Abstract
Aims
To quantify the association of self-reported walking pace and handgrip strength with all-cause, cardiovascular, and cancer mortality.
Methods and results
A total of 230 670 women and 190 057 men free from prevalent cancer and cardiovascular disease were included from UK Biobank. Usual walking pace was self-defined as slow, steady/average or brisk. Handgrip strength was assessed by dynamometer. Cox-proportional hazard models were adjusted for social deprivation, ethnicity, employment, medications, alcohol use, diet, physical activity, and television viewing time. Interaction terms investigated whether age, body mass index (BMI), and smoking status modified associations. Over 6.3 years, there were 8598 deaths, 1654 from cardiovascular disease and 4850 from cancer. Associations of walking pace with mortality were modified by BMI. In women, the hazard ratio (HR) for all-cause mortality in slow compared with fast walkers were 2.16 [95% confidence interval (CI): 1.68–2.77] and 1.31 (1.08–1.60) in the bottom and top BMI tertiles, respectively; corresponding HRs for men were 2.01 (1.68–2.41) and 1.41 (1.20–1.66). Hazard ratios for cardiovascular mortality remained above 1.7 across all categories of BMI in men and women, with modest heterogeneity in men. Handgrip strength was associated with cardiovascular mortality in men only (HR tertile 1 vs. tertile 3 = 1.38; 1.18–1.62), without differences across BMI categories, while associations with all-cause mortality were only seen in men with low BMI. Associations for walking pace and handgrip strength with cancer mortality were less consistent.
Conclusion
A simple self-reported measure of slow walking pace could aid risk stratification for all-cause and cardiovascular mortality within the general population.
Keywords: Cardiorespiratory fitness, Handgrip strength, Mortality, Walking pace
Introduction
Physical fitness, composed of cardiorespiratory fitness and muscle strength, is an important predictor of mortality and an established target for cardiovascular disease prevention.1–3 Cardiorespiratory fitness, in particular, has shown a strong graded association with the risk of all-cause and cardiovascular mortality and is advocated as a clinical vital sign with importance for risk classification.1,4 Therefore, pragmatic methods of harnessing cardiorespiratory fitness for risk stratification are warranted. Studies have found an association between objectively assessed walking pace and mortality, mostly in older adult,5–7 with other studies suggesting self-reported walking pace may act as an important marker of cardiovascular health.8,9 Thus, self-reported walking pace could function as a pragmatic measure of physical fitness and a simple index of mortality risk. However, in order to have clinical and public health relevance, research is needed to examine the extent to which pragmatic self-reported measures of walking pace are associated with cardiorespiratory fitness and whether the associations with all-cause and cause-specific mortality are maintained after adjustment for potential confounding variables or vary across potential effect modifiers such as age, smoking status, or body mass index (BMI).
Muscle strength is also an important and reproducible measure of physical fitness and has been associated with all-cause and cardiovascular mortality.2 Handgrip strength is correlated with overall muscle strength and is associated with functional and health outcomes including frailty, falls, nutritional status and mortality.10–15 Investigating whether associations persist after adjustment for other pragmatic measures of physical fitness, such as walking pace, and maintained across potential effect modifiers will further help elucidate the potential clinical importance of handgrip strength within the general population.
The aim of this analysis was to quantify the associations of self-reported walking pace and handgrip strength with all-cause, cardiovascular, and cancer mortality in men and women from UK Biobank and to investigate whether associations are maintained across categories of age, BMI, and smoking status.
Methods
UK Biobank
This analysis used data from 502 639 participants within UK Biobank, the methods and aim of which have been reported elsewhere.16 In brief, UK Biobank is a large prospective cohort of middle-aged adults designed to support biomedical analysis focused on improving the prevention, diagnosis, and treatment of chronic disease. Between March 2006 and July 2010, individuals living within 25 miles of one of the 22 study assessment centres located throughout England, Scotland, and Wales were recruited and provided comprehensive data on a broad range of demographic, clinical, lifestyle, and social outcomes. All participants provided written informed consent and the study was approved by the NHS National Research Ethics Service (Ref: 11/NW/0382).
For each variable reported in this analysis, we provide the UK Biobank Data-Field (DF) number. Each DF is linked to detailed information on measurement procedures through the UK Biobank website.17
Measures of physical function
The primary exposures of interest were self-reported walking pace and objectively assessed handgrip strength. Walking pace was assessed by the following single item question: ‘How would you describe your usual walking pace? (i) Slow pace, (ii) Steady/average pace, and (iii) Brisk pace’ (DF 924). Handgrip strength was assessed through the use of a hydraulic hand dynamometer (Jamar J00105) while sitting (DF 46 and 47). The elbow of the arm holding the dynamometer was placed against the side of the body and bent to a 90° angle with the forearm placed on an armrest. Participants were instructed to squeeze the handle of the dynamometer as hard as they could for 3 s. Both left and right hand strengths were measured. The values for the right and left hand were summed and divided by 2 to estimate an average.
Cardiorespiratory fitness
A subsample of UK Biobank undertook a 6 min graded submaximal fitness test on a cycle ergometer (DF 6025). We utilized data from this subsample to inform the main analyses by investigating the association of cardiorespiratory fitness with walking pace and handgrip strength. The start and end workload of the graded fitness test was set according to age, height, weight, resting heart rate, and sex to ensure a similar relative intensity across the population. Heart rate was monitored before, during, and after the exercise test via a four-lead electrocardiogram. Maximal cardiorespiratory fitness was estimated by: (i) Fitting a linear regression line between heart rate and power output for each stage of the test; (ii) The regression line was extrapolated to the age-predicted maximal heart rate with the formula 208 − 0.7 × age18; (iii) Maximal oxygen uptake was estimated from the regression equation for the relationship between work rate (power) and oxygen uptake [oxygen uptake (mL·kg−1·min−1) = 7 + [10.8 × work rate (Watts)]/body mass (kg)].19 Individuals without cancer and cardiovascular disease who completed at least one stage of the fitness test were included for this sub-analysis (n = 51 282).
Data on potential confounders, mediators, and effect modifiers
This study utilized the following covariates: anthropometric (BMI); demographic [age, sex, ethnicity, social deprivation (Townsend index), and employment status]; health (number of medications); and lifestyle [smoking (never, past, current), alcohol, diet, television (TV) viewing time, and self-reported physical activity]. Diet was assessed through a food frequency questionnaire; alcohol (DF 1558), fresh fruit (DF 1309), cooked vegetable (DF 1289), and processed meat (DF 1349) were included. Physical activity was assessed as weekly frequency of walking (DF 864), moderate (DF 884), or vigorous (DF 904) intensity physical activity lasting at least 10 min in duration. Further details for each variable are available on the UK Biobank Website.20
Mortality status follow-up
UK Biobank undertook comprehensive data linkage for mortality status. Date and cause of death were obtained from the National Health Service (NHS) Information Centre for participants from England and Wales, and from the NHS Central Register, Scotland, for participants from Scotland. Detailed information about the linkage procedure is available online.20 Linkage captured all deaths occurring until 31 January 2016 for England and 30 November 2015 for Scotland. We defined cancer and cardiovascular disease mortality using the International Classification of Diseases edition 10 (ICD-10): C00-C97 for cancer death and I00-I79 for cardiovascular death.
Participants included
From the initial sample of 502 639 participants, those with prevalent cancer [number of self-reported cancers (DF 134); n = 41 706] or prevalent cardiovascular disease [defined as myocardial infarction, stroke, or angina (DF 6150); n = 27 573] were excluded from the analysis. From the remaining sample, only subjects with information available for all covariates were included (missing data were less than 2% for all included covariates), leaving 420 727 individuals who at baseline were free from cancer and cardiovascular disease and had complete walking pace, handgrip strength (both left and right measures) and covariate data (see Supplementary material online, Figure S1).
Statistical analysis
In the subsample with cardiorespiratory fitness data, the association between walking pace, handgrip strength, and cardiorespiratory fitness was tested using analysis of variance models adjusted for age and stratified by sex.
In the full cohort, Cox-proportional hazard models were used to investigate the association of walking pace and handgrip strength with mortality using age as time scale. Handgrip strength was categorized into sex-specific tertiles and separate models were undertaken for all-cause mortality, cardiovascular mortality, and cancer mortality. The proportional hazards assumption was verified for all variables by inspection of the plots of the Schoenfeld residual for covariates. Models were arranged a priori to investigate the impact of incremental adjustment. Model 1 was unadjusted. Model 2 adjusted for ethnicity, social deprivation, employment status, and number of prescribed medications. Model 3 additionally adjusted for smoking, alcohol consumption, and fruit, vegetable, and processed meat intake. Model 4 additionally adjusted for BMI, TV viewing, and frequency of walking, moderate intensity, and vigorous intensity physical activity. Lastly, Model 5 was additionally mutually adjusted for walking pace and handgrip strength. For the regression models including all covariates in Model 4 plus walking pace or handgrip strength, the C-indexes were calculated and calibration plots displayed.
Interaction terms were fitted to Model 5 to assess whether BMI (as tertiles), age (tertiles), and smoking (never, past, current) modified associations with mortality; in addition, the interaction between walking pace and grip strength was also tested. A sensitivity analysis was also conducted to investigate the effect of removing all deaths occurring within the first 24 months of follow-up (women = 424, men = 797) to reduce the possible impact of reverse causation.
All analyses were performed with Stata V14.1 (Stata Corporation, College Station, TX, USA). Results stratified by sex are reported with 95% confidence interval; a two-sided P-value < 0.05 was considered statistically significant.
Results
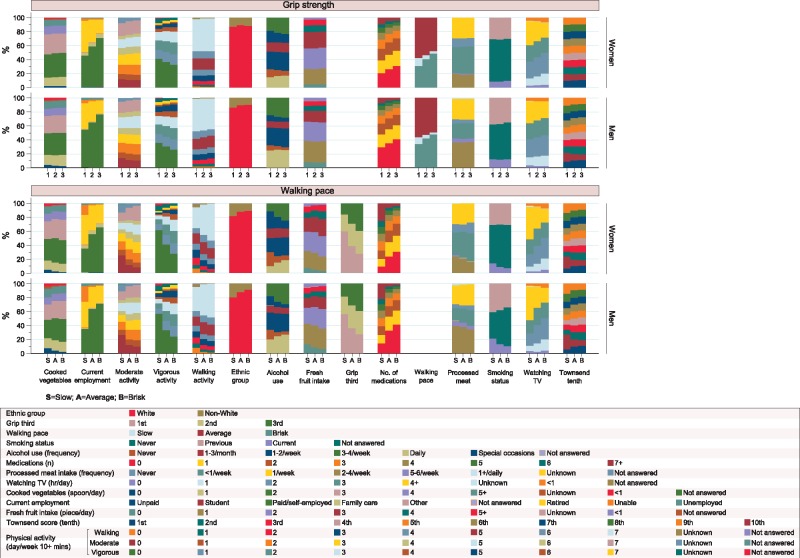
The mean (range) age of included participants was 56.4 (38.9–73.7) years. The distribution of the categorical covariates for the 230 670 women and 190 057 men included in the analyses are displayed in Figure 1. Table 1 shows BMI, age, and mean handgrip strength values across categories of walking pace and handgrip strength. Categories of walking pace were similarly distributed between men and women. However, mean values of handgrip strength for sex-specific tertiles showed little overlap between men and women; those in the highest tertile for women had a mean (standard deviation) value of 30.4 (3.3) kg whereas men in the lowest tertile had a mean value of 30.6 (4.9) kg (Table 1).
Figure 1.
Distribution in participant characteristics for men and women across tertiles of handgrip strength and categories of walking pace.
Table 1.
Age and body mass index characteristics across categories of walking pace and handgrip strength
| Women |
Men |
|||||
|---|---|---|---|---|---|---|
| Handgrip strength |
||||||
| Tertile 1 | Tertile 2 | Tertile 3 | Tertile 1 | Tertile 2 | Tertile 3 | |
| Age (years) | 59.1 (7.3) | 56.7 (7.8) | 53.1 (7.7) | 58.8 (7.9) | 56.8 (8.0) | 53.8 (7.9) |
| Body mass index (kg/m2) | 27.3 (5.2) | 26.8 (5.0) | 26.8 (5.1) | 27.6 (4.4) | 27.6 (4.1) | 28.0 (4.0) |
| Grip strength (kg) | 17.2 (3.6) | 23.9 (1.4) | 30.4 (3.3) | 30.6 (4.9) | 39.9 (2.0) | 49.3 (4.8) |
| Walking pace | ||||||
| Slow | 9850 (11.8%) | 3935 (5.4%) | 2636 (3.5%) | 6370 (9.9%) | 2956 (4.8%) | 2055 (3.2%) |
| Average | 47 557 (57.2%) | 38 744 (53.6%) | 35 980 (47.9%) | 36 250 (56.1%) | 32 244 (52.5%) | 31 227 (48.8%) |
| Brisk | 25 759 (31.0%) | 29 648 (41.0%) | 36 561 (48.6%) | 22 014 (34.1%) | 26 180 (42.7%) | 30 761 (48.0%) |
|
Walking pace |
||||||
| Slow | Average | Brisk | Slow | Average | Brisk | |
| Age (years) | 58.2 (7.7) | 57.0 (8.0) | 55.2 (8.0) | 58.8 (7.8) | 57.1 (8.2) | 55.4 (8.2) |
| Body mass index (kg/m2) | 31.9 (7.1) | 27.7 (5.0) | 25.1 (3.8) | 30.3 (5.8) | 28.2 (4.2) | 26.7 (3.5) |
| Grip strength (kg) | 19.6 (6.9) | 23.2 (6.0) | 24.9 (5.9) | 34.7 (10.0) | 39.4 (8.6) | 41.2 (8.4) |
Numbers are mean (SD) or number (%).
SD, standard deviation.
In the subsample who undertook the fitness test, self-reported walking pace showed a strong graded association with cardiorespiratory fitness in men and women (Table 2). Slow walkers achieved an average fitness level of 28.1 (95% CI: 27.7–28.6) mL·kg−1·min−1 and 35.9 (35.3–36.5) mL·kg−1·min−1 in women and men, respectively; whereas brisk walkers achieved levels of 34.0 (33.8–34.1) mL·kg−1·min−1 and 42.0 (41.8–42.2) in women and men, respectively. Associations between handgrip strength and cardiorespiratory fitness were less pronounced.
Table 2.
Age-adjusted cardiorespiratory fitness across walking speed and handgrip categories
| Women (n = 26 593) | ||||
|---|---|---|---|---|
| Walking speed | Slow | Steady | Brisk | P-value |
| Mean fitness (mL·kg-1·min-1) | 28.1 (27.7–28.6) | 30.9 (30.7–31.0) | 34.0 (33.8–34.1) | < 0.001 |
| Handgrip strength | Tertile 1 | Tertile 2 | Tertile 3 | |
| Mean fitness (mL·kg-1·min-1) | 31.4 (31.2–31.6) | 32.2 (32.0–32.4) | 32.9 (32.7–33.0) | < 0.001 |
|
Men (n = 24 689) | ||||
| Walking speed | Slow | Steady | Brisk | P-value |
| Mean fitness (mL·kg-1·min-1) | 35.9 (35.3–36.5) | 39.3 (39.1–39.5) | 42.0 (41.8–42.2) | < 0.001 |
| Handgrip strength | Tertile 1 | Tertile 2 | Tertile 3 | |
| Mean fitness (mL·kg-1·min-1) | 39.5 (39.3–39.7) | 40.6 (40.4–40.8) | 41.1 (40.9–41.3) | <0.001 |
Data as marginal mean (adjusted for age) (95% CI).
CI, confidence interval.
Table 3 reports the number of deaths for men and women by walking pace group and tertile of handgrip strength. During a median (interquartile range) follow-up of 6.3 [7.0–7.6] years [2 926 917 person-years], 8598 (2.0%) all-cause deaths, 4850 (1.2%) cancer deaths, and 1654 (0.4%) cardiovascular deaths occurred. The number of all-cause, cancer, and cardiovascular deaths were greater in men (5184, 2682, and 1188, respectively) than women (3414, 2168, and 466 respectively).
Table 3.
Number of mortality events across categories of walking pace and handgrip strength in women and men
| Women |
Men |
|||||||
|---|---|---|---|---|---|---|---|---|
| Mortality events | Mortality events | |||||||
| Exposure | Participants | All-cause | Cancer | Cardiovascular | Participants | All-cause | Cancer | Cardiovascular |
| Handgrip strength | ||||||||
| First third | 83 166 | 1644 (2.0%) | 967 (1.2%) | 244 (0.3%) | 64 634 | 2465 (3.8%) | 1151 (1.8%) | 591 (0.9%) |
| Second third | 72 327 | 1032 (1.4%) | 680 (0.9%) | 128 (0.2%) | 61 380 | 1535 (2.5%) | 825 (1.3%) | 363 (0.6%) |
| Third third | 75 177 | 738 (1.0%) | 521 (0.7%) | 94 (0.1%) | 64 043 | 1184 (1.8%) | 706 (1.1%) | 234 (0.4%) |
| Walking pace | ||||||||
| Slow | 16 421 | 563 (3.4%) | 239 (1.5%) | 108 (0.7%) | 11 381 | 853 (7.5%) | 287 (2.5%) | 245 (2.2%) |
| Average | 122 281 | 1866 (1.5%) | 1237 (1.0%) | 247 (0.2%) | 99 721 | 2898 (2.9%) | 1553 (1.6%) | 660 (0.7%) |
| Brisk | 91 968 | 985 (1.1%) | 692 (0.8%) | 111 (0.1%) | 789 55 | 1433 (1.8%) | 842 (1.1%) | 283 (0.4%) |
| Total | 230 670 | 3414 (1.5%) | 2168 (0.9%) | 466 (0.2%) | 190 057 | 5184 (2.7%) | 2682 (1.4%) | 1188 (0.6%) |
| Excluding first 2 years | 230 246 | 2990 (1.3%) | 1936 (0.8%) | 382 (0.2%) | 189 260 | 4387 (2.3%) | 2338 (1.2%) | 960 (0.5%) |
Number (%).
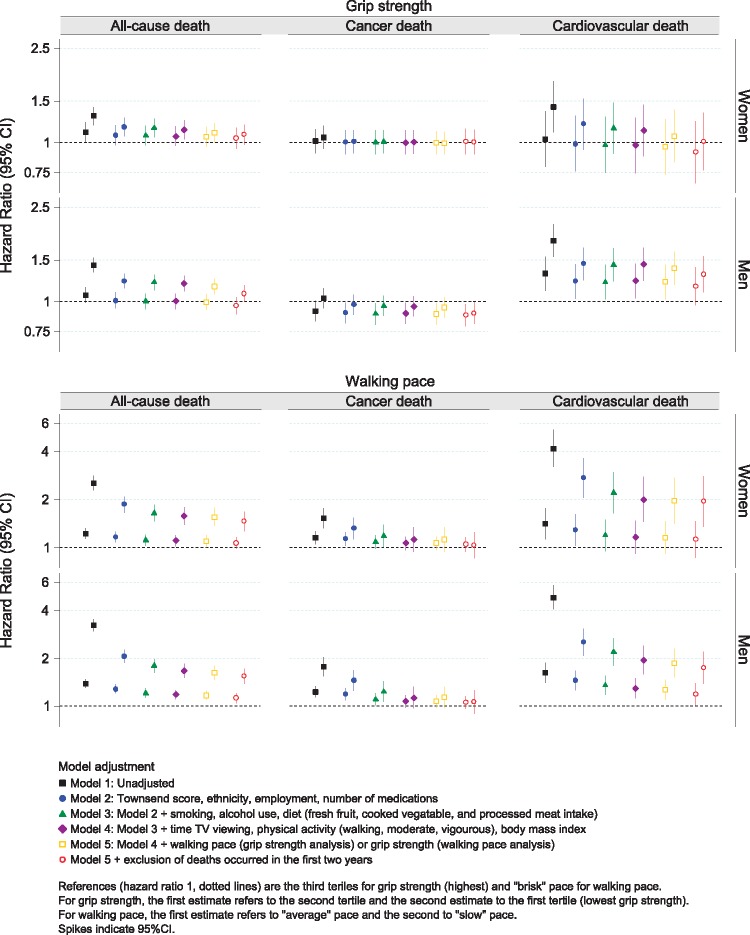
The hazard ratios (HRs) for walking pace and handgrip strength are shown graphically in Figure 2 with corresponding estimates presented in Supplementary material online, Tables S1 and S2. Walking pace was associated with all-cause and cardiovascular mortality, but not cancer mortality, in women and men across all models. Handgrip strength was associated with cardiovascular mortality in men and with all-cause mortality in women and men, although results for women were attenuated after removing deaths occurring within the first 24 months. Handgrip strength was not associated with cancer mortality in women, whereas in men a weaker handgrip appeared to be associated with a lower risk of cancer, particularly after removing deaths occurring within the first 24 months (Figure 2 and Supplementary material online, Table S2).
Figure 2.
Association of walking pace and handgrip strength with all-cause, cancer, and cardiovascular mortality.
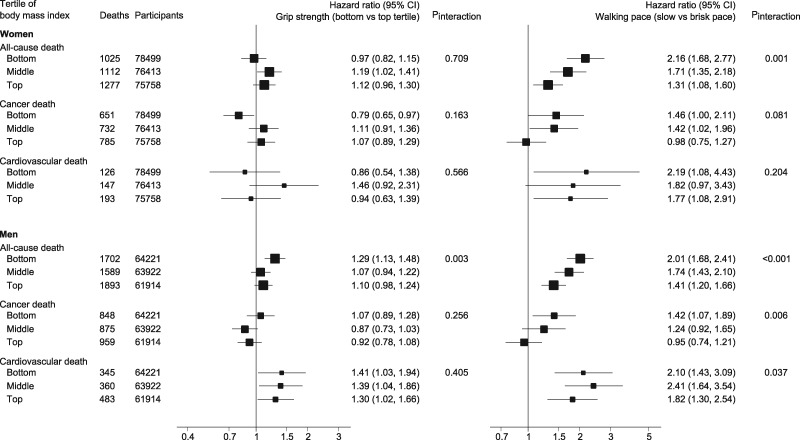
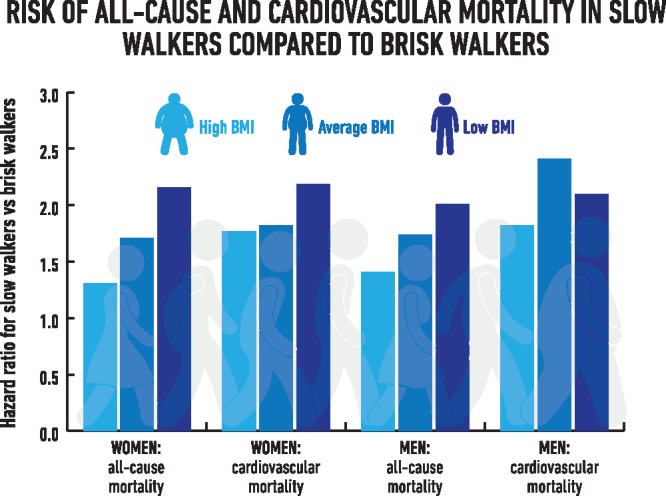
Body mass index was found to modify associations for walking pace in men and women and handgrip strength in men. Interaction tests and stratified results are displayed in Figure 3. For women, the HR for all-cause mortality in slow walkers compared with fast walkers ranged from 2.16 (1.68–2.77) to 1.31 (1.08–1.60) for participants in the bottom and top BMI tertiles, respectively. Corresponding HRs for men were 2.01 (1.68–2.41) and 1.41 (1.20–1.66). Hazard ratios for cardiovascular mortality for slow walkers compared with fast walkers remained above 1.7 across all categories of BMI in men and women, with a modest heterogeneity in men. A slow walking pace was also associated with cancer mortality in women and men with low BMI. Weak handgrip strength was only associated with a higher risk of all-cause mortality in men with low BMI (1.29; 1.13–1.48) whereas associations between handgrip strength and cardiovascular mortality were not affected by BMI status.
Figure 3.
Association of walking pace and handgrip strength with all-cause, cardiovascular, and cancer mortality across categories of BMI. Adjusted for Model 5.
Interaction analysis revealed the association of walking pace with all-cause (P = 0.572 for women; P = 0.626 for men) and cardiovascular mortality (P = 0.068 for women; P = 0.634 for men) was not modified by handgrip strength or vice-versa. In addition, the association of walking pace and handgrip strength with all-cause or cardiovascular morality was not modified by age or smoking status.
The C-indexes for the most adjusted models containing walking pace or handgrip strength demonstrated moderate discrimination (ranging from 0.63 to 0.71), with good calibration (Supplementary material online, Figure S2).
Discussion
Within a large national sample of UK adults free from cancer and cardiovascular disease, a simple measure of self-perceived walking pace was associated with a higher risk of all-cause and cardiovascular mortality; the strength of association persisted after adjustment for handgrip strength and was modified by levels of BMI. For those with low BMI, slow walkers had over twice the risk of cardiovascular and all-cause mortality compared with fast walkers in men and women. Associations were weaker at higher levels of BMI, yet slow walkers still had over 1.7 times the risk of cardiovascular mortality compared with fast walkers. Handgrip strength was not consistently associated with mortality in women, whereas in men a weak handgrip was associated with a higher risk of all-cause mortality within the lowest tertile of BMI and with cardiovascular mortality across all categories of BMI. There was no clear pattern of association of walking pace or handgrip strength with cancer mortality, although a slow walking pace was associated with a higher risk of cancer mortality in women and men with low BMI.
The strength of association of self-reported walking pace with all-cause mortality reported in the present study is consistent with previous research in smaller cohorts using objective measures, primarily in older adults. A recent meta-analysis including a total of 12 901 subjects across 9 studies reported a higher risk of all-cause mortality for the slowest walkers compared with the fastest walkers (HR = 1.89 [1.46–2.46]) when measured objectively.6 The Whitehall II study reported a similar finding in middle-aged adults.5 Other studies have also investigated the association between self-reported walking pace and cardiovascular events in occupational cohorts.8,9,21 For example, in the Women’s Health Study of health care professionals in the USA and Puerto Rico, those reporting a fast walking pace had around half the risk of a cardiovascular event compared with slow walkers.9 Similar results were seen for men in the Health Professionals’ Follow-up Study.8 Self-reported measures of physical fitness have also been associated with frailty and health outcomes in community dwelling cohorts or older adults.22,23
Our findings extend previous evidence by reporting the novel finding that a simple semi-quantitative measure of walking pace that asks individuals to rate their walking pace as slow, steady/average, or brisk is associated with objectively measured cardiorespiratory fitness and by evidencing that this simple measure is associated with mortality within the general population. A further novel finding revealed the strength of the association of walking pace with all-cause (men and women), cardiovascular (men), and cancer (men) mortality was modified by BMI, with stronger associations seen in the lowest tertile of BMI. Low levels of BMI are associated with malnutrition and higher levels of sarcopenia,24,25 therefore our results suggest the potential importance of self-reported walking pace in differentiating between healthy and unhealthy forms of low BMI.
Associations between handgrip strength and mortality are somewhat weaker than those reported in smaller studies which did not stratify by sex.13,26,27 As this study demonstrated, there is very little overlap in the distribution of handgrip strength between men and women. Therefore, conventional methods of adjustment for sex may not fully account for this difference.
This study did not find a clear pattern of association for walking pace or handgrip strength with the risk of cancer mortality in men or women. However, unexpectedly a weaker handgrip appeared to be associated with a slightly lower risk of cancer in men, a finding that was strengthened after removing deaths occurring within the first 24 months. To date, the literature in this area has been discrepant. Some studies have reported an association between higher levels of physical fitness and lower cancer mortality risk,2,28–30 while others have reported no association despite finding clear associations with cardiovascular mortality.14,15,31 Of note, another large population study also reported the finding that weaker handgrip strength was associated with a lower risk of cancer mortality.13 The equivocal nature of the evidence may reflect factors like age and the predominant types of cancer within the measured population; however, based on this study and the literature to date it is likely that simple indices of physical performance, such as walking pace and handgrip strength, are more reflective of cardiovascular health within the general population. The lower risk of cancer mortality with lower muscular strength reported for men in this study and in men and women in another large study warrants further investigation.13
Our results are supported by interventional research. Physical fitness, which was strongly associated with walking pace in the present study, is modifiable with exercise training. Although inter-individual responses to exercise training are heterogeneous, the majority gain some improvement. For example, in a population of healthy, pre-frail, and frail older adults (>65 years), all individual increased their functional capacity following an exercise intervention.32 Other studies have shown that the majority of adults respond with increased cardiorespiratory fitness following aerobic exercise training.33,34 Importantly, exercise training has also been shown to reduce the risk of chronic disease and improve cardiometabolic risk factors,35 including type 2 diabetes,36 blood pressure,37,38 and cholesterol.39 The fact that physical fitness is modifiable through exercise intervention and that exercise interventions in turn improve health status provide a robust mechanism that supports the association between measures of physical fitness and cardiovascular mortality reported in this study.
The present study has important strengths, including the large sample size and number of deaths which allowed stratification by sex. The extensively phenotyped population also allowed a comprehensive investigation into possible confounding or mediating influences on the association between walking pace, handgrip strength and mortality. However, important limitations remain. Although participants with cancer, myocardial infarction, stroke, or angina were excluded from the analysis, other potentially relevant conditions, such as a history of coronary artery bypass grafting or revascularization were not captured and reverse causality remains a possible explanation of our findings, including the interactions with BMI. Moreover, although we adjusted for a wide range of demographic, clinical and lifestyle factors, many covariates were self-reported, thus residual confounding due limited measurement precision also remains a possibility. This analysis was also limited to mortality end points.
Conclusions
In conclusion, this study found that a slow self-reported walking pace was associated with a higher risk of all-cause and cardiovascular mortality in women and men with associations remaining robust across multiple layers of adjustment. Associations were stronger in those with low BMI. Self-reported walking pace could therefore represent an important measure of physical fitness with potential applicability for risk stratification within the general population, particularly those presenting with low BMI. Future research is needed to investigate the extent to which walking pace can be used to improve the prognostic performance of common cardiovascular risk scores across different categories of BMI. Compared with self-reported walking pace, handgrip strength appeared to be a less generalizable marker of risk within the general population.
Take-home figure.

Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
National Institute for Health Research (NIHR) Leicester Biomedical Research Centre, Leicester, UK to T.Y. and M.D.; NIHR Collaboration for Leadership in Applied Health Research and Care—East Midlands to K.K; F.Z. is a clinical research fellow funded with an unrestricted Educational Grant to the University of Leicester from Sanofi-Aventis; the funding source had no role in study design, data collection, data analysis, data interpretation or writing of the report. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Data source: This research has been conducted using the UK Biobank Resource under Application Number 18815.
Conflicts of interest: none declared.
Supplementary Material
References
- 1. Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y.. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA 2009;301:2024–2035. [DOI] [PubMed] [Google Scholar]
- 2. Ruiz JR, Sui X, Lobelo F, Morrow JR, Jackson AW, Sjöström M, Blair SN.. Association between muscular strength and mortality in men: prospective cohort study. BMJ 2008;337:a439.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Authors/Task Force Members Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FD, Lochen ML, Lollgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WM Additional Contributor Simone Binno (Italy) Document Reviewers De Backer G, Roffi M, Aboyans V, Bachl N, Bueno H, Carerj S, Cho L, Cox J, De Sutter J, Egidi G, Fisher M, Fitzsimons D, Franco OH, Guenoun M, Jennings C, Jug B, Kirchhof P, Kotseva K, Lip GY, Mach F, Mancia G, Bermudo FM, Mezzani A, Niessner A, Ponikowski P, Rauch B, Ryden L, Stauder A, Turc G, Wiklund O, Windecker S, Zamorano JL.. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur J Prev Cardiol 2016;23:NP1–NP96. [DOI] [PubMed] [Google Scholar]
- 4. Ross R, Blair SN, Arena R, Church TS, Després J, Franklin BA, Haskell WL, Kaminsky LA, Levine BD, Lavie CJ.. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation 2016;134:e653–e699. [DOI] [PubMed] [Google Scholar]
- 5. Elbaz A, Sabia S, Brunner E, Shipley M, Marmot M, Kivimaki M, Singh-Manoux A.. Association of walking speed in late midlife with mortality: results from the Whitehall II cohort study. Age 2013;35:943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu B, Hu X, Zhang Q, Fan Y, Li J, Zou R, Zhang M, Wang X, Wang J.. Usual walking speed and all-cause mortality risk in older people: a systematic review and meta-analysis. Gait Posture 2016;44:172–177. [DOI] [PubMed] [Google Scholar]
- 7. Cooper R, Kuh D, Hardy R; Mortality Review Group; FALCon and HALCyon Study Teams. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ 2010;341:c4467.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB.. Exercise type and intensity in relation to coronary heart disease in men. JAMA 2002;288:1994–2000. [DOI] [PubMed] [Google Scholar]
- 9. Manson JE, Hu FB, Rich-Edwards JW, Colditz GA, Stampfer MJ, Willett WC, Speizer FE, Hennekens CH.. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N Engl J Med 1999;341:650–658. [DOI] [PubMed] [Google Scholar]
- 10. Norman K, Stobäus N, Gonzalez MC, Schulzke J, Pirlich M.. Hand grip strength: outcome predictor and marker of nutritional status. Clin Nutr 2011;30:135–142. [DOI] [PubMed] [Google Scholar]
- 11. Sayer AA, Syddall HE, Martin HJ, Dennison EM, Anderson FH, Cooper C.. Falls, sarcopenia, and growth in early life: findings from the Hertfordshire cohort study. Am J Epidemiol 2006;164:665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Purser JL, Kuchibhatla MN, Fillenbaum GG, Harding T, Peterson ED, Alexander KP.. Identifying frailty in hospitalized older adults with significant coronary artery disease. J Am Geriatr Soc 2006;54:1674–1681. [DOI] [PubMed] [Google Scholar]
- 13. Leong DP, Teo KK, Rangarajan S, Lopez-Jaramillo P, Avezum A, Orlandini A, Seron P, Ahmed SH, Rosengren A, Kelishadi R.. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015;386:266–273. [DOI] [PubMed] [Google Scholar]
- 14. Nofuji Y, Shinkai S, Taniguchi Y, Amano H, Nishi M, Murayama H, Fujiwara Y, Suzuki T.. Associations of walking speed, grip strength, and standing balance with total and cause-specific mortality in a general population of Japanese elders. J Am Med Dir Assoc 2016;17:184.e1–184.e7. [DOI] [PubMed] [Google Scholar]
- 15. Kishimoto H, Hata J, Ninomiya T, Nemeth H, Hirakawa Y, Yoshida D, Kumagai S, Kitazono T, Kiyohara Y.. Midlife and late-life handgrip strength and risk of cause-specific death in a general Japanese population: the Hisayama Study. J Epidemiol Community Health 2014;68:663–668. [DOI] [PubMed] [Google Scholar]
- 16. UK Biobank. UK Biobank: protocol for a large-scale prospective epidemiological resource. 2007. http://www.ukbiobank.ac.uk/wp-content/uploads/2011/11/UK-Biobank-Protocol.pdf (12 January 2017).
- 17. UK Biobank. UK Biobank data showcase. 2017. http://biobank.ctsu.ox.ac.uk/crystal/search.cgi (19 March 2017).
- 18. Tanaka H, Monahan KD, Seals DR.. Age-predicted maximal heart rate revisited. J Am Coll Cardiol 2001;37:153–156. [DOI] [PubMed] [Google Scholar]
- 19. Swain DP. Energy cost calculations for exercise prescription. Sports Med 2000;30:17–22. [DOI] [PubMed] [Google Scholar]
- 20. UK Biobank. UK Biobank website. 2017. https://www.ukbiobank.ac.uk/. (20 January 2017).
- 21. Lee I, Rexrode KM, Cook NR, Manson JE, Buring JE.. Physical activity and coronary heart disease in women: is no pain, no gain passé? JAMA 2001;285:1447–1454. [DOI] [PubMed] [Google Scholar]
- 22. Syddall HE, Westbury LD, Cooper C, Sayer AA.. Self-reported walking speed: a useful marker of physical performance among community-dwelling older people? J Am Med Dir Assoc 2015;16:323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jotheeswaran A, Bryce R, Prina M, Acosta D, Ferri CP, Guerra M, Huang Y, Rodriguez JJL, Salas A, Sosa AL.. Frailty and the prediction of dependence and mortality in low-and middle-income countries: a 10/66 population-based cohort study. BMC Med 2015;13:138.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim H, Suzuki T, Kim M, Kojima N, Yoshida Y, Hirano H, Saito K, Iwasa H, Shimada H, Hosoi E.. Incidence and predictors of sarcopenia onset in community-dwelling elderly Japanese women: 4-year follow-up study. J Am Med Dir Assoc 2015;16:85.e1–85.e8. [DOI] [PubMed] [Google Scholar]
- 25. Iannuzzi-Sucich M, Prestwood KM, Kenny AM.. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci 2002;57:M772–M777. [DOI] [PubMed] [Google Scholar]
- 26. Sasaki H, Kasagi F, Yamada M, Fujita S.. Grip strength predicts cause-specific mortality in middle-aged and elderly persons. Am J Med 2007;120:337–342. [DOI] [PubMed] [Google Scholar]
- 27. Chainani V, Shaharyar S, Dave K, Choksi V, Ravindranathan S, Hanno R, Jamal O, Abdo A, Rafeh NA.. Objective measures of the frailty syndrome (hand grip strength and gait speed) and cardiovascular mortality: a systematic review. Int J Cardiol 2016;215:487–493. [DOI] [PubMed] [Google Scholar]
- 28. Farrell SW, Finley CE, McAuley PA, Frierson GM.. Cardiorespiratory fitness, different measures of adiposity, and total cancer mortality in women. Obesity 2011;19:2261–2267. [DOI] [PubMed] [Google Scholar]
- 29. Laukkanen JA, Pukkala E, Rauramaa R, Mäkikallio TH, Toriola AT, Kurl S.. Cardiorespiratory fitness, lifestyle factors and cancer risk and mortality in Finnish men. Eur J Cancer 2010;46:355–363. [DOI] [PubMed] [Google Scholar]
- 30. Schmid D, Leitzmann MF.. Cardiorespiratory fitness as predictor of cancer mortality: a systematic review and meta-analysis. Ann Oncol 2015;26:272–278. [DOI] [PubMed] [Google Scholar]
- 31. Dumurgier J, Elbaz A, Ducimetiere P, Tavernier B, Alperovitch A, Tzourio C.. Slow walking speed and cardiovascular death in well functioning older adults: prospective cohort study. BMJ 2009;339:b4460.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Churchward-Venne TA, Tieland M, Verdijk LB, Leenders M, Dirks ML, de Groot LC, van Loon LJ.. There are no nonresponders to resistance-type exercise training in older men and women. J Am Med Dir Assoc 2015;16:400–411. [DOI] [PubMed] [Google Scholar]
- 33. Hautala AJ, Kiviniemi AM, Mäkikallio TH, Kinnunen H, Nissilä S, Huikuri HV, Tulppo MP.. Individual differences in the responses to endurance and resistance training. Eur J Appl Physiol 2006;96:535–542. [DOI] [PubMed] [Google Scholar]
- 34. Skinner JS, Jaskolski A, Jaskolska A, Krasnoff J, Gagnon J, Leon AS, Rao DC, Wilmore JH, Bouchard C; HERITAGE Family Study. Age, sex, race, initial fitness, and response to training: the HERITAGE Family Study. J Appl Physiol (1985) 2001;90:1770–1776. [DOI] [PubMed] [Google Scholar]
- 35. Lin X, Zhang X, Guo J, Roberts CK, McKenzie S, Wu WC, Liu S, Song Y.. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc 2015;4:doi:10.1161/JAHA.115.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, Hsu RT, Khunti K.. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ 2007;334:299.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cornelissen VA, Smart NA.. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc 2013;2:e004473.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cornelissen VA, Fagard RH.. Effects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factors. Hypertension 2005;46:667–675. [DOI] [PubMed] [Google Scholar]
- 39. Kodama S, Tanaka S, Saito K, Shu M, Sone Y, Onitake F, Suzuki E, Shimano H, Yamamoto S, Kondo K.. Effect of aerobic exercise training on serum levels of high-density lipoprotein cholesterol: a meta-analysis. Arch Intern Med 2007;167:999–1008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.