This editorial refers to ‘Rates and predictors of hospital readmission after transcatheter aortic valve implantation’†, by A. Franzone et al., on page 2211.
Readmissions represent an important outcome for patients and healthcare systems alike. From the patient’s perspective, recent hospitalizations are associated with a period of increased risk for adverse events.1 From the perspective of hospitals and healthcare systems, readmissions represent potentially preventable and costly events. For these reasons, there has been substantial research aimed at understanding the causes and consequences of readmission among a diverse array of conditions and procedures.
Transcatheter aortic valve implantation (TAVI) is a first-line treatment option for symptomatic aortic stenosis in patients with prohibitive, high, or intermediate risk for surgical aortic valve replacement.2,3 By definition, most patients have significant co-morbid conditions putting them at increased risk for adverse events including readmission, leading to a recent focus on understanding readmissions after TAVI. In order to prevent readmissions, we must understand the causes and predictors of this costly event.
In this issue of the journal, Franzone and colleagues4 have done just that by describing the rates, causes, and predictors of readmission within 1 year after TAVI at a single centre in Switzerland. The authors prospectively followed 900 consecutive patients with aortic stenosis who underwent TAVI between August 2007 and June 2014. Of those, 868 patients were discharged alive and at risk for readmission during the first year after TAVI. A total of 221 patients (25%) were readmitted within 1 year after discharge. Most readmissions were for non-cardiovascular causes (54%), including non-cardiac surgery (12%), gastrointestinal disease (10%), and ‘other’ causes (17%) such as falls and immunological disorders. The most common cardiovascular reason for readmission was heart failure (39%), similar to other common cardiovascular discharge diagnoses including acute myocardial infarction and congestive heart failure.5 Notably, valve-related causes of readmission were rare in the current study, accounting for only 2.8% of cardiovascular readmissions and 1.3% of all readmissions.
Using multivariable regression techniques that account for the competing risk of death, the authors identified male gender and in-hospital acute kidney injury as independent risk factors for all-cause readmission, whereas a history of myocardial infarction and in-hospital life-threatening bleeding were associated with an increased risk of cardiovascular-related readmission. Interestingly, post-procedural echocardiographic variables such as mean transprosthetic gradient, indexed aortic valve area, left ventricular ejection fraction, aortic regurgitation, and moderate or severe mitral regurgitation were not significantly associated with the risk of readmission. Finally, the authors discovered that early hospital readmission (within 30 days of discharge) was associated with significantly increased risks of all-cause and cardiovascular mortality, further highlighting the need to better understand the causes and effects of post-TAVI readmissions.
The authors should be commended on this important study, providing detailed, clinically adjudicated outcomes on a large number of patients who underwent TAVI at a single centre. They used sophisticated statistical techniques to predict the risk of readmission while accounting for the competing risk of death, an issue that is often present and variably accounted for in many studies.6 They also provide a breakdown of the causes of readmissions not only for the initial readmission but for subsequent readmissions as well.
This study also should be interpreted in the context of some limitations, the most significant of which may be related to the single-centre setting of the study, limiting the generalizability of the findings. However, the authors disclose a 1-year readmission rate that, when placed in the context of other recent observations, is remarkable.
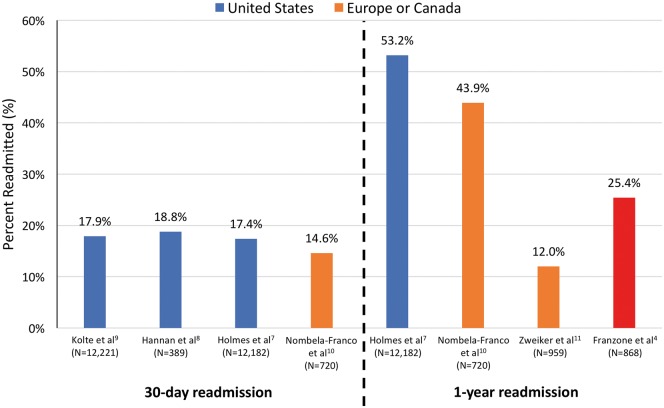
In contrast, in a large observational study using the US STS/ACC Transcatheter Valve Registry, Holmes et al. reported a 1-year readmission rate that is more than twice the rate reported in the current study (53.2% vs. 25.4%; Figure 1).4,7 Furthermore, two recent US studies reported that ∼18% of patients discharged alive after TAVI were readmitted within 30 days,8,9 whereas the current study reports 25% readmitted within 1 year. Although the current study does not provide a 30-day readmission rate, based upon the reported median and interquartile range (IQR) for the time to first readmission, ∼13% and ∼6% of patients, respectively, were readmitted within 70 days and 23 days of discharge after TAVI. These rates are substantially lower than the 30-day readmission rates reported by the US studies mentioned above (Figure 1).
Figure 1.
Presentation of 30-day and 1-year readmission rates after transcatheter aortic valve implantation (TAVI) from select published observational studies in the USA (blue) and Europe/Canada (orange) compared with the 1-year readmission rate reported by Franzone et al.4 (red).
Even in other countries there is substantial variation in published 1-year readmission rates. For example, in a study from a Spanish and a Canadian centre, Nombela-Franco et al. reported a 1-year readmission rate of 43.9%,10 whereas a recent study of 11 Austrian sites reported a 1-year readmission rate of 12.0%.11 Of course, variation does not only exist between countries. Within the USA, Kolte and colleagues found hospital 30-day readmission rates after TAVI that ranged between 0% and 50%.9
Readmissions were also important endpoints for many of the landmark randomized controlled trials evaluating TAVI. The 1-year readmission rates in the TAVI treatment arms of the PARTNER 1A,12 PARTNER 1B,13 PARTNER 2A,14 and SURTAVI15 trial cohorts were 18.2, 22.3, 14.8, and 8.5%, respectively. However, unlike the current study which examined all-cause readmission, the recurrent hospitalization endpoints used in these randomized controlled trials generally included only hospital admissions for signs and symptoms of aortic valve disease or complications from the procedure (i.e. infection, renal failure, etc.). Due to the differing definitions of readmission, it is difficult to place the findings of the current study in context with these important randomized controlled trials.
What could account for these differences between studies? Put another way: what are they doing right? And how can we do it better? As the authors point out, there are potentially numerous answers to these questions, including: (i) differences in the assessment and definition of readmission; (ii) patient clinical features; (iii) organization of healthcare systems; (iv) procedural protocols; and (v) management of post-interventional care. With respect to the first two factors, the definitions and methods of assessment for readmission indeed differed between studies. Whereas Kolte et al.9 utilized administrative claims and ICD-9 (International Classification of Dieases, 9th revision) codes to identify and classify readmissions, Franzone et al.4 clinically adjudicated their outcomes in a prospective fashion through clinic visits, phone calls, and clinical records. Also, the current study defined readmission as a hospitalization lasting >24 h, which may not have been the case in other studies. Individual patient risk may also vary between studies and potentially explain the variation in readmission rates. However, the study by Holmes et al. had a median STS predicted risk of mortality that was only marginally higher than the mean score in the current study (median 7.1%, IQR 4.8–10.8% vs. mean 6.6%, SD ±4.3%).4,7
This brings us to the latter three reasons as to why variation in readmission rates may exist. Arguably, these are the most important factors to understand, as they represent actionable targets from a healthcare delivery perspective. Is there something about the Swiss healthcare system in general that may partially explain variation between countries? Is there greater access to urgent outpatient follow-up or better co-ordination of post-TAVI care? Such questions warrant further study, as the optimal healthcare system remains elusive.
Could differences in procedural technique, protocols, and post-procedural management explain some of the variability in readmission rates? In the current study, post-procedural acute kidney injury and life-threatening bleeding were associated with significantly increased risks of readmission. Although there is obvious incentive to reduce the rate of all post-procedural complications, considering the findings from the current study, it is also possible that, by doing so, we may reduce downstream events such as readmissions. From a healthcare systems’ perspective, reducing post-procedural complications would carry an immediate benefit to patients and may also reduce long-term costs, although the latter remains unproven. Ultimately, these findings should drive hospitals not only to adopt protocols to reduce the occurrence of post-procedural complications but also to identify patients at high risk of readmission, and implement strategies to prevent future admissions.
Lastly, it might be wrong to assert that all readmissions are bad. Some patients are readmitted because they warrant close medical attention and treatment, hopefully resulting in improved health outcomes. Therefore, it is critical that further research identifies factors associated with potentially preventable readmissions after TAVI, so that upstream strategies can be designed and implemented to prevent such readmissions in the future.
In the coming years, outcomes after TAVI should continue to improve due to advances in device technology and procedural technique. However, it is imperative that we seek to understand the sources of variation in post-TAVI outcomes such as readmission between sites (and studies). Exploring the reasons why certain sites have remarkably low readmission rates compared with others should inform the development of novel strategies to improve care and reduce costly, preventable readmissions.
Funding
D.S. is supported by the National Institutes of Health T32 postdoctoral research training grant (T32-HL007853).
Conflict of interest: none declared.
References
- 1. Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med 2013;368:100–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O’Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A.. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;in press. [DOI] [PubMed] [Google Scholar]
- 3. Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS); Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schafers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M.. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451–2496. [DOI] [PubMed] [Google Scholar]
- 4. Franzone A,, Pilgrim T,, Arnold N,, Heg D,, Langhammer B,, Piccolo R,, Roost E,, Praz F,, Räber L,, Valgimigli M,, Wenaweser P,, Jüni P,, Carrel T,, Windecker S,, Stortecky S.. Rates and predictors of hospital readmission after transcatheter aortic valve implantation. Eur Heart J 2017;38:2211–2217. [DOI] [PubMed] [Google Scholar]
- 5. Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, Barreto-Filho JA, Kim N, Bernheim SM, Suter LG, Drye EE, Krumholz HM.. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA 2013;309:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koller MT, Raatz H, Steyerberg EW, Wolbers M.. Competing risks and the clinical community: irrelevance or ignorance? Stat Med 2012;31:1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holmes DR Jr, Brennan JM, Rumsfeld JS, Dai D, O’Brien SM, Vemulapalli S, Edwards FH, Carroll J, Shahian D, Grover F, Tuzcu EM, Peterson ED, Brindis RG, Mack MJ, STS/ACC TVT Registry. Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA 2015;313:1019–1028. [DOI] [PubMed] [Google Scholar]
- 8. Hannan EL, Samadashvili Z, Jordan D, Sundt TM 3rd, Stamato NJ, Lahey SJ, Gold JP, Wechsler A, Ashraf MH, Ruiz C, Wilson S, Smith CR.. Thirty-day readmissions after transcatheter aortic valve implantation versus surgical aortic valve replacement in patients with severe aortic stenosis in New York state. Circ Cardiovasc Interv 2015;8:e002744. [DOI] [PubMed] [Google Scholar]
- 9. Kolte D, Khera S, Sardar MR, Gheewala N, Gupta T, Chatterjee S, Goldsweig A, Aronow WS, Fonarow GC, Bhatt DL, Greenbaum AB, Gordon PC, Sharaf B, Abbott JD.. Thirty-day readmissions after transcatheter aortic valve replacement in the United States: insights from the nationwide readmissions database. Circ Cardiovasc Interv 2017;10:e004472. [DOI] [PubMed] [Google Scholar]
- 10. Nombela-Franco L, del Trigo M, Morrison-Polo G, Veiga G, Jimenez-Quevedo P, Abdul-Jawad Altisent O, Campelo-Parada F, Biagioni C, Puri R, DeLarochelliere R, Dumont E, Doyle D, Paradis JM, Quiros A, Almeria C, Gonzalo N, Nunez-Gil I, Salinas P, Mohammadi S, Escaned J, Fernandez-Ortiz A, Macaya C, Rodes-Cabau J.. Incidence, causes, and predictors of early (≤30 days) and late unplanned hospital readmissions after transcatheter aortic valve replacement. JACC Cardiovasc Interv 2015;8:1748–1757. [DOI] [PubMed] [Google Scholar]
- 11. Zweiker D, Maier R, Lamm G, Maurer E, Heigert M, Neunteufl T, Zeindlhofer E, Grund M, Aichinger J, Huber K, Schneider J, Pollak J, Luha O, Zweiker R, Austrian Society of Cardiology, Committee on Interventional Cardiology. The Austrian transcatheter aortic valve implantation (TAVI) Registry—3 years’ data. Int J Cardiol 2014;177:114–116. [DOI] [PubMed] [Google Scholar]
- 12. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ, PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187–2198. [DOI] [PubMed] [Google Scholar]
- 13. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S, PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597–607. [DOI] [PubMed] [Google Scholar]
- 14. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG, PARTNER Trial Investigators. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 15. Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Sondergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, Chetcuti S, Gleason T, Heiser J, Lange R, Merhi W, Oh JK, Olsen PS, Piazza N, Williams M, Windecker S, Yakubov SJ, Grube E, Makkar R, Lee JS, Conte J, Vang E, Nguyen H, Chang Y, Mugglin AS, Serruys PW, Kappetein AP, SURTAVI Investigators. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med 2017;376:1321–1331. [DOI] [PubMed] [Google Scholar]