Abstract
Tumour necrosis factor alpha (TNFα) is a cytokine that is pivotal in the inflammatory response. Blockade of TNFα has been shown to be effective in a number of human autoimmune diseases, including rheumatoid arthritis, raising the question of whether this approach may be effective in inflammatory kidney disease, such as ANCA-associated vasculitis (AAV). In AAV, there is considerable evidence for the role of TNFα in the pathophysiology of disease, including increased expression of TNFα mRNA in leucocytes and in renal tissue. Importantly, TNFα can induce leucocyte cell membrane expression of the autoantigens involved in vasculitis [proteinase 3 and myeloperoxidase (MPO)], thus priming cells for the effects of ANCA. In rodent models of anti-GBM disease (nephrotoxic nephritis), TNFα enhances glomerular injury and TNFα blockade using soluble TNFα receptor or anti-TNFα antibody ameliorates disease. Mice deficient in TNFα are protected from nephrotoxic nephritis and this effect is dependent mainly on intrinsic renal cells. A mouse model of anti-MPO antibody-induced glomerulonephritis is enhanced by LPS, and this effect is blocked by anti-TNFα antibody. In a rat model of AAV induced by MPO (experimental autoimmune vasculitis), anti-TNFα antibody improves renal pathology and also reduces leucocyte transmigration, as shown by intravital microscopy. In clinical studies, the Wegener's Granulomatosis Etanercept Trial (WGET) showed no benefit of additional etanercept versus standard therapy. However, there are several reasons why the results of the WGET study do not rule out the use of anti-TNFα antibody in acute renal AAV, including the study design and the considerable biological differences between the effects of etanercept and anti-TNFα antibody. There are several clinical studies demonstrating a response to anti-TNFα antibody in patients with AAV refractory to conventional treatment, and in some of these, the addition of anti-TNFα antibody was the only change in treatment. We suggest that further investigation of TNFα blockade in AAV is warranted.
Keywords: ANCA, glomerulonephritis, TNFα, TNFα blockade, vasculitis
INTRODUCTION
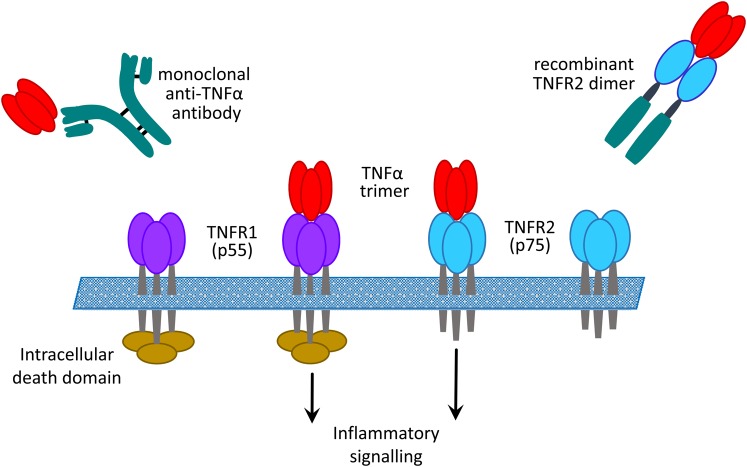
Tumour necrosis factor alpha (TNFα) is a pleiotropic cytokine that plays a central role in inflammation and leads to the production of a wide range of other pro-inflammatory cytokines and chemokines. It also has important immune regulatory functions and can induce a number of anti-inflammatory and regulatory cytokines. TNFα is a 26 kDa transmembrane protein that can be cleaved by TNFα-converting enzyme into a soluble 17 kDa form. TNFα binds to two cell surface receptors, TNFR1 (CD120A, p55) and TNFR2 (CD120B, P75). These receptors engage different signalling pathways, with TNFR1 having a death domain on its intracellular region, whereas TNFR2 does not. Both receptors can also be cleaved from the cell surface and may circulate on their own or bound to TNFα. It has been suggested that they might act as decoys for TNFα, although they might have their own immunomodulatory functions. Binding of TNFα to its receptors can be inhibited by either monoclonal antibodies or soluble forms of the receptor (Figure 1). Early clinical and experimental studies in rheumatoid arthritis (RA) led to the identification of the TNFα-dependent cytokine cascade and subsequent trials of TNFα blockade in patients with treatment resistant disease. This approach rapidly proved effective, and since then TNFα blockade has been approved in RA and a wide range of other chronic inflammatory diseases [1]. Several studies have implicated TNFα or TNFRs in a range of different kidney diseases, and this topic has been previously reviewed [2, 3].
FIGURE 1:
Inhibition of binding of TNFα to its receptors by monoclonal anti-TNFα antibody or soluble recombinant receptor.
There has been particular interest in the possible role of TNFα blockade in ANCA-associated vasculitis (AAV) and other forms of rapidly progressive glomerulonephritis (RPGN) [4, 5]. There is considerable evidence for the role of TNFα in pathogenesis of AAV from both clinical and experimental studies. For example, it has been shown that TNFα mRNA is upregulated in both peripheral blood mononuclear cells and in renal tissue from patients with granulomatosis with polyangiitis (GPA) [6, 7]. In particular, CD4 T cells in the granulomas found in GPA secrete large amounts of Th1 cytokines, including TNFα [8]. In vitro studies have shown that TNFα is important in sensitizing neutrophils and monocytes to the effects of ANCA. Priming these cells with TNFα induces cell membrane expression of proteinase 3 (PR3) and myeloperoxidase (MPO) where they are accessible to binding with ANCA [9]. This subsequently leads to pro-inflammatory consequences, including neutrophil degranulation and production of reactive oxygen species, and also dysregulation of apoptosis and the development of netosis, which contributes to tissue damage and perpetuation of the autoimmune response [10]. TNFα primes endothelial cells and promotes leucocyte endothelial adhesion by inducing expression of selectins and integrins [11]. TNFα therefore plays a part in the endothelial damage induced by ANCA-activated neutrophils. The TNFRs are also overexpressed at sites of inflammation in AAV [12] and increased levels of TNFRs have been associated with progression of other types of glomerulonephritis (GN) [13, 14]. Although both types of TNFR contribute to glomerular injury, studies in knockout mice suggest that TNFR1 is more important in activation of intrinsic renal cells by soluble TNFα [15]. We will consider whether TNFα blockade might be an effective approach in AAV and GN by reviewing its role in experimental models and clinical studies.
ROLE OF TNFα IN EXPERIMENTAL GLOMERULONEPHRITIS AND VASCULITIS
Investigation of the effects of TNFα in experimental GN was originally prompted by clinical observations that intercurrent infection exaggerated renal injury in several types of GN, including IgA nephropathy and AAV. In one early study, it was shown that the administration of even small doses of TNFα increased glomerular damage in the heterologous phase of nephrotoxic nephritis (NTN) in the Sprague-Dawley (SD) rat [16], a model that is not dependent on the development of ANCA, but that may be used to study the mechanisms of renal inflammation in crescentic nephritis. In subsequent experiments, it was shown that treatment with soluble TNF receptor (sTNFr p55) reduced glomerular injury in LPS-enhanced NTN. This was accompanied by a reduction in glomerular IL-1β expression [17]. In another early study, TNF-binding protein, a dimeric form of the soluble receptor, was found to reduce glomerular injury in accelerated NTN in the SD rat, and decreased glomerular expression and circulating levels of macrophage migration inhibitory factor (MIF) [18]. TNFα blockade can therefore reduce acute glomerular inflammation and also modulate production of other pro-inflammatory cytokines (Table 1).
Table 1.
Tumour necrosis factor alpha (TNFα) blockade in experimental glomerulonephritis (GN) and vasculitis
| Author | Model | Agent | Outcome |
|---|---|---|---|
| Karkar [17] | SD rat NTN | sTNFr p55 | Reduced glomerular inflammation and IL-1β expression |
| Lan [18] | SD rat NTN | TNF binding protein | Reduced glomerular inflammation and MIF expression |
| Karkar [19] | WKY rat NTN | sTNFr p55 | Prevention: abrogation of crescents Treatment: reduced crescents and IL-1β |
| Khan [20] | WKY rat NTN | Anti-TNFα mAb | Prevention: reduced crescents and urine MCP-1 Treatment: reduced glomerular injury and interstitial scarring |
| Le Hir [21] | TNF−/− mouse NTN | – | Knockout protected from crescents |
| Timoshanko [22] | TNF−/− chimeric mice NTN | – | Intrinsic renal cells major source of TNF with contribution from leucocytes |
| Little [23] | WKY rat EAV | Anti-TNFα mAb | Abrogation of crescents Improvement lung haemorrhage Inhibition leucocyte transmigration |
| Huugen [24] | Mouse anti-MPO GN | Anti-TNFα mAb | Reduced glomerular inflammation |
SD, Sprague-Dawley; WKY, Wistar Kyoto; NTN, nephrotoxic nephritis; EAV, experimental autoimmune vasculitis; MPO, myeloperoxidase; MIF, migration inhibitory factor; sTNFr, soluble TNF receptor; MCP-1, monocyte chemoattractant protein 1.
The role of TNFα was subsequently investigated in a more robust model of crescentic nephritis induced by nephrotoxic serum in the Wistar Kyoto (WKY) rat. In this model, preventive treatment with sTNFr p55 significantly improved glomerular disease and completely prevented crescent formation. If treatment was delayed until 4 days after induction of nephritis, when hypercellularity is well established, there was still a marked reduction in albuminuria and crescent formation. This was again accompanied by reduced glomerular expression of IL-1β [19]. A blocking monoclonal antibody to rat TNFα has also been investigated in the same model. When given from day 0 or day 4 to day 14, there was improved renal function together with a significant reduction in crescents. When treatment was extended from day 4 to day 28, there was preservation of renal function and significantly less glomerular and tubulointerstitial scarring. Even when treatment was delayed until day 14, the time of peak crescent formation, there was still preserved renal function and reduced interstitial scarring by day 28. This was accompanied throughout by a reduction in urinary monocyte chemoattractant protein 1 (MCP-1) [20]. This work demonstrated the benefit of blocking endogenous TNFα in severe crescentic GN and, importantly, in the subsequent development of interstitial fibrosis.
Although nephrotoxic nephritis in mice does not produce such robust crescent formation, it does allow investigation of knockout strains. In an early study, accelerated NTN was induced in TNFα knockout and wild-type mice. The TNFα knockout mice developed significantly less glomerular pathology and in particular showed less T-cell infiltration and significantly fewer crescents [21]. In subsequent work, bone marrow transplantation was used to investigate the importance of circulating leucocytes versus intrinsic renal cells. In accelerated NTN, TNFα knockout animals showed greatest protection from disease, while wild-type to knockout chimeras showed less disease than knockout to wild-type chimeras [22]. This study suggests that intrinsic renal cells are the major cellular source of TNFα in this model, although circulating leucocytes also contribute.
More recently, the role of TNFα blockade has been investigated in rodent models of AAV that may more accurately reproduce the biology of human disease. Experimental autoimmune vasculitis (EAV) can be induced by immunizing WKY rats with human MPO in adjuvant. Animals develop circulating anti-MPO antibodies reactive with rat neutrophils, together with pauci-immune focal crescentic GN and alveolar haemorrhage. Using intravital microscopy of mesenteric vessels, we showed that anti-MPO antibodies in EAV enhanced leucocyte adhesion and transmigration in vivo in response to the chemokine CXCL1. A blocking monoclonal antibody to rat TNFα significantly reduced leucocyte transmigration and this was accompanied by abrogation of crescent formation and reduction in lung haemorrhage [23]. This work demonstrates one possible mechanism of action of TNFα blockade in systemic vasculitis.
A model of anti-MPO antibody-induced GN has been developed in mice. In this model, anti-MPO antibodies raised in MPO knockout mice are transferred to naive recipients, which go on to develop pauci-immune necrotizing GN. The severity of renal injury in this model can be enhanced by LPS and the severity of glomerular injury in the enhanced model can be reduced by administration of an anti-TNFα monoclonal antibody [24]. In attempts to induce a similar model using anti-PR3 antibodies, although there was no glomerular injury, the intradermal injection of TNFα triggered local inflammation in these animals [25]. These studies further support the role of TNFα in ANCA-associated inflammation.
The role of TNFα in murine models of systemic lupus erythematosus (SLE) is more complicated. In NZB/W mice, which show reduced production of TNFα, administration of recombinant TNFα early in life has a protective effect against lupus nephritis [26]. It is likely that this is due to regulatory effects on the autoimmune response. However, later in the disease course in NZB/W mice, TNFα has the effect of worsening the renal disease, presumably due to its pro-inflammatory effects [27]. In the MRL/lpr mouse, TNFα is overexpressed and levels of TNFα correlate with the degree of renal injury [28]. Anti-TNFα antibody was shown to be of benefit in several clinical features of this model. It also reduces the severity of nephritis in the C3H.SW mouse model of SLE induced by injection of anti-DNA antibody [29].
CLINICAL STUDIES OF ANTI-TNFα THERAPIES IN ANCA-ASSOCIATED VASCULITIS AND GLOMERULONEPHRITIS
ANCA-associated vasculitis
A number of anti-TNFα therapies are now approved for clinical use in non-renal autoimmune and inflammatory conditions, such as RA, the seronegative arthritides and inflammatory bowel disease (IBD) (Table 2). The use of three agents—adalimumab, etanercept and infliximab—has been reported in AAV, the latter drug being most commonly employed (Table 3). The majority of reports are of compassionate, off-label use in resistant or refractory disease, where anti-TNFα therapy has been added to conventional treatment with corticosteroids and immunosuppression. While these small series have clear limitations, being uncontrolled, including a diverse case mix, and employing variable definitions of refractory disease and remission, many appeared to suggest that anti-TNFα therapy has biological activity and potential clinical benefit in AAV. One of the largest prospective cohort studies, for example, showed that introduction of infliximab was associated with attaining disease remission in 88% of patients with severe disease, or who had previously shown an incomplete response to steroids and cytotoxic treatment [36]. In the latter group, addition of infliximab was the only change in therapy prior to improvement. A parallel mechanistic study suggested that infliximab treatment was associated with improvements in vascular endothelial dysfunction and circulating markers of disease activity [43]. Similarly, the use of adalimumab, as adjuvant therapy with cyclophosphamide and corticosteroids, was associated with high rates of early remission (∼80% at 3 months) and the ability to significantly reduce steroid dose during induction treatment [40]. Infectious episodes, occasionally severe, were observed in these series, though in patients with a burden of disease-related damage and previous immunosuppressive exposure; these cannot be wholly attributed to anti-TNFα treatment.
Table 2.
Anti-tumour necrosis factor alpha (TNFα) therapies in current clinical use
| Agent | Description | EMA licenced indications |
|---|---|---|
| Infliximab (Remicade®) | Mouse–human chimeric monoclonal antibody | Rheumatoid arthritis Ankylosing spondylitis Psoriatic arthritis Psoriasis Inflammatory bowel disease |
| Adalimumab (Humira®) | Human monoclonal antibody | Rheumatoid arthritis Juvenile idiopathic arthritis Ankylosing spondylitis Psoriatic arthritis Psoriasis Inflammatory bowel disease Hidradenitis suppurativa |
| Etanercept (Enbrel®) | Soluble TNFα-receptor-Fc′ fusion protein | Rheumatoid arthritis Juvenile idiopathic arthritis Ankylosing spondylitis Psoriatic arthritis Psoriasis |
| Golimumab (Simponi®) | Human monoclonal antibody | Rheumatoid arthritis Ankylosing spondylitis Psoriatic arthritis Ulcerative colitis |
| Certolizumab (Cimzia®) | Humanized mouse monoclonal Fab′ fragment; polyethylene glycolated | Rheumatoid arthritis |
EMA, European Medicines Agency.
Table 3.
Clinical studies of anti-tumour necrosis factor alpha (TNFα) therapies in ANCA-associated vasculitis (AAV)
| Study, design | Agent, dose, duration | N | Indication | Key outcomes | Adverse events and other comments |
|---|---|---|---|---|---|
| Stone [30] Open-label, prospective cohort study |
ETN 25 mg Twice per week for 6 months |
20 All GPA Renal involvement in 4/20 |
Active (n= 6) or persistent disease (n= 14) ETN added to CS and other IS |
|
|
| Booth [31] Case series |
IFX 200 mg Monthly for 3 months |
6 3 GPA, 3 MPA Proportion with renal involvement not reported |
Refractory disease IFX added to CS and other IS (not specified) |
|
|
| Bartolucci [32] Open-label, uncontrolled pilot study |
IFX 5 mg/kg Weeks 0, 2, 6 Then every 8 weeks |
10 2 RA, 7 GPA, 1 MC No GPA with renal involvement |
Refractory disease IFX added to CS for first 6 weeks, then added other IS (included CYC/AZA/MTX/MMF) |
|
|
| Lamprecht [33] Cases series |
IFX 3–5 mg/kg Weeks 0, 2, 6 Then every 4 weeks |
6 All GPA Renal involvement in 4/6 |
Refractory disease IFX added to CS and CYC |
|
|
| Zaenker [34] Case report |
IFX 4 mg/kg Weeks 0, 2, 6, 10 |
1 MPA With renal involvement |
Active disease IFX added to iv and oral CS only; AZA for maintenance |
|
|
| Kleinert [35] Case report |
IFX 3 mg/kg 6 doses in 6 months |
1 GPA With renal involvement |
Refractory disease IFX added to CS and CYC/AZA |
|
|
| Booth [36] Open-label, prospective cohort study |
IFX 5 mg/kg Weeks 0, 2, 6, 10 |
Study 1: 16 7 GPA, 9 MPA 13/16 with renal involvement Study 2: 16 12 GPA, 4 MPA Proportion with renal involvement not stated |
Study 1: Adjuvant therapy (with CYC and CS) for remission induction in new or relapsing active disease Study 2: Additional therapy for persistent disease despite 3 month therapy with CS and CYC/AZA/MTX/MMF |
|
|
| WGET [37] Prospective, randomized, placebo-controlled trial |
ETN 25 mg Twice per week for study duration |
89 (versus 92 in control group) All GPA 53% with renal involvement |
Remission maintenance ETN as adjuvant therapy with CS and CYC/MTX |
|
|
| Josselin [39] Case series |
IFX 5 mg/kg Weeks 0, 2, 6 Then 4–6 weekly |
15 10 GPA, 1 MPA 3 RA, 1 MC (Includes nine patients previously reported by Bartolucci) No AAV cases had renal involvement |
Refractory disease IFX added to CS and other IS |
|
|
| Laurino [40] Open-label, prospective cohort study |
ADA 40 mg Every 2 weeks for 3 months |
14 9 GPA, 5 MPA All had renal involvement |
Active disease ADA as adjuvant therapy with CS and conventional IS |
|
|
| Morgan [41] Open-label, non-randomized prospective cohort study |
IFX 5 mg/kg weeks 0, 2, 6, 10 |
16 11 GPA, 5 MPA 11/16 with renal involvement |
Active disease IFX with CS and CYC/MMF |
|
|
| de Menthon [42] Open-label, prospective, randomized trial of IFX versus RTX |
IFX 3–5 mg/kg weeks 0, 2, 6 Then every 4 weeks |
9 All GPA 3/9 with renal involvement (8 GPA with RTX) |
Refractory disease IFX added to CS and other IS (including CYC/AZA/MTX/CMX) |
|
|
IFX, infliximab; ETN, etanercept; ADA, adalimumab; GPA, granulomatosis with polyangiitis; MPA, microscopic polyangiitis; RA, rheumatoid arthritis; MC, mixed essential cryoglobulinaemia; CYC, cyclophosphamide; AZA, azathioprine; MTX, methotrexate; MMF, mycophenolate mofetil; RTX, rituximab; PEX, plasma exchange; CS, corticosteroids; IS, immunosuppression; CMX, co-trimoxazole; BVAS, Birmingham Vasculitis Activity Score; WG, Wegener's granulomatosis; MI, myocardial infarction.
The promising observations of these early series, however, were not fully borne out in the two randomized trials of anti-TNFα therapy in AAV that have been conducted to date. A small randomized study compared the use of infliximab and rituximab in refractory GPA. Approximately one-third of patients had a treatment response to infliximab, although response rates to rituximab were higher (50%), and thus this study is regarded as having negative outcome for the use of infliximab [42]. A much larger randomized, placebo-controlled study examined the use of etanercept as add-on therapy to conventional treatment with corticosteroids and either methotrexate or cyclophosphamide, for maintenance of remission, in a large cohort of patients with GPA (the WGET study) [37]. This study found no benefit of etanercept in this context. Reassuringly, there was no difference in the rate of infectious complications between the intervention and placebo groups, though of concern when the study was first reported, an excess of solid cancers was observed in the etanercept-treated group.
Based on the findings of these studies, and in particular the WGET trial, many now regard anti-TNFα therapy as a failed strategy in AAV. The important limitations of these trials should, however, be considered before abandoning this therapeutic approach. Since the publication of the former study [42], rituximab has gone on to become an established and effective treatment in AAV, for both remission induction and remission maintenance. Failure to demonstrate superiority to rituximab, therefore, may not necessarily imply an absence of any biologic or therapeutic effect. In the WGET study [37], etanercept was used as ‘add-on’ therapy, along with standard treatment, in a cohort of patients with moderately severe disease (only 50% had renal involvement and Birmingham Vasculitis Activity Scores were relatively low at enrolment). Thus, the potential effect of anti-TNFα treatment may not have been apparent in a group of patients that was likely to respond well to conventional therapy. In addition, differences in disease response have been observed with different anti-TNFα agents in other conditions—it is recognized, for example, that etanercept is less effective than infliximab in granulomatous diseases, including Crohn's disease [44], and WGET enrolled only patients with GPA and none with MPA. This variation in disease response may be due to differences in pharmacokinetics and dynamics between the different drugs. For example, etanercept can only interact with soluble TNFα, whereas infliximab may interact with cell membrane-bound forms, and other functional binding differences are described [45]. Thus, the efficacy, or lack thereof, of etanercept should not be used to determine the validity of this general approach with other agents, and it is notable that the uncontrolled studies that suggested benefit of anti-TNFα treatment in refractory disease all employed monoclonal antibody preparations rather than the soluble fusion protein. Finally, concerns regarding the risk of malignancy with anti-TNFα treatment in AAV may have been overstated—a subsequent analysis of the WGET study, with an extended period of follow-up [38], suggested that the increased risk of cancer was not different between the intervention and placebo groups when compared with the general population, and that this risk could not be attributed solely to etanerept treatment, as overall duration of disease, past history of malignancy prior to trial enrolment and previous cyclophosphamide exposure also played a significant role. In addition, there is conflicting evidence regarding the increased risk of malignancy following anti-TNFα therapy in much larger cohorts of patients with rheumatoid arthritis [46, 47].
For these reasons, it remains possible that anti-TNFα therapy has a role in the management of AAV, potentially for refractory disease, or as a steroid sparing agent during the induction phase of treatment. A recent trial (NCT01363388), for example, studied the use of a C5a receptor antagonist as a substitute for corticosteroids, rather than as ‘add-on’ therapy, and a similar approach could be employed using anti-TNFα treatment. Alternatively, it may be that specific disease manifestations should be considered for anti-TNFα therapy. Recent reviews of the ophthalmology literature, for example, indicate that anti-TNFα treatment may be of particular benefit in scleritis and retinal vasculitis [48, 49], and experience at our centre suggests that anti-TNFα may be a useful strategy for resistant ocular manifestations in AAV. In the future, well-designed clinical studies, along with identification of biomarkers or other disease features that predict response to targeted anti-TNFα therapy, may allow us to better define how these agents could be used in the management of AAV.
Anti-TNFα therapy in other glomerulonephritides
Systemic lupus erythematosus
While preclinical data strongly implicate TNFα in the pathogenesis of crescentic GN and AAV, the rationale for targeting TNFα in SLE is less clear, given the protective role for TNFα described in some murine models of lupus, and the reported development of lupus-like serology, sometimes associated with clinical manifestations, in large cohorts of patients treated with anti-TNFα drugs for other indications (discussed further below). Taken together, these studies suggest that TNFα may have a role in suppressing the development of autoimmunity. Conversely, circulating TNFα levels are increased in patients with SLE and levels correlate with disease activity, and intra-renal TNFα expression appears to correlate with the severity of lupus nephritis (reviewed in [50]), suggesting that it may have a role in mediating end-organ damage once autoimmunity is established, and on which basis it may represent a therapeutic target. Three small, uncontrolled, open-label, prospective studies of anti-TNFα treatment in SLE have been published [51–53]. All employed infliximab in addition to conventional therapy, and all reported moderate clinical responses following short-term treatment, including in patients with active lupus nephritis. Notably, increases in anti-dsDNA and anti-cardiolipin antibody levels were seen in some patients. They did not appear to correlate with disease activity or relapse risk, although thrombotic events were reported. Two randomized controlled trials of anti-TNFα treatment for lupus nephritis were initiated in 2006/2007, although both were terminated before completion: one study failed to recruit well (NCT00368264) and the other was terminated due to concerns regarding risk–benefit ratio of treatment (NCT00447265). Given the number of other biologic targets currently under investigation in SLE, and concerns regarding the potential for anti-TNFα to exacerbate or trigger disease flares, it seems unlikely that anti-TNFα agents will be extensively investigated for the treatment of lupus in the future.
Focal segmental glomerulosclerosis
TNFα has been implicated in the pathogenesis of focal segmental glomerulosclerosis (FSGS) since the description of elevated plasma and urine levels of TNFα in patients with nephrotic syndrome secondary to FSGS and minimal change disease in the 1990s [54]. In a subsequent study, spontaneous TNFα production by cultured peripheral blood mononuclear cells isolated from patients with FSGS correlated with the severity of proteinuria and the degree of glomerulosclerosis observed on renal biopsy [55]. Successful treatment, using infliximab and etanercept, of recurrent FSGS in a paediatric renal transplant recipient has been described in an isolated case report [56], and long-term follow-up of a phase I study of adalimumab in resistant FGSG suggested attenuation of renal function decline in a proportion of patients [57]. However, a subsequent phase 2 study failed to recruit well, or to identify any benefit in those patients analysed [58]. To the best of our knowledge, there are no ongoing studies of anti-TNFα therapy in FSGS.
Safety of anti-TNFα therapy
The development of circulating autoantibodies after anti-TNFα therapy is a well-recognized occurrence. The detection of antinuclear antibodies, for example, in patients treated with anti-TNFα therapy for RA or IBD in clinical trials, has been reported at rates of 29–77% after infliximab, 11–36% with etanercept and 13% with adalinumab [59]. Anti-double-stranded DNA and anti-phospholipid antibodies are also reported, but at lower frequencies. It has been suggested that TNFα inhibition skews the immune response towards a Th2 response that favours IL-10 and autoantibody production. In addition, anti-TNFα therapy may induce apoptosis and the release of autoantigens from inflammatory cells, thus promoting autoantibody production [60]. Reliable data on the frequency of clinical manifestations associated with these autoantibodies are scarce, however, mostly being limited to anecdotal case reports and series. A registry-initiated study identified 22 cases of drug-associated lupus syndromes in an estimated population of 10 000 patients treated with either infliximab or etanercept for inflammatory arthritis in France until 2003, though as a retrospective, survey-based analysis, it may underestimate the true incidence of this phenomenon [61]. In this cohort, cutaneous disease manifestations predominated (no patients had lupus nephritis), developing on average 9 months into anti-TNFα therapy and resolving in almost all cases with cessation of treatment. A subsequent literature review identified 105 cases of anti-TNFα therapy-associated lupus, with a similar time and pattern of clinical presentation, and low rates of renal involvement (∼5%) [62]. In both studies, both monoclonal antibody and soluble receptor therapies were equally implicated in the development of lupus-like disease. The latter review also identified several other autoimmune conditions associated with anti-TNFα therapy, including cutaneous vasculitis, interstitial lung disease and psoriasis. There are also cases of various other renal and glomerular disorders associated with anti-TNFα therapy, including minimal change disease, membranous nephropathy, IgA nephropathy and pauci-immune necrotizing GN [63–65]. It may be difficult to confirm causality in these cases, since many of the underlying inflammatory diseases for which anti-TNFα therapy is prescribed are themselves associated with other autoimmune and glomerular disorders. We suggest that patients should be carefully monitored for the development of new autoimmune phenomena and renal disease after initiating anti-TNFα therapy, particularly in the first 12 months, and that treatment should be withdrawn should these develop. In the future, systematic, prospective, registry-based studies may define the true extent of autoimmune syndromes associated with anti-TNFα therapy.
The risk of infectious complications, particularly mycobacterial infection [66], following anti-TNFα therapy in non-renal disorders is well recognized, and should not be forgotten when adopting this approach in AAV. Such patients are increasingly likely to have been exposed to prior immunosuppressive therapy, including biologic agents, and a significant proportion will have additional, functional immune-paresis related to chronic kidney disease, and already heightened risk of mycobacterial infection [67]. However, as discussed above, concerns about the development of malignancy have probably been overstated.
CONCLUSIONS
The available experimental and clinical data provide strong evidence that TNFα contributes to the pathogenesis of various forms of GN, and AAV in particular. Despite demonstrating remarkable efficacy in non-renal diseases such as rheumatoid arthritis and inflammatory bowel disease, anti-TNFα therapies have not found an established role in the treatment of glomerular disease or vasculitis. Uncontrolled studies suggest that anti-TNFα agents have biological activity and potential therapeutic benefit in AAV (and lupus nephritis and FSGS), though the only large randomized, controlled trial to date (WGET) failed to show any benefit in preventing disease relapse in AAV. This negative outcome, the subsequent explosion in other drug targets and biologic therapies, and potential concerns about their role in inducing autoimmunity have meant that anti-TNFα agents are now largely discounted as a therapeutic option in AAV. This conclusion, however, may have been premature: limitations in trial design, differences in bio-activity of individual drugs and failure to consider specific diseases or disease phenotypes that are most likely to respond to therapy may mean that we have not yet identified how best to use these agents. A clearer understanding of the role of TNFα in the pathogenesis of the spectrum of GN may, in the future, open new avenues for clinical studies and prospective trials. Within the field of AAV in particular, future studies might also address whether anti-TNFα drugs have a role in treating refractory disease, as steroid or cytotoxic sparing agents, or in managing specific disease manifestations. They might also clarify concerns regarding the potential induction of autoimmunity using these drugs. Pending such studies, we highlight the published experience of anti-TNFα use in refractory disease, and remind clinicians that this remains a possible approach in the management of critical disease unresponsive to the conventional repertoire of immunosuppressant treatment.
ACKNOWLEDGEMENTS
We acknowledge support from the NIHR Imperial Biomedical Research Centre.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Feldmann M, Pusey CD. Is there a role for TNF-alpha in anti-neutrophil cytoplasmic antibody-associated vasculitis? Lessons from other chronic inflammatory diseases. J Am Soc Nephrol 2006; 17: 1243–1252 [DOI] [PubMed] [Google Scholar]
- 2. Speeckaert MM, Speeckaert R, Laute M, et al. Tumor necrosis factor receptors: biology and therapeutic potential in kidney diseases. Am J Nephrol 2012; 36: 261–270 [DOI] [PubMed] [Google Scholar]
- 3. Vielhauer V, Mayadas TN. Functions of TNF and its receptors in renal disease: distinct roles in inflammatory tissue injury and immune regulation. Semin Nephrol 2007; 27: 286–308 [DOI] [PubMed] [Google Scholar]
- 4. Jarrot PA, Kaplanski G. Anti-TNF-alpha therapy and systemic vasculitis. Mediators Inflamm 2014; 2014: 493593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mukhtyar C, Luqmani R. Current state of tumour necrosis factor {alpha} blockade in Wegener's granulomatosis. Ann Rheum Dis 2005; 64 (Suppl 4): iv31–iv36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deguchi Y, Shibata N, Kishimoto S. Enhanced expression of the tumour necrosis factor/cachectin gene in peripheral blood mononuclear cells from patients with systemic vasculitis. Clin Exp Immunol 1990; 81: 311–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Noronha IL, Kruger C, Andrassy K, et al. In situ production of TNF-alpha, IL-1 beta and IL-2R in ANCA-positive glomerulonephritis. Kidney Int 1993; 43: 682–692 [DOI] [PubMed] [Google Scholar]
- 8. Komocsi A, Lamprecht P, Csernok E, et al. Peripheral blood and granuloma CD4(+)CD28(-) T cells are a major source of interferon-gamma and tumor necrosis factor-alpha in Wegener's granulomatosis. Am J Pathol 2002; 160: 1717–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Csernok E, Ernst M, Schmitt W, et al. Activated neutrophils express proteinase 3 on their plasma membrane in vitro and in vivo. Clin Exp Immunol 1994; 95: 244–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kessenbrock K, Krumbholz M, Schonermarck U, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 2009; 15: 623–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mackay F, Loetscher H, Stueber D, et al. Tumor necrosis factor alpha (TNF-alpha)-induced cell adhesion to human endothelial cells is under dominant control of one TNF receptor type, TNF-R55. J Exp Med 1993; 177: 1277–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hasegawa M, Nishii C, Ohashi A, et al. Expression of tumor necrosis factor receptors on granulocytes in patients with myeloperoxidase anti-neutrophil cytoplasmic autoantibody-associated vasculitis. Nephron Clin Pract 2009; 113: c222–c233 [DOI] [PubMed] [Google Scholar]
- 13. Sonoda Y, Gohda T, Suzuki Y, et al. Circulating TNF receptors 1 and 2 are associated with the severity of renal interstitial fibrosis in IgA nephropathy. PLoS One 2015; 10: e0122212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee SM, Yang S, Cha RH, et al. Circulating TNF receptors are significant prognostic biomarkers for idiopathic membranous nephropathy. PLoS One 2014; 9: e104354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Taubitz A, Schwarz M, Eltrich N, et al. Distinct contributions of TNF receptor 1 and 2 to TNF-induced glomerular inflammation in mice. PLoS One 2013; 8: e68167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tomosugi NI, Cashman SJ, Hay H, et al. Modulation of antibody-mediated glomerular injury in vivo by bacterial lipopolysaccharide, tumor necrosis factor, and IL-1. J Immunol 1989; 142: 3083–3090 [PubMed] [Google Scholar]
- 17. Karkar AM, Tam FW, Steinkasserer A, et al. Modulation of antibody-mediated glomerular injury in vivo by IL-1ra, soluble IL-1 receptor, and soluble TNF receptor. Kidney Int 1995; 48: 1738–1746 [DOI] [PubMed] [Google Scholar]
- 18. Lan HY, Yang N, Metz C, et al. TNF-alpha up-regulates renal MIF expression in rat crescentic glomerulonephritis. Mol Med 1997; 3: 136–144 [PMC free article] [PubMed] [Google Scholar]
- 19. Karkar AM, Smith J, Pusey CD. Prevention and treatment of experimental crescentic glomerulonephritis by blocking tumour necrosis factor-alpha. Nephrol Dial Transplant 2001; 16: 518–524 [DOI] [PubMed] [Google Scholar]
- 20. Khan SB, Cook HT, Bhangal G, et al. Antibody blockade of TNF-alpha reduces inflammation and scarring in experimental crescentic glomerulonephritis. Kidney Int 2005; 67: 1812–1820 [DOI] [PubMed] [Google Scholar]
- 21. Le Hir M, Haas C, Marino M, et al. Prevention of crescentic glomerulonephritis induced by anti-glomerular membrane antibody in tumor necrosis factor-deficient mice. Lab Invest 1998; 78: 1625–1631 [PubMed] [Google Scholar]
- 22. Timoshanko JR, Sedgwick JD, Holdsworth SR, et al. Intrinsic renal cells are the major source of tumor necrosis factor contributing to renal injury in murine crescentic glomerulonephritis. J Am Soc Nephrol 2003; 14: 1785–1793 [DOI] [PubMed] [Google Scholar]
- 23. Little MA, Bhangal G, Smyth CL, et al. Therapeutic effect of anti-TNF-alpha antibodies in an experimental model of anti-neutrophil cytoplasm antibody-associated systemic vasculitis. J Am Soc Nephrol 2006; 17: 160–169 [DOI] [PubMed] [Google Scholar]
- 24. Huugen D, Xiao H, van Esch A, et al. Aggravation of anti-myeloperoxidase antibody-induced glomerulonephritis by bacterial lipopolysaccharide: role of tumor necrosis factor-alpha. Am J Pathol 2005; 167: 47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pfister H, Ollert M, Frohlich LF, et al. Antineutrophil cytoplasmic autoantibodies against the murine homolog of proteinase 3 (Wegener autoantigen) are pathogenic in vivo. Blood 2004; 104: 1411–1418 [DOI] [PubMed] [Google Scholar]
- 26. Jacob CO, McDevitt HO. Tumour necrosis factor-alpha in murine autoimmune ‘lupus’ nephritis. Nature 1988; 331: 356–358 [DOI] [PubMed] [Google Scholar]
- 27. Brennan DC, Yui MA, Wuthrich RP, et al. Tumor necrosis factor and IL-1 in New Zealand Black/White mice. Enhanced gene expression and acceleration of renal injury. J Immunol 1989; 143: 3470–3475 [PubMed] [Google Scholar]
- 28. Yokoyama H, Kreft B, Kelley VR. Biphasic increase in circulating and renal TNF-alpha in MRL-lpr mice with differing regulatory mechanisms. Kidney Int 1995; 47: 122–130 [DOI] [PubMed] [Google Scholar]
- 29. Segal R, Dayan M, Zinger H, et al. Suppression of experimental systemic lupus erythematosus (SLE) in mice via TNF inhibition by an anti-TNFalpha monoclonal antibody and by pentoxiphylline. Lupus 2001; 10: 23–31 [DOI] [PubMed] [Google Scholar]
- 30. Stone JH, Uhlfelder ML, Hellmann DB, et al. Etanercept combined with conventional treatment in Wegener's granulomatosis: a six-month open-label trial to evaluate safety. Arthritis Rheum 2001; 44: 1149–1154 [DOI] [PubMed] [Google Scholar]
- 31. Booth AD, Jefferson HJ, Ayliffe W, et al. Safety and efficacy of TNFalpha blockade in relapsing vasculitis. Ann Rheum Dis 2002; 61: 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bartolucci P, Ramanoelina J, Cohen P, et al. Efficacy of the anti-TNF-alpha antibody infliximab against refractory systemic vasculitides: an open pilot study on 10 patients. Rheumatology 2002; 41: 1126–1132 [DOI] [PubMed] [Google Scholar]
- 33. Lamprecht P, Voswinkel J, Lilienthal T, et al. Effectiveness of TNF-alpha blockade with infliximab in refractory Wegener's granulomatosis. Rheumatology 2002; 41: 1303–1307 [DOI] [PubMed] [Google Scholar]
- 34. Zaenker M, Arbach O, Helmchen U, et al. Crescentic glomerulonephritis associated with myeloperoxidase-antineutrophil-cytoplasmic antibodies: first report on the efficacy of primary anti-TNF-alpha treatment. Int J Tissue React 2004; 26: 85–92 [PubMed] [Google Scholar]
- 35. Kleinert J, Lorenz M, Kostler W, et al. Refractory Wegener's granulomatosis responds to tumor necrosis factor blockade. Wien Klin Wochenschr 2004; 116: 334–338 [DOI] [PubMed] [Google Scholar]
- 36. Booth A, Harper L, Hammad T, et al. Prospective study of TNFalpha blockade with infliximab in anti-neutrophil cytoplasmic antibody-associated systemic vasculitis. J Am Soc Nephrol 2004; 15: 717–721 [DOI] [PubMed] [Google Scholar]
- 37. Wegener's Granulomatosis Etanercept Trial Research Group. Etanercept plus standard therapy for Wegener's granulomatosis. N Engl J Med 2005; 352: 351–361 [DOI] [PubMed] [Google Scholar]
- 38. Silva F, Seo P, Schroeder DR, et al. Solid malignancies among etanercept-treated patients with granulomatosis with polyangiitis (Wegener's): long-term followup of a multicenter longitudinal cohort. Arthritis Rheum 2011; 63: 2495–2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Josselin L, Mahr A, Cohen P, et al. Infliximab efficacy and safety against refractory systemic necrotising vasculitides: long-term follow-up of 15 patients. Ann Rheum Dis 2008; 67: 1343–1346 [DOI] [PubMed] [Google Scholar]
- 40. Laurino S, Chaudhry A, Booth A, et al. Prospective study of TNFalpha blockade with adalimumab in ANCA-associated systemic vasculitis with renal involvement. Nephrol Dial Transplant 2010; 25: 3307–3314 [DOI] [PubMed] [Google Scholar]
- 41. Morgan MD, Drayson MT, Savage CO, et al. Addition of infliximab to standard therapy for ANCA-associated vasculitis. Nephron Clin Pract 2011; 117: c89–c97 [DOI] [PubMed] [Google Scholar]
- 42. de Menthon M, Cohen P, Pagnoux C, et al. Infliximab or rituximab for refractory Wegener's granulomatosis: long-term follow up. A prospective randomised multicentre study on 17 patients. Clin Exp Rheumatol 2011; 29(1 Suppl 64): S63–S71 [PubMed] [Google Scholar]
- 43. Booth AD, Jayne DR, Kharbanda RK, et al. Infliximab improves endothelial dysfunction in systemic vasculitis: a model of vascular inflammation. Circulation 2004; 109: 1718–1723 [DOI] [PubMed] [Google Scholar]
- 44. Sandborn WJ, Hanauer SB, Katz S, et al. Etanercept for active Crohn's disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology 2001; 121: 1088–1094 [DOI] [PubMed] [Google Scholar]
- 45. Scallon B, Cai A, Solowski N, et al. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J Pharmacol Exp Ther 2002; 301: 418–426 [DOI] [PubMed] [Google Scholar]
- 46. Thompson AE, Rieder SW, Pope JE. Tumor necrosis factor therapy and the risk of serious infection and malignancy in patients with early rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheum 2011; 63: 1479–1485 [DOI] [PubMed] [Google Scholar]
- 47. Bongartz T, Sutton AJ, Sweeting MJ, et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. J Am Med Assoc 2006; 295: 2275–2285 [DOI] [PubMed] [Google Scholar]
- 48. de Fidelix TS, Vieira LA, de Freitas D, et al. Biologic therapy for refractory scleritis: a new treatment perspective. Int Ophthalmol 2015; 35: 903–912 [DOI] [PubMed] [Google Scholar]
- 49. Rosenbaum JT, Sibley CH, Lin P. Retinal vasculitis. Curr Opin Rheumatol 2016; 28: 228–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aringer M, Smolen JS. Therapeutic blockade of TNF in patients with SLE-promising or crazy? Autoimmun Rev 2012; 11: 321–325 [DOI] [PubMed] [Google Scholar]
- 51. Aringer M, Graninger WB, Steiner G, et al. Safety and efficacy of tumor necrosis factor alpha blockade in systemic lupus erythematosus: an open-label study. Arthritis Rheum 2004; 50: 3161–3169 [DOI] [PubMed] [Google Scholar]
- 52. Uppal SS, Hayat SJ, Raghupathy R. Efficacy and safety of infliximab in active SLE: a pilot study. Lupus 2009; 18: 690–697 [DOI] [PubMed] [Google Scholar]
- 53. Matsumura R, Umemiya K, Sugiyama T, et al. Anti-tumor necrosis factor therapy in patients with difficult-to-treat lupus nephritis: a prospective series of nine patients. Clin Exp Rheumatol 2009; 27: 416–421 [PubMed] [Google Scholar]
- 54. Suranyi MG, Guasch A, Hall BM, et al. Elevated levels of tumor necrosis factor-alpha in the nephrotic syndrome in humans. Am J Kidney Dis 1993; 21: 251–259 [DOI] [PubMed] [Google Scholar]
- 55. Bakr A, Shokeir M, El-Chenawi F, et al. Tumor necrosis factor-alpha production from mononuclear cells in nephrotic syndrome. Pediatr Nephrol 2003; 18: 516–520 [DOI] [PubMed] [Google Scholar]
- 56. Leroy S, Guigonis V, Bruckner D, et al. Successful anti-TNFalpha treatment in a child with posttransplant recurrent focal segmental glomerulosclerosis. Am J Transplant 2009; 9: 858–861 [DOI] [PubMed] [Google Scholar]
- 57. Peyser A, Machardy N, Tarapore F, et al. Follow-up of phase I trial of adalimumab and rosiglitazone in FSGS: III. Report of the FONT study group. BMC Nephrol 2010; 11: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Trachtman H, Vento S, Herreshoff E, et al. Efficacy of galactose and adalimumab in patients with resistant focal segmental glomerulosclerosis: report of the font clinical trial group. BMC Nephrol 2015; 16: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Atzeni F, Sarzi-Puttini P. Autoantibody production in patients treated with anti-TNF-alpha. Expert Rev Clin Immunol 2008; 4: 275–280 [DOI] [PubMed] [Google Scholar]
- 60. Atzeni F, Talotta R, Salaffi F, et al. Immunogenicity and autoimmunity during anti-TNF therapy. Autoimmun Rev 2013; 12: 703–708 [DOI] [PubMed] [Google Scholar]
- 61. De Bandt M, Sibilia J, Le Loet X. et al. Systemic lupus erythematosus induced by anti-tumour necrosis factor alpha therapy: a French national survey. Arthritis Res Ther 2005; 7: R545–R551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ramos-Casals M, Brito-Zeron P, Soto MJ, et al. Autoimmune diseases induced by TNF-targeted therapies. Best Pract Res Clin Rheumatol 2008; 22: 847–861 [DOI] [PubMed] [Google Scholar]
- 63. Piga M, Chessa E, Ibba V, et al. Biologics-induced autoimmune renal disorders in chronic inflammatory rheumatic diseases: systematic literature review and analysis of a monocentric cohort. Autoimmun Rev 2014; 13: 873–879 [DOI] [PubMed] [Google Scholar]
- 64. Stokes MB, Foster K, Markowitz GS, et al. Development of glomerulonephritis during anti-TNF-alpha therapy for rheumatoid arthritis. Nephrol Dial Transplant 2005; 20: 1400–1406 [DOI] [PubMed] [Google Scholar]
- 65. Doulton TW, Tucker B, Reardon J, et al. Antineutrophil cytoplasmic antibody-associated necrotizing crescentic glomerulonephritis in a patient receiving treatment with etanercept for severe rheumatoid arthritis. Clin Nephrol 2004; 62: 234–238 [DOI] [PubMed] [Google Scholar]
- 66. Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl JMed 2001; 345: 1098–1104 [DOI] [PubMed] [Google Scholar]
- 67. British Thoracic Society Standards of Care Committee and Joint Tuberculosis Committee, Milburn H, et al. Guidelines for the prevention and management of Mycobacterium tuberculosis infection and disease in adult patients with chronic kidney disease. Thorax 2010; 65: 557–570 [DOI] [PubMed] [Google Scholar]