ABSTRACT
Background: Aortic stiffness increases with age and increases pulsatile stress in the microcirculation. Abnormalities in kidney microvascular structure and function may contribute to development or progression of chronic kidney disease in older people.
Methods: We performed a longitudinal analysis of 629 community-dwelling elderly Icelandic adults from the Age, Gene/Environment Susceptibility-Reykjavik Study with two visits over a mean follow-up of 5.3 years. We evaluated the associations of carotid-femoral pulse wave velocity (CFPWV), carotid pulse pressure (CPP) and augmentation index (AI), with the change in estimated glomerular filtration rate (eGFR) and urine albumin-to-creatinine ratio (UACR) assessed as annual change and dichotomized as large changes. Models were adjusted for age, sex, height, heart rate, traditional cardiovascular disease risk factors and baseline kidney measures.
Results: When eGFR was analyzed as a continuous variable, higher baseline CFPWV and CPP, but not AI, were significantly associated with a larger annual decline in eGFR in models adjusted for age, sex, height, heart rate and baseline eGFR, but not after additional adjustment for the mean arterial pressure. When eGFR was analyzed as a categorical variable, higher CFPWV was significantly associated with a decrease in eGFR of ≥3 mL/min/1.73 m2/year [odds ratio (OR) 1.53, 95% confidence interval (CI) 1.11–2.13] and higher AI was associated with 30% eGFR decline during follow-up (OR 1.44 and 95% CI 1.03–2.00) in fully adjusted models. None of the tonometry measures was associated with change in UACR.
Conclusions: Abnormalities in vascular health may play a role in large declines in eGFR beyond the traditional cardiovascular disease risks in this older Icelandic cohort.
Keywords: albuminuria, aortic stiffness, chronic kidney disease, estimated glomerular filtration, older people
INTRODUCTION
Chronic kidney disease (CKD) is a common health problem [1–4], especially in older people [3–8]. With aging, a decrease in vascular health is observed with decreased elasticity, decreased compliance and increased wall thickness, leading to higher aortic stiffness [9, 10]. Previous studies have shown that increased aortic stiffness and excessive pressure and flow pulsatility are associated with abnormalities in microvascular structure and function, especially in high-flow organs such as the kidney and the brain [11–13]. In the kidney, the resistance in the glomerular efferent arterioles is higher than in the afferent arterioles and, thus, glomerular capillaries are exposed to high pressure pulsatility in the presence of aortic stiffness. It is suggested that vascular health may offer one of the possible underlying mechanisms for development or progression of CKD in elderly adults in whom specific etiologies of disease are not apparent.
In cross-sectional work in elderly community-dwelling adults from the Age, Gene/Environment, Susceptibility-Reykjavik Study (AGES-RS), an association between carotid pulse pressure (CPP) and albuminuria, a marker of kidney damage, but not estimated glomerular filtration rate (eGFR), was found [14]. Yet, in another cross-sectional study in the same cohort, higher carotid-femoral pulse wave velocity (CFPWV) was associated with lower measured GFR (mGFR) [15]. In this study, we examined the association of baseline measures of aortic stiffness, pressure pulsatility and central arterial waveform with longitudinal changes in eGFR with urinary albumin-to-creatinine ratio (UACR) over an approximate follow-up of 5 years in AGES-RS. We hypothesized that measures of aortic stiffness may be associated with long-term changes in kidney measures.
MATERIALS AND METHODS
Study design and population
We studied participants in AGES-RS who underwent tonometry at the first visit and had serum creatinine and cystatin C, and UACR measurements at this visit and at a follow-up visit (mean interval, 5.3 ± 0.2 years). AGES-RS originates from the Reykjavik Study, which was a community-based cohort study established in 1967 to prospectively investigate cardiovascular disease (CVD) in 30 795 participants in Iceland [15–17]. AGES-RS began in 2002 as a follow-up to the Reykjavik Study to examine risk factors, genetic components and gene–environment interactions for disease in older adults. A total of 5764 participants attended the first study visit in 2002–2006; of these, 1082 participants had arterial tonometry performed and 940 had complete data [14, 18]. The second visit of the AGES-RS was a repeat examination between 2007 and 2011. Of the 940 participants with complete tonometry at the baseline visit, we excluded 310 who did not attend the second study visit and one participant who had missing data on serum creatinine and serum cystatin C at the second visit, resulting in a sample size of 629 for the present analysis.
Arterial tonometry measures
Arterial tonometry was performed using a standardized protocol that has been described previously [14, 19]. Tonometry waveforms were obtained from the brachial, radial, femoral and carotid arteries with simultaneous electrocardiography using a custom transducer (Cardiovascular Engineering, Inc., Norwood, MA, USA). Transit distances were assessed by body surface measurements from the suprasternal notch to each pulse-recording site. CFPWV, CPP and the augmentation index (AI) were used as the primary metrics. Due to the skewed distribution, CFPWV was transformed to a negative inverse scale (iCFPWV) using the formula iCFPWV = −1000/CFPWV.
Kidney measures
eGFR was calculated with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) 2012 creatinine-cystatin C equation [20]. Serum creatinine was measured using the Roche Hitachi-P-Module instrument with Roche Creatinine Plus assay [coefficient of variation (CV) was 2.3% for creatinine assay], which is traceable to National Institute Standardized Technology creatinine standard reference material 909b [21]. Serum cystatin C was measured on the Siemens BN100 Nephelometer using a particle-enhanced immuno-nephelometric assay (PENIA, CV of 2.7 and 5.5% for intra-assay and inter-assay imprecision, respectively), with assays traceable to the International Federation for Clinical Chemists Working Group for the Standardization of Serum Cystatin C and the Institute for Reference Materials and Measurements certified reference materials [22–24].
Urine albumin was measured on a fresh morning urine sample on a Hitachi 912 using the Tina-quant® immunoturbidimetric assay (intra-assay CV = 7.2%; Roche Diagnostics, Mannheim, Germany) at the Icelandic Heart Association. Urine creatinine was measured on a Hitachi 912 using HiCo Creatinine Jaffe method (intra-assay CV = 4.2%; Roche Diagnostics). UACR was transformed to natural log scale due to its skewed distribution.
We defined the annual rate of change in eGFR as the difference between the final value and the baseline value divided by the follow-up duration in years. We defined the annual change in UACR as the natural logarithm of difference in UACR at the two visits divided by the follow-up duration in years, and parameter estimates in logistic models were then exponentiated to express the change as a geometric mean ratio. To verify whether there was a threshold effect in the associations, we also examined changes in eGFR and UACR as binary outcomes. Consistent with previous reports on associations of large changes in eGFR with outcomes, we defined large changes in eGFR as decline in eGFR ≥3 mL/min/1.73 m2/year [25], ≥30% decrease in eGFR over the follow-up duration compared with baseline eGFR [26] and development of eGFR <60 mL/min/1.73 m2 in those with eGFR ≥60 mL/min/1.73 m2 at the first visit (n = 506). There are no established standards as to what constitutes a large change in UACR. We defined a large increase in UACR as a 10-fold increase in UACR during follow-up or the development of UACR ≥30 mg/g among those with UACR <30 mg/g at the first visit (n = 568).
Covariates
Fasting concentrations of serum high-density lipoprotein (HDL) cholesterol, hemoglobin A1c (HbA1c) and C-reactive protein (CRP) were measured. Hypertension was defined as responding positively to the question, ‘Has a doctor or other health care provider ever told you that you had high blood pressure or hypertension?’ and participants on medication as well as those with measurements of systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg. Diabetes was defined by self-report, fasting glucose ≥126 mg/dL or medication use for diabetes. Coronary heart disease was defined by International Classification of Diseases codes for myocardial infarction, ischemic heart disease, coronary aneurysm or dissection and atherosclerosis.
Statistical analysis
We first evaluated the bivariate association of aortic stiffness measurements and kidney function outcomes using scatter plots and assessed for nonlinearity. Linear regression was used to evaluate the relationship between arterial tonometry measures and continuous annual change in eGFR and annual change in the geometric mean ratio of UACR. From the linear regression, we report the parameter estimates (referred to as beta coefficients or β) expressed as change in the outcome variable per standard deviation (SD) of the continuous predictor variable. Logistic regression was used to assess the association of arterial tonometry measures with decline in eGFR ≥3 mL/min/1.73 m2/year, ≥30% decrease in eGFR during follow-up, development of eGFR <60 mL/min/1.73 m2, 10-fold increase in UACR or development of UACR ≥30 mg/g. We evaluated unadjusted associations as well as multivariable models with sequential adjustment for potential confounders. Adjusted models included the following: demographic characteristics (age, sex), heart rate, height and baseline kidney measures; further adjustment by addition of mean arterial pressure (MAP) and fully adjusted by the addition of CVD risk factors (HbA1c, CRP and HDL cholesterol). Heart rate and height are included in these models as they influence the tonometry measures [27]. We used MAP because aortic stiffness is sensitive to distending pressure and because aortic stiffness may affect the microcirculation, potentially resulting in an increase in MAP and a vicious cycle [10]. We hypothesized that MAP may be a mediator in the relation between aortic stiffness and eGFR. Hence, we performed mediation analysis based on the method of Barron and Kenny to examine relations of MAP to aortic stiffness and eGFR, with adjustment for baseline age, sex, height, heart rate and baseline eGFR [28]. A P-value <0.05 was considered statistically significant. All analyses were performed in R (version 3.1.0; Free Software Foundation Inc., Vienna, Austria, www.r-project.org).
Ethics
AGES-RS was approved by the National Bioethics Committee in Iceland (VSN-00-063) and by the National Institute on Aging Intramural Institutional Review Board. Our investigation of aortic stiffness, kidney function and aging in the AGES-RS cohort was approved by the Institutional Review Board of Tufts Medical Center (IRB #9222).
RESULTS
Baseline characteristics of the sample
In the overall cohort, the mean age was 74.8 (SD 3.7) years, 9.9% had diabetes and 58% had hypertension (Table 1). Comparison of baseline characteristics between those who did and did not attend the AGES-RS second visit showed that those who did attend were younger, had higher eGFR and lower UACR, CFPWV and CPP, but had similar CVD risk factor profiles (Supplementary data, Table S1). The mean (SD) annual change in eGFR was −0.9 (2.1) mL/min/1.73 m2/year and the annual geometric mean ratio in UACR was 1.3 (0.3). A total of 411 (65.3%) experienced a decline in eGFR and 541 (89.2%) experienced an increase in UACR during follow-up (Supplementary data, Figure S1).
Table 1.
Baseline study sample characteristics and changes in kidney function and aortic stiffness over the study period
| Total (n = 629) | |
|---|---|
| Age (years) | 74.8 ± 3.7 |
| Male (n, %) | 282 (44.8) |
| Diabetes (n, %) | 62 (9.9) |
| Hypertension (n, %) | 365 (58) |
| Current smoker (n, %) | 55 (8.7) |
| History of CHD (n, %) | 121 (19.4) |
| Height (cm) | 168.6 ± 9.1 |
| Weight (kg) | 76.8 ± 13.1 |
| Heart rate (bpm) | 62.0 ± 10.4 |
| SBP (mmHg) | 138 ± 19 |
| DBP (mmHg) | 67 ± 9 |
| MAP (mmHg) | 94 ± 12 |
| Total cholesterol (mg/dL) | 5.4 ± 1.1 |
| HDL cholesterol (mmol/L) | 1.6 ± 0.4 |
| LDL cholesterol (mmol/L) | 3.2 ± 1.0 |
| HbA1c (%) | 5.6 ± 0.5 |
| Fasting blood glucose (mmol/L) | 5.7 ± 1.1 |
| CRP (mg/L) | 3.0 ± 5.6 |
| Baseline kidney measures | |
| eGFRcr-cys (mL/min/1.73 m2) | 71.9 ± 14.8 |
| eGFRcr-cys <60 mL/min/1.73 m2 (n, %) | 123 (19.6) |
| UACR (mg/g), median (IQR) | 2.9 (1.7, 6.3) |
| UACR ≥30 mg/g (n, %) | 17 (2.8) |
| Baseline tonometry measures | |
| CFPWV (m/s) | 12.6 ± 4.1 |
| CPP (mmHg) | 67.9 ± 21.2 |
| AI (%) | 14.9 ± 15.1 |
| Central SBP (mmHg) | 135.0 ± 22.7 |
| Central DBP (mmHg) | 67.0 ± 9.3 |
| Baseline medications | |
| Statin (n, %) | 174 (27.6) |
| RAS inhibition (n, %) | 188 (29.9) |
| Aspirin (n, %) | 201 (32.0) |
Values are means ± SD unless otherwise noted.
CHD, coronary heart disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL, low-density lipoprotein; eGFRcr-cys, estimated glomerular filtration rate from creatinine and cystatin C; RAS, renin angiotension system.
Association of baseline arterial tonometry measures and change in eGFR
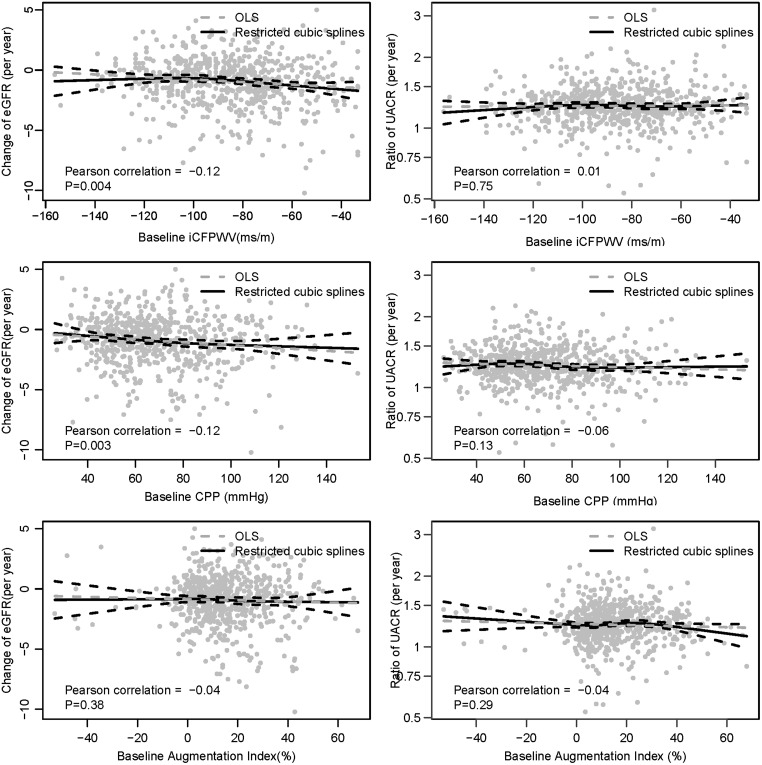
Figure 1 shows the associations of continuous change in arterial tonometry and annual rate of change in eGFR. For aortic stiffness and pressure pulsatility measures, there was a significant association (P = 0.003 and 0.003 for iCFPWV and CPP, respectively), whereas the relationship with AI was not significant (P = 0.38). Table 2 shows these associations unadjusted and then adjusted for covariates. In univariate models, higher CFPWV was associated with declining eGFR (β = −0.24 mL/min/1.73 m2/year per SD higher baseline iCFPWV, 95% CI −0.40 to −0.08, P = 0.004). This association persisted with adjustment for age, sex, heart rate, height and baseline eGFR (β = −0.23 mL/min/1.73 m2/year, 95% CI −0.41 to −0.05, P = 0.01), but was attenuated and was no longer significant after additional adjustment for MAP (β = −0.16 mL/min/1.73 m2/year, 95% CI −0.36 to 0.04, P = 0.11), with further attenuation after adjustment for HDL cholesterol, HbA1c and CRP (β = −0.07 mL/min/1.73 m2/year, 95% CI −0.27 to 0.13, P = 0.51, Table 2). Similarly, higher CPP was significantly associated with an annual decline in eGFR in the univariate model (β = −0.25 mL/min/1.73 m2/year per SD higher baseline CPP, 95% CI −0.41 to −0.09, P = 0.002) and with adjustment for age, sex, heart rate, height and baseline eGFR, but the association was attenuated when MAP was added to the model (β = −0.18 mL/min/1.73 m2/year per SD higher baseline CPP, 95% CI −0.39 to 0.03, P = 0.10, Table 2). AI was not significantly associated with eGFR change in the univariate model or in models with multivariable adjustment (Table 2).
FIGURE 1.
Association of baseline arterial tonometry measures and the annual change of eGFR and UACR. OLS, ordinary least squares.
Table 2.
Association of arterial tonometry measures with change in eGFR
| Aortic stiffness parameters | Models | eGFR change (mL/min/1.73 m2/year), β (95% CI) | Decrease in eGFR (≥3 mL/min/1.73 m2/year), OR (95% CI) | Percentage decrease in eGFR ≥30%, OR (95% CI) | Progression to eGFR <60 mL/min/1.73 m2, OR (95% CI) |
|---|---|---|---|---|---|
| iCFPWV (per SD) | Model 1 | −0.24 (−0.40,−0.08) | 1.45 (1.14, 1.85) | 1.41 (1.05, 1.91) | 1.61 (1.28, 2.04) |
| Model 2 | −0.23 (−0.41,−0.05) | 1.72 (1.30, 2.29) | 1.66 (1.17, 2.37) | 1.50 (1.14, 1.99) | |
| Model 3 | −0.16 (−0.36, 0.04) | 1.72 (1.27, 2.36) | 1.57 (1.08, 2.32) | 1.34 (0.99, 1.83) | |
| Model 4 | −0.07 (−0.27, 0.13) | 1.53 (1.11, 2.13) | 1.33 (0.88, 2.00) | 1.16 (0.84, 1.61) | |
| CPP (per SD) | Model 1 | −0.25 (−0.41,−0.09) | 1.37 (1.09, 1.75) | 1.40 (1.06, 1.83) | 1.34 (1.09, 1.66) |
| Model 2 | −0.25 (−0.42,−0.08) | 1.37 (1.08, 1.75) | 1.38 (1.02, 1.86) | 1.38 (1.07, 1.77) | |
| Model 3 | −0.18 (−0.39, 0.03) | 1.36 (1.00, 1.84) | 1.28 (0.87, 1.84) | 1.18 (0.86, 1.62) | |
| Model 4 | −0.13 (−0.33, 0.08) | 1.26 (0.92, 1.71) | 1.13 (0.76, 1.65) | 1.08 (0.78, 1.50) | |
| AI (per SD) | Model 1 | −0.07 (−0.23, 0.09) | 1.21 (0.95, 1.53) | 1.51 (1.12, 2.01) | 1.04 (0.84, 1.30) |
| Model 2 | −0.09 (−0.26, 0.08) | 1.18 (0.92, 1.52) | 1.46 (1.07, 2.00) | 1.13 (0.88, 1.46) | |
| Model 3 | −0.05 (−0.22, 0.12) | 1 .14 (0.88, 1.48) | 1.40 (1.02, 1.93) | 1.08 (0.83, 1.39) | |
| Model 4 | −0.05 (−0.22, 0.12) | 1.16 (0.89, 1.51) | 1.44 (1.03, 2.00) | 1.06 (0.81, 1.38) |
For the outcome, progression to eGFR <60 mL/min/1.73 m2, only 506 patients were included in the analysis as those who had baseline eGFR <60 mL/min/1.73 m2 were excluded. CFPWV was negative inverse transformed in the model: CFPWV = −1000/CFPWV.
Models: Model 1: unadjusted; Model 2: adjusted for age, sex, heart rate, height and baseline eGFR; Model 3: Model 2 + MAP; Model 4: Model 3 + HDL cholesterol, HbA1c and CRP.
SD for iCFPWV 0.24 s/m, CPP 21.2 mmHg, 15.1 for AI.
Bold indicates significance at P < 0.05.
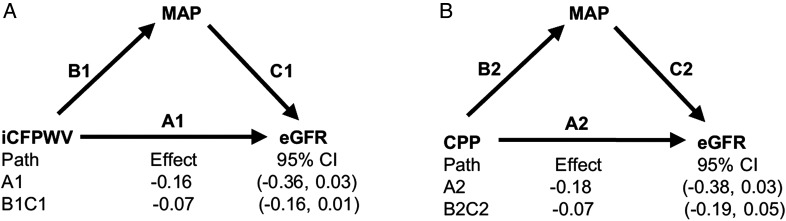
Figure 2 shows the results of an analysis to explore whether MAP was a mediator in the link of aortic stiffness and change in eGFR. We did not observe significant mediation by MAP for either iCFPWV (Figure 2A, pathway B1C1; P = 0.15 for the association with eGFR change mediated by MAP) or CPP (Figure 2B, pathway B2C2; P = 0.30 for the association with eGFR change mediated by MAP).
FIGURE 2.
Analysis of MAP as a mediator of the association between (A) CFPWV and change in eGFR and (B) CPP and change in eGFR. OLS, ordinary least squares. Units of eGFR are ml/min/1.73 m2
We next examined the associations of categorical outcomes of change in eGFR. In the overall cohort, 80 participants (12.7%) experienced an eGFR decline ≥3 mL/min/1.73 m2/year and 48 participants (7.6%) had ≥30% decline in eGFR during the follow-up period. In the 506 participants with eGFR ≥60 mL/min/1.73 m2 at baseline, 101 (20%) developed eGFR <60 mL/min/1.73 m2. iCFPWV and CPP but not AI were significantly associated with eGFR decline ≥3 mL/min/1.73 m2. The effect remained significant even after adjustment for age, sex, heart rate, height, baseline eGFR and MAP, but only iCFPWV remained significant after additional adjustment for CVD risk factors [odds ratio (OR) per SD higher baseline iCFPWV 1.53, 95% CI 1.11, 2.13, Table 2]. All three measures were associated with ≥30% decline in eGFR after initial adjustment, but only associations with AI remained significant after adjustment for MAP and CVD risk factors (OR 1.44 per SD higher baseline AI, 95% CI 1.03, 2.00, Table 2). iCFPWV and CPP but not AI were significantly associated with development of eGFR <60 mL/min/1.73 m2 after adjustment for age, sex, heart rate, height and baseline eGFR; however, these associations did not persist after adjustment for MAP and CVD risk factors (Table 2).
Association of baseline arterial tonometry measures and change in UACR
No significant correlation was found between the three tonometry measures and annual change in the geometric mean ratio of UACR (Figure 1, right panels, P = 0.75, 0.29 and 0.77 for iCFPWV, CPP and AI, respectively). In continuous analyses, no significant associations were observed between iCFPWV, CPP or AI with continuous annual change in the geometric mean ratio of UACR either in univariate models or with additional sequential model adjustment for potential confounders (Table 3). Overall, 9.9% of participants experienced a rapid increase in UACR. Of the 568 participants with UACR <30 mg/g at baseline, 9.9% developed moderate albuminuria. None of the aortic stiffness measures was associated with a rapid increase in UACR or with the development of moderate albuminuria (Table 3).
Table 3.
Associations of arterial tonometry measures with change in UACR
| Aortic stiffness parameters | Models | Geometric mean ratio of UACR (per year) | UACR ratio ≥10, OR (95% CI) | Development of UACR ≥30 mg/g, OR (95% CI) |
|---|---|---|---|---|
| iCFPWV (per SD) | Model 1 | 1.00 (0.99, 1.02) | 1.04 (0.79, 1.36) | 1.28 (0.97, 1.69) |
| Model 2 | 1.01 (0.99, 1.02) | 0.91 (0.65, 1.27) | 0.99 (0.71, 1.38) | |
| Model 3 | 1.01 (1.00, 1.03) | 1.03 (0.71, 1.49) | 115 (0.80, 1.65) | |
| Model 4 | 1.01 (0.99, 1.03) | 0.95 (0.64, 1.39) | 1.07 (0.73, 1.56) | |
| CPP (per SD) | Model 1 | 0.99 (0.97, 1.00) | 1.01 (0.77, 1.31) | 1.05 (0.80, 1.38) |
| Model 2 | 1.00 (0.98, 1.01) | 1.01 (0.74, 1.36) | 0.93 (0.66, 1.29) | |
| Model 3 | 1.00 (0.99, 1.02) | 1.27 (0.87, 1.84) | 1.15 (0.76, 1.71) | |
| Model 4 | 1.00 (0.99, 1.02) | 1.31 (0.89, 1.90) | 1.11 (0.74, 1.66) | |
| AI (per SD) | Model 1 | 0.99 (−0.98, 1.01) | 0.96 (0.74, 1.26) | 1.04 (0.79, 1.37) |
| Model 2 | 1.00 (0.99, 1.01) | 1.01 (0.76, 1.37) | 1.07 (0.77, 1.48) | |
| Model 3 | 1.00 (0.99, 1.02) | 1.03 (0.77, 1.40) | 1.15 (0.82, 1.60) | |
| Model 4 | 1.00 (0.99, 1.02) | 1.03 (0.77, 1.40) | 1.16 (0.83, 1.62) |
LogUACR change was calculated as follows: (follow-up log UACR-baseline log UACR)/year; development of moderate albuminuria was defined as development of UACR ≥30 mg/g among those with UACR <30 mg/g at the first visit (n = 568).
Models: Model 1: unadjusted; Model 2: adjusted for age, sex, heart rate, height and baseline logUACR; Model 3: Model 2 + MAP; Model 4: Model 3 + HDL cholesterol, HbA1c and CRP.
SD for iCFPWV 0.24 s/m, CPP 21.2 mmHg, 15.1 for AI.
Bold indicates significance at P < 0.05.
DISCUSSION
In the present community-based study, when eGFR was analyzed as a continuous variable, both higher baseline CFPWV and CPP were associated with change in eGFR, even after adjustment for demographic characteristics but not after additional adjustment for MAP or CVD risk factors. When eGFR was analyzed as a categorical variable, higher baseline CFPWV was associated with more frequent decreases in eGFR of ≥3 mL/min/1.73 m2/year and higher baseline AI was associated with more frequent 30% eGFR decline during follow-up independent of demographic characteristics, MAP and other traditional CVD risks. None of the vascular measures was found to be associated with change in UACR as a continuous or categorical variable. Overall, we conclude that the measures of aortic stiffness are associated with large declines in eGFR, but not increases in albuminuria.
The finding of a significant association of CFPWV or AI with large declines in eGFR as a categorical variable, but not as a continuous variable, suggest a nonlinear or threshold effect. This could explain why the prior cross-sectional analysis in this same cohort did not show significant associations between higher CFPWV or higher CPP and eGFR after adjustment for age and CVD risk factors [14]. Reports of studies in other cohorts could be consistent with a threshold effect. A study of older adults (mean age of 69 years) with CKD stage 3–4 aortic stiffness was independently associated with large changes in eGFR, defined by combined endpoint of ≥25% decline in eGFR or start of renal replacement therapy [29]. In contrast, in the Framingham Offspring cohort, measures of arterial stiffness were not associated with prevalent eGFR <60 mL/min/1.73 m2 in cross-sectional analyses or with the development of eGFR <60 mL/min/1.73 m2 in longitudinal analyses over a 7- to 10-year follow-up period [30]; associations with large changes in eGFR, however, were not examined, as we have done here. Potential explanations for the different findings between our study and others might be that participants in our study were older and had greater magnitude of stiffening of the aorta, thus leading to more function loss and categorical events and allowing greater statistical power to explore the relationship, or that the presence of aortic stiffness leads to progression rather than development of decreased eGFR. Recently, a report from the Rotterdam Study showed that higher brachial pulse pressure (n = 2950) was associated with larger annual eGFR decline and higher risk of incident CKD and that higher CFPWV (n = 2665) was associated with increased risk of incident CKD over a median follow-up of 11 years [31]. In contrast to our study, these associations were statistically significant even with adjustment for MAP, which may be due to the larger sample size and longer length of follow-up, and support our overall findings of associations between measures of aortic stiffness and decline in eGFR.
We also observed some inconsistency among the aortic stiffness measures in their associations with eGFR change. Associations with aortic stiffness, measured using CFPWV, and pressure pulsatility, measured using CPP, for the most part were similar, but differed from measures of relative wave reflection, as assessed by AI. It is believed that CFPWV and CPP depend on the wall thickness and elasticity of the central arteries and are specifically influenced by proximal aortic wall elasticity and diameter [31, 32]. In older people with a stiffened aorta, loss of impedance mismatch between the aorta and muscular arteries renders AI more sensitive to peripheral arterial resistance and left ventricular ejection pattern [33]. Consequently, AI is considered as a relatively imprecise measure of any particular property of the arterial system. Nevertheless, in the Framingham Offspring cohort, while the associations of CFPWV and CPP with incident CKD or with continuous eGFR from creatinine and cystatin C, were not significant, there was a significant relationship between AI and incident CKD [30].
Aortic stiffness is thought to cause hypertrophic remodeling and increased tone in the microcirculation in response to excessive pressure pulsatility, leading to secondary elevation of MAP [10]. In this study, we noted attenuation in the association of aortic stiffness and change of eGFR when MAP was introduced into the adjusted linear regression models. While this attenuation may provide evidence that MAP confounds the true relationship between aortic stiffness and eGFR, it could also suggest that MAP is a mediator of the effect of aortic stiffness on kidney function. To evaluate this, we performed a mediation analysis of MAP as a mediator in the association of CFPWV and CPP with change in eGFR. We observed that associations mediated by MAP were not statistically significant, although our study may have been underpowered to detect these associations given the sample size. In a prior cross-sectional analysis in a subset of this cohort, the association between CFPWV and mGFR was attenuated substantially when pulsatility index, arterial blood volume in the cortex and increase in renal vascular resistance were introduced into the adjusted model. In that study, mediation analysis showed that these factors were mediators of mGFR [12, 13].
For albuminuria, a prior cross-sectional analysis in this cohort showed that higher CPP was modestly associated with UACR, even after adjustment for age, sex, height, heart rate, MAP, HDL cholesterol, HbA1c, CRP and baseline eGFR [14]. In the current study, no significant associations were observed between aortic stiffness and change in UACR either as continuous or categorical variables. Consistent with our findings, in cross-sectional analyses in other cohorts, significant relationships between aortic stiffness measures and albuminuria beyond blood pressure, blood cholesterol and other CVD risk factors have been observed [34, 35]. In the Framingham Offspring cohort, several aortic stiffness measures were noted to be related to albuminuria in cross-sectional analyses but not incident albuminuria over a 7- to 10-year interval in a multivariable-adjusted model [30]. In contrast to our finding, in cohorts with diabetes [36] and hypertension [37], higher aortic stiffness measures were associated with development of albuminuria in longitudinal analysis. Possibly, the association between albuminuria and arterial stiffness is stronger in people with diabetes or hypertension than in people without these disorders [35]. Moreover, it has been observed that patients with higher CFPWV are less likely to have a regression of albuminuria than patients with lower CFPWV, suggesting that aortic stiffness may predict not only progression but also regression of albuminuria [36]. Larger-scale studies using the albumin excretion rate rather than UACR may be needed to clarify the association between aortic stiffness and kidney damage.
There are several strengths in this study. First, the study population is a large community-based elderly cohort. Second, this is a well-characterized cohort, allowing us to account for several potential confounders. Third, participants were followed over 5 years, which is sufficient to capture changes of kidney measures. Fourth, we employed a state-of-the-art method for determination of eGFR using standardized assays and the use of gold standard tonometry technique for assessment of vascular health. However, our study also has limitations. First, our study sample consisted only of white participants of Icelandic heritage, which may limit the generalizability of our findings to other racial or ethnic groups. Second, participants included in this study all survived to and agreed to participate in the second visit; participants included in this analysis are younger and healthier than those who did not survive or were not included, which would likely bias our results toward the null. Thus, our findings may underestimate the relation between aortic stiffness and kidney disease. Third, the numbers of subjects with events were not large enough to adjust for more confounders and could be overfit for some categorical endpoints. Fourth, even though we computed eGFR using the CKD-EPI equation based on serum creatinine and cystatin C combined, which is superior to other equations in this cohort, we do not have mGFR for both visits, and non-GFR determinants of creatinine and cystatin C may have influenced our observed associations of aortic stiffness and eGFR [38]. Fifth, our change in eGFR and albuminuria were based on measurements captured at only two time points.
In conclusion, associations between large decline in eGFR and CFPWV and AI were significant even after adjustments by other traditional CVD risk factors. None of the aortic stiffness parameters was associated with change in UACR. These analyses emphasize that aortic stiffness may play a role in development or progression of CKD beyond the traditional CVD risks in older Icelandic adults. Large, prospective studies are needed to further determine the contribution of aortic stiffness and subsequent microvascular damage to CKD in the elderly.
SU PPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
Supplementary Material
ACKNOWLEDGEMENTS
G.F.M. serves as a consultant to and receives honoraria from Novartis, Merck and Servier and is funded by research grants from the National Institutes of Health. L.A.I reports funding to Tufts Medical Center for research and contracts with the National Institutes of Health, National Kidney Foundation, Pharmalink AB and Gilead Sciences, a consulting agreement with Otsuka, and has a provisional patent [Coresh, Inker and Levey] filed 8/15/2014—precise estimation of glomerular filtration rate from multiple biomarkers. The technology is not licensed in whole or in part to any company. A.S.L. reports funding to Tufts Medical Center for research and contracts with the National Institutes of Health, National Kidney Foundation, Amgen, Pharmalink AB, Gilead Sciences and has a provisional patent [Coresh, Inker and Levey] filed 8/15/2014—precise estimation of glomerular filtration rate from multiple biomarkers. The technology is not licensed in whole or in part to any company.
CONFLICT OF INTEREST STATEMENT
G.F.M. is the owner of Cardiovascular Engineering, Inc., a company that develops and manufactures devices to measure vascular stiffness. T.B.H and L.J.L. work for the National Institute on Aging (NIA). N.H., M.C.F., M.B.A., G.E., H.G., R.P. and V.G. declare that they have no conflicts of interest.
REFERENCES
- 1. Coresh J, Selvin E, Stevens LA et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298: 2038–2047 [DOI] [PubMed] [Google Scholar]
- 2. Zhang L, Wang F, Wang L et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 2012; 379: 815–822 [DOI] [PubMed] [Google Scholar]
- 3. Takahashi S, Okada K, Yanai M. The Kidney Early Evaluation Program (KEEP) of Japan: results from the initial screening period. Kidney Int Suppl 2010; 116: S17–S23 [DOI] [PubMed] [Google Scholar]
- 4. Gambaro G, Yabarek T, Graziani MS et al. Prevalence of CKD in northeastern Italy: results of the INCIPE study and comparison with NHANES. Clin J Am Soc Nephrol 2010; 5: 1946–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davies DF, Shock NW. Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J Clin Invest 1950; 29: 496–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levey AS, Coresh J. Chronic kidney disease. Lancet 2012; 379: 165–180 [DOI] [PubMed] [Google Scholar]
- 7. Anderson S, Halter JB, Hazzard WR et al. Prediction, progression, and outcomes of chronic kidney disease in older adults. J Am Soc Nephrol 2009; 20: 1199–1209 [DOI] [PubMed] [Google Scholar]
- 8. Stevens LA, Li S, Wang C et al. Prevalence of CKD and comorbid illness in elderly patients in the United States: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 2010; 55: S23–S33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mitchell GF, Parise H, Benjamin EJ et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension 2004; 43: 1239–1245 [DOI] [PubMed] [Google Scholar]
- 10. Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol (1985) 2008; 105: 1652–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mitchell GF, Vita JA, Larson MG et al. Cross-sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness: the Framingham Heart Study. Circulation 2005; 112: 3722–3728 [DOI] [PubMed] [Google Scholar]
- 12. Cheung N, Sharrett AR, Klein R et al. Aortic distensibility and retinal arteriolar narrowing: the multi-ethnic study of atherosclerosis. Hypertension 2007; 50: 617–622 [DOI] [PubMed] [Google Scholar]
- 13. Liao D, Wong TY, Klein R et al. Relationship between carotid artery stiffness and retinal arteriolar narrowing in healthy middle-aged persons. Stroke 2004; 35: 837–842 [DOI] [PubMed] [Google Scholar]
- 14. Michener KH, Mitchell GF, Noubary F et al. Aortic stiffness and kidney disease in an elderly population. Am J Nephrol 2015; 41: 320–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Woodard T, Sigurdsson S, Gotal JD et al. Mediation analysis of aortic stiffness and renal microvascular function. J Am Soc Nephrol 2015; 26: 1181–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harris TB, Launer LJ, Eiriksdottir G et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol 2007; 165: 1076–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fan L, Levey AS, Gudnason V et al. Comparing GFR estimating equations using cystatin C and creatinine in elderly individuals. Am J Epidemiol 2015; 26: 1982–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mitchell GF, Gudnason V, Launer LJ et al. Hemodynamics of increased pulse pressure in older women in the community-based Age, Gene/Environment Susceptibility-Reykjavik Study. Hypertension 2008; 51: 1123–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mitchell GF, Hwang SJ, Vasan RS et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 2010; 121: 505–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Inker LA, Schmid CH, Tighiouart H et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012; 367: 20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levey AS, Coresh J, Greene T et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 2007; 53: 766–772 [DOI] [PubMed] [Google Scholar]
- 22. Grubb A, Blirup-Jensen S, Lindstrom V et al. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med 2010; 48: 1619–1621 [DOI] [PubMed] [Google Scholar]
- 23. Blirup-Jensen S, Grubb A, Lindstrom V et al. Standardization of cystatin C: development of primary and secondary reference preparations. Scand J Clin Lab Invest Suppl 2008; 241: 67–70 [DOI] [PubMed] [Google Scholar]
- 24. Selvin E, Juraschek SP, Eckfeldt J et al. Calibration of cystatin C in the National Health and Nutrition Examination Surveys (NHANES). Am J Kidney Dis 2013; 61: 353–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rifkin DE, Katz R, Chonchol M et al. Blood pressure components and decline in kidney function in community-living older adults: the Cardiovascular Health Study. Am J Hypertens 2013; 26: 1037–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levey AS, Inker LA, Matsushita K et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis 2014; 64: 821–835 [DOI] [PubMed] [Google Scholar]
- 27. Lieb W, Larson MG, Benjamin EJ et al. Multimarker approach to evaluate correlates of vascular stiffness: the Framingham Heart Study. Circulation 2009; 119: 37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosseel Y. lavaan: an R Package for structural equation modeling. J Stat Softw 2012; 48: 1–36 [Google Scholar]
- 29. Ford ML, Tomlinson LA, Chapman TP et al. Aortic stiffness is independently associated with rate of renal function decline in chronic kidney disease stages 3 and 4. Hypertension 2010; 55: 1110–1115 [DOI] [PubMed] [Google Scholar]
- 30. Upadhyay A, Hwang SJ, Mitchell GF et al. Arterial stiffness in mild-to-moderate CKD. J Am Soc Nephrol 2009; 20: 2044–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mitchell GF. Triangulating the peaks of arterial pressure. Hypertension 2006; 48: 543–545 [DOI] [PubMed] [Google Scholar]
- 32. Laurent S, Cockcroft J, Van Bortel L et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27: 2588–2605 [DOI] [PubMed] [Google Scholar]
- 33. Torjesen AA, Wang N, Larson MG et al. Forward and backward wave morphology and central pressure augmentation in men and women in the Framingham Heart Study. Hypertension 2014; 64: 259–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kohara K, Tabara Y, Tachibana R et al. Microalbuminuria and arterial stiffness in a general population: the Shimanami Health Promoting Program (J-SHIPP) study. Hypertens Res 2004; 27: 471–477 [DOI] [PubMed] [Google Scholar]
- 35. Liu CS, Pi-Sunyer FX, Li CI et al. Albuminuria is strongly associated with arterial stiffness, especially in diabetic or hypertensive subjects—a population-based study (Taichung Community Health Study, TCHS). Atherosclerosis 2010; 211: 315–321 [DOI] [PubMed] [Google Scholar]
- 36. Bouchi R, Babazono T, Mugishima M et al. Arterial stiffness is associated with incident albuminuria and decreased glomerular filtration rate in type 2 diabetic patients. Diabetes Care 2011; 34: 2570–2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Munakata M, Miura Y, Yoshinaga K et al. Higher brachial-ankle pulse wave velocity as an independent risk factor for future microalbuminuria in patients with essential hypertension: the J-TOPP study. J Hypertens 2009; 27: 1466–1471 [DOI] [PubMed] [Google Scholar]
- 38. Fan L, Levey AS, Gudnason V et al. Comparing GFR estimating equations using cystatin C and creatinine in elderly individuals. J Am Soc Nephrol 2015; 26: 1982–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.