T, Pietinen P, Virtanen M. et al. Intake of flavonols and flavones and risk of coronary heart disease in male smokers. Epidemiology 2001;12:62–67. [DOI] [PubMed] [Google Scholar]
- 103. Liu S, Lee IM, Ajani U, Cole SR, Buring JE, Manson JE. Intake of vegetables rich in carotenoids and risk of coronary heart disease in men: The Physicians’ Health Study. Int J Epidemiol 2001;30:130–35. [DOI] [PubMed] [Google Scholar]
- 104. Rebello SA, Koh H, Chen C. et al. Amount, type, and sources of carbohydrates in relation to ischemic heart disease mortality in a Chinese population: a prospective cohort study. Am J Clin Nutr 2014;100:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hertog MG, Sweetnam PM, Fehily AM, Elwood PC, Kromhout D. Antioxidant flavonols and ischemic heart disease in a Welsh population of men: the Caerphilly Study. Am J Clin Nutr 1997;65:1489–94. [DOI] [PubMed] [Google Scholar]
- 106. Dilis V, Katsoulis M, Lagiou P, Trichopoulos D, Naska A, Trichopoulou A. Mediterranean diet and CHD: the Greek European Prospective Investigation into Cancer and Nutrition cohort. Br J Nutr 2012;108:699–709. [DOI] [PubMed] [Google Scholar]
- 107. Oude Griep LM, Verschuren WM, Kromhout D, Ocke MC, Geleijnse JM. Raw and processed fruit and vegetable consumption and 10-year stroke incidence in a population-based cohort study in the Netherlands. Eur J Clin Nutr 2011;65:791–99. [DOI] [PubMed] [Google Scholar]
- 108. Gillman MW, Cupples LA, Gagnon D. et al. Protective effect of fruits and vegetables on development of stroke in men. JAMA 1995;273:1113–17. [DOI] [PubMed] [Google Scholar]
- 109. Larsson SC, Virtamo J, Wolk A. Total and specific fruit and vegetable consumption and risk of stroke: a prospective study. Atherosclerosis 2013;227:147–52. [DOI] [PubMed] [Google Scholar]
- 110. Joshipura KJ, Ascherio A, Manson JE. et al. Fruit and vegetable intake in relation to risk of ischemic stroke. JAMA 1999;282:1233–39. [DOI] [PubMed] [Google Scholar]
- 111. Johnsen SP, Overvad K, Stripp C, Tjonneland A, Husted SE, Sorensen HT. Intake of fruit and vegetables and the risk of ischemic stroke in a cohort of Danish men and women. Am J Clin Nutr 2003;78:57–64. [DOI] [PubMed] [Google Scholar]
- 112. Keli SO, Hertog MG, Feskens EJ, Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch Intern Med 1996;156:637–42. [PubMed] [Google Scholar]
- 113. Yokoyama T, Date C, Kokubo Y, Yoshiike N, Matsumura Y, Tanaka H. Serum vitamin C concentration was inversely associated with subsequent 20-year incidence of stroke in a Japanese rural community. The Shibata study. Stroke 2000;31:2287–94. [DOI] [PubMed] [Google Scholar]
- 114. Sauvaget C, Nagano J, Allen N, Kodama K. Vegetable and fruit intake and stroke mortality in the Hiroshima/Nagasaki Life Span Study. Stroke 2003;34:2355–60. [DOI] [PubMed] [Google Scholar]
- 115. Pham TM, Fujino Y, Tokui N. et al. Mortality and risk factors for stroke and its subtypes in a cohort study in Japan. Prev Med 2007;44:526–30. [DOI] [PubMed] [Google Scholar]
- 116. Zhang Y, Tuomilehto J, Jousilahti P, Wang Y, Antikainen R, Hu G. Lifestyle factors on the risks of ischemic and hemorrhagic stroke. Arch Intern Med 2011;171:1811–18. [DOI] [PubMed] [Google Scholar]
- 117. Sharma S, Cruickshank JK, Green DM, Vik S, Tome A, Kolonel LN. Impact of diet on mortality from stroke: results from the U.S. multiethnic cohort study. J Am Coll Nutr 2013;32:151–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bos MJ, Koudstaal PJ, Hofman A, Ikram MA. Modifiable etiological factors and the burden of stroke from the Rotterdam study: a population-based cohort study. PLoS Med 2014;11:e1001634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Qiu D, Mei J, Tanihata T, Kawaminami K, Minowa M. A cohort study on cerebrovascular disease in middle-aged and elderly population in rural areas in Jiangxi Province, China. J Epidemiol 2003;13:149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Oude Griep LM, Verschuren WM, Kromhout D, Ocke MC, Geleijnse JM. Colors of fruit and vegetables and 10-year incidence of stroke. Stroke 2011;42:3190–95. [DOI] [PubMed] [Google Scholar]
- 121. Cassidy A, Rimm EB, O’Reilly EJ. et al. Dietary flavonoids and risk of stroke in women. Stroke 2012;43:946–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Manuel DG, Tuna M, Perez R. et al. Predicting stroke risk based on health behaviours: development of the Stroke Population Risk Tool (SPoRT). PLoS One 2015;10:e0143342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Buijsse B, Feskens EJ, Kwape L, Kok FJ, Kromhout D. Both alpha- and beta-carotene, but not tocopherols and vitamin C, are inversely related to 15-year cardiovascular mortality in Dutch elderly men. J Nutr 2008;138:344–50. [DOI] [PubMed] [Google Scholar]
- 124. Joshipura KJ, Hung HC, Li TY. et al. Intakes of fruits, vegetables and carbohydrate and the risk of CVD. Public Health Nutr 2009;12:115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Belin RJ, Greenland P, Allison M. et al. Diet quality and the risk of cardiovascular disease: the Women’s Health Initiative (WHI). Am J Clin Nutr 2011;94:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Misirli G, Benetou V, Lagiou P, Bamia C, Trichopoulos D, Trichopoulou A. Relation of the traditional Mediterranean diet to cerebrovascular disease in a Mediterranean population. Am J Epidemiol 2012;176:1185–92. [DOI] [PubMed] [Google Scholar]
- 127. Fitzgerald KC, Chiuve SE, Buring JE, Ridker PM, Glynn RJ. Comparison of associations of adherence to a Dietary Approaches to Stop Hypertension (DASH)-style diet with risks of cardiovascular disease and venous thromboembolism. J Thromb Haemost 2012;10:189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Wie GA, Cho YA, Kang HH. et al. Red meat consumption is associated with an increased overall cancer risk: a prospective cohort study in Korea. Br J Nutr 2014;112:238–47. [DOI] [PubMed] [Google Scholar]
- 129. Jacques PF, Cassidy A, Rogers G, Peterson JJ, Dwyer JT. Dietary flavonoid intakes and CVD incidence in the Framingham Offspring Cohort. Br J Nutr 2015;114:1496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Wang L, Lee IM, Zhang SM, Blumberg JB, Buring JE, Sesso HD. Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle-aged and older women. Am J Clin Nutr 2009;89:905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kouris-Blazos A, Gnardellis C, Wahlqvist ML, Trichopoulos D, Lukito W, Trichopoulou A. Are the advantages of the Mediterranean diet transferable to other populations? A cohort study in Melbourne, Australia. Br J Nutr 1999;82:57–61. [DOI] [PubMed] [Google Scholar]
- 132. Tucker KL, Hallfrisch J, Qiao N, Muller D, Andres R, Fleg JL. The combination of high fruit and vegetable and low saturated fat intakes is more protective against mortality in aging men than is either alone: the Baltimore Longitudinal Study of Aging. J Nutr 2005;135:556–61. [DOI] [PubMed] [Google Scholar]
- 133. Gonzalez S, Huerta JM, Fernandez S, Patterson AM, Lasheras C. Differences in overall mortality in the elderly may be explained by diet. Gerontology 2008;54:232–37. [DOI] [PubMed] [Google Scholar]
- 134. Bellavia A, Larsson SC, Bottai M, Wolk A, Orsini N. Fruit and vegetable consumption and all-cause mortality: a dose-response analysis. Am J Clin Nutr 2013;98:454–59. [DOI] [PubMed] [Google Scholar]
- 135. Strandhagen E, Hansson PO, Bosaeus I, Isaksson B, Eriksson H. High fruit intake may reduce mortality among middle-aged and elderly men. The Study of Men Born in 1913. Eur J Clin Nutr 2000;54:337–41. [DOI] [PubMed] [Google Scholar]
- 136. Tognon G, Rothenberg E, Eiben G, Sundh V, Winkvist A, Lissner L. Does the Mediterranean diet predict longevity in the elderly? A Swedish perspective. Age (Dordr) 2011;33:439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Prinelli F, Yannakoulia M, Anastasiou CA. et al. Mediterranean diet and other lifestyle factors in relation to 20-year all-cause mortality: a cohort study in an Italian population. Br J Nutr 2015;113:1003–11. [DOI] [PubMed] [Google Scholar]
- 138. Roswall N, Sandin S, Lof M. et al. Adherence to the healthy Nordic food index and total and cause-specific mortality among Swedish women. Eur J Epidemiol 2015;30:509–17. [DOI] [PubMed] [Google Scholar]
- 139. Boggs DA, Ban Y, Palmer JR, Rosenberg L. Higher diet quality is inversely associated with mortality in African-American women. J Nutr 2015;145:547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Joshipura KJ, Hu FB, Manson JE. et al. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med 2001;134:1106–14. [DOI] [PubMed] [Google Scholar]
- 141. Sesso HD, Liu S, Gaziano JM, Buring JE. Dietary lycopene, tomato-based food products and cardiovascular disease in women. J Nutr 2003;133:2336–41. [DOI] [PubMed] [Google Scholar]
- 142. Mink PJ, Scrafford CG, Barraj LM. et al. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr 2007;85:895–909. [DOI] [PubMed] [Google Scholar]
- 143. Iso H, Kubota Y. Nutrition and disease in the Japan Collaborative Cohort Study for Evaluation of Cancer (JACC). Asian Pac J Cancer Prev 2007;(Suppl 8):35–80. [PubMed] [Google Scholar]
- 144. Sesso HD, Gaziano JM, Jenkins DJ, Buring JE. Strawberry intake, lipids, C-reactive protein, and the risk of cardiovascular disease in women. J Am Coll Nutr 2007;26:303–10. [DOI] [PubMed] [Google Scholar]
- 145. Lin J, Rexrode KM, Hu F. et al. Dietary intakes of flavonols and flavones and coronary heart disease in US women. Am J Epidemiol 2007;165:1305–13. [DOI] [PubMed] [Google Scholar]
- 146. Yamada T, Hayasaka S, Shibata Y. et al. Frequency of citrus fruit intake is associated with the incidence of cardiovascular disease: the Jichi Medical School cohort study. J Epidemiol 2011;21:169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Bendinelli B, Masala G, Saieva C. et al. Fruit, vegetables, and olive oil and risk of coronary heart disease in Italian women: the EPICOR Study. Am J Clin Nutr 2011;93:275–83. [DOI] [PubMed] [Google Scholar]
- 148. Jacques PF, Lyass A, Massaro JM, Vasan RS, D’Agostino RB Sr. Relationship of lycopene intake and consumption of tomato products to incident CVD. Br J Nutr 2013;110:545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Knekt P, Isotupa S, Rissanen H. et al. Quercetin intake and the incidence of cerebrovascular disease. Eur J Clin Nutr 2000;54:415–17. [DOI] [PubMed] [Google Scholar]
- 150. Hirvonen T, Virtamo J, Korhonen P, Albanes D, Pietinen P. Intake of flavonoids, carotenoids, vitamins C and E, and risk of stroke in male smokers. Stroke 2000;31:2301–06. [DOI] [PubMed] [Google Scholar]
- 151. Gaziano JM, Manson JE, Branch LG, Colditz GA, Willett WC, Buring JE. A prospective study of consumption of carotenoids in fruits and vegetables and decreased cardiovascular mortality in the elderly. Ann Epidemiol 1995;5:255–60. [DOI] [PubMed] [Google Scholar]
- 152. Sesso HD, Gaziano JM, Liu S, Buring JE. Flavonoid intake and the risk of cardiovascular disease in women. Am J Clin Nutr 2003;77:1400–08. [DOI] [PubMed] [Google Scholar]
- 153. Colditz GA, Branch LG, Lipnick RJ. et al. Increased green and yellow vegetable intake and lowered cancer deaths in an elderly population. Am J Clin Nutr 1985;41:32–36. [DOI] [PubMed] [Google Scholar]
- 154. von Ruesten A, Feller S, Bergmann MM, Boeing H. Diet and risk of chronic diseases: results from the first 8 years of follow-up in the EPIC-Potsdam study. Eur J Clin Nutr 2013;67:412–19. [DOI] [PubMed] [Google Scholar]
- 155. Cutler GJ, Nettleton JA, Ross JA. et al. Dietary flavonoid intake and risk of cancer in postmenopausal women: the Iowa Women’s Health Study. Int J Cancer 2008;123:664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Li WQ, Kuriyama S, Li Q. et al. Citrus consumption and cancer incidence: the Ohsaki cohort study. Int J Cancer 2010;127:1913–22. [DOI] [PubMed] [Google Scholar]
- 157. Hirayama T. A large scale cohort study on cancer risks by diet – with special reference to the risk reducing effects of green-yellow vegetable consumption. Princess Takamatsu Symp 1985;16:41–53. [PubMed] [Google Scholar]
- 158. Olsen A, Stripp C, Christense J, Thomsen BL, Overvad K, Tjonneland A. Re: Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst 2005;97:1307–38. [DOI] [PubMed] [Google Scholar]
- 159. Aasheim ET, Sharp SJ, Appleby PN. et al. Tinned fruit consumption and mortality in three prospective cohorts. PLoS One 2015;10:e0117796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Odegaard AO, Koh WP, Yuan JM, Pereira MA. Beverage habits and mortality in Chinese adults. J Nutr 2015;145:595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Letois F, Mura T, Scali J, Gutierrez LA, Feart C, Berr C. Nutrition and mortality in the elderly over 10 years of follow-up: the Three-City study. Br J Nutr 2016;116:882–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Goetz ME, Judd SE, Hartman TJ, McClellan W, Anderson A, Vaccarino V. Flavanone intake is inversely associated with risk of incident ischemic stroke in the REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. J Nutr 2016;146:2233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Goetz ME, Judd SE, Safford MM, Hartman TJ, McClellan WM, Vaccarino V. Dietary flavonoid intake and incident coronary heart disease: the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Am J Clin Nutr 2016;104:1236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Larsson SC, Wolk A. Potato consumption and risk of cardiovascular disease: 2 prospective cohort studies. Am J Clin Nutr 2016;104:1245–52. [DOI] [PubMed] [Google Scholar]
- 165. Aune D, Chan DS, Vieira AR. et al. Fruits, vegetables and breast cancer risk: a systematic review and meta-analysis of prospective studies. Breast Cancer Res Treat 2012;134:479–93. [DOI] [PubMed] [Google Scholar]
- 166. Vieira AR, Vingeliene S, Chan DS. et al. Fruits, vegetables, and bladder cancer risk: a systematic review and meta-analysis. Cancer Med 2015;4:136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Vieira AR, Abar L, Vingeliene S. et al. Fruits, vegetables and lung cancer risk: a systematic review and meta-analysis. Ann Oncol 2016;27:81–96. [DOI] [PubMed] [Google Scholar]
- 168. Chuang SC, Norat T, Murphy N. et al. Fibre intake and total and cause-specific mortality in the European Prospective Investigation into Cancer and Nutrition cohort. Am J Clin Nutr 2012;96:164–74. [DOI] [PubMed] [Google Scholar]
- 169. Leenders M, Boshuizen HC, Ferrari P. et al. Fruit and vegetable intake and cause-specific mortality in the EPIC study. Eur J Epidemiol 2014;29:639–52. [DOI] [PubMed] [Google Scholar]
- 170. Hemila H, Kaprio J, Pietinen P, Albanes D, Heinonen OP. Vitamin C and other compounds in vitamin C rich food in relation to risk of tuberculosis in male smokers. Am J Epidemiol 1999;150:632–41. [DOI] [PubMed] [Google Scholar]
- 171. Li L, Werler MM. Fruit and vegetable intake and risk of upper respiratory tract infection in pregnant women. Public Health Nutr 2010;13:276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Buyken AE, Flood V, Empson M. et al. Carbohydrate nutrition and inflammatory disease mortality in older adults. Am J Clin Nutr 2010;92:634–43. [DOI] [PubMed] [Google Scholar]
- 173. Tabak C, Smit HA, Heederik D, Ocke MC, Kromhout D. Diet and chronic obstructive pulmonary disease: independent beneficial effects of fruits, whole grains, and alcohol (the MORGEN study). Clin Exp Allergy 2001;31:747–55. [DOI] [PubMed] [Google Scholar]
- 174. Walda IC, Tabak C, Smit HA. et al. Diet and 20-year chronic obstructive pulmonary disease mortality in middle-aged men from three European countries. Eur J Clin Nutr 2002;56:638–43. [DOI] [PubMed] [Google Scholar]
- 175. Oskarsson V, Sadr-Azodi O, Orsini N, Andren-Sandberg A, Wolk A. Vegetables, fruit and risk of non-gallstone-related acute pancreatitis: a population-based prospective cohort study. Gut 2013;62:1187–92. [DOI] [PubMed] [Google Scholar]
- 176. Bohn SK, Myhrstad MC, Thoresen M. et al. Blood cell gene expression associated with cellular stress defense is modulated by antioxidant-rich food in a randomised controlled clinical trial of male smokers. BMC Med 2010;8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Anderson JW, Baird P, Davis RH Jr. et al. Health benefits of dietary fibre. Nutr Rev 2009;67:188–205. [DOI] [PubMed] [Google Scholar]
- 178. Lampe JW. Health effects of vegetables and fruit: assessing mechanisms of action in human experimental studies. Am J Clin Nutr 1999;70(Suppl 3):475–90S. [DOI] [PubMed] [Google Scholar]
- 179. Broekmans WM, Klopping-Ketelaars IA, Schuurman CR. et al. Fruits and vegetables increase plasma carotenoids and vitamins and decrease homocysteine in humans. J Nutr 2000;130:1578–83. [DOI] [PubMed] [Google Scholar]
- 180. Macready AL, George TW, Chong MF. et al. Flavonoid-rich fruit and vegetables improve microvascular reactivity and inflammatory status in men at risk of cardiovascular disease – FLAVURS: a randomized controlled trial. Am J Clin Nutr 2014;99:479–89. [DOI] [PubMed] [Google Scholar]
- 181. Threapleton DE, Greenwood DC, Evans CE. et al. Dietary fibre intake and risk of first stroke: a systematic review and meta-analysis. Stroke 2013;44:1360–68. [DOI] [PubMed] [Google Scholar]
- 182. Threapleton DE, Greenwood DC, Evans CE. et al. Dietary fibre intake and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 2013;347:f6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Steinmetz KA, Potter JD. Vegetables, fruit, and cancer. II. Mechanisms. Cancer Causes Control 1991;2:427–42. [DOI] [PubMed] [Google Scholar]
- 184. He K, Hu FB, Colditz GA, Manson JE, Willett WC, Liu S. Changes in intake of fruits and vegetables in relation to risk of obesity and weight gain among middle-aged women. Int J Obes Relat Metab Disord 2004;28:1569–74. [DOI] [PubMed] [Google Scholar]
- 185. Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Schwingshackl L, Hoffmann G, Kalle-Uhlmann T, Arregui M, Buijsse B, Boeing H. Fruit and vegetable consumption and changes in anthropometric variables in adult populations: a systematic review and meta-analysis of prospective cohort studies. PLoS One 2015;10:e0140846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Kaiser KA, Brown AW, Bohan Brown MM, Shikany JM, Mattes RD, Allison DB. Increased fruit and vegetable intake has no discernible effect on weight loss: a systematic review and meta-analysis. Am J Clin Nutr 2014;100:567–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Lanas F, Avezum A, Bautista LE. et al. Risk factors for acute myocardial infarction in Latin America: the INTERHEART Latin American study. Circulation 2007;115:1067–74. [DOI] [PubMed] [Google Scholar]
- 189. Yusuf S, Hawken S, Ounpuu S. et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–52. [DOI] [PubMed] [Google Scholar]
- 190. Bradford Hill A. The environment and disease: association or causation?. Proc R Soc Med 1965;58:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Miller V, Yusuf S, Chow CK. et al. Availability, affordability, and consumption of fruits and vegetables in 18 countries across income levels: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet Glob Health 2016;4:e695–703. [DOI] [PubMed] [Google Scholar]
- 192. Aune D, Keum N, Giovannucci E. et al. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ 2016;353:i2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Aune D, Keum N, Giovannucci E. et al. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective studies. BMC Med 2016;14:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Springmann M, Godfray HC, Rayner M, Scarborough P. Analysis and valuation of the health and climate change cobenefits of dietary change. Proc Natl Acad Sci USA 2016;113:4146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.