Abstract
The capacity of the mammalian heart to regenerate cardiomyocytes has been debated over the last decades. However, limitations in existing techniques to track and identify nascent cardiomyocytes have often led to inconsistent results. Radiocarbon (14C) birth dating, in combination with other quantitative strategies, allows to establish the number and age of human cardiomyocytes, making it possible to describe their age distribution and turnover dynamics. Accurate estimates of cardiomyocyte generation in the adult heart can provide the foundation for novel regenerative strategies that aim to stimulate cardiomyocyte renewal in various cardiac pathologies.
Keywords: Retrospective radiocarbon dating, Cardiomyocyte proliferation, Dynamics of renewal
Introduction
Cardiomyopathy and consequential heart failure are leading causes of death worldwide.1–3 The main hallmark of heart failure is a loss of cardiomyocytes, which is often followed by scar formation and compensatory alterations of remote cardiac tissue, also known as adverse cardiac remodelling. Conceptually, the ideal therapeutic strategy would be to replace dead myocardium with newly formed cardiomyocytes that are electromechanically coupled to the pre-existing healthy tissue. However, today’s treatment options are still focused on myocardial salvage rather than replacement.4 Recently, substantial effort has been invested in studying various stem and progenitor cell populations from external sources to assess their capacity to help the failing heart tissue. Cell transplantation strategies aiming to administer autologous bone marrow cells were proven safe but resulted in only modest functional improvements.5–7 To date, two clinical studies have used intracoronary injection of c-kit+ cardiac cells (SCIPIO study)8 and cardiosphere-derived cells (CADUCEUS study)9 in patients after myocardial infarction. While both studies showed significantly reduced cardiac scar formation, the CADUCEUS study described no substantial functional benefits, while the SCIPIO study documented a modest but significant increase in the ejection fraction.8,9 However, considering our current knowledge, the reported effects of the infused cells might be more due to indirect paracrine interactions than to de novo cardiomyogenesis.10,11 Several other clinical trials are underway, which will further clarify the mechanism of action of cell therapy. In addition, cardiomyocytes that have differentiated from various sources, such as induced pluripotent stem cells (iPSCs), embryonic stem cells (ESCs) and directly reprogrammed cells, have been successfully used in animal models of heart disease,12–14 but the safety and efficacy of these strategies need to be demonstrated in human trials.
Although the adult mammalian heart has been traditionally viewed as a terminally differentiated organ, several species, including certain amphibians and zebrafish, retain the ability to promote cardiomyogenesis well into adulthood.15–19 Beyond injury-induced myocyte renewal, emerging evidence supports the notion that new cardiomyocytes are continuously born in the adult mammalian heart under homeostatic circumstances, although controversial results have been published regarding the possible sources and generation rates of these cells, which have been fuelling active scientific debate.20 Accordingly, another attractive approach for direct therapeutic intervention in cardiac disease would be to promote the innate cardiac regenerative capacity of the mammalian heart by stimulating the yet undiscovered molecular pathways and cellular mechanisms underlying this phenomenon.21–24 To determine whether this strategy is rational and realistic, it is essential to explore the magnitude and dynamics of cardiomyocyte renewal in the human heart.
Cycling cardiomyocytes
Studying cardiomyocyte renewal in humans has been technically challenging and has led to controversial results; therefore, there is an ongoing debate about the magnitude of this process. Mitotic figures in human cardiomyocytes were already reported in the 1920s.25,26 However, these studies did not change the prevailing view of the human heart as a post-mitotic organ, mainly because of the absence of a substantial regenerative response to cardiac injuries. In the late 1990s these findings were revived by reports demonstrating an increase in cell cycle activity in diseased hearts based on the presence of mitotic spindles and cell cycle markers, such as Ki-67.27,28 These results suggested that the human heart can completely renew within 5 years.29 However, neither cell cycle markers nor mitotic spindles provide definite evidence for cardiomyocyte proliferation.20,30 Instead, non-productive cell cycle activity, leading to polyploidy and binucleation but not to effective cytokinesis, can be observed during physiological heart growth as well as in several cardiac diseases, which can substantially bias estimates of the turnover rates that exclusively rely on the detection of the above-mentioned markers.31–35 The functional significance of cardiomyocyte endoreplication in homeostasis and disease remains unclear; nevertheless, it is possible that the increased amount of genomic DNA contributes to a more efficient transcription machinery, providing proteins that support the enhanced metabolic activity and structural needs of cardiac cells undergoing hypertrophic transformation.36
A different yet equally problematic approach is to estimate myocyte renewal by quantifying apoptotic and necrotic myocytes.37 Although there is no doubt that human cardiomyocytes undergo apoptosis in the diseased myocardium and during homeostasis,38–40 the length of time that the apoptotic phenotype is present has to be precisely determined to establish the magnitude of cell death and thus the replacement required to maintain a constant number of cardiomyocytes. Estimates of apoptotic durations range from a few hours to days, making it impossible to accurately predict the renewal rates of myocytes.41–43 While the number of studies showing cardiomyocyte proliferation has been growing, inconsistent data on its magnitude and time course have made it difficult to evaluate the therapeutic potential of this process.20 To overcome the limitations of these strategies, 14C birth dating has helped provide a more integrated model for the dynamics of cell generation in human hearts.22,44 The existence of an innate proliferative capacity, which has been supported by studies based on this novel methodology, might be the basis for future therapeutic strategies that activate regenerative pathways and enhance cardiomyocyte renewal in the human heart.
Radiocarbon dating establishes the age of human cardiomyocytes
Radiocarbon (14C) dating was developed and successfully applied by Willard Libby in the late 1940s to determine the age of biological samples. Since then, archaeologists have utilized this method to estimate the age of dead organic material—the Shroud of Turin 45 and the Ötztal Ice Man,46 among others—based on the radioactive decay of 14C.47 Radiocarbon incorporated in the tissues of a living organism decays at a rate determined by the half-life of the isotope (∼5740 years). Therefore, the age of biological materials can be estimated based on this decay value and the amount of 14C remaining in the organic sample at a certain time point. Retrospective birth dating of human cells with a lifetime of less than a century would not be possible given the long half-life of 14C. However, the dramatic increase in the atmospheric 14C caused by above-ground nuclear bomb tests conducted by the superpowers in the 1950s and 1960s increased the sensitivity of radiocarbon dating to a degree that allows for age determination with a temporal resolution of 1–2 years.48,49 This method has been proven adequate to establish the age of various biological materials, including carotid plaques,50 hair, bones,51 and dental enamel,52,53 for forensic purposes.
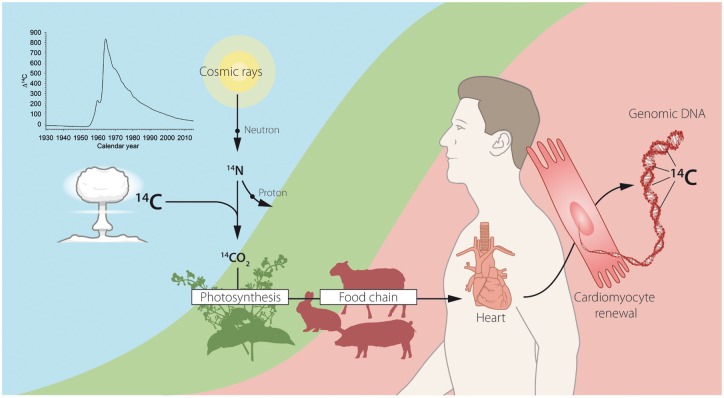
Formed when cosmic rays interact with molecular nitrogen, 14C is naturally present in the Earth’s atmosphere. In the next reaction step, radioactive carbon dioxide is formed, which enters the food chain via plants that convert carbon dioxide into organic material during photosynthesis. Radiocarbon eventually reaches the human body and is incorporated into genomic DNA upon each cell division. As all living animals, including humans, constantly exchange carbon with the biosphere, the genomic 14C concentrations parallel the atmospheric 14C levels with a negligible food lag of less than 1.5 years54 (Figure 1). When a cell undergoes its last cell division, this 14C circulation stops and the amount of 14C integrated in the genomic DNA remains virtually constant. The precise date when a certain cell population was generated can thus be determined by comparing its genomic 14C concentration with the environmental levels, making use of the unique, spike-like shape of the atmospheric 14C curve around the time of the nuclear bomb tests55–57 (Figure 2A and B). Radiocarbon isotope can be measured with accelerated mass spectrometry, which normally requires carbon levels in the range of milligrams. However, carbon masses in genomic DNA obtained from sorted nucleus populations are typically around a few micrograms, therefore sample preparation techniques need to be optimized for this purpose.58 Moreover, due to the low abundance of 14C even after the above ground nuclear bomb tests (on average, only one 14C isotope is present in every 15th cell), it will never be possible to establish the age at the single-cell level with this method.55
Figure 1.
Retrospective radiocarbon (14C) birth dating of human cells in the heart. Although 14C is naturally generated in the atmosphere by the action of cosmic rays on nitrogen, above-ground nuclear tests conducted during the Cold War significantly contributed to its environmental level. In the atmosphere, 14C reacts with oxygen to form CO2, which then gets incorporated into plants through photosynthesis. The carbon content of plants is transferred into the human body via the food chain such that the 14C levels in tissues mirror the atmospheric 14C concentrations. With every cell division, 14C is integrated into newly synthetized DNA in amounts proportional to its atmospheric level at any given time. Due to the stability of nuclear DNA, the measured genomic 14C concentration can be used to establish the age of human cardiomyocytes.
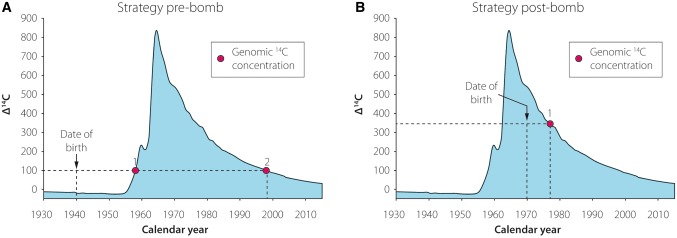
Figure 2.
Strategy for radiocarbon (14C) birth dating of distinct cell populations. (A–B) 14C content in the genomic DNA of the cell population of interest is compared with the recorded atmospheric 14C levels, as shown by the blue graph. The date of birth of the studied individual is indicated by a vertical dashed bar, while the horizontal dashed line corresponds to the 14C value measured in the DNA samples. The age of the sampled cell population can be inferred from the intersection of the horizontal line and the atmospheric 14C curve. (A) Genomic 14C values gained from individuals born before the nuclear bomb tests correspond to two distinct time points (Points 1 and 2). Since the incorporation of environmental 14C might have occurred during the rise and/or the fall of the curve, the precise age of the cell population cannot be determined with certainty based exclusively on these results. (B) However, in individuals born after the nuclear tests, the measured 14C levels unambiguously determine the age of the cell population (Point 1).
Cardiomyocyte turnover in humans
Radiocarbon birth dating could successfully establish, for the first time, the age of cardiac cells.22,44 Analysing hearts from patients born before the sudden increase in atmospheric 14C, we could clearly demonstrate that heart muscle renewal continues at least until the third decade of life. Because cardiomyocytes are surrounded by other cell types with a much higher proliferative capacity, unequivocal identification and stringent isolation of cardiomyocytes was a prerequisite for accurate age determination of these cells. We chose to isolate cardiomyocyte nuclei instead of intact cells because this strategy was the most efficient when using archived frozen material.34,59,60 We successfully validated three independent markers to identify and separate cardiomyocyte nuclei in human and rodent heart samples.34,59,60 Nuclear-localized cardiac troponins I and T44 and pericentriolar material 1 (PCM-1)60 identify most, if not all, cardiomyocyte nuclei in neonatal and adult mammalian hearts.61–66 Using either of these isolation strategies, 14C birth dating demonstrated that cardiomyocyte DNA was younger than the respective individual.22,44 However, DNA synthesis with premature cycle exit, resulting in polyploidy, has been reported to occur during physiological heart growth22,24,33 and in cardiac pathologies, including ischaemic heart disease,32 pathological hypertrophy,67 and congenital heart disease.68 Therefore, it was critical to include the time course of polyploidization in the mathematical model to estimate renewal rates. Additionally, to obtain a direct measure of cardiomyocyte formation, we selectively enriched diploid cardiomyocyte nuclei to estimate the rate of cell renewal independent of polyploidy.22,44 Data gained from this type of 14C analysis and a comprehensive time course analysis of binucleation in human cardiomyocytes provided compelling evidence for cardiomyocyte turnover in the adult human heart.22,44
Dynamics of cardiac cell turnover in humans
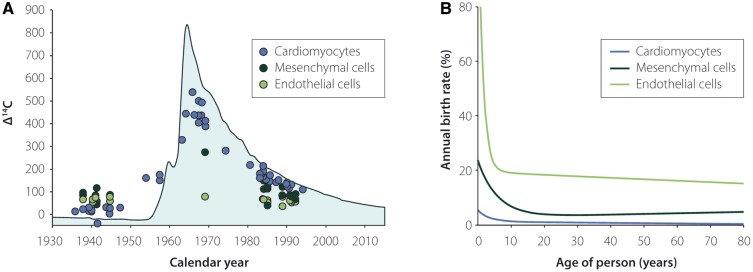
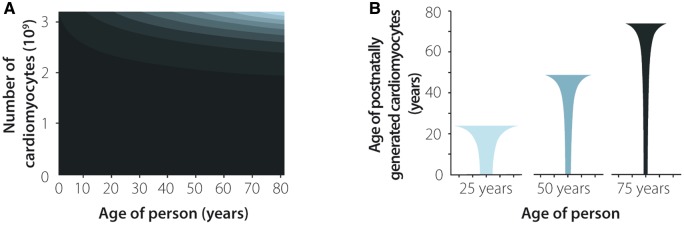
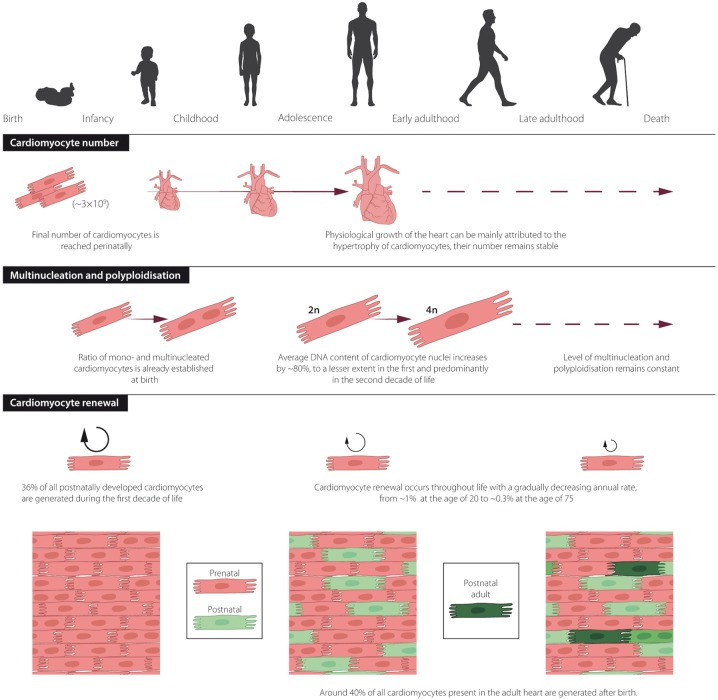
We performed 14C birth dating and design-based stereology to establish a comprehensive model of the generation and exchange dynamics of cardiomyocytes in the human heart.22 The number of cardiomyocytes in the left human ventricle estimated by design-based stereology reached the maximum value (3.2 ± 0.75 billion cells) 1 month after birth22; therefore, the later increase in volume and weight during physiological heart growth can mainly be attributed to hypertrophic growth of cardiomyocytes. This finding agrees with earlier stereological studies that show no further increase in cardiomyocyte number after the perinatal period.69 The unique possibility of establishing the age of cells through 14C birth dating allows for the generation of mathematical models to describe age distributions within cell populations, which can be used to infer their turnover dynamics.70 These mathematical scenarios are based on the birth and death rates of cells in the analysed population. Each scenario defines a set of parameters, such as the change in cell number, preferential cell death or renewal. The scenario that best fits the experimental data gained from heart nucleus samples has been chosen as the model that most accurately describes the dynamics of cell turnover22,44,71,72 (Figure 3A and B). Based on our results, we predicted that endothelial and mesenchymal cells are rapidly exchanged in young adults with birth rates of 20% per year for the former and 5% per year for the latter. In contrast, we found much lower age-dependent renewal of cardiomyocytes with the highest turnover during the first two decades of life, corresponding to rates of approximately 1% per year at the age of 20, declining to lower than 0.5% per year in elderly individuals22,44 (Figure 3B). Although the average renewal rates are relatively low during a human’s lifetime, approximately 39% of all cardiomyocytes are replaced by post-natally generated myocytes in the left ventricle, and 36% of these cells are already exchanged by the age of 10 years22 (Figure 4A and B).
Figure 3.
Retrospective radiocarbon (14C) birth dating and mathematical modelling of the turnover of different cell types in the heart. (A) Genomic 14C values of cardiomyocytes (blue dots), cardiac endothelial (light green dots), and cardiac mesenchymal cells (dark green dots) from different individuals are plotted against the person’s date of birth. Data points lying on the atmospheric 14C curve indicate no renewal of the cells after birth. The degree of deviation from the atmospheric 14C curve indicates the intensity of DNA synthesis and turnover in the post-natal period, childhood, and adulthood. (B) Applying mathematical modelling, the annual renewal rates of the investigated cell populations can be calculated. While endothelial (light green line) and mesenchymal cells (dark green line) are rapidly exchanged in young adults, with birth rates of 20% per year for the former and 5% per year for the latter, human cardiomyocytes (blue line) are replaced with annual rates of approximately 1% per year at the age of 20, declining to rates of < 0.5% per year in elderly individuals. This figure was adapted from Bergmann et al.22
Figure 4.
Cell generation and renewal dynamics in human cardiomyocytes. The number and cell turnover dynamics of human cardiomyocytes were established using a mathematical model combining results from radiocarbon (14C) birth dating and stereological strategies.22 (A) The number of human cardiomyocytes is already set during the perinatal period. Significant exchange of post-natal cardiomyocytes can mainly be observed during the first decades of life. Distinct shades of grey indicate cardiomyocyte subpopulations born during different decades of life. The black area shows cardiomyocytes that were already present at the time of birth.22 (B) Age distribution of post-natally generated cardiomyocytes in 25-, 50-, and 75-year-old individuals. This figure was adapted from Bergmann et al.22
In contrast to our data, a recent study using a similar stereological strategy found that physiological heart growth is accompanied by cardiomyocyte number expansion.24 Our results, combining 14C birth dating and stereology, support a continuous renewal of the cardiomyocyte population in which cell birth is counterbalanced by cell death, maintaining a constant cardiomyocyte number throughout an individual’s adult life. Challenges of cardiomyocyte labelling and sampling strategies might account for the discrepancies between the two reports.22 Moreover, our findings disagree with earlier data reporting turnover rates up to 50% per year.29,73,74 As discussed before, these previous approaches relied on assumptions based on the frequency and duration of apoptosis and cell cycle activity of cardiomyocytes,29 number of cells expressing putative stem cell markers,74 or incorporation of thymidine analogues in patients with malignant diseases.73 These approaches have substantial shortcomings, as previously described20,44,75; therefore, they need to be interpreted with caution.
With 14C birth dating, we could show that the human heart retains its ability to generate new cardiomyocytes, even in middle-aged and older individuals. This finding has important consequences for our understanding of heart homeostasis and disease. Tissue plasticity in the heart is not only based on myocyte hypertrophy often linked to post-mitotic cardiomyocyte endoreplication but also depends on a continuous exchange of cardiomyocytes. A potential loss of the heart’s capacity to generate new cardiomyocytes would result in a loss of more than one billon myocytes over a human’s lifetime. To compensate for this loss, pre-existing cardiomyocytes should substantially increase in size to maintain the contractility of the heart. This process, however, would only be efficient for a limited amount of time, and gradual exhaustion of the myocardium would quickly lead to the development of heart failure. Future studies need to assess whether such changes in cardiomyocyte renewal may be the cause or consequence of certain types of cardiomyopathies.
Sources of new cardiomyocytes
While cardiomyocyte renewal shown by 14C birth dating appears to be insufficient to compensate for the extreme loss of functional heart muscle tissue following myocardial infarction, investigating the underlying mechanisms and sources of postnatally born myocytes may offer new therapeutic strategies targeting these endogenous cardiomyocyte regeneration pathways.
According to our current knowledge, there are two potential sources of newly formed cardiomyocytes: pre-existing cardiomyocytes that undergo dedifferentiation and duplication, and stem or progenitor cells that give rise to de novo cardiomyocytes. Animal studies provide evidence for either mechanism, although the biological significance of these regenerative processes has been debated.20,30 Myocyte duplication has been documented in injured amphibian and zebrafish hearts as well as in murine hearts, albeit at a much lower rate. Long-term infusion of the non-toxic nucleotide analogue 15N-thymidine allowed for labelling and identification of cycling cardiomyocytes in mouse hearts through multi-isotope imaging mass spectrometry.21 Excluding binucleation and polyploidy events, Senyo et al.21 demonstrated that 0.76% of all cardiomyocytes in the left ventricle are exchanged within 1 year in the young adult animal. This process was augmented after cardiac infarction, suggesting activation of regenerative pathways upon injury. Myocyte renewal in the mouse heart seems to be age related, as it is in the human heart.22 The highest renewal rates were observed in neonatal mice21,35,63 with improved regenerative capacity and reduced scarring within the first post-natal week upon apical resection and ischaemic injury.23,76–78 Any injury induced later leads to substantial scar formation and impaired heart function. Although the regenerative response in the neonatal mouse heart has been successfully demonstrated by several research groups,15,20–22,21,63 technical discrepancies in the surgical procedure and resection of larger portions of the myocardium have led to differences in the amount of scarring and recovery.75,79,80 The existence of this regenerative window and its duration have yet to be examined in large animals and human neonates.
Apart from cycling cardiomyocytes, several other cell types, including cardiac progenitor cells, have been suggested as potential sources of this renewal process. Several endogenous cell populations that harbour stem cell characteristics have been described in the mammalian heart and could contribute to its endogenous regenerative capacity.81 Cardiac progenitor cells comprise distinct cell types characterized by the expression of c-kit, Sca-1, ABCG2 (SP cells), Islet-1, or Tbx18 (epicardial progenitor cells);82–86 however, there is overlap between these populations across different studies, which makes the interpretation of experimental data problematic. Although these various cell populations have been described to possess the ability to give rise to cardiomyocytes, as well as to endothelial and smooth muscle cells post-natally,83,84 recent genetic lineage tracing studies have challenged this view. These fate-mapping experiments, using constitutive and inducible genetic cell tracing systems, demonstrated a minimal contribution of endogenous Sca-1+87 or c-kit+ cells to the adult cardiomyocyte population.88–90
Stimulating cardiomyogenesis
Increasing evidence supports that differentiated cardiomyocytes of the functional myocardium contribute to the generation of new cardiac cells through dedifferentiation or re-entry into the cell cycle.21 Several promoting factors have emerged as potential initiators and drivers of the dedifferentiation process and cell cycle re-entry in mammalian cardiomyocytes.91–93
Direct manipulation of the cell cycle through the modification of activity or expression of well-known regulatory proteins offers an obvious yet challenging way to enhance proliferation of distinct cell types. Overexpression of several different members of the cell cycle machinery has been studied in the context of myocardial regeneration lately.94 In a recent study, overexpression of cyclin A2 in transgenic mice increased the rate of cardiomyocyte mitoses during early post-natal development and resulted in better systolic function following cardiac infarction compared with wild-type controls.95–98 Moreover, when cyclin A2 was introduced by adenoviral transfection to the infarct border zone following experimental cardiac infarction in adult rats and in a porcine model, substantial cardiomyocyte regeneration and functional improvement could be observed.95,96,99
Recent studies have demonstrated that changes in oxygen concentration and, consequentially, in the level of reactive oxygen species in cardiomyocytes impact their regenerative potential.100 Hypoxic cardiomyocytes were genetically labelled and traced using the oxygen-dependent degradation domain of Hif-1α in the adult mouse heart. These hypoxic myocytes show a higher capacity to proliferate and contribute to turnover in the adult myocardium.101 Moreover, when adult mice are exposed to gradual chronic hypoxia, cardiomyocyte proliferation is induced, leading to improved recovery following myocardial infarction.102
Regardless of their molecular characteristics, the method of delivery and subsequent kinetics of potential pro-proliferative compounds are of primary importance. A recent advancement has been the introduction of a novel delivery method, the injection of modified RNA (modRNA). These molecules contain substituted nucleotides that are responsible for the increased transfection efficiency and lower cytotoxicity of modRNA in primary cells.103 In an important study aiming to investigate the applicability of these compounds in treating ischaemic heart diseases, vascular endothelial growth factor (VEGF) modRNA was injected into murine hearts at the time of experimental myocardial infarction, reducing the infarct size, enhancing neovascularization, and improving heart function at 3 weeks post-infarction and survival for over 1 year.104
Growing evidence suggests that the otherwise quiescent epicardium becomes reactivated upon injury and contributes to the regenerative response.105 Recently, follistatin-like 1 (Fstl1) has been identified as a secretion product of epicardial mesothelial cells.66 When human Fstl1 protein is applied via an epidural patch over the ischaemic myocardium, cell cycle re-entry and division can be observed in pre-existing cardiomyocytes in mice and swine models. Future studies need to demonstrate whether administration of Fstl1 may be an effective therapeutic strategy following myocardial infarction in humans.
Some of the previously mentioned approaches rely on forced expression of certain proteins, which requires genetic manipulation of cardiomyocytes or their potential progenitor cells. The administration of external nucleotide sequences coding well-defined transcription factors—often in the form of viral particles—has been the traditional approach for modifying and differentiating cardiomyocytes from iPSCs. However, several groups have recently described the use of small molecules to induce targeted differentiation of iPSCs. Different combinations, or so-called ‘cocktails’, of small molecular compounds have been found to chemically induce the formation of cardiomyocyte-like cells in vitro, which hold the potential to be transplanted into living animals.106 Additionally, direct transdifferentiation of fibroblasts into cardiomyocytes has also been described using such compounds, which potentially obviates the need for the intermediate, dedifferentiated iPSC stage.107,108 Recently, a computational method was designed, which was aimed at discovering effective combinations of such small molecules to drive cardiomyocyte differentiation.109
Conclusions
In recent years, a growing body of evidence demonstrated that, in contrast to our classical view, the adult heart is not a post-mitotic organ; instead, it retains its capacity to generate new cardiomyocytes throughout adulthood.
Although the age and turnover dynamics of human cardiomyocytes could be established using 14C birth dating and mathematical modelling (Figure 5), little is known about how de novo cardiomyogenesis is regulated in the adult human heart.21,32 The abundance of experimental approaches aimed at stimulating endogenous cardiomyocyte renewal illustrates this increasing interest. Unveiling the physiological processes that underlie the endogenous regenerative potential of the human heart could provide a basis for therapeutic approaches to promote significant cardiomyocyte renewal in various cardiovascular pathologies.
Figure 5.
The human heart throughout life. Overview of age-related changes in cardiomyocyte number, multinucleation, polyploidy, and cardiomyocyte renewal in the human heart.
Conflict of interest: none declared.
Funding
DFG-Center for Regenerative Therapies; Karolinska Institutet; Swedish Research Council; Ragnar Söderberg Foundation; Åke Wiberg Foundation; and Jeanssons Foundations to O.B.; NIH 1R01HL131778 to H.A.S.
References
- 1. Writing Group M, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 2016;133:e38–e60. [DOI] [PubMed] [Google Scholar]
- 2. Townsend N, Nichols M, Scarborough P, Rayner M.. Cardiovascular disease in Europe—epidemiological update 2015. Eur Heart J 2015;36:2696–2705. [DOI] [PubMed] [Google Scholar]
- 3. Ohira T, Iso H.. Cardiovascular disease epidemiology in Asia: an overview. Circ J 2013;77:1646–1652. [DOI] [PubMed] [Google Scholar]
- 4. Task Force on the management of STseamiotESoC, Steg PG, James SK, Atar D, Badano LP, Blomstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van 'T Hof A, Widimsky P, Zahger D.. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;33:2569–2619. [DOI] [PubMed] [Google Scholar]
- 5. Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Werner N, Haase J, Neuzner J, Germing A, Mark B, Assmus B, Tonn T, Dimmeler S, Zeiher AM.. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur Heart J 2006;27:2775–2783. [DOI] [PubMed] [Google Scholar]
- 6. Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM.. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med 2006;355:1210–1221. [DOI] [PubMed] [Google Scholar]
- 7. Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de Werf F.. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet 2006;367:113–121. [DOI] [PubMed] [Google Scholar]
- 8. Bolli R, Chugh AR, D'amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P.. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 2011;378:1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9. Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marban E.. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 2012;379:895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tang XL, Li Q, Rokosh G, Sanganalmath SK, Chen N, Ou Q, Stowers H, Hunt G, Bolli R.. Long-term outcome of administration of c-kit(POS) cardiac progenitor cells after acute myocardial infarction: transplanted cells do not become cardiomyocytes, but structural and functional improvement and proliferation of endogenous cells persist for at least one year. Circ Res 2016;118:1091–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoon C-H, Koyanagi M, Iekushi K, Seeger F, Urbich C, Zeiher AM, Dimmeler S.. Mechanism of improved cardiac function after bone marrow mononuclear cell therapy. Role of cardiovascular lineage commitment. Circulation 2010;21:2001–2011. [DOI] [PubMed] [Google Scholar]
- 12. Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D.. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 2010;142:375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE.. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014;510:273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shiba Y, Gomibuchi T, Seto T, Wada Y, Ichimura H, Tanaka Y, Ogasawara T, Okada K, Shiba N, Sakamoto K, Ido D, Shiina T, Ohkura M, Nakai J, Uno N, Kazuki Y, Oshimura M, Minami I, Ikeda U.. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 2016;538:388–391. [DOI] [PubMed] [Google Scholar]
- 15. Laflamme MA, Murry CE.. Heart regeneration. Nature 2011;473:326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kikuchi K. Dedifferentiation, transdifferentiation, and proliferation: mechanisms underlying cardiac muscle regeneration in zebrafish. Curr Pathobiol Rep 2015;3:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Singh BN, Koyano-Nakagawa N, Garry JP, Weaver CV.. Heart of newt: a recipe for regeneration. J Cardiovasc Transl Res 2010;3:397–409. [DOI] [PubMed] [Google Scholar]
- 18. Jopling C, Sleep E, Raya M, Marti M, Raya A, Izpisua Belmonte JC.. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 2010;464:606–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD.. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature 2010;464:601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bergmann O, Jovinge S.. Cardiac regeneration in vivo: mending the heart from within? Stem Cell Res 2014;13:523–531. [DOI] [PubMed] [Google Scholar]
- 21. Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu T-D, Guerquin-Kern J-L, Lechene CP, Lee RT.. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 2013;493:433–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C, Malm T, Andra M, Jashari R, Nyengaard JR, Possnert G, Jovinge S, Druid H, Frisen J.. Dynamics of cell generation and turnover in the human heart. Cell 2015;161:1566–1575. [DOI] [PubMed] [Google Scholar]
- 23. Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA.. Transient regenerative potential of the neonatal mouse heart. Science 2011;331:1078–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mollova M, Bersell K, Walsh S, Savla J, Das LT, Park S-y, Silberstein LE, Dos Remedios CG, Graham D, Colan S, Kühn B.. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci USA 2013;110:1446–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Warthin AS. The myocardial lesions of diphtheria. J Infect Dis 1924;35:32–66. [Google Scholar]
- 26. Macmahon HE. Hyperplasia and regeneration of the myocardium in infants and in children. Am J Pathol 1937;13:845–854 5. [PMC free article] [PubMed] [Google Scholar]
- 27. Kajstura J, Leri A, Finato N, Di Loreto C, Beltrami CA, Anversa P.. Myocyte proliferation in end-stage cardiac failure in humans. Proc Natl Acad Sci USA 1998;95:8801–8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beltrami CA, Di Loreto C, Finato N, Rocco M, Artico D, Cigola E, Gambert SR, Olivetti G, Kajstura J, Anversa P.. Proliferating cell nuclear antigen (PCNA), DNA synthesis and mitosis in myocytes following cardiac transplantation in man. J Mol Cell Cardiol 1997;29:2789–2802. [DOI] [PubMed] [Google Scholar]
- 29. Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA, Anversa P.. Evidence that human cardiac myocytes divide after myocardial infarction. New Engl J Med 2001;344:1750–1757. [DOI] [PubMed] [Google Scholar]
- 30. Zebrowski DC, Becker R, Engel FB.. Towards regenerating the mammalian heart: challenges in evaluating experimentally induced adult mammalian cardiomyocyte proliferation. Am J Physiol Heart Circ Physiol 2016;310:H1045–H1054. [DOI] [PubMed] [Google Scholar]
- 31. Adler CP, Friedburg H, Herget GW, Neuburger M, Schwalb H.. Variability of cardiomyocyte DNA content, ploidy level and nuclear number in mammalian hearts. Virchows Arch 1996;429:159–164. [DOI] [PubMed] [Google Scholar]
- 32. Herget GW, Neuburger M, Plagwitz R, Adler CP.. DNA content, ploidy level and number of nuclei in the human heart after myocardial infarction. Cardiovasc Res 1997;36:45–51. [DOI] [PubMed] [Google Scholar]
- 33. Adler CP. The development and regenerative potential of cardiac muscle In Oberpriller JO, Oberpriller JC, Mauro A, eds. The Development and Regenerative Potential of Cardiac Muscle. London: HAP; 1991, pp. 227–252. [Google Scholar]
- 34. Bergmann O, Zdunek S, Alkass K, Druid H, Bernard S, Frisén J.. Identification of cardiomyocyte nuclei and assessment of ploidy for the analysis of cell turnover. Exp Cell Res 2011;327:188–194. [DOI] [PubMed] [Google Scholar]
- 35. Soonpaa MH, Kim KK, Pajak L, Franklin M, Field LJ.. Cardiomyocyte DNA synthesis and binucleation during murine development. Am J Physiol 1996;271(5 Pt 2):H2183–H2189. [DOI] [PubMed] [Google Scholar]
- 36. Edgar BA, Zielke N, Gutierrez C.. Endocycles: a recurrent evolutionary innovation for post-mitotic cell growth. Nat Rev Mol Cell Biol 2014;15:197–210. [DOI] [PubMed] [Google Scholar]
- 37. Anversa P, Kajstura J, Rota M, Leri A.. Regenerating new heart with stem cells. J Clin Invest 2013;123:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38. Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara J, Quaini E, Di Loreto C, Beltrami Ca Krajewski S, Reed JC, Anversa P.. Apoptosis in the failing human heart. New Engl J Med 1997;336:1131–1141. [DOI] [PubMed] [Google Scholar]
- 39. Mallat Z, Fornes P, Costagliola R, Esposito B, Belmin J, Lecomte D, Tedgui A.. Age and gender effects on cardiomyocyte apoptosis in the normal human heart. J Gerontol A Biol Sci Med Sci 2001;56:M719–M723. [DOI] [PubMed] [Google Scholar]
- 40. Saraste A, Pulkki K, Kallajoki M, Heikkilä P, Laine P, Mattila S, Nieminen MS, Parvinen M, Voipio-Pulkki LM.. Cardiomyocyte apoptosis and progression of heart failure to transplantation. Eur J Clin Invest 1999;29:380–386. [DOI] [PubMed] [Google Scholar]
- 41. Takemura G, Kanoh M, Minatoguchi S, Fujiwara H.. Cardiomyocyte apoptosis in the failing heart—a critical review from definition and classification of cell death. Int J Cardiol 2013;167:2373–2386. [DOI] [PubMed] [Google Scholar]
- 42. Rodriguez M, Schaper J.. Apoptosis: measurement and technical issues. J Mol Cell Cardiol 2005;38:15–20. [DOI] [PubMed] [Google Scholar]
- 43. De Saint-Hubert M, Prinsen K, Mortelmans L, Verbruggen A, Mottaghy FM.. Molecular imaging of cell death. Methods (San Diego, Calif) 2009;48:178–187. [DOI] [PubMed] [Google Scholar]
- 44. Bergmann O, Bhardwaj R, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz B, Druid H, Jovinge S, Frisen J.. Evidence for cardiomyocyte renewal in humans. Science 2009;324:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Damon PE, Donahue DJ, Gore BH, Hatheway AL, Jull AJT, Linick TW, Sercel PJ, Toolin LJ, Bronk CR, Hall ET, Hedges REM, Housley R, Law IA, Perry C, Bonani G, Trumbore S, Woelfli W, Ambers JC, Bowman SGE, Leese MN, Tite MS.. Radiocarbon dating of the Shroud of Turin. Nature 1989;337:611–615. [Google Scholar]
- 46. Bonani G, Ivy SD, Hajdas I, Niklaus TR, Suter M.. AMS 14C age determinations of tissue, bone and grass samples from the Ötztal ice man. Radiocarbon 1994;36:247–250. [Google Scholar]
- 47. Libby WF, Anderson EC, Arnold JR.. Age determination by radiocarbon content: world-wide assay of natural radiocarbon. Science 1949;109:227–228. [DOI] [PubMed] [Google Scholar]
- 48. Levin I, Kromer B.. The tropospheric 14CO2 level in Mid-Latitudes of the Northern Hemisphere (1959-2003). Radiocarbon 2004;46:1261–1272. [Google Scholar]
- 49. Levin I, Naegler T, Kromer B, Diehl M, Francey RJ, Gomez-Pelaez AJ, Steele LP, Wagenbach D, Weller R, Worthy DE.. Observations and modelling of the global distribution and long-term trend of atmospheric 14CO2. Tellus 2010;63b:26–46. [Google Scholar]
- 50. Hagg S, Salehpour M, Noori P, Lundstrom J, Possnert G, Takolander R, Konrad P, Rosfors S, Ruusalepp A, Skogsberg J, Tegner J, Bjorkegren J.. Carotid plaque age is a feature of plaque stability inversely related to levels of plasma insulin. PloS One 2011;6:e18248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wild EM, Arlamovsky KA, Golser R, Kutschera W, Priller A, Puchegger S, Rom W, Steier P, Vycudilik W.. 14C dating with the bomb peak: an application to forensic medicine. Nucl Instrum Meth Phys Res B Beam Interact Mater Atoms 2000;172:944–950. [Google Scholar]
- 52. Spalding KL, Buchholz BA, Bergman LE, Druid H, Frisen J.. Forensics: age written in teeth by nuclear tests. Nature 2005;437:333–334. [DOI] [PubMed] [Google Scholar]
- 53. Alkass K, Saitoh H, Buchholz BA, Bernard S, Holmlund G, Senn DR, Spalding KL, Druid H.. Analysis of radiocarbon, stable isotopes and DNA in teeth to facilitate identification of unknown decedents. PloS One 2013;8:e69597.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Georgiadou E, Stenström KE, Uvo CB, Nilsson P, Skog G, Mattsson S.. Bomb-pulse 14C analysis combined with 13C and 15N measurements in blood serum from residents of Malmö, Sweden. Radiat Environ Biophys 2013;52:175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Spalding K, Bhardwaj RD, Buchholz B, Druid H, Frisén J.. Retrospective birth dating of cells in humans. Cell 2005;122:133–143. [DOI] [PubMed] [Google Scholar]
- 56. Libby WF, Berger R, Mead JF, Alexander GV, Ross JF.. Replacement rates for human tissue from atmospheric radiocarbon. Science 1964;146:1170–1172. [DOI] [PubMed] [Google Scholar]
- 57. Harkness DD. Further investigations of the transfer of bomb 14 C to man. Nature 1972;240:302–303. [DOI] [PubMed] [Google Scholar]
- 58. Salehpour M, Håkansson K, Possnert G.. Accelerator mass spectrometry of ultra-small samples with applications in the biosciences. Nucl Instrum Meth Phys Res B Beam Interact Mater Atoms 2013;294:97–103. [Google Scholar]
- 59. Thienpont B, Aronsen JM, Robinson EL, Okkenhaug H, Loche E, Ferrini A, Brien P, Alkass K, Tomasso A, Agrawal A, Bergmann O, Sjaastad I, Reik W, Roderick HL.. The H3K9 dimethyltransferases EHMT1/2 protect against pathological cardiac hypertrophy. J Clin Invest 2017;127:335–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bergmann O, Jovinge S.. Isolation of cardiomyocyte nuclei from post-mortem tissue. J Vis Exp 2012;65:4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Srsen V, Fant X, Heald R, Rabouille C, Merdes A.. Centrosome proteins form an insoluble perinuclear matrix during muscle cell differentiation. BMC Cell Biol 2009;10:28.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zebrowski DC, Vergarajauregui S, Wu CC, Piatkowski T, Becker R, Leone M, Hirth S, Ricciardi F, Falk N, Giessl A, Just S, Braun T, Weidinger G, Engel FB.. Developmental alterations in centrosome integrity contribute to the post-mitotic state of mammalian cardiomyocytes. eLife 2015;4:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Alkass K, Panula J, Westman M, Wu TD, Guerquin-Kern JL, Bergmann O.. No evidence for cardiomyocyte number expansion in preadolescent mice. Cell 2015;163:1026–1036. [DOI] [PubMed] [Google Scholar]
- 64. Gilsbach R, Preissl S, Gruning BA, Schnick T, Burger L, Benes V, Wurch A, Bonisch U, Gunther S, Backofen R, Fleischmann BK, Schubeler D, Hein L.. Dynamic DNA methylation orchestrates cardiomyocyte development, maturation and disease. Nat Commun 2014;5:5288.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Raulf A, Horder H, Tarnawski L, Geisen C, Ottersbach A, Röll W, Jovinge S, Fleischmann BK, Hesse M.. Transgenic systems for unequivocal identification of cardiac myocyte nuclei and analysis of cardiomyocyte cell cycle status. Basic Res Cardiol 2015;110:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wei K, Serpooshan V, Hurtado C, Diez-Cunado M, Zhao M, Maruyama S, Zhu W, Fajardo G, Noseda M, Nakamura K, Tian X, Liu Q, Wang A, Matsuura Y, Bushway P, Cai W, Savchenko A, Mahmoudi M, Schneider MD, van den Hoff MJ, Butte MJ, Yang PC, Walsh K, Zhou B, Bernstein D, Mercola M, Ruiz-Lozano P.. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature 2015;525:479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Adler CP, Friedburg H.. Myocardial DNA content. ploidy level and cell number in geriatric hearts: postmortem examinations of human myocardium in old age. Mol Cell Cardiol 1986;18:3953. [DOI] [PubMed] [Google Scholar]
- 68. Adler CP. DNA in growing hearts of children. Biochemical and cytophotometric investigations. Beitr Pathol 1976;158:173–202. [PubMed] [Google Scholar]
- 69. Mayhew TM, Pharaoh A, Austin A, Fagan DG.. Stereological estimates of nuclear number in human ventricular cardiomyocytes before and after birth obtained using physical disectors. J Anat 1997;191(Pt 1):107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bernard S, Frisén J, Spalding KL.. A mathematical model for the interpretation of nuclear bomb test derived 14C incorporation in biological systems. Nucl Instrum Meth Phys Res B Beam Interact Mater Atoms 2010;268:1295–1298. [Google Scholar]
- 71. Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Boström E, Westerlund I, Vial C, Buchholz B, Possnert G, Mash DC, Druid H, Frisén J.. Dynamics of hippocampal neurogenesis in adult humans. Cell 2013;153:1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yeung MS, Zdunek S, Bergmann O, Bernard S, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Brundin L, Druid H, Frisen J.. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell 2014;159:766–774. [DOI] [PubMed] [Google Scholar]
- 73. Kajstura J, Urbanek K, Perl S, Hosoda T, Zheng H, Ogórek B, Ferreira-Martins J, Goichberg P, Rondon C, D'amario D, Rota M, Del Monte F, Orlic D, Tisdale J, Leri A, Anversa P.. Cardiomyogenesis in the adult human heart. Circ Res 2010;107:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 74. Kajstura J, Gurusamy N, Ogorek B, Goichberg P, Clavo-Rondon C, Hosoda T, D'amario D, Bardelli S, Beltrami AP, Cesselli D, Bussani R, del Monte F, Quaini F, Rota M, Beltrami CA, Buchholz BA, Leri A, Anversa P.. Myocyte turnover in the aging human heart. Circ Res 2010;107:1374–1386. [DOI] [PubMed] [Google Scholar]
- 75. Bryant DM, O'meara CC, Ho NN, Gannon J, Cai L, Lee RT.. A systematic analysis of neonatal mouse heart regeneration after apical resection. J Mol Cell Cardiol 2015;79:315–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Porrello ER, Mahmoud AI, Simpson E, Johnson Ba Grinsfelder D, Canseco D, Mammen PP, Rothermel Ba Olson EN, Sadek H.. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc Natl Acad Sci USA 2013;110:187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Haubner BJ, Adamowicz-Brice M, Khadayate S, Tiefenthaler V, Metzler B, Aitman T, Penninger JM.. Complete cardiac regeneration in a mouse model of myocardial infarction. Aging (Milano) 2012;4:966–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. van den Bos EJ, Mees BM, de Waard MC, de Crom R, Duncker DJ.. A novel model of cryoinjury-induced myocardial infarction in the mouse: a comparison with coronary artery ligation. Am J Physiol Heart Circ Physiol 2005;289:H1291–H1300. [DOI] [PubMed] [Google Scholar]
- 79. Andersen Ditte C, Ganesalingam S, Jensen Charlotte H, Sheikh Søren P.. Do neonatal mouse hearts regenerate following heart apex resection? Stem Cell Reports 2014;2:406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sen S, Sadek HA.. Neonatal heart regeneration: mounting support and need for technical standards. J Am Heart Assoc 2015;4:e001727.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Garbern JC, Lee RT.. Cardiac stem cell therapy and the promise of heart regeneration. Cell Stem Cell 2013;12:689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, Goetsch SC, Gallardo TD, Garry DJ.. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol 2004;265:262–275. [DOI] [PubMed] [Google Scholar]
- 83. Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD.. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA 2003;100:12313–12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P.. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 2003;114:763–776. [DOI] [PubMed] [Google Scholar]
- 85. Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR.. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature 2005;433:647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, Zhang X, Stallcup WB, Denton CP, McCulloch A, Chen J, Evans SM.. A myocardial lineage derives from Tbx18 epicardial cells. Nature 2008;454:104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Uchida S, De Gaspari P, Kostin S, Jenniches K, Kilic A, Izumiya Y, Shiojima I, Kreymborg Karsten G, Renz H, Walsh K, Braun T.. Sca1-derived cells are a source of myocardial renewal in the murine adult heart. Stem Cell Reports 2013;1:397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marban E, Molkentin JD.. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature 2014;509:337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Liu Q, Yang R, Huang X, Zhang H, He L, Zhang L, Tian X, Nie Y, Hu S, Yan Y, Zhang L, Qiao Z, Wang QD, Lui KO, Zhou B.. Genetic lineage tracing identifies in situ Kit-expressing cardiomyocytes. Cell Res 2016;26:119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sultana N, Zhang L, Yan J, Chen J, Cai W, Razzaque S, Jeong D, Sheng W, Bu L, Xu M, Huang GY, Hajjar RJ, Zhou B, Moon A, Cai CL.. Resident c-kit(+) cells in the heart are not cardiac stem cells. Nat Commun 2015;6:8701.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kuhn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT.. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med 2007;13:962–969. [DOI] [PubMed] [Google Scholar]
- 92. Bersell K, Arab S, Haring B, Kuhn B.. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell 2009;138:257–270. [DOI] [PubMed] [Google Scholar]
- 93. D'Uva G, Aharonov A, Lauriola M, Kain D, Yahalom-Ronen Y, Carvalho S, Weisinger K, Bassat E, Rajchman D, Yifa O, Lysenko M, Konfino T, Hegesh J, Brenner O, Neeman M, Yarden Y, Leor J, Sarig R, Harvey RP, Tzahor E.. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat Cell Biol 2015;17:627–638. [DOI] [PubMed] [Google Scholar]
- 94. Ahuja P, Sdek P, MacLellan WR.. Cardiac myocyte cell cycle control in development, disease, and regeneration. Physiol Rev 2007;87:521–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Woo YJ, Panlilio CM, Cheng RK, Liao GP, Atluri P, Hsu VM, Cohen JE, Chaudhry HW.. Therapeutic delivery of cyclin A2 induces myocardial regeneration and enhances cardiac function in ischemic heart failure. Circulation 2006;114(1 Suppl):I206–I213. [DOI] [PubMed] [Google Scholar]
- 96. Woo YJ, Panlilio CM, Cheng RK, Liao GP, Suarez EE, Atluri P, Chaudhry HW.. Myocardial regeneration therapy for ischemic cardiomyopathy with cyclin A2. J Thorac Cardiovasc Surg 2007;133:927–933. [DOI] [PubMed] [Google Scholar]
- 97. Chaudhry HW, Dashoush NH, Tang H, Zhang L, Wang X, Wu EX, Wolgemuth DJ.. Cyclin A2 mediates cardiomyocyte mitosis in the postmitotic myocardium. J Biol Chem 2004;279:35858–35866. [DOI] [PubMed] [Google Scholar]
- 98. Cheng RK, Asai T, Tang H, Dashoush NH, Kara RJ, Costa KD, Naka Y, Wu EX, Wolgemuth DJ, Chaudhry HW.. Cyclin A2 induces cardiac regeneration after myocardial infarction and prevents heart failure. Circ Res 2007;100:1741–1748. [DOI] [PubMed] [Google Scholar]
- 99. Shapiro SD, Ranjan AK, Kawase Y, Cheng RK, Kara RJ, Bhattacharya R, Guzman-Martinez G, Sanz J, Garcia MJ, Chaudhry HW.. Cyclin A2 induces cardiac regeneration after myocardial infarction through cytokinesis of adult cardiomyocytes. Sci Transl Med 2014;6:224ra27.. [DOI] [PubMed] [Google Scholar]
- 100. Puente Bao N, Kimura W, Muralidhar Shalini A, Moon J, Amatruda James F, Phelps Kate L, Grinsfelder D, Rothermel Beverly A, Chen R, Garcia Joseph A, Santos Celio X, Thet S, Mori E, Kinter Michael T, Rindler Paul M, Zacchigna S, Mukherjee S, Chen David J, Mahmoud Ahmed I, Giacca M, Rabinovitch Peter S, Aroumougame A, Shah Ajay M, Szweda Luke I, Sadek Hesham A.. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell 2014;157:565–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kimura W, Xiao F, Canseco DC, Muralidhar S, Thet S, Zhang HM, Abdulrahman Y, Chen R, Garcia J, Shelton JM, Richardson J, Ashour AM, Asaithamby A, Liang H, Xing C, Lu Z, Zhang CC, Sadek H.. Hypoxia fate mapping identifies cycling cardiomyocytes in the adult heart. Nature 2015;523:226–230. [DOI] [PubMed] [Google Scholar]
- 102. Nakada Y, Canseco DC, Thet S, Abdisalaam S, Asaithamby A, Santos CX, Shah AM, Zhang H, Faber JE, Kinter MT, Szweda LI, Xing C, Hu Z, Deberardinis RJ, Schiattarella G, Hill JA, Oz O, Lu Z, Zhang CC, Kimura W, Sadek HA.. Hypoxia induces heart regeneration in adult mice. Nature 2017;541:222–227. [DOI] [PubMed] [Google Scholar]
- 103. Kormann MS, Hasenpusch G, Aneja MK, Nica G, Flemmer AW, Herber-Jonat S, Huppmann M, Mays LE, Illenyi M, Schams A, Griese M, Bittmann I, Handgretinger R, Hartl D, Rosenecker J, Rudolph C.. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat Biotechnol 2011;29:154–157. [DOI] [PubMed] [Google Scholar]
- 104. Zangi L, Lui KO, von Gise A, Ma Q, Ebina W, Ptaszek LM, Spater D, Xu H, Tabebordbar M, Gorbatov R, Sena B, Nahrendorf M, Briscoe DM, Li RA, Wagers AJ, Rossi DJ, Pu WT, Chien KR.. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat Biotechnol 2013;31:898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Smart N, Bollini S, Dube KN, Vieira JM, Zhou B, Davidson S, Yellon D, Riegler J, Price AN, Lythgoe MF, Pu WT, Riley PR.. De novo cardiomyocytes from within the activated adult heart after injury. Nature 2011;474:640–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, Lan F, Diecke S, Huber B, Mordwinkin NM, Plews JR, Abilez OJ, Cui B, Gold JD, Wu JC.. Chemically defined generation of human cardiomyocytes. Nat Methods 2014;11:855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Fu Y, Huang C, Xu X, Gu H, Ye Y, Jiang C, Qiu Z, Xie X.. Direct reprogramming of mouse fibroblasts into cardiomyocytes with chemical cocktails. Cell Res 2015;25:1013–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cao N, Huang Y, Zheng J, Spencer CI, Zhang Y, Fu JD, Nie B, Xie M, Zhang M, Wang H, Ma T, Xu T, Shi G, Srivastava D, Ding S.. Conversion of human fibroblasts into functional cardiomyocytes by small molecules. Science 2016;352:1216–1220. [DOI] [PubMed] [Google Scholar]
- 109. KalantarMotamedi Y, Peymani M, Baharvand H, Nasr-Esfahani MH, Bender A.. Systematic selection of small molecules to promote differentiation of embryonic stem cells and experimental validation for generating cardiomyocytes. Cell Death Discov 2016;2:16007.. [DOI] [PMC free article] [PubMed] [Google Scholar]