Abstract
Background
Although shared decision-making (SDM) can better align patient preferences with treatment, barriers remain incompletely understood and the impact on patient satisfaction is unknown.
Methods
This is a qualitative study with semistructured interviews. A purposive sample of prevalent dialysis patients ≥65 years of age at two facilities in Greater Boston were selected for diversity in time from initiation, race, modality and vintage. A codebook was developed and interrater reliability was 89%. Codes were discussed and organized into themes.
Results
A total of 31 interviews with 23 in-center hemodialysis patients, 1 home hemodialysis patient and 7 peritoneal dialysis patients were completed. The mean age was 76 ± 9 years. Two dominant themes (with related subthemes) emerged: decision-making experiences and satisfaction, and barriers to SDM. Subthemes included negative versus positive decision-making experiences, struggling for autonomy, being a ‘good patient’ and lack of choice. In spite of believing that dialysis initiation should be the patient's choice, no patients perceived that they had made a choice. Patients explained that this is due to the perception of imminent death or that the decision to start dialysis belonged to physicians. Clinicians and family frequently overrode patient preferences, with patient autonomy honored mostly to select dialysis modality. Poor decision-making experiences were associated with low treatment satisfaction.
Conclusions
Despite recommendations for SDM, many older patients were unaware that dialysis initiation was voluntary, held mistaken beliefs about their prognosis and were not engaged in decision-making, resulting in poor satisfaction. Patients desired greater information, specifically focusing on the acuity of their choice, prognosis and goals of care.
Keywords: decision-making, dialysis, geriatric nephrology, informed consent, older adults, patient-centered outcomes, qualitative methods, shared decision-making
INTRODUCTION
Between 2000 and 2012, the incident dialysis population in the USA increased by nearly 60%, most dramatically in adults ≥75 years of age [1]. Adults >75 years of age treated with dialysis have 1- and 3-year adjusted survivals of 63 and 33%, respectively, similar to conservative management [2, 3]. Dialysis is an optional, preference-sensitive treatment [4–6] that affects quality of life and is often irreversible. Accordingly, the decision to start dialysis should incorporate shared decision-making (SDM), promoting patient autonomy and helping patients make informed treatment decisions aligned with their preferences and values [3, 7–11]. Despite guidelines calling for the use of SDM in dialysis decision-making [12], many patients initiate dialysis without understanding the long-term implications of their decision. Qualitative studies demonstrate that many older patients feel unprepared for dialysis decision-making and may experience regret following initiation [3, 4, 13–16]. Informed decision-making is significantly less likely to occur in adults >65 years of age compared with younger adults [17, 18]. Although studies demonstrate that patients may not perceive a choice [19], the reasons are not fully understood [20, 21].
Several systematic reviews and qualitative studies indicate that older patients seldom discuss their prognosis with nephrologists and may not identify dialysis initiation as a decision [17, 18, 21–23]. Harwood et al. [24] concluded that patients did not perceive any option as superior and that decision-making should be individualized, informed by values, context and preferences [24]. Examining SDM in qualitative studies of dialysis patients, Hussain et al. [25] determined that while quality of life and survival considerations were important to patients, clinicians tended to focus mostly on biomedical factors and the desire to prolong life [25]. This review demonstrates that patients only appreciated the realities of dialysis after prolonged periods on dialysis, at which point patients questioned their past choices. By then, however, patients were physically dependent on treatment. These reviews highlight an important gap, namely, understanding how SDM affects patient satisfaction and confidence in treatment selection.
We conducted a qualitative study to examine patient perspectives of the dialysis initiation process (i.e. decision to start dialysis) and the relationship between patient engagement and treatment satisfaction, which is inadequately studied in the US context [16]. Older dialysis patients were asked to reflect in extended narrative interviews on the sequence leading them to initiate dialysis and the relationship between decision-making experiences, decisional autonomy and subsequent satisfaction.
MATERIALS AND METHODS
Design, setting and participants
We completed 31 semistructured in-depth interviews of older dialysis patients at two dialysis clinics in Greater Boston between August 2014 and June 2015. Inclusion criteria were receiving maintenance outpatient dialysis (>1 month), age ≥65 years, English speaking and capacity to consent. Participants were selected to ensure diversity in demographics and potential patient experience, sampling by sex, age (70s or younger, 80s and 90s), dialysis vintage (first 3 months, 3 months–2 years and >2 years), modality and race/ethnicity [26]. Our response rate was 86%. Recruitment was conducted in conjunction with analysis and continued until interviews did not yield new insights [27]. Medical record data reflect demographics and treatment at the time of the interview or the most proximate laboratory values drawn routinely for care.
Interviews
K.L. and S.K.W., experts in qualitative methods [28–31], and D.E.W., a nephrologist, developed the semistructured interview guide. Open-ended questions explored how patients learned about and initiated dialysis; whether decisions were informed and autonomous; and treatment implications, advice for future patients and suggestions for improving SDM. Specific probes examined information, prior knowledge about dialysis and end-stage renal disease and decision-making interactions. K.L. and S.K.W. trained research assistants in in-depth interviewing, coding and theme development. Interviewers completed multiple practice interviews with the investigators, were observed during their first interviews and met weekly with K.L. to review interviews. Trained interviewers conducted private face-to-face interviews at dialysis facilities and kept field notes. Interviews were audiotaped and transcribed verbatim. K.L. audited a subsample of transcripts from each interviewer. Interviews lasted ∼1 h. The study was approved by the Tufts University Institutional Review Board.
Qualitative analyses
K.L. and S.K.W. created a preliminary codebook based on the interview's questioning structure. K.L. and S.K.W. then independently coded the first three transcripts using line-by-line coding [32]. Coding differences, refinement and emergent codes were documented and consensus was reached through discussion. The revised codebook was then used by two research assistants who had conducted the interviews. They read and independently coded the initial three transcripts and an additional random sample of five transcripts. These were reviewed with K.L. and S.K.W. The coding team discussed coding discrepancies and amended code descriptions using a team-based consensus process. This final codebook was then applied iteratively to the remaining transcripts. To ensure uniformity in coding, interrater reliability (IRR) was assessed. Fifteen randomly selected transcripts were independently coded, and based on the percentage of characters in a source that the two coders agreed upon, IRR was 89%. These codes were organized into themes and subthemes through iterative deliberation led by S.K.W. and K.L. with D.E.W. and interviewers/coders [32]. We used a combination of inductive and deductive coding of emergent concepts and themes and subthemes, using both open and focused codes. We followed the consolidated criteria for reporting qualitative research (COREQ) [33]. NVivo version 10 (QSR International) was used for coding and analysis. Our study is guided by the core principles of biomedical ethics (autonomy, beneficence, nonmaleficence and justice) and by the socio-ecological model related to SDM [34, 35].
RESULTS
A total of 36 patients were approached; 5 declined, while 1 completed a partial interview (86% participation rate). Of these, 16 were women, 7 received peritoneal dialysis (PD), 23 received in-center hemodialysis (HD) and 1 received home HD (Table 1). All lived at home; 29 were retired (due to age or disability) and 2 still worked. Dialysis vintage ranged from 2 months to 10 years. Two dominant themes emerged: (i) decision-making experiences and treatment satisfaction and (ii) barriers to SDM. The first theme relates decision-making experiences and treatment satisfaction (negative and positive experiences), whereas the second theme describes key barriers to SDM (subthemes: views about choice, struggling for autonomy and being a ‘good patient’). Exemplar quotes are presented in Tables 2 and 3.
Table 1.
Sample characteristics
| Hemodialysis | Peritoneal dialysis | Total | |
|---|---|---|---|
| (n = 24) | (n = 7) | (n = 31) | |
| Age (years) | 77.7 ± 9.8 | 70.9 ± 5.8 | 76.2 ± 9.4 |
| Female sex (%) | 50 | 57 | 52 |
| Dialysis vintage (months) | 24 (13, 54) | 25 (16, 32) | 25 (13, 49) |
| Race (%) | |||
| White | 75 | 71 | 75 |
| African American (%) | 21 | 14 | 19 |
| Asian | 4 | 14 | 6 |
| Hispanic | 8 | 0 | 6 |
| Insurance status (%) | |||
| Medicare primary | 58 | 57 | 58 |
| Dual eligible | 25 | 29 | 26 |
| Private/veterans | 17 | 14 | 16 |
| Primary cause of ESRD (%) | |||
| Diabetes | 57 | 43 | 52 |
| Hypertension | 17 | 29 | 19 |
| Return from transplant | 0 | 29 | 6 |
| Other | 25 | 0 | 23 |
| Laboratory results | |||
| Albumin (g/dL) | 3.8 ± 0.4 | 3.7 ± 0.3 | 3.8 ± 0.3 |
| Hemoglobin (g/dL) | 10.6 ± 0.7 | 11.4 ± 1.3 | 10.8 ± 0.9 |
| Phosphorus (mg/dL) | 5.1 ± 1.2 | 4.7 ± 1.1 | 5.0 ± 1.2 |
One hemodialysis patient performed home hemodialysis. All Medicare primary insurance patients had secondary insurance. Data are mean ± standard deviation or median (25th, 75th percentile) unless otherwise indicated.
Table 2.
Quotes reflecting poor decision-making experiences and patient dissatisfaction with treatment outcomes
| Theme 1: Barriers to SDM |
|---|
| Perceived lack of choice |
| Perceived threat of imminent death |
| ‘It was the only way, to go on dialysis. That was it or I was going to die’ (65–70-year-old woman, PD). |
| ‘… they told me if I didn't have it [dialysis], I'd be dead by morning time… And they told me I did, that I was gonna be dead by morning and everything. So I agreed’ (<65-year-old woman, HD). |
| Perception that is not the patient's role |
| ‘[Doctors] said I needed the treatment… So I had no choice’ (80–90-year-old woman, HD). |
| ‘I was just told to go on dialysis. That was it… I had to do it. There was no ifs, ands about it’ (80–90-year-old man, HD). |
| ‘[Doctor] said that it's time for you to have dialysis. I say okay. He says that's the only way you're going to survive’ (>90-year-old man, HD). |
| ‘My doctor… told me that I had to. I had to do dialysis’ (80–90-year-old woman, PD). |
| Struggle for autonomy |
| Assertive physicians |
| ‘I thought… I do not want to do this. I do not want to do this… I was quite upset. I thought, I'm never gonna do this. I wonder how painful it is, just to forget it and go’ (80–90-year-old woman, PD). |
| ‘I knew myself that if I did not go on dialysis, I wasn't going to live for much longer. So what [nephrologist] told me about it, he asked me, [if] I mind dying, and I said I wasn't afraid of dying. We all die one day. Then he sent me to the clinic’ (75–80-year-old woman, HD). |
| ‘They had a hard time convincing me to take it. I finally did agree, said that's it, because I wasn't well. They kept after me … It was a good couple of months before they convinced me. I said oh well, what can I do now’ (80–90-year-old woman, HD). |
| ‘I didn't really want it, but [nephrologist] said so’ (80–90-year-old man, HD). |
| Limited information |
| ‘The only way I'm able to cope is to have knowledge… Which [physicians] think, you know, if you don't know, that's how you're going to be able to cope’ (65–70-year-old woman, HD). |
| Being a ‘good patient’ |
| ‘I'm here. I'm into the ritual. I'm into it’ (80–90-year-old man, HD). |
| ‘Coming in here, it's basically like a full-time job’ (65–70-year-old woman, HD). |
| ‘I have family, and they're all good to me…I don't want to disappoint them’ (80–90-year-old woman, HD). |
| Theme 2: Lack of SDM and poor patient satisfaction |
| ‘I was in the hospital and they discovered that all of the sudden out of the blue I had a kidney problem. The kidney doctor is the one that said I should have dialysis, and I don't know…lying on a bed three hours a day is not my way of living’ (80–90-year-old woman, HD). |
| ‘I have two arms that I really can't do much with, because bending it up, it hurts… I got two arms I can't do anything with’ (<65-year-old woman, HD). |
| ‘It's not easy to keep up the life all locked up in the house. I cannot work anymore. I have to pick up life in a hard way. Solitary’ (70–75-year-old man, HD). |
| ‘Socially, I kind of dropped out of being around as many people… I don't always feel the best, so I just don't go out like I used to. I think it slowed me down a lot, because I was one of those people that was always on the go…very social, I always had people at my house, card games, dinner parties, but not now. I just don't do it anymore’ (65–70-year-old woman, HD). |
| ‘I have to accept that I'm at home, my kids can't take me. I can't drive, I've accepted what I have. Which isn't much, but at least I'm alive, they say. Such as it is…’ (80–90-year-old woman, HD). |
| ‘More about how it makes you feel, everything feels different you know. I didn't realize I was going to be so drained at the end of dialysis’ (65–70-year-old woman, HD). |
Table 3.
Exemplary quotes: SDM and satisfaction with treatment outcomes
| Theme 1: Facilitators of SDM |
|---|
| Perceived choice |
| ‘I had to do dialysis, and it was clear to me that I wanted to do peritoneal dialysis after finding out about it… I knew I had to do dialysis, and I certainly didn't want to do hemodialysis if I could away without doing it’ (70–75-year-old woman, PD). |
| ‘I had to do dialysis. I had to make the decision whether it would be PD or hemo’ (80–90-year-old woman, PD). |
| ‘So that's what I chose [PD], and glad I did because it seems to be more convenient than the other’ (75–80-year-old man, PD). |
| Patient autonomy and discussions with others |
| ‘Chose this one [PD], and probably there isn't any difference, but in my heart, I felt as though that this was better. And so did my primary [care physician], when I talked to him too, and I've known him for years’ (65–70-year-old man, PD). |
| ‘The doctors were very helpful in explaining dialysis… I was the one who chose to come here [in-center HD vs PD]… I felt good about [decision]’ (65–70-year-old woman, HD). |
| ‘It was kinda the way [nephrologist] told me things, it didn't make me really alarmed, really afraid of doing the whole process. He explained the whole process, so it seemed like it was going to be a lot of work, but it …wasn't going to be invasive. He did a good job explaining’ (65–70-year-old woman, HD). |
| ‘We talked about [HD versus PD] and I expressed my thoughts about it. [My wife] agrees with me. She would agree with me if I chose the other way too.’ |
| ‘I made the decision. I didn't want to give that to the boys, [they] have a lot on their mind and I have to make the decision myself. Because whatever they say wouldn't matter. It's up to me. I had to say it’ (80–90-year-old woman, HD). |
| ‘My family, and church, were very supportive in getting me to accept that this is something that is going to take place, and that they'd do anything to support me. So, I'm very lucky, very lucky’ (65–70-year-old woman, HD). |
| Theme 2: Decision-making experiences and patient satisfaction |
| ‘[..I chose] PD, I don't need to go to the hospital every time, so it's at home…It's convenient for me… I think it is the right decision because you don't need to spend a long time at the hospital’ (65–70-year-old woman, PD). |
| ‘Because, they say dialysis will prolong your life, and I got my great grandkids, and see them grow up as much as I can. I made the right decision, it's good’ (80–90-year-old woman, HD). |
| ‘I guess it's keeping me alive, so that's a big thing’ (75–80-year-old woman, PD). |
| ‘I really like the people here a lot, so that was a pleasant outcome that I hadn't really anticipated, so I'm glad about that. They work really hard, they're very confident, and they're really nice. So that's a great combination’ (65–70-year-old woman, HD). |
| ‘Didn't take long after starting dialysis that I felt better, talking on the phone people said I sounded better, definitely felt better and had more energy and was able to socialize, I couldn't really socialize before I was so exhausted, and now I make plans cautiously, and go out to lunch, and do things with other people, so that's a real improvement’ (65–70-year-old woman, HD). |
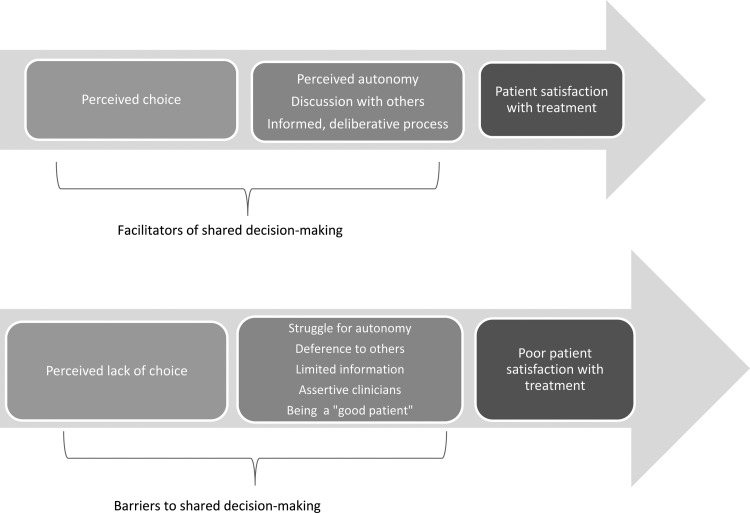
Theme 1: decision-making experiences and satisfaction with treatment
This theme highlights the linkage between SDM experiences and treatment satisfaction. Successes were found largely in modality selection, where patients expressed pride and confidence in decisions. In contrast, dialysis initiation decisions were characterized by perceived powerlessness, unaddressed concerns and subsequently poor satisfaction (Figure 1).
FIGURE 1.
Relationship between SDM and treatment satisfaction among older dialysis patients.
Negative experiences of dialysis initiation
Inadequately presented choices: Patients uniformly did not view dialysis initiation as their choice (Table 2). They described doctors making decisions and perceived that dialysis initiation was not a patient's decision. ‘I didn't make the decision. It wasn't mine.’ (80–90-year-old woman, HD); and, ‘If the doctor said I had to do it, what choice would I have?’ (75–80-year-old man, PD). ‘There was no choice really. Choice is you go on, or probably not be here’ (80–90-year-old man, HD). No patient described conservative management as an option.
Limited engagement: Most patients described accepting physician recommendations without much discussion. Few patients discussed preferences with nephrologists. Many cited clinician time constraints and lack of engagement as important barriers to fully considering quality of life implications. Even patients who had repeated conversations with nephrologists recalled only brief, rushed discussions about initiation.
Some attempted to engage physicians, asking mostly about prognosis and quality of life with limited success. ‘I did not want to [start dialysis]. I was quarreling with my physician, because he was really… interested in having my dialysis. I didn't like it… They didn't give me very much information’ (>90-year-old man, HD). Patients described unsuccessful efforts obtaining prognostic information. ‘When they started me on dialysis… I asked the nurses, what is the lifespan on [dialysis], because … that scares me. Am I gonna be on it forever? Do people come off of it? There's a lot of questions I need answering to…it's really hard’ (65–70-year-old woman, HD). This woman described feeling powerless and uninformed despite requesting information from numerous clinicians. Subsequently she described her dialysis experience negatively, focusing on unexpected setbacks. ‘It's not something that you wanna do. I'm really not independent, I need help.’
Lack of engagement and treatment dissatisfaction: Patients who described less-engaged decision-making faced challenges reconciling their values with dialysis and felt that their preferences had been overlooked. ‘I didn't expect it to be so disruptive, you become centered around your dialysis, and everything else comes after that. I'm surprised at that’ (65–70-year-old woman, HD). While patients stressed the importance of independence, travel, lack of invasive treatment, ability to retain occupations and responsibilities and social participation on their well-being, these factors often conflicted with their dialysis experience. ‘I thought if I take [PD], I could … go to work. But you can't, because it takes too much out of you… I miss working… Being home is killing me’ (65–70-year-old man, PD). Many described travel as central to fulfilling their life plans, especially in the context of long-anticipated retirement and reuniting with family and friends. Patients described disappointment with outcomes, including loss of purpose and identity, isolation, feeling constrained and social and mobility limitations.
Patients were distressed by unexpected outcomes not discussed during decision-making. One remarked that he had expected his health to improve but was surprised that dialysis left him weak and lacking appetite. Another concluded, ‘That's been the majority of the upset in my life, is that chunk of time is taken away, and then arranging to get that chunk of time taken away from you. Kind of a dichotomy’ (65–70-year-old woman, HD).
Positive experiences of modality selection
Although patients did not recognize dialysis initiation as a decision, most actively choose between modalities (HD and PD) (Figure 1, Table 2). ‘I had to make the decision whether it would be PD or hemo. It was after I spoke with [clinicians] … that I made the decision’ (80–90-year-old woman, PD). Another clarified, ‘I knew that dialysis was something I had to do… there was no discussion there. It was the modality I'd be using that was really the issue’ (65–70-year-old man, PD). Patients described active choices characterized by many nuanced deliberations with clinicians and family, reading educational materials and attending informational sessions. Most felt that doctors presented modalities fairly and equally, though two concluded that doctors prefer PD to reduce costs. Discussions were seen as instrumental to clarifying which modality aligns best with preferences and goals. Patients considered quality of life and demands on loved ones with clinicians and families. Partners and children were often instrumental in selecting a modality, particularly due to their caregiving role.
Generally, when reflecting on the success of their modality choice, many patients (HD and PD) described meaningful participation, including seeing family and friends, playing games, using computers and staying active. ‘The positive aspect is that it's helping me to live a fairly normal life’ (80–90-year-old woman, PD). Some described weight loss, feeling better and improved energy as positive outcomes. Satisfaction with modality decisions was high, with some patients even stating that they would withdraw from dialysis if they had to change modality.
Patients who described active decision-making appeared more confident and satisfied with decisions than those who described being pushed into a choice, some of whom described regret. ‘I chose this one [PD], and probably there isn't any difference, but in my heart, I felt as though that this was better. And so did my primary, when I talked to him too, and I've known him for years’ (65–70-year-old man, PD). In the single case in which a patient changed modalities, the patient felt uninformed and not listened to during decision-making discussions. ‘Unfortunately, my doctor's office kinda pushed me toward … PD. [After starting], they told me that I couldn't have the cats in the room when I was on. I have 3 cats … My home just turned into like a hospital room’ (65–70-year-old woman, HD).
Theme 2: barriers to SDM
Although patients experienced greater satisfaction when they engaged in SDM, numerous barriers prevented their involvement in dialysis initiation decisions that were overcome in modality selection. Three subthemes (views about choice, struggling for autonomy, being a ‘good patient’) provide insight into key barriers to SDM. These subthemes may also reflect a paternalistic approach of some health care professionals when presenting dialysis as a necessary rather than optional step.
Views about choice
This subtheme encompasses patients' views that lack of decision-making was either due to the perception dialysis was needed to prevent imminent death or that initiation was not their (patient) decision.
Many described an acute need for action that was rooted in fear and anxiety. ‘They said if I didn't do dialysis, I might as well plan my funeral’ (75–80-year-old woman, HD). Others ascribed lack of engagement to their perceived patient role, deferring instead to nephrologists. ‘My doctor told me I had to go on dialysis… It's not a question. You don't decide… Period’ (65–70-year-old woman, HD). Others stated, ‘I was just going by what the doctor told me… I didn't feel I had a choice’ (80–90-year-old man, HD). This belief permeated the narratives of all patients.
Numerous patients regretted the lack of timely discussions and not realizing the decision. Many recalled a desire for more discussion and information from clinicians during their decision-making process and emphasized the importance of this for future patients.
When asked what advice they would offer to future patients, participants supported SDM. Despite not perceiving having made a decision, patients preferred that dialysis initiation be the patient's choice. Many suggested, ‘It's [the patients'] decision’ (70–75-year-old man, PD) and ‘They've got to find what fits for them’ (65–70-year-old woman, HD). Others emphasized the importance of the patient voice, active participation and people to talk to when navigating the decision as central components of SDM.
Struggling for autonomy
Unlike modality decisions, patients described a lack of autonomy in the context of dialysis initiation.
Assertive doctors: Patients described doctors ‘convincing’ them to initiate dialysis, which was generally perceived negatively. ‘The kidney doctor is the one that said I should have dialysis, [but] lying on the bed three hours a day is not my way of living’ (80–90-year-old woman, HD). Patients seemed resigned to having their initial preferences overturned. ‘They had a hard time convincing me. I finally did agree… Sometimes you don't have a choice’ (80–90-year-old woman, HD).
Limited information: Patients desired prognostic information for making life plans and selecting treatments. One said, ‘They never told me how long I'd live’ (>90-year-old man, HD). Many were never given a prognosis, despite requesting it. Another stated, ‘Doctors don't usually answer questions like this…’ (70–75-year-old man, HD). Patients interpreted nephrologists' reluctance to discuss prognosis as an indication that patients should not request information or ‘push’ for answers. Most patients felt uncomfortable broaching the subject and were unaware of conservative management as a therapeutic alternative.
Some successes: Some patients did not experience the same level of struggle for autonomy. Patients who discussed dialysis with physicians were more satisfied with the decision-making process, even if they did not identify initiation as a decision. ‘I was kind of shocked, but [the doctor] explained it, so it didn't seem like it was such a big deal… he did a good job explaining’ (65–70-year-old woman, HD). They expressed a personal connection to their clinical team and satisfaction and appreciation for their care.
Being a ‘good patient’
This describes deference to clinicians and families, revealing that patients assume a ‘patient identity’, mediating limited choice and poor patient satisfaction.
In adapting to the demands of dialysis, patients described an acute shift in life priorities in the context of ‘being a good patient’ as opposed to goals and values held prior to dialysis. Dialysis required relinquishing major parts of their identity, including occupation, caregiver and active social participant. Instead, patients described achievement and satisfaction in the context of praise from clinical staff, adhering to appointments, diet and exercise.
One patient described how proud her nephrologist was and how happy her treatment was making him. Another described her initial refusal and subsequent acquiescence, exemplifying the difficulty patients face having their wishes respected. ‘[they said], ‘Nana, you have to live for us… That's what made my mind up. Cause I did wanna go… I'm too old, if I die, so what?’ But they wanted me to live’ (>90-year-old woman, HD).
DISCUSSION
Aligning treatment decisions with patient preferences and goals of care is the gold standard for patient-centered care [36]. Our study links decision-making experiences and patient satisfaction, illustrating key barriers in the process, beginning with patients not identifying dialysis initiation as a decision and continuing with understanding how the patient voice is obscured during decision-making and how physicians and families may convince patients to pursue treatment. Expanding upon prior studies [16, 37, 38], our data illustrate how and why patients do not perceive dialysis initiation as a choice, namely due to the perception of imminent death or because choice was attributed to physicians. Perceptions of imminent death may reflect inaccurate prognostic information, as several observational studies have demonstrated average survival without dialysis of up to 24 months [39–41].
In recalled discussions, physicians emphasized survival benefits over patient values of independence, travel and social participation and withheld information about prognosis, complications and conservative management. Patients with unmet informational needs were less satisfied with treatment, frequently expressing surprise at fatigue, quality of life and recovery time. Finally, many patients expressed disappointment about how the decision had transpired, and some expressed regret. In contrast, patients who engaged in SDM described (mostly modality selection) greater satisfaction with the process and outcomes, exhibiting confidence and pride in their thorough, deliberative decision-making process.
Patients consistently recounted that dialysis was required for survival and had unaddressed concerns about quality of life, side effects (e.g. fatigue, recovery time), treatment requirements (e.g. repeated needle sticks, fistula placement and maintenance, lifelong need) and prognosis. This is consistent with research showing that patients often are unprepared for dialysis [19, 42, 43] and may experience regret [13, 44]. Many patients directly requested prognostic information and suggested that future patients be advised of prognosis. Consistent with the SDM literature [45–48], patients preferred deliberative conversations with clinicians, although mostly described prescriptive interactions.
We did not examine nephrologists' perspectives. However, research suggests that nephrologists are often optimistic about how individual patients will fare [3, 49], uncomfortable in broaching prognosis with their patients [49–51] and may find that initiating dialysis is simpler, more expedient and financially incentivized [37]. Interestingly, patients in our study were keenly aware of nephrologists' reluctance to engage in discussions of prognosis, inferring that they were not supposed to request such information. Viewed as a whole, this neglects patients' desire to learn about prognosis and treatment options and highlights the need for tools to facilitate these discussions [52]. Importantly, nephrologists may be wary that patients may decide against trying dialysis. While attempting to steer patients toward dialysis with the altruistic belief that this is the optimal treatment, clinicians may be affected by their local culture of care and may be unduly paternalistic [4].
Patient engagement in decision-making is associated with better patient-centered outcomes, including quality of life and satisfaction [44, 53]. We found examples of SDM when patients described choosing a dialysis modality. Indeed, patients cited their decisions between HD and PD and discussions with physicians and family members about the benefits and burdens as central to their belief that they chose the right treatment. Active participation also seemed to buffer potential negative consequences of dialysis. Patients who felt convinced to initiate dialysis more often described treatment hardship, even when acknowledging longevity benefits. In the UK, patients in centers where conservative management pathways are more mature were more apt to avoid dialysis, maintaining their end-of-life values [4].
Our study is not without limitations. Although we interviewed a diverse sample of older dialysis patients from Greater Boston, our study should be replicated to ensure generalizability. Patients may also experience recall bias when reflecting on past decision-making. With a mean dialysis time of 2 years, we may have selected a population of dialysis survivors who exceeded prognostic expectations. Because these patients have derived increased life expectancy from dialysis, they may be more satisfied with having initiated dialysis, potentially biasing the results of the study. Alternatively, these patients may have, after initial improved health, begun to decline, potentially affecting their responses. This is not supported by reasonably high serum albumin levels among participants. Currently, comprehensive conservative care for kidney failure is likely underutilized, particularly in the USA. Future studies should evaluate individuals with advanced Chronic Kidney Disease prospectively to evaluate the process of dialysis decision-making. Finally, we do not know whether these themes apply to patients who chose conservative care.
Our findings underscore that patients desire an opportunity to know about the implications of the choices that they face and to actively engage in the decision. Realizing that they may not have seen these choices clearly at the time was undoubtedly difficult for some, as was dissatisfaction with dialysis and its consequences for daily life. However, as evidenced by the advice offered to future patients, improving SDM and quality of care requires the patient's voice. Current patients remained optimistic about the potential for improving SDM and the potential for clinicians to engage patients and provide prognostic and quality of life information. Even with difficult choices between modalities, patients appreciated the opportunity to deliberate, learn more through discussion and educational resources and to engage in discussions with clinicians and loved ones.
CONCLUSIONS
Many older patients do not perceive dialysis as their choice, do not actively engage in decision-making and remain unaware of conservative management. However, patients who engaged in SDM perceived dialysis more positively. Future studies should explore ways to promote SDM and to respect patient preferences [28, 54, 55]. Our findings demonstrate the importance of SDM for patient autonomy in ensuring that patients are making informed decisions about their treatment. The findings also clarify that without SDM there is the potential for harm due to the greater potential for regret [13], contradicting the bioethical principle of nonmaleficence [35].
AUTHORS’ CONTRIBUTIONS
Research idea and study design: K.L., D.E.W. and S.K.W. Recruitment: D.E.W., E.H., N.L., G.Z. and K.L. Interviews: K.L., E.H., G.Z. and N.L. Data analysis and interpretation: K.L., S.K.W. and D.E.W. Mentorship and supervision: D.E.W., S.K.W. and K.L. Each author contributed important intellectual content during the manuscript drafting and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. K.L. takes responsibility that this study has been reported honestly, accurately and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained. Any remaining errors are our own.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Klemens Meyer, MD, for assistance in recruitment and Katie Buttafaro and Victoria Oliva for their contributions to the study. We are extremely grateful for the generous contributions of the nurses, social workers, technicians, other staff and the patients at the Dialysis Clinic, Inc. units who extended their hospitality to us, enabling us to complete this study. We are grateful to participants of the Occupational Therapy and Health Research Seminar at Tufts University for insightful comments. K.L. gratefully acknowledges financial support from the National Center for Advancing Translational Sciences, National Institutes of Health, Award Number KL2TR001063 and the Neubauer Faculty Fellowship at Tufts University. D.E.W. receives indirect salary support for research projects from Dialysis Clinic, Inc. paid through Tufts Medical Center.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. United States Renal Data System. 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: USRDS, 2014 [Google Scholar]
- 2. Collins AJ, Foley RN, Chavers B. et al. US Renal Data System 2013 annual data report. Am J Kidney Dis 2014; 63: A7. [DOI] [PubMed] [Google Scholar]
- 3. Schell JO, Da Silva-Gane M, Germain MJ. Recent insights into life expectancy with and without dialysis. Curr Opin Nephrol Hypertens 2013; 22: 185–192 [DOI] [PubMed] [Google Scholar]
- 4. Tonkin-Crine S, Okamoto I, Leydon GM. et al. Understanding by older patients of dialysis and conservative management for chronic kidney failure. Am J Kidney Dis 2015; 65: 443–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. White Y, Fitzpatrick G. Dialysis: prolonging life or prolonging dying? Ethical, legal and professional considerations for end of life decision making. EDTNA ERCA J 2006; 32: 99–103 [DOI] [PubMed] [Google Scholar]
- 6. Galla JH. Clinical practice guideline on shared decision-making in the appropriate initiation of and withdrawal from dialysis. The Renal Physicians Association and the American Society of Nephrology. J Am Soc Nephrol 2000; 11: 1340–1342 [DOI] [PubMed] [Google Scholar]
- 7. Renal Physicians Association. Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis, 2nd edn Rockville, MD: Renal Physicians Association, 2010 [Google Scholar]
- 8. Dasgupta I, Rayner HC. In good conscience—safely withholding dialysis in the elderly. Semin Dial 2009; 22: 476–479 [DOI] [PubMed] [Google Scholar]
- 9. Moss AH. Too many patients who are too sick to benefit start chronic dialysis nephrologists need to learn to “just say no”. Am J Kidney Dis 2003; 41: 723–727 [DOI] [PubMed] [Google Scholar]
- 10. Vachharajani TJ, Moist LM, Glickman MH. et al. Elderly patients with CKD—dilemmas in dialysis therapy and vascular access. Nat Rev Nephrol 2014; 10: 116–122 [DOI] [PubMed] [Google Scholar]
- 11. van de Luijtgaarden MW, Noordzij M, van Biesen W. et al. Conservative care in Europe—nephrologists’ experience with the decision not to start renal replacement therapy. Nephrol Dial Transplant 2013; 28: 2604–2612 [DOI] [PubMed] [Google Scholar]
- 12. Covic A, Bammens B, Lobbedez T. et al. Educating end-stage renal disease patients on dialysis modality selection: clinical advice from the European Renal Best Practice (ERBP) Advisory Board. Nephrol Dial Transplant 2010; 25: 1757–1759 [DOI] [PubMed] [Google Scholar]
- 13. Davison SN. End-of-life care preferences and needs: perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol 2010; 5: 195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaufman SR, Shim JK, Russ AJ. Old age, life extension, and the character of medical choice. J Gerontol B Psych Sci Soc Sci 2006; 61: S175–S184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Russ AJ, Shim JK, Kaufman SR. “Is there life on dialysis?”: time and aging in a clinically sustained existence. Med Anthropol 2005; 24: 297–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Van Biesen W, van der Veer SN, Murphey M. et al. Patients’ perceptions of information and education for renal replacement therapy: an independent survey by the European Kidney Patients’ Federation on information and support on renal replacement therapy. PLoS One 2014; 9: e103914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Song MK, Ward SE. The extent of informed decision-making about starting dialysis: does patients’ age matter? J Nephrol 2014; 27: 571–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Song MK, Lin FC, Gilet CA. et al. Patient perspectives on informed decision-making surrounding dialysis initiation. Nephrol Dial Transplant 2013; 28: 2815–2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schell JO, Patel UD, Steinhauser KE. et al. Discussions of the kidney disease trajectory by elderly patients and nephrologists: a qualitative study. Am J Kidney Dis 2012; 59: 495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Russ AJ, Kaufman SR. Discernment rather than decision-making among elderly dialysis patients. Semin Dial 2012; 25: 31–32 [DOI] [PubMed] [Google Scholar]
- 21. Russ AJ, Shim JK, Kaufman SR. The value of “life at any cost”: talk about stopping kidney dialysis. Soc Sci Med 2007; 64: 2236–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morton RL, Tong A, Howard K. et al. The views of patients and carers in treatment decision making for chronic kidney disease: systematic review and thematic synthesis of qualitative studies. BMJ 2010; 340: c112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Walker RC, Hanson CS, Palmer SC. et al. Patient and caregiver perspectives on home hemodialysis: a systematic review. Am J Kidney Dis 2015; 65: 451–463 [DOI] [PubMed] [Google Scholar]
- 24. Harwood L, Clark AM. Understanding pre-dialysis modality decision-making: a meta-synthesis of qualitative studies. Int J Nurs Stud 2013; 50: 109–120 [DOI] [PubMed] [Google Scholar]
- 25. Hussain JA, Flemming K, Murtagh FE. et al. Patient and health care professional decision-making to commence and withdraw from renal dialysis: a systematic review of qualitative research. Clin J Am Soc Nephrol 2015; 10: 1201–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Patton M. Qualitative Evaluation and Research Methods. Beverly Hills, CA: Sage, 1990 [Google Scholar]
- 27. Hamberg K, Johansson E, Lindgren G. et al. Scientific rigour in qualitative research—examples from a study of women's health in family practice. Fam Pract 1994; 11: 176–181 [DOI] [PubMed] [Google Scholar]
- 28. Ladin K, Weiner DE. Better informing older patients with kidney failure in an era of patient-centered care. Am J Kidney Dis 2015; 65: 372–374 [DOI] [PubMed] [Google Scholar]
- 29. Koch-Weser S, Dejong W, Rudd RE. Medical word use in clinical encounters. Health Expect 2009; 12: 371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koch-Weser S, Rudd RE, Dejong W. Quantifying word use to study health literacy in doctor-patient communication. J Health Comm 2010; 15: 590–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Emmons KM, Koch-Weser S, Atwood K. et al. A qualitative evaluation of the Harvard Cancer Risk Index. J Health Comm 1999; 4: 181–193 [DOI] [PubMed] [Google Scholar]
- 32. Saldaña J. The Coding Manual for Qualitative Researchers. Beverly Hills, CA: Sage, 2012 [Google Scholar]
- 33. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007; 19: 349–357 [DOI] [PubMed] [Google Scholar]
- 34. Joseph-Williams N, Elwyn G, Edwards A. Knowledge is not power for patients: a systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision making. Patient Educ Couns 2014; 94: 291–309 [DOI] [PubMed] [Google Scholar]
- 35. Beauchamp T, Childress J. Principles of Biomedical Ethics, 7th edn New York: Oxford University Press, 2012 [Google Scholar]
- 36. Davison SN, Levin A, Moss AH. et al. Executive summary of the KDIGO Controversies Conference on Supportive Care in Chronic Kidney Disease: developing a roadmap to improving quality care. Kidney Int 2015; 88: 447–459 [DOI] [PubMed] [Google Scholar]
- 37. Davison SN, Jhangri GS, Holley JL. et al. Nephrologists’ reported preparedness for end-of-life decision-making. Clin J Am Soc Nephrol 2006; 1: 1256–1262 [DOI] [PubMed] [Google Scholar]
- 38. Brown E. Epidemiology of renal palliative care. J Palliat Med 2007; 10: 1248–1252 [DOI] [PubMed] [Google Scholar]
- 39. Chandna SM, Da Silva-Gane M, Marshall C. et al. Survival of elderly patients with stage 5 CKD: comparison of conservative management and renal replacement therapy. Nephrol Dial Transplant 2011; 26: 1608–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carson RC, Juszczak M, Davenport A. et al. Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol 2009; 4: 1611–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brown MA, Collett GK, Josland EA. et al. CKD in elderly patients managed without dialysis: survival, symptoms, and quality of life. Clin J Am Soc Nephrol 2015; 10: 260–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fine A, Fontaine B, Kraushar MM. et al. Nephrologists should voluntarily divulge survival data to potential dialysis patients: a questionnaire study. Perit Dial Int 2005; 25: 269–273 [PubMed] [Google Scholar]
- 43. Walker R, Fassett RG, Morton RL. Research issues in elderly patients: gaps in knowledge and suggested directions. Nephrology 2013Apr 16. doi:10.1111/nep.12084 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 44. Heyland DK, Allan DE, Rocker G. et al. Discussing prognosis with patients and their families near the end of life: impact on satisfaction with end-of-life care. Open Med 2009; 3: e101–e110 [PMC free article] [PubMed] [Google Scholar]
- 45. Moss AH, Armistead NC. Improving end-of-life care for ESRD patients: an initiative for professionals. Nephrol News Issues 2013; 27: 30–32 [PubMed] [Google Scholar]
- 46. Muthalagappan S, Johansson L, Kong WM. et al. Dialysis or conservative care for frail older patients: ethics of shared decision-making. Nephrol Dial Transplant 2013; 28: 2717–2722 [DOI] [PubMed] [Google Scholar]
- 47. Singh P, Germain MJ, Cohen L. et al. The elderly patient on dialysis: geriatric considerations. Nephrol Dial Transplant 2014; 29: 990–996 [DOI] [PubMed] [Google Scholar]
- 48. Thamer M, Kaufman JS, Zhang Y. et al. Predicting early death among elderly dialysis patients: development and validation of a risk score to assist shared decision making for dialysis initiation. Am J Kidney Dis 2015; 66: 1024–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wachterman MW, Marcantonio ER, Davis RB. et al. Relationship between the prognostic expectations of seriously ill patients undergoing hemodialysis and their nephrologists. JAMA Intern Med 2013; 173: 1206–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Grubbs V, Moss AH, Cohen LM. et al. A palliative approach to dialysis care: a patient-centered transition to the end of life. Clin J Am Soc Nephrol 2014; 9: 2203–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. van Biesen W, van de Luijtgaarden MW, Brown EA. et al. Nephrologists’ perceptions regarding dialysis withdrawal and palliative care in Europe: lessons from a European Renal Best Practice survey. Nephrol Dial Transplant 2015; 30: 1951–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Holley JL, Carmody SS, Moss AH. et al. The need for end-of-life care training in nephrology: national survey results of nephrology fellows. Am J Kidney Dis 2003; 42: 813–820 [DOI] [PubMed] [Google Scholar]
- 53. Zhang B, Wright AA, Huskamp HA. et al. Health care costs in the last week of life: associations with end-of-life conversations. Arch Intern Med 2009; 169: 480–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Song MK, Ward SE, Fine JP. et al. Advance care planning and end-of-life decision making in dialysis: a randomized controlled trial targeting patients and their surrogates. Am J Kidney Dis 2015; 66: 813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schell JO, Cohen RA. A communication framework for dialysis decision-making for frail elderly patients. Clin J Am Soc Nephrol 2014; 9: 2014–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]