Abstract
Background
Consistent evidence at high levels of water arsenic (≥100 µg/l), and growing evidence at low-moderate levels (<100 µg/l), support a link with cardiovascular disease (CVD). The shape of the dose-response across low-moderate and high levels of arsenic in drinking water is uncertain and critical for risk assessment.
Methods
We conducted a systematic review of general population epidemiological studies of arsenic and incident clinical CVD (all CVD, coronary heart disease (CHD) and stroke) with three or more exposure categories. In a dose-response meta-analysis, we estimated the pooled association between log-transformed water arsenic (log-linear) and restricted cubic splines of log-transformed water arsenic (non-linear) and the relative risk of each CVD endpoint.
Results
Twelve studies (pooled N = 408 945) conducted at high (N = 7) and low-moderate (N = 5) levels of water arsenic met inclusion criteria, and 11 studies were included in the meta-analysis. Compared with 10 µg/l, the estimated pooled relative risks [95% confidence interval (CI)] for 20 µg/l water arsenic, based on a log-linear model, were 1.09 (1.03, 1.14) (N = 2) for CVD incidence, 1.07 (1.01, 1.14) (N = 6) for CVD mortality, 1.11 (1.05, 1.17) (N = 4) for CHD incidence, 1.16 (1.07, 1.26) (N = 6) for CHD mortality, 1.08 (0.99, 1.17) (N = 2) for stroke incidence and 1.06 (0.93, 1.20) (N = 6) for stroke mortality. We found no evidence of non-linearity, although these tests had low statistical power.
Conclusions
Although limited by the small number of studies, this analysis supports quantitatively including CVD in inorganic arsenic risk assessment, and strengthens the evidence for an association between arsenic and CVD across low-moderate to high levels.
Keywords: Arsenic, cardiovascular disease, dose-response, meta-analysis
Key Messages
This systematic review and meta-analysis of epidemiological studies is the first to quantitatively examine log-linear and non-linear dose-response associations between chronic arsenic exposure and the relative risk of cardiovascular disease (CVD) across low-moderate (<100 µg/l) to high levels (≥100 µg/l) of arsenic in drinking water.
We identified 12 studies of chronic arsenic exposure and incident CVD endpoints in the general population with three or more exposure categories to evaluate log-linear and non-linear associations.
In a dose-response meta-analysis, we found no statistical evidence of a non-linear dose-response association between log relative risk and log-transformed water arsenic concentrations modelled with flexible splines, although statistical power was limited.
This analysis strengthens the evidence of an association between arsenic and CVD across low to high levels in drinking water, and supports the inclusion of CVD endpoints in future inorganic arsenic risk assessment.
Introduction
More than 200 million people live in areas of the world where arsenic in drinking water exceeds the World Health Organization (WHO) standard (10 µg/l).1 In the USA, around five million people drink water with arsenic levels above the US Environmental Protection Agency (EPA) Maximum Contaminant Level (MCL) (> 10 µg/l), either from community water systems2 or from private wells.3–5 In areas where groundwater is naturally contaminated with moderate to high levels of inorganic arsenic (arsenite and arsenate), drinking water is the primary source of arsenic exposure in the general population.6 At low levels of arsenic in drinking water, arsenic in food (such as rice and rice products, some fruit juices and poultry) can be an important source of arsenic exposure.5,7–10
Chronic exposure to high arsenic levels (≥100 µg/l in drinking water) is associated with cardiovascular disease (CVD), particularly peripheral arterial disease (PAD) and coronary heart disease (CHD), according to the National Research Council (NRC) Committee on Inorganic Arsenic.11 Recent studies of populations exposed to low arsenic levels (<50 µg/l) from the USA,12–14 China15 and Italy16 have found increased risks of CHD and stroke, although the association was limited to smokers in one study.14 Further, studies with a subset of participants exposed to drinking water arsenic between 50 and 100 µg/l in Bangladesh have observed elevated, but not statistically significantly increased, relative risks of CHD and stroke.17,18
When the EPA last revised the drinking water standard for inorganic arsenic in 2001, few epidemiological studies of CVD existed and CVD outcomes were only qualitatively considered in the risk assessment.19,20 Considering the strengthening of the evidence linking arsenic to CHD at low to moderate levels of arsenic in drinking water (<100 µg/l), the 2013 National Research Council (NRC) committee recommended that the EPA designate CHD as a critical endpoint in the ongoing risk assessment of inorganic arsenic.11
The determination of the dose-response association is an essential step in the risk assessment process.21,22 For arsenic, where the majority of the evidence on adverse health effects comes from populations exposed to high levels of arsenic in drinking water (≥100 µg/l), a linear extrapolation of risk to the much lower levels common in the USA is controversial.11,23 Instead of selecting a single study, the 2013 NRC committee recommended the use of meta-analysis to explore the dose-response association across the range of arsenic exposures relevant to populations worldwide.11 Dose-response meta-analysis uses published categorical associations to examine the dose-response across studies, and allows for the statistical assessment of non-linearity.24 To our knowledge, no previous meta-analysis has quantitatively examined the dose-response association between arsenic and CVD endpoints.
Our goal was to conduct a systematic review and a dose-response meta-analysis of the association between chronic exposure to arsenic across populations exposed to low, moderate and high levels in drinking water and the risk of incident clinical CVD endpoints (overall CVD, CHD, stroke and PAD). Based on previous epidemiological studies, we evaluated the association between log-transformed arsenic concentrations and the relative risk of CVD endpoints, allowing for a log-linear or non-linear dose-response.
Methods
Search strategy
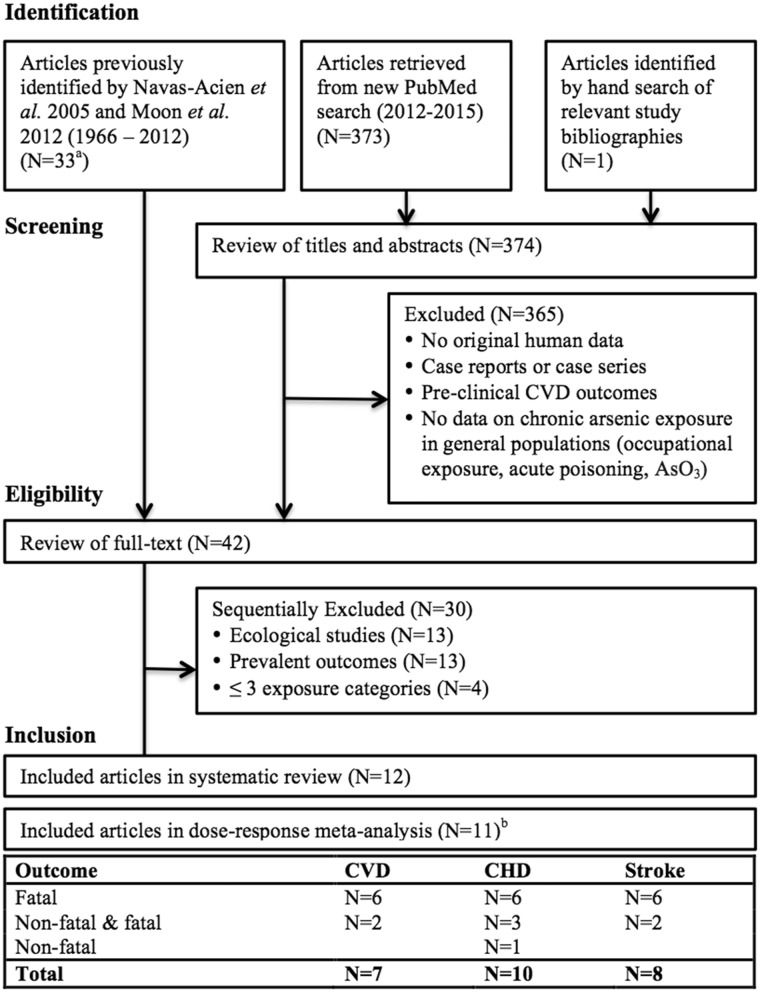
We defined clinical CVD as CHD (myocardial infarction and ischaemic heart disease), stroke (cerebrovascular disease, ischaemic and haemorrhagic stroke) and PAD. We identified studies that assessed the association between arsenic exposure and clinical CVD in the adult general population (i.e. excluding studies of childhood exposure, occupational exposure and arsenic compounds uncommon in the general population, such as arsenic trioxide) from previous systematic reviews25,26 and an updated search in PubMed (Figure 1) using the keywords found in Supplementary Table 1 (available as Supplementary data at IJE online). The updated PubMed search was limited to the period between June 2012 (slightly before the end of search period in a previous systematic review25) and December 2015. We also conducted a hand search of bibliographies of relevant studies and recent review articles. There were no language restrictions; however, no non-English articles were identified in the updated search. We conducted the literature search and review process in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria27 and Meta-Analysis of Observational Studies in Epidemiology (MOOSE)28 guidelines. Two authors, K.M. and S.O., independently searched the literature, applied eligibility criteria and abstracted study results. A.N-A. resolved any discrepancies. K.M. and A.N-A. independently assessed study quality and resolved differences through discussion.
Figure 1.
Study exclusion criteria. a13 general population epidemiological studies (14 articles) were identified in Navas-Acien et al. 2005 (Tsai et al. 1999 and Wu et al. 1989 were considered as the same study population). bWang et al. (2005) was included in the meta-analysis only as a sensitivity analysis.
Study eligibility
Based on a review of the titles and abstracts of identified articles, we first excluded studies if they met any of the following criteria: no human data, case reports or case series, non-clinical CVD outcomes or no data on chronic arsenic exposure in the general population (Figure 1). After reviewing the full text of the remaining articles, we sequentially excluded ecological studies (or studies analysed at the group level), cross-sectional studies with only prevalent events and studies that reported results with fewer than the three exposure categories (necessary to estimate potential non-linear dose-response associations). Only four studies (three unique populations) were excluded because there were too few exposure categories.29–32 Of these four studies, two reported only unadjusted mean arsenic concentrations in urine and hair among the same 130 myocardial infarction patients and 60 healthy controls,29,30 and the other two studies, of CVD mortality in Taiwan,31,32 included fewer participants with less follow-up time than another study in the same population already included.33 We contacted the study corresponding author before excluding a study for this reason. Only one study ascertained incident PAD;16 therefore, PAD was not included as an endpoint in the main analysis.
We identified one prospective cohort study of combined fatal CHD and stroke33 that reported the results of additional follow-up of two previously described cohorts34,35 from the arsenic-endemic areas of Taiwan combined with a cohort of Taiwan residents exposed to low levels of arsenic in drinking water. We included this study in the systematic review because of the importance of Taiwan data for arsenic risk assessment, but excluded it from the meta-analysis because the study was not peer-reviewed and did not report the number of person-years necessary to calculate the correlations between categorical relative risks compared with a common reference exposure. In a sensitivity analysis, we included the study by Wang and colleagues in the meta-analysis (CVD mortality) by making assumptions about the distribution of person-years across exposure categories.
Data abstraction
For each study, we abstracted descriptive information including population source, participant characteristics, exposure assessment, outcome ascertainment and adjustment for confounding. For each exposure category, we abstracted the number of cases, number of non-cases (if case-control study) or person-years (if incidence rate) or persons (if cumulative incidence), the measure of association (e.g. rate ratio, hazard ratio, odds ratio) and a measure of statistical uncertainty of the measure of association (standard error or confidence interval). We abstracted the mean and median arsenic concentration of each exposure category, and contacted study authors if this information was not provided.
The arsenic exposure categories, mean and median of arsenic within the exposure categories and measures of association from each study are presented in Supplementary Table 2, available as Supplementary data at IJE online. All measures of association (e.g. rate ratio, hazard ratio, odds ratio) were pooled together as approximations of the relative risk. If results were reported separately for men and women,16 we calculated a pooled relative risk for each exposure category using a random-effects (Dersimonian and Laird) model. For one study where relative risks were reported separately for heart disease and stroke,36 we estimated a pooled relative risk for overall CVD. We abstracted the model adjusted with the most potential confounders, excluding potential mediators (diabetes, hypertension and lipids) if available. If confidence intervals or standard errors were not reported,35 we conservatively approximated the standard error using the reported P-values. We assigned the median arsenic concentration within each exposure category as the exposure level for all participants in that category. If the median was not available, we assigned the mean37 or the midpoint of the category boundaries,33,35 calculated according to common practice.38 To calculate the midpoint of exposure categories with no minimum (e.g. < 10), we used the detection limit as the lower boundary. If the maximum was not defined (e.g. > 100), we assumed that the width of the highest exposure category was equal to the next lowest category.
Quality assessment
We assessed study quality using a combination of criteria from the Newcastle-Ottawa scale for cohort and case-control studies39 and criteria adapted from Longnecker et al.40 (Supplementary Table 3, available as Supplementary data at IJE online). We did not calculate a summary quality score, which can obscure important variation in potential biases.41
Common exposure metric
For studies with measured water arsenic concentrations, we selected the relative risks estimated from water arsenic. For two studies at low-moderate levels of arsenic in drinking water that measured arsenic using urine13 and toenails,14 we estimated water arsenic concentrations. For the New Hampshire Skin Cancer study,14 with toenail arsenic concentrations, we estimated water arsenic concentrations using linear regression coefficients from a model of water arsenic on toenail arsenic, adjusted for age, intake (glasses/day), sex, smoking, season, and year of interview (Karagas M, unpublished data) estimated from data from the overall case-control study42 (Supplementary Table 4, available as Supplementary data at IJE online). For the Strong Heart Study,13 where only urine arsenic concentrations were available, we estimated water arsenic concentrations as if the measured urine arsenic exposure had come solely from water, by multiplying each creatinine-corrected urine arsenic concentration (micrograms per gram creatinine) by the overall mean creatinine concentration in the population (grams per litre) (Supplementary Table 5, available as Supplementary data at IJE online). In the same units (grams per litre), urine arsenic concentrations were approximately equal to water arsenic concentrations at high levels in drinking water in Taiwan (mean 600 µg/l)43 and at more moderate levels (60% samples < 50 µg/l) in the USA.44 In a sensitivity analysis of the conversion of urine to water arsenic in the Strong Heart Study (rural Arizona, Oklahoma and North/South Dakota),13 we calculated water arsenic concentrations based on published linear regression coefficients from a US study in rural Utah with slightly higher levels of arsenic in drinking water compared with the Strong Heart Study44 (Supplementary Table 6, available as Supplementary data at IJE online).
Dose-response meta-analysis
We conducted a two-stage random-effects dose-response meta-analysis24,45 of water arsenic concentrations and the log relative risk of each CVD endpoint, assuming both a constant log-linear association (log-transformed arsenic concentrations) and a flexible non-linear association (restricted cubic splines with knots at the 10th, 50th and 90th percentiles of log-transformed arsenic). In the first stage, we fitted a log-linear or restricted cubic spline model to each study’s published results using generalized least squares, accounting for the correlation of relative risks within each study.46 In the second stage, we calculated a pooled relative risk by combining the study-specific log relative risks and variance-covariance matrices using restricted maximum likelihood in a multivariate random-effects meta-analysis.47 We evaluated possible non-linear associations by testing that the non-linear restricted cubic spline coefficient was different from zero. Pooled log-linear and non-linear relative risks for each endpoint were estimated for water arsenic concentrations of 20, 50, 100 and 200 µg/l, using 10 µg/l of arsenic in drinking water as the reference exposure.
We examined each endpoint (all CVD, CHD and stroke) in separate models and stratified by relative risks estimated from combined non-fatal and fatal events (CVD incidence) compared with only fatal events (CVD mortality). We assessed the amount of statistical heterogeneity across studies using the I2 statistic, which describes the proportion of total variation due to between-study heterogeneity, and Cochran’s Q-statistic.48,49P-values for heterogeneity were considered statistically significant if less than 0.10.48 Otherwise, P-values less than 0.05 were considered statistically significant. Our evaluation of sources of heterogeneity was limited because of the small number of studies and the difficulty of distinguishing between strongly related study characteristics (e.g. by arsenic levels of drinking water, exposure assessment method or other study characteristics). However, we examined stratified models by low-moderate vs high water arsenic levels and calculated P-values for subgroup heterogeneity for CVD, CHD and stroke mortality models that had at least two studies within each subgroup. Final model goodness-of-fit was assessed using deviance, coefficient of determination (R2) and decorrelated residuals-vs-exposure plots.50 We examined potential publication bias with funnel plots for all CVD endpoints and Egger’s test of funnel plot asymmetry for CVD endpoint models with greater than two studies.51 Influential studies were assessed using Baujat plots (Supplementary Figure 4, available as Supplementary data at IJE online).52
We evaluated an alternative method (Hamling) to estimate the correlations between relative risks reported in relation to a common reference group,53 and we assigned the mean rather than the median arsenic concentration if available. Finally, we conducted a sensitivity analysis that included three ecological studies that had sufficient data available to estimate both a linear and a non-linear dose-response association in relation to water arsenic exposure (Supplementary Table 7, available as Supplementary data at IJE online).54–56 Statistical analyses were conducted in Stata (glst) and R (dosresmeta, metafor).57,58 Figures were created using ggplot259 in R.58
Results
Systematic review
We identified 12 studies that examined categorical dose-response associations between arsenic exposure and incident CVD endpoints, including eight studies of all CVD (incidence, N = 2; mortality, N = 7), 10 studies of CHD (incidence, N = 4; mortality, N = 6), and eight studies of stroke (incidence, N = 2; mortality, N = 6) (Table 1). Seven studies were conducted in populations with at least one exposure category at high arsenic levels in drinking water (≥100 µg/l) in Taiwan,33,35 Bangladesh17,18,37,60 and Inner Mongolia, China.36 Five studies were conducted in populations exposed to low-moderate arsenic in drinking water (<100 µg/l) in Inner Mongolia, China,15 Italy,16 and the USA.12–14 There were four prospective cohort studies,13,17,35,37 one prospective cohort study with an external comparison group,33 two prospective case-cohort studies,12,18 three retrospective cohort studies,16,36,60 a prospective analysis of a population-based case-control study of non-melanoma skin cancer14 and a hospital-based case-control study.15
Table 1.
Epidemiological studies of arsenic and incident overall cardiovascular disease, coronary heart disease, and stroke
| Author and year | Design | Population | N (follow-up)a | Exposure assessment | Exposure categories | Outcome | Outcome ascertainment | Adjustment factors |
|---|---|---|---|---|---|---|---|---|
| High arsenic in drinking water (at least one exposure group ≥ 100 µg/l) | ||||||||
| Rahman et al. 2014 | Prospective cohort | Matlab, Bangladesh ≥18 y 43% men | 61074 (∼7 y) | TWA individual drinking water (μg/l) | <10 10–49 ≥50 | Fatal stroke | Verbal autopsy (ICD-10: I61–69) | Age, sex, education, SES |
| Chen et al. 2013 | Prospective case-cohort | Araihazar, Bangladesh 18–75 y 43% men | 1109 (∼6 y) | TWA household drinking water (µg/l) | 0.1–25 25.1–107 108–864 | Non-fatal and fatal CVD, CHD, and stroke | Non-fatal: clinical exam (ECG and cardiac enzymes) and medical records Fatal: verbal autopsy, medical records (ICD-10: I00–99) | Age, sex, education, BMI, smoking, hypertension, diabetes, change in arsenic between visits |
| Chen et al. 2011 | Prospective cohort | Araihazar, Bangladesh 18–75 y 43% men | 11746 (∼6 y) | Urine total arsenic (µg/g creatinine) | 6.6–105.9 106.0–199.0 199.1–351.8 351.9–1100 | Fatal CVD, CHD, and stroke | Verbal autopsy, medical records (ICD-10: I00–99) | Age, sex, education, BMI, smoking, change in arsenic between visits |
| TWA household drinking water (µg/l) | 0.1–12 12–62.0 62.1–148.0 148.1–864 | |||||||
| Sohel et al. 2009 | Retrospective cohort | Matlab, Bangladesh ≥15 y 49% men | 115903 (∼10 y) | TWA household drinking water (µg/l) | <10 10–49 50–149 150–299 > 300 | Fatal CVD | Verbal autopsy (modified ICD-8, including acute MI, acute but ill-defined CVD including stroke, and complications of acute but ill-defined CVD) | Age, sex, education, asset score |
| Wade et al. 2009 | Retrospective cohort | Inner Mongolia, China 0 > 80 y 50% men | ∼12334 (∼6 y) | Household drinking water (µg/l) | <0.1–5 5.1–20 20.1–100 100.1–300 > 300 | Fatal CVD, CHD and strokec | Verbal autopsy, medical records and physician interview (ICD-10: I00–09, I11, I13, I20–51; I60–69) | Age, sex, education, smoking, alcohol use, farm work |
| Wang et al. 2005b | Prospective cohort with external comparison group | SW and NE Taiwan, and low-arsenic townships in Taiwan > 40 y 50% men | 29972 (∼12 y) | Village, household and municipal water (µg/l) | <10 10–49 50–499 ≥500 | Fatal CVD | National death registry (ICD-9: 410–414; 430–438) | Age, sex, smoking |
| Chen et al. 1996 | Prospective cohort | SW Taiwan 40–≥70 y 52% men | 2556 (∼5 y) | Village drinking water | <10 10–500 ≥510 | Fatal CHD | National death registry (ICD-9: 410–414) | Age, sex, smoking, BMI, lipids, hypertension, diabetes |
| Low-moderate arsenic in drinking water (all exposure groups < 100 µg/l) | ||||||||
| D’Ippoliti et al. 2015 | Administrative retrospective cohort | Lazio, Italy Mean 33 y 50% men | 165609 (∼40 y) | Predicted TWA household drinking water (µg/l) | <10 10–20 > 20 | Fatal CVD, CHD, stroke, PAD | Death certificate registry (ICD-9: 390–459; 410–414; 430, 431, 434, 436) | Age, sex, calendar period, occupation in the ceramic industry; SES, smoking sales and radon exposure (municipal level) |
| James et al. 2015 | Prospective case-cohort | Colorado, USA 20–74 y 46% men | 555 (∼<10–14 y) | Predicted TWA household drinking water (µg/l) | 1–10 10–20 20–30 35–45 45–88 | Non-fatal and fatal CHD | Non-fatal: self-report and medical record review Fatal: obituary monitoring and death certificate searches (ICD-9: 410–414) | Age, sex, income, Hispanic ethnicity, smoking, alcohol use, BMI, sedentary physical activity, family history of CHD, diabetes, LDL cholesterol, TG, HDL cholesterol, folate, selenium |
| Wade et al. 2015 | Hospital-based case-control | Inner Mongolia, China 18–70 y 70% men | 298 cases/275 controls | Household drinking water (µg/l) | <10 10–39 > 40–99 | Non-fatal CHDd | Standard clinical criteria (angina, ECG, echocardiogram and cardiac enzymes) | Age, sex, education, smoking, BMI, occupation, alcohol use, family history of hypertension, diabetes, or heart disease |
| Farzan et al. 2015 | Prospective analysis of population-based non-melanoma skin cancer case-control study | New Hampshire, USA Median 61 y 56% men | 3939 (∼14 y) | Toenail (µg/g) | <0.01–0.07 0.07–0.11 0.11–3.26 | Fatal CVD, CHD and stroke | National death index (ICD-10: I00–99, I20–25, I60–69) | Age, sex, education, smoking (pack-years), cancer status (case vs control) |
| Moon et al. 2013 | Prospective cohort | Arizona, Oklahoma, and North/South Dakota, USA 45–74 y 40% men | 3575 (∼15 y) | Urine sum of inorganic and methylated metabolites (µg/g creatinine) | <5.8 5.8–9.7 9.8–15.7 > 15.7 | Non-fatal and fatal CVD, CHD and stroke | Non-fatal: self-report, clinical follow-up exams, medical records Fatal: death certificate searches, medical records, informant interviews (ICD-9: 410–414; 431, 432, 434, 436, 437) | Age, sex, education, smoking, BMI, LDL cholesterol |
TWA, time-weighted average; CVD, cardiovascular disease; CHD, coronary heart disease; ICD, International Classification of Disease; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG, triglycerides; BMI, body mass index; SES, socioeconomic status; SW, Southwest; NE, Northeast; RR, relative risk; ECG, electrocardiogram.
aNumber of persons at baseline for cohort studies, number of persons in the sub-cohort at baseline for case-cohort studies and number of cases and non-cases for case-control studies.
bWang et al. 2005 was only included as a sensitivity analysis in the meta-analysis. This non-peer-reviewed study combined two ‘exposed’ cohorts from arsenic-endemic areas in Southwest (described in Chen et al. (1996) and Northeast Taiwan (described in Chiou et al. 1997) and an ‘unexposed’ cohort in other areas of Taiwan with lower arsenic levels in drinking water. The majority (∼86%) of participants in the lowest reference category (<10 µg/l) were from the ‘unexposed’ cohort.
cBoth the relative risk of stroke and cerebrovascular disease were reported in Wade et al. (2009). We selected the relative risk for cerebrovascular disease as more consistent with the majority of studies of fatal stroke and because the outcome of cerebrovascular disease may be more relevant to ascertainment via death certificates.
dWade et al. (2015) included 16 cases of cardiomyopathy (<4%) in addition to acute or previous myocardial infarction. Cases must have survived after initial hospitalization to enrol.
The majority of studies met most of the quality criteria, although there was considerable variation across studies in potential for selection bias, exposure assessment, outcome assessment and adjustment for confounding (Supplementary Table 3). Most studies assessed arsenic exposure at the individual or household level, measuring speciated urine arsenic,13 total urine arsenic,17 toenail arsenic14,15 or arsenic in household or shared wells15,17,18,36,37,60 (Table 1). Other studies relied on village and municipal drinking water measurements,33,35 a spatial prediction model of lifetime exposure using residential history and municipal drinking water monitoring data16 or a spatial prediction model of lifetime exposure using residential history, household drinking water samples (only available in 64% of participants) and historical well measurements.12 Whereas many studies with measured water arsenic used a single measurement as a proxy for chronic exposure, several studies used time-weighted averages to assess arsenic exposure over the past several years17,18,37,60 or the lifetime.12,16 Limitations in exposure assessment add additional uncertainty to the estimation of the association between arsenic and CVD. In principle, if exposure misclassification is non-differential by disease status, the association would result in an underestimation of the association.
There was considerable heterogeneity in the definition of CVD endpoints and quality of outcome ascertainment methods across studies (Table 1). Only three studies systematically ascertained both non-fatal and fatal events12,13,18 and only non-fatal cases were included in a hospital-based case-control study because cases must have survived until enrolment.15 Studies identified non-fatal CVD events through medical record review and clinical examination12,13,18 or clinical examination only.15 Studies identified CVD deaths via active surveillance of death certificates and medical record review,12,13 death certificate registry linkage14,16,33,35 or verbal autopsy (a method to identify possible causes of death in the absence of medical records).17,18,36,37,60 One study using death registry linkage also examined a subset of death certificates,14 and three studies using verbal autopsy also reviewed medical records for hospital deaths.17,18,36 Studies of fatal CHD generally used consistent International Classification of Disease (ICD) codes,16–18,35 although one study used a broad case definition (less than half of events were acute MI: heart attack).36 Fatal stroke was defined broadly as cerebrovascular disease in all studies14,16–18,36,37 except one where cases were limited to ischaemic or haemorrhagic stroke.13
All but four studies16,33,37,60 adjusted for at least one lifestyle or clinical cardiovascular risk factor in addition to age, sex and either education or socioeconomic status. Of these four studies, one adjusted for age, sex and smoking,33 two adjusted for age, sex and education or socioeconomic status37, 60 and one adjusted only for age and sex.16
Pooled association between arsenic and cardiovascular disease endpoints
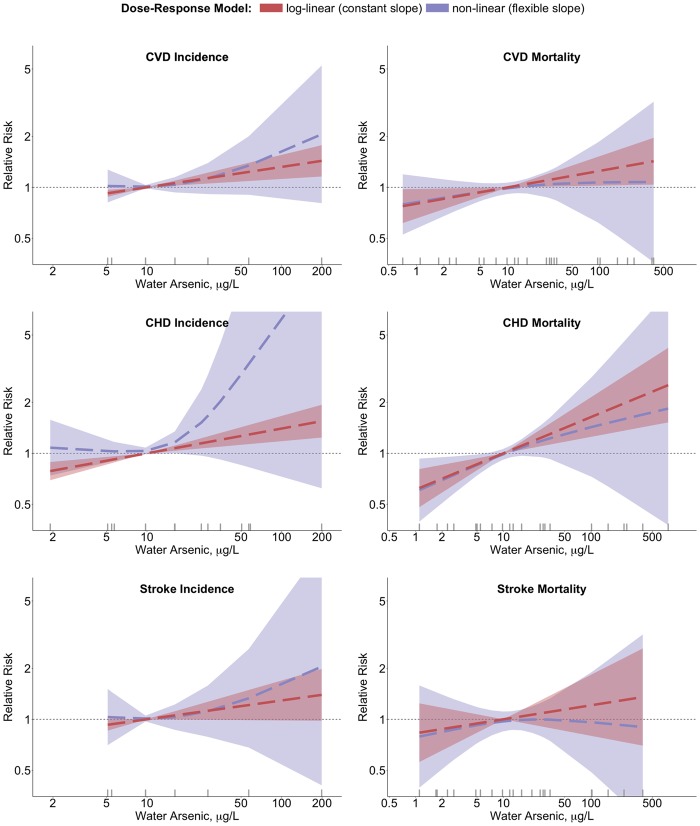
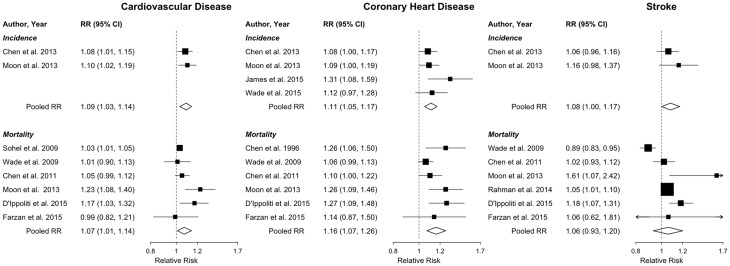
For the dose-response meta-analysis, we excluded one non-peer-reviewed study of CVD mortality without complete data,33 leaving two studies with all CVD incidence, six studies with all CVD mortality, five studies with CHD incidence, six studies with CHD mortality, two studies with stroke incidence and six studies with stroke mortality. Individual dose-response data are presented in Supplementary Figure 1, available as Supplementary data at IJE online. Compared with 10 µg/l, the pooled relative risks (95% confidence intervals) for exposure to 20 µg/l water arsenic assuming a constant log-linear dose-response association (i.e. per doubling of water arsenic) and the relative risk of CVD endpoints were 1.09 (1.03, 1.14) for CVD incidence, 1.07 (1.01, 1.14) for CVD mortality, 1.11 (1.05, 1.17) for CHD incidence, 1.16 (1.07, 1.26) for CHD mortality, 1.08 (0.99, 1.17) for stroke incidence and 1.06 (0.93, 1.20) for stroke mortality (Table 2 and Figure 2). In analyses where water arsenic was modelled as restricted cubic splines of log-transformed arsenic concentrations, we found no statistical evidence of a departure from a constant dose-response association (all P-values for non-linear trend > 0.05). The corresponding non-linear pooled relative risks for exposure to 20 µg/l water arsenic, compared with 10 µg/l, were 1.05 (0.90, 1.21) for CVD incidence, 1.07 (1.00, 1.14) for CVD mortality, 1.14 (1.01, 1.29) for CHD incidence, 1.16 (1.05, 1.27) for CHD mortality, 1.04 (0.81, 1.33) for stroke incidence and 1.07 (0.97, 1.18) for stroke mortality.
Table 2.
Pooled relative risks (95% confidence intervals) for cardiovascular disease, coronary heart disease and stroke in relation to water arsenic concentrations
| CVD incidence | CVD mortality | CHD incidence | CHD mortality | Stroke incidence | Stroke mortality | |||
|---|---|---|---|---|---|---|---|---|
| # Studies (# relative risks)a | 2 (5) | 6 (18) | 4 (10) | 6 (16) | 2 (5) | 6 (16) | ||
| Log-linear or constant dose-response association model (log-transformed arsenic) | ||||||||
| 10 µg/l arsenic | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||
| 20 µg/l arsenic | 1.09 (1.03, 1.14) | 1.07 (1.01, 1.14) | 1.11 (1.05, 1.17) | 1.16 (1.07, 1.26) | 1.08 (1.00, 1.17) | 1.06 (0.93, 1.20) | ||
| 50 µg/l arsenic | 1.21 (1.08, 1.36) | 1.17 (1.02, 1.34) | 1.27 (1.12, 1.43) | 1.41 (1.17, 1.71) | 1.20 (0.99, 1.45) | 1.14 (0.85, 1.53) | ||
| 100 µg/l arsenic | 1.32 (1.12, 1.56) | 1.25 (1.02, 1.53) | 1.40 (1.18, 1.66) | 1.64 (1.25, 2.15) | 1.29 (0.98, 1.69) | 1.21 (0.80, 1.83) | ||
| 200 µg/l arsenic | 1.43 (1.16, 1.78) | 1.34 (1.03, 1.74) | 1.55 (1.24, 1.94) | 1.90 (1.34, 2.70) | 1.40 (0.98, 1.99) | 1.28 (0.75, 2.20) | ||
| P-linear trendb | 0.001 | 0.029 | <0.001 | <0.001 | 0.065 | 0.37 | ||
| I2 (95% CI)c | 0 (0, 0) | 64 (0, 91) | (0, 0) | 51 (0, 83) | 0 (0, 0) | 87 (0, 96) | ||
| P-heterogeneityd | 0.66 | 0.041 | 0.33 | 0.087 | 0.35 | <0.001 | ||
| Non-linear or flexible dose-response association model (restricted cubic splines of log-transformed arsenic) | ||||||||
| 10 µg/l arsenic | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||
| 20 µg/l arsenic | 1.05 (0.90, 1.21) | 1.07 (1.00, 1.14) | 1.14 (1.01, 1.29) | 1.16 (1.05, 1.27) | 1.04 (0.81, 1.33) | 1.07 (0.97, 1.18) | ||
| 50 µg/l arsenic | 1.25 (0.92, 1.72) | 1.17 (1.02, 1.33) | 1.83 (1.05, 3.19) | 1.40 (1.13, 1.73) | 1.24 (0.73, 2.12) | 1.11 (0.98, 1.26) | ||
| 100 µg/l arsenic | 1.53 (0.90, 2.60) | 1.25 (1.00, 1.57) | 2.81 (1.05, 7.52) | 1.62 (1.15, 2.28) | 1.53 (0.61, 3.80) | 1.11 (0.89, 1.38) | ||
| 200 µg/l arsenic | 1.92 (0.86, 4.31) | 1.35 (0.96, 1.90) | 4.33 (1.05, 17.80) | 1.87 (1.13, 3.10) | 1.92 (0.47, 7.83) | 1.10 (0.76, 1.59) | ||
| P-non-linear trende | 0.30 | 0.91 | 0.091 | 0.99 | 0.52 | 0.51 | ||
aSum of the non-reference exposure categories across studies; the total number of relative risks in each model.
bP-value for linear trend from a Wald test of the coefficient for log-transformed water arsenic concentrations.
cProportion of total variance due to between-study heterogeneity.
dP-value for heterogeneity is chi-square P-value of the Q-statistic.
eNon-linear trend P-value for the non-linear spline coefficient in a model with arsenic concentrations entered as a restricted cubic spline with knots at 10th, 50th and 90th percentiles of log-transformed water arsenic.
Figure 2.
Pooled log-linear and non-linear relative risks of incident overall cardiovascular disease, coronary heart disease and stroke in relation to estimated water arsenic. Pooled linear (red) and non-linear (blue) relative risks of CVD endpoints (overall CVD, CHD and stroke, stratified by studies of incidence and mortality) were estimated for drinking water arsenic concentrations in reference to 10 µg/l. Dashed lines correspond to pooled relative risks, and shaded regions correspond to the 95% confidence intervals of the pooled relative risks. Log-linear associations were estimated from models with log-transformed estimated water arsenic concentrations. Non-linear associations were estimated from models with restricted cubic splines of log-transformed estimated water arsenic concentrations with knots at the 10th, 50th and 90th percentiles of log-transformed arsenic (exact knot locations vary by model; for CHD incidence, knots were placed at 5.1, 20.5 and 58.7 µg/l). A rug plot along the x-axis provides the median estimated water arsenic concentrations included in each model.
The individual study relative risks of CVD overall, CHD and stroke for 20 µg/l water arsenic in comparison with 10 µg/l are presented in Figure 3. In models assuming a log-linear dose-response association, we found little evidence of heterogeneity among studies of all CVD, CHD and stroke incidence (all heterogeneity P-values > 0.10 and I2 = 0%), although there were few studies with both non-fatal and fatal cases (Table 2 and Figure 3). There was evidence of heterogeneity among studies of all CVD and CHD mortality (I2 = 64% and 51%, P-values for heterogeneity = 0.041 and 0.087, respectively) and among studies of stroke mortality (I2 = 87%, P-value for heterogeneity < 0.001).
Figure 3.
Individual study and pooled log-linear relative risks (95% confidence intervals) of incident overall cardiovascular disease, coronary heart disease and stroke, comparing 20 µg/l with 10 µg/l water arsenic. Forest plot of individual study pooled relative risks (95% confidence intervals) and overall pooled relative risks (95% confidence intervals) for CVD, CHD and stroke (stratified by incidence and mortality) comparing 20 µg/l with 10 µg/l (i.e. per doubling) of water arsenic. Individual study pooled linear relative risks were estimated in the first stage of the two-stage dose-response meta-analysis, in which a generalized least-squares linear model was fitted to each study’s categorical dose-response data. In the second stage, the individual study pooled linear relative risks were pooled together in a multivariate random-effects meta-analysis to estimate the overall pooled relative risks. Within each model, studies were ordered by average water arsenic levels, from highest to lowest. The sizes of the individual study relative risk squares were weighted by the inverse variance of the log relative risk within each model.
For CVD and CHD endpoints and for stroke incidence, the deviance test did not detect lack of fit (deviance P-values > 0.05) (Supplementary Table 8, available as Supplementary data at IJE online). For stroke mortality, the deviance test indicated an overall lack of fit for both linear (P = 0.001) and non-linear (P = 0.002) dose-response models. For all endpoints, there was a slight decrease in deviance and R2 values in non-linear models, compared with linear models, but the deviance test did not detect differences in the model goodness-of-fit across dose-response models (all difference in deviance P-values > 0.05). The decorrelated residuals plots suggested some differences in model fit across water arsenic levels (Supplementary Figure 2, available as Supplementary data at IJE online). Funnel plots and Egger’s test for funnel plot asymmetry suggested potential bias only for CHD mortality (Pp = 0.015) (Supplementary Figure 3 and Supplementary Table 9, available as Supplementary data at IJE online). For each endpoint model, different studies were influential in the overall pooled relative risks and in their contribution to heterogeneity (Supplementary Figure 4, available as Supplementary data at IJE online).
In stratified analyses, we found evidence of significant heterogeneity by arsenic levels in drinking water (low-moderate vs high levels in water), with stronger relative risks among studies conducted at lower levels of arsenic in drinking water (<100 µg/l) compared with studies of populations exposed to higher levels (≥100 µg/l) for CVD mortality (P-value for heterogeneity = 0.006) and CHD mortality (P-value for heterogeneity = 0.017) (Supplementary Table 10, available as Supplementary data at IJE online). Compared with overall models, heterogeneity remained for stroke mortality in studies at high arsenic levels, whereas heterogeneity was reduced for other outcomes. We found similar results when we added data from Taiwan by Wang and colleagues33 to the model for overall CVD mortality (Supplementary Table 11, available as Supplementary data at IJE online). Including ecological studies for CVD, CHD and stroke mortality models resulted in similar estimates for pooled linear relative risks, with higher estimates of heterogeneity (Supplementary Table 12, available as Supplementary data at IJE online). Non-linear dose-response models including ecological studies differed from the main analysis, but there was no statistical evidence of non-linearity. We found that for CHD incidence, using an alternative method to estimate water concentrations in one study with urine concentrations was suggestive of a non-linear association (P-value for non-linearity, <0.001) (Supplementary Table 13, available as Supplementary data at IJE online). Using the mean arsenic concentrations, instead of the median, we observed almost no differences in the pooled log-linear models but found slightly less steep slopes in pooled non-linear models compared with the main analysis (Supplementary Figure 4, available as Supplementary data at IJE online). We found little difference in pooled relative risks estimated using the Hamling method to estimate the covariance of categorical relative risks (results not shown).
Discussion
In a systematic review, we identified 12 epidemiological studies examining the association between chronic, general population exposure to arsenic and the incidence of clinical CVD outcomes (overall CVD, CHD or stroke). We excluded ecological studies and studies without sufficient information to estimate a non-linear dose-response association (i.e. three or more categories of arsenic exposure). In a dose-response meta-analysis, we found a consistent positive association between arsenic exposure and overall CVD, CHD and stroke. Compared with 10 µg/l, the magnitude of the estimated relative risk of CVD endpoints at 20 µg/l water arsenic ranged from a 6% increase for risk of stroke mortality to a 16% increase in risk of CHD mortality. We found no statistical evidence of a non-linear dose-response association, although we cannot rule out non-linear associations because of our limited sample size.
Previous systematic reviews and meta-analyses of arsenic and clinical CVD, CHD and stroke have identified strong evidence for an association between arsenic and clinical CVD endpoints at high levels of arsenic in drinking water (≥100 µg/l),25,26,61,62 and some have observed growing evidence for an association at low-moderate levels (<100 µg/l).25 The dose-response association, however, has been evaluated only descriptively. Further, these previous analyses calculated the relative risk of CVD endpoints comparing only the highest with the lowest arsenic exposure levels, without considering a more flexible dose-response association. Incorporating recently published prospective cohort studies, including three at low to moderate (<100 µg/l)12,13,16 and three at high (≥100 µg/l)17,18,37 levels of arsenic in drinking water, this study quantitatively estimated both log-linear and non-linear pooled dose-response associations between arsenic exposure and incident CVD. Some studies reported relative risks at arsenic exposure between 5 and 10 µg/l,13,14,36 although the number of studies was relatively small. Additional research is needed at water arsenic levels below 10 µg/l, to improve the estimation of the effect of arsenic on CVD at low exposure levels in drinking water and food. We excluded cross-sectional studies and ecological studies in this analysis to best inform the dose-response assessment, although these studies remain useful for the overall evaluation of the association between arsenic and incident clinical CVD endpoints.
The majority of included studies observed associations between chronic arsenic exposure and CVD endpoints, at both low-moderate and high levels of arsenic in drinking water. The observed heterogeneity in relative risks is likely related to both true differences in underlying population characteristics (i.e. susceptibility) and differences in methodology, including study design, exposure assessment, outcome ascertainment and adjustment for confounding. Studies suggest that the association between arsenic and CVD endpoints could vary by pre-existing disease (e.g. diabetes),13 smoking status,13,14,17 diet and nutritional sufficiency,63 arsenic metabolism18,64,65 or other genetic susceptibility factors.66,67 Additionally, the time period captured by arsenic exposure assessment varied across studies, and studies ascertaining exposure during different aetiological periods may result in different magnitudes of association.
The methodological limitations of the underlying epidemiological studies must be considered. Study quality was generally higher for incident endpoint models, although there were fewer studies than in fatal endpoint models. Most studies ascertained only fatal outcomes, which do not capture most incident CVD events. In populations exposed to high levels of arsenic in drinking water, ascertainment of CVD was largely limited to verbal autopsy and death certificates, which are more vulnerable to measurement error, although some studies identified cases through medical record review and standard clinical criteria. At low-moderate levels, several studies also relied solely on death certificates. Most studies adjusted for age, sex, either education or socioeconomic status and at least some measures of clinical or lifestyle risks of CVD (e.g. smoking, body mass index, lipids), but adjustment for confounders varied considerably and one study only adjusted for individual-level age and sex.16 Residual confounding, potentially from geographical factors or diet, could also have introduced bias. In addition, hypertension and diabetes could be on the causal pathway from arsenic exposure to CVD.68,69
Studies with village-level or predicted arsenic exposure assessment may have greater measurement error than individual or household-level exposure assessment, most likely resulting in an underestimation of the true association. Assessing arsenic exposure via a biomarker of internal dose (e.g. urine or toenail arsenic) is important, particularly at low to moderate levels of arsenic in drinking water, because these biomarkers capture both drinking water and dietary sources.70,71 Biomarkers, however, should be collected prospectively. In addition to inorganic arsenic species, dietary arsenic also contains organic arsenic species, such as from seafood, which are generally thought to be nontoxic.6
Although urine arsenic is excreted within days to weeks (depending on the species),72,73 urine arsenic is often used as a biomarker of chronic exposure because studies of arsenic concentrations in drinking water have generally found little change over time, with no intervention or change in water source.74–76 Toenail arsenic, in contrast, measures ingested arsenic over the past 2 to 18 months.77,78 Repeated water measurements are needed to capture changes in water arsenic concentrations over time or changes in water sources, but repeated biomarker measurements can capture within-individual changes over time in water sources, water consumption, water use (drinking, cooking and irrigation), and diet that affect total exposure.79 The association between a biomarker of arsenic (urine or toenail) and arsenic measured in drinking water is complex, depending not only on the magnitude, frequency and duration of arsenic in drinking water and other sources, but also on individual variability related to body weight, metabolism, urine dilution and toenail growth.70,71
In this meta-analysis of published group-level data with arsenic exposure measured in water, urine and toenails, we pooled studies with different exposure media and we estimated median water arsenic concentrations from reported median toenail arsenic and urine arsenic concentrations. This approach could have resulted in exposure misclassification, particularly at low water arsenic levels where both diet and water can contribute substantially to total arsenic exposure. However, some assumptions are necessary to pool studies with different exposure metrics, and our sensitivity analyses with varying assumptions resulted in similar conclusions. In the New Hampshire Skin Cancer Study,14 we were able to use measured water and toenail arsenic concentrations from the overall cohort to predict the concentration of water arsenic from toenail arsenic concentrations. For the Strong Heart Study,13 we did not have detailed information on water arsenic concentrations in the population. At the time of the study, arsenic levels in public drinking water systems were less than 10 µg/l to 61 µg/l in Arizona, less than 10 µg/l in Oklahoma, and less than 1 µg/l to 21 µg/l in North and South Dakota.80 Dose-response meta-analysis of aggregate data can be sensitive to the assignment of a single value for all participants within an exposure category, particularly when exposure categories are broad and no information is available about the distribution of exposures.38,81 In this meta-analysis, we were able to use the true median concentrations of arsenic within published exposure categories for almost all included studies, and sensitivity analyses using the mean rather than the median did not substantially alter the results.
Individual studies found no evidence for non-linearity whether arsenic concentrations were log-transformed13 or not,17,18 but one recent study found that the dose-response relationship between arsenic and fatal CVD was steeper at lower arsenic concentrations.16 In this meta-analysis, we found no statistical evidence that the dose-response model fit for arsenic and the relative risk of CVD was improved by allowing a more flexible slope compared with a log-linear model. However, the observed differences in shape and magnitude of the dose-response comparing flexible and constant slope models may indicate true non-linearity. This meta-analysis was underpowered to discern statistical evidence of a departure from a constant log-linear dose-response association, due to the small number of studies and few exposure categories in each study. Dose-response modelling of CVD incidence and stroke incidence was particularly limited, with only two studies each for these outcomes. Further, funnel plots and statistical tests of publication bias are limited when there are few studies, and distinguishing between potential reasons for funnel plot asymmetry, including other sources of heterogeneity, is challenging. Subgroup analyses supported differences in the dose-response across populations exposed to low-moderate vs high water arsenic levels, and goodness-of-fit residual plots indicated potential differences in model fit across water arsenic levels, but these findings should be interpreted cautiously given the observed heterogeneity and closely related potential sources of heterogeneity across studies (differences in water arsenic levels, exposure assessments and population characteristics). Overall, given the remaining uncertainty, additional studies with sufficient data to estimate the dose-response relationship for CVD are needed.
Meta-analyses based on published data have inherent limitations to estimate dose-response associations, and large cohort studies or pooled analysis of individual participant data may be needed to fully discard non-linear dose-response associations and to evaluate sources of heterogeneity. Pooled analyses of individual participant data have several advantages over traditional aggregate meta-analysis, including allowing for the standardization of the modelling strategy (e.g. modelling arsenic as log-transformed in all studies) and adjustment for confounding.82
This analysis, like most meta-analyses, was conducted largely from published results. To aid in secondary data analysis, future individual-level studies of arsenic and adverse health effects should comprehensively explore and report non-linear associations using categorical analyses and flexible splines. Studies with careful measurement of both drinking water arsenic and a biomarker of arsenic internal dose are important to comprehensively assess the relative contribution of arsenic from water vs other sources, mainly the diet. Urine is the most commonly available biomarker, and urine arsenic can be speciated to distinguish between species related to inorganic arsenic exposure compared with much less toxic organic arsenic exposure.6 In addition, physiologically based pharmacokinetic models that incorporate both toenail and urine measurements would be informative for both epidemiology and risk assessment.
About 1.8 million US residents are exposed to arsenic in public drinking water above 10 µg/l2, and approximately 3 million people are exposed to arsenic in private wells above the arsenic MCL of 10 µg/l (about 7% of private wells).3–5 Although arsenic levels in the USA are largely below 50 µg/l in drinking water,3,83 hot spots of high arsenic concentrations have been found in the West, Midwest and Northeast USA.84 The federal Safe Drinking Water Act does not regulate private wells, and the responsibility of testing and mitigation falls on the well owner.3 Additionally, at the low-moderate levels of arsenic in drinking water common in the USA, dietary sources can be a major contributor to arsenic exposure in the general population.5,7,10 Rice has been the subject of particular concern because it can contain relatively high concentrations of arsenic and because it is a common staple food.9,85–87 Future studies should include sufficient numbers of participants exposed to very low levels of arsenic, including both food and water sources of exposure.
Conclusion
This meta-analysis found increased pooled relative risks of CVD and CHD in relation to log-transformed arsenic concentrations across low-moderate to high levels of arsenic in drinking water. We found no statistical evidence of a non-linear dose-response association, although we were underpowered to examine non-linear associations. The results of this dose-response meta-analysis may be useful to the forthcoming EPA risk assessment of inorganic arsenic. The NRC recommendation that CHD be included as a critical endpoint to the inorganic arsenic risk assessment, in addition to lung, skin and bladder cancer, has important implications for public health. Approximately 1.5 million adults in the USA have a heart attack or stroke each year and CVD accounts for about 1 in 3 deaths in the USA.88 Reducing arsenic exposures, even below the current EPA standard of 10 µg/l, could have significant public health benefits at the population level, due to the widespread exposure to arsenic in drinking water and food.
Supplementary Data
Supplementary data are available at IJE online.
Funding
This work was supported by the National Institute of Environmental Health Sciences at the National Institutes of Health (1R01ES025216, R01ES021367, P42ES010349, P30ES009089). K.A.M. was supported by the National Heart Lung and Blood Institute (5T32HL007024).
Conflict of interest: None declared.
Supplementary Material
References
- 1. Naujokas MF, Anderson B, Ahsan H, et al. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect 2013;121:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. U.S. Environmental Protection Agency. Fiscal Year 2011 Drinking Water and Ground Water Statistics . EPA 816-R-13–003. March 2013. 2013. http://water.epa.gov/scitech/datait/databases/drink/sdwisfed/howtoaccessdata.cfm (18 November 2015, date last accessed). [Google Scholar]
- 3. Ayotte JD, Gronberg JM, Apodaca LE. Trace Elements and Radon in Groundwater Across the United States, 1992–2003. Scientific Investigations Report 2011–5059. 2011. http://pubs.usgs.gov/sir/2011/5059/pdf/sir2011–5059_report-covers_508.pdf (18 November 2015, date last accessed). [Google Scholar]
- 4. Hutson SS, Barber NL, Kenney JF, Linsey KS, Lumia DS, Maupin MA. Estimated Use of Water in the United States in 2000:U.S. Geological Survey Circular 1268. 2004:http://pubs.usgs.gov/circ/2004/circ1268/ (18 November 2015, date last accessed). [Google Scholar]
- 5. Stanton BA, Caldwell K, Congdon CB, et al. MDI Biological Laboratory Arsenic Summit: Approaches to Limiting Human Exposure to Arsenic. Curr Environ Health Rep 2015;2:329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Research Council. Arsenic in Drinking Water. Subcommittee on Arsenic in Drinking Water, National Research Council. Washington DC: National Academy Press, 1999. [Google Scholar]
- 7. Nachman KE, Baron PA, Raber G, Francesconi KA, Navas-Acien A, Love DC. Roxarsone, inorganic arsenic, and other arsenic species in chicken: a US-based market basket sample. Environ Health Perspect 2013;121:818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xue J, Zartarian V, Wang SW, Liu SV, Georgopoulos P. Probabilistic modeling of dietary arsenic exposure and dose and evaluation with 2003–2004 NHANES Data. Environ Health Perspect 2010;118:345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gilbert-Diamond D, Cottingham KL, Gruber JF, et al. Rice consumption contributes to arsenic exposure in US women. Proc Natl Acad Sci U S A 2011;108:20656–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Navas-Acien A, Nachman KE. Public health responses to arsenic in rice and other foods. JAMA Intern Med 2013;173:1395–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Research Council. Critical Aspects of EPA’s IRIS Assessment of Inorganic Arsenic: Interim Report. Washington DC: National Academies Press, 2013. [Google Scholar]
- 12. James KA, Byers T, Hokanson JE, Meliker JR, Zerbe GO, Marshall JA. Association between lifetime exposure to inorganic arsenic in drinking water and coronary heart disease in Colorado residents. Environ Health Perspect 2015;123:128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moon KA, Guallar E, Umans JG, et al. Low to moderate arsenic exposure and incident cardiovascular disease: the Strong Heart Study. Ann Intern Med 2013;159649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Farzan SF, Chen Y, Rees JR, Zens MS, Karagas MR. Risk of death from cardiovascular disease associated with low-level arsenic exposure among long-term smokers in a US population-based study. Toxicol Appl Pharmacol 2015;287:93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wade TJ, Xia Y, Mumford J, et al. Cardiovascular disease and arsenic exposure in Inner Mongolia, China: a case control study. Environ Health 2015;14:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. D’Ippoliti D, Santelli E, De Sario M, Scortichini M, Davoli M, Michelozzi P. Arsenic in drinking water and mortality for cancer and chronic diseases in central Italy, 1990–2010. PLoS One 2015;10:e0138182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen Y, Graziano JH, Parvez F, et al. Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: prospective cohort study. BMJ 2011;342:d2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen Y, Wu F, Liu M, et al. A prospective study of arsenic exposure, arsenic methylation capacity, and risk of cardiovascular disease in Bangladesh. Environ Health Perspect 2013;121:832–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. U.S. Environmental Protection Agency. National primary drinking water regulations; arsenic and clarifications to compliance and new source contaminants monitoring; final rule. Federal Register 2001:6976–7066. [Google Scholar]
- 20. National Research Council. Arsenic in Drinking Water. 2001 Update. Washington DC: National Academy Press; 2001. [Google Scholar]
- 21. National Research Council. Science and Decisions: Advancing Risk Assessment. Washington DC: National Academies Press, 2009. [PubMed] [Google Scholar]
- 22. National Research Council. Review of EPA’s Integrated Risk Information System (IRIS) Process. Washington, DC: National Academies Press, 2014. [PubMed] [Google Scholar]
- 23. Schmidt CW. Low-dose arsenic: in search of a risk threshold. Environ Health Perspect;2014;122:A130–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 2012;175:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moon K, Guallar E, Navas-Acien A. Arsenic exposure and cardiovascular disease: an updated systematic review. Curr Atheroscler Rep 2012;14:542–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Navas-Acien A, Sharrett AR, Silbergeld EK, et al. Arsenic exposure and cardiovascular disease: a systematic review of the epidemiologic evidence. Am J Epidemiol 2005;162:1037–49. [DOI] [PubMed] [Google Scholar]
- 27. Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 29. Afridi HI, Kazi TG, Kazi N, et al. Association of environmental toxic elements in biological samples of myocardial infarction patients at different stages. Biol Trace Elem Res 2011;141:26–40. [DOI] [PubMed] [Google Scholar]
- 30. Afridi HI, Kazi TG, Kazi N, et al. Evaluation of toxic elements in scalp hair samples of myocardial infarction patients at different stages as related to controls. Biol Trace Elem Res 2010;134:1–12. [DOI] [PubMed] [Google Scholar]
- 31. Liao YT, Chen CJ, Li WF, et al. Elevated lactate dehydrogenase activity and increased cardiovascular mortality in the arsenic-endemic areas of southwestern Taiwan. Toxicol Appl Pharmacol 2012;262:232–37. [DOI] [PubMed] [Google Scholar]
- 32. Wu MM, Chiou HY, Chen CL, et al. GT-repeat polymorphism in the heme oxygenase-1 gene promoter is associated with cardiovascular mortality risk in an arsenic-exposed population in northeastern Taiwan. Toxicol Appl Pharmacol 2010;248:226–33. [DOI] [PubMed] [Google Scholar]
- 33. Wang C-H, Chen C-L, Hsu L-I, et al. Chronic Arsenic Exposure Increases Mortality from Ischemic Heart Disease and Stroke: A Follow-up Study on 26, 851 Residents in Taiwan. 2005. https://www.researchgate.net/publication/251423451_Chronic_Arsenic_Exposure_Increases_Mortality_from_Ischemic_Heart_Disease_and_Stroke_A_Follow-up_Study_on_26851_Residents_in_Taiwan (18 November 2015, date last accessed). [Google Scholar]
- 34. Chiou HY, Huang WI, Su CL, Chang SF, Hsu YH, Chen CJ. Dose-response relationship between prevalence of cerebrovascular disease and ingested inorganic arsenic. Stroke 1997;28:1717–23. [DOI] [PubMed] [Google Scholar]
- 35. Chen CJ, Chiou HY, Chiang MH, Lin LJ, Tai TY. Dose-response relationship between ischemic heart disease mortality and long-term arsenic exposure. Arterioscler Thromb Vasc Biol 1996;16:504–10. [DOI] [PubMed] [Google Scholar]
- 36. Wade TJ, Xia Y, Wu K, et al. Increased mortality associated with well-water arsenic exposure in Inner Mongolia, China. Int J Environ Res Public Health 2009;6:1107–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rahman M, Sohel N, Yunus M, et al. A prospective cohort study of stroke mortality and arsenic in drinking water in Bangladeshi adults. BMC Public Health 2014;14:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hartemink N, Boshuizen HC, Nagelkerke NJD, Jacobs MAM, van Houwelingen HC. Combining risk estimates from observational studies with different exposure cutpoints: a meta-analysis on body mass index and diabetes type 2. Am J Epidemiol 2006;163:1042–52. [DOI] [PubMed] [Google Scholar]
- 39. Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (1 May 2014, date last accessed). [Google Scholar]
- 40. Longnecker MP, Berlin JA, Orza MJ, Chalmers TC. A meta-analysis of alcohol consumption in relation to risk of breast cancer. JAMA 1988;260:652–56. [PubMed] [Google Scholar]
- 41. Greenland S. Invited commentary: a critical look at some popular meta-analytic methods. Am J Epidemiol 1994;140:290–96. [DOI] [PubMed] [Google Scholar]
- 42. Karagas MR, Tosteson TD, Blum J, et al. Measurement of low levels of arsenic exposure:a comparison of water and toenail concentrations. Am J Epidemiol 2000;152:84–90. [DOI] [PubMed] [Google Scholar]
- 43. Smith AH, Ercumen A, Yuan Y, Steinmaus CM. Increased lung cancer risks are similar whether arsenic is ingested or inhaled. J Expo Sci Environ Epidemiol 2009;19:343–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Calderon RL, Hudgens E, Le XC, Schreinemachers D, Thomas DJ. Excretion of arsenic in urine as a function of exposure to arsenic in drinking water. Environ Health Perspect 1999;107:663–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu Q, Cook NR, Bergström A, Hsieh C-C. A two-stage hierarchical regression model for meta-analysis of epidemiologic nonlinear dose–response data. Comput Stat Data Anal 2009;53:4157–67. [Google Scholar]
- 46. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301–39. [DOI] [PubMed] [Google Scholar]
- 47. Jackson D, White IR, Thompson SG. Extending DerSimonian and Laird’s methodology to perform multivariate random effects meta-analyses. Stat Med 2010;29:1282–97. [DOI] [PubMed] [Google Scholar]
- 48. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jackson D, White IR, Riley RD. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med 2012;31:3805–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Discacciati A, Crippa A, Orsini N. Goodness of fit tools for dose-response meta-analysis of binary outcomes. Res Synth Methods 2017;8:149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Baujat B, Mahé C, Pignon JP, Hill C. A graphical method for exploring heterogeneity in meta-analyses: application to a meta-analysis of 65 trials. Stat Med 2002;21:2641–52. [DOI] [PubMed] [Google Scholar]
- 53. Hamling J, Lee P, Weitkunat R, Ambuhl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med 2008;27:954–70. [DOI] [PubMed] [Google Scholar]
- 54. Wu MM, Kuo TL, Hwang YH, Chen CJ. Dose-response relation between arsenic concentration in well water and mortality from cancers and vascular diseases. Am J Epidemiol 1989;130:1123–32. [DOI] [PubMed] [Google Scholar]
- 55. Medrano MAJ, Boix R, Pastor-Barriuso R, et al. Arsenic in public water supplies and cardiovascular mortality in Spain. Environ Res 2010;110:448–54. [DOI] [PubMed] [Google Scholar]
- 56. Engel RR, Smith AH. Arsenic in drinking water and mortality from vascular disease: an ecologic analysis in 30 counties in the United States. Arch Environ Health 1994;49:418–27. [DOI] [PubMed] [Google Scholar]
- 57. StataCorp. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP, 2011. [Google Scholar]
- 58. R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing, 2015. [Google Scholar]
- 59. Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York, NY: Springer, 2009. [Google Scholar]
- 60. Sohel N, Persson LA, Rahman M, et al. Arsenic in drinking water and adult mortality: a population-based cohort study in rural Bangladesh. Epidemiology 2009;20:824–30. [DOI] [PubMed] [Google Scholar]
- 61. Wang C-H, Hsiao CK, Chen C-L, et al. A review of the epidemiologic literature on the role of environmental arsenic exposure and cardiovascular diseases. Toxicol Appl Pharmacol 2007;222:315–26. [DOI] [PubMed] [Google Scholar]
- 62. Tsuji JS, Perez V, Garry MR, Alexander DD. Association of low-level arsenic exposure in drinking water with cardiovascular disease: A systematic review and risk assessment. Toxicology 2014;323:78–94. [DOI] [PubMed] [Google Scholar]
- 63. Chen Y, Factor-Litvak P, Howe GR, et al. Arsenic exposure from drinking water, dietary intakes of B vitamins and folate, and risk of high blood pressure in Bangladesh: a population-based, cross-sectional study. Am J Epidemiol 2007;165:541–52. [DOI] [PubMed] [Google Scholar]
- 64. Tseng CH, Huang YK, Huang YL, et al. Arsenic exposure, urinary arsenic speciation, and peripheral vascular disease in blackfoot disease-hyperendemic villages in Taiwan. Toxicol Appl Pharmacol 2005;206:299–308. [DOI] [PubMed] [Google Scholar]
- 65. Huang Y-L, Hsueh Y-M, Huang Y-K, Yip P-K, Yang M-H, Chen C-J. Urinary arsenic methylation capability and carotid atherosclerosis risk in subjects living in arsenicosis-hyperendemic areas in southwestern Taiwan. Sci Total Environ 2009;407:2608–14. [DOI] [PubMed] [Google Scholar]
- 66. Hsieh Y-C, Lien L-M, Chung W-T, et al. Significantly increased risk of carotid atherosclerosis with arsenic exposure and polymorphisms in arsenic metabolism genes. Environ Res 2011;111:804–10. [DOI] [PubMed] [Google Scholar]
- 67. Wu F, Jasmine F, Kibriya MG, et al. Interaction between arsenic exposure from drinking water and genetic susceptibility in carotid intima-media thickness in Bangladesh. Toxicol Appl Pharmacol 2014;276:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gribble MO, Howard BV, Umans JG, et al. Arsenic exposure, diabetes prevalence, and diabetes control in the Strong Heart Study. Am J Epkdemiol 2012;176:865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kuo CC, Howard BV, Umans JG, et al. Arsenic exposure, arsenic metabolism, and incident diabetes in the Strong Heart Study. Diabetes Care 2015;38:620–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Marchiset-Ferlay N, Savanovitch C, Sauvant-Rochat MP. What is the best biomarker to assess arsenic exposure via drinking water? Environ Int 2012;39:150–71. [DOI] [PubMed] [Google Scholar]
- 71. Hughes MF. Biomarkers of exposure: a case study with inorganic arsenic. Environ Health Perspect 2006;114:1790–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pomroy C, Charbonneau SM, McCullough RS, Tam GK. Human retention studies with 74As. Toxicol Appl Pharmacol 1980;53:550–56. [DOI] [PubMed] [Google Scholar]
- 73. Cullen WR, Reimer KJ. Arsenic speciation in the environment. Chem Rev 1989;89:713–64. [Google Scholar]
- 74. Steinmaus CM, Yuan Y, Smith AH. The temporal stability of arsenic concentrations in well water in western Nevada. Environ Res 2005;99:164–68. [DOI] [PubMed] [Google Scholar]
- 75. Karagas MR, Le CX, Morris S, et al. Markers of low level arsenic exposure for evaluating human cancer risks in a US population. Int J Occup Med Environ Health 2001;14:171–75. [PubMed] [Google Scholar]
- 76. Ryan PB, Huet N, MacIntosh DL. Longitudinal investigation of exposure to arsenic, cadmium, and lead in drinking water. Environ Health Perspect 2000;108:731–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Button M, Jenkin GR, Harrington CF, Watts MJ. Human toenails as a biomarker of exposure to elevated environmental arsenic. J Environ Monit 2009;11:610–17. [DOI] [PubMed] [Google Scholar]
- 78. Slotnick MJ, Nriagu JO. Validity of human nails as a biomarker of arsenic and selenium exposure: A review. Environ Res 2006;102:125–39. [DOI] [PubMed] [Google Scholar]
- 79. Cubadda F, Jackson BP, Cottingham KL, Van Horne YO, Kurzius-Spencer M. Human exposure to dietary inorganic arsenic and other arsenic species: State of knowledge, gaps and uncertainties. Sci Total Environ 2017;579:1228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Navas-Acien A, Umans JG, Howard BV, et al. Urine arsenic concentrations and species excretion patterns in American Indian communities over a 10-year period: the Strong Heart Study. Environ Health Perspect 2009;117:1428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Berlin JA, Longnecker MP, Greenland S. Meta-analysis of epidemiologic dose-response data. Epidemiology 1993;4:218–28. [DOI] [PubMed] [Google Scholar]
- 82. Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ 2010;340:c221. [DOI] [PubMed] [Google Scholar]
- 83. Focazio MJ, Welch AH, Watkins SA, Helsel DR, Horn MA. A Retrospective Analysis on the Occurrence of Arsenic in Ground-Water Resources of the United States and Limitations in Drinking-Water-Supply Characterizations. 2000. http://pubs.usgs.gov/wri/wri994279/pdf/wri994279.pdf (18 November 2015, date last accessed). [Google Scholar]
- 84. DeSimone LA. Quality of Water From Domestic Wells in Principal Aquifers of the United States, 1991–2004:U.S. Geological Survey Scientific Investigations Report 2008–5227. 2009. http://pubs.usgs.gov/sir/2008/5227 (18 November 2015, date last accessed). [Google Scholar]
- 85. EFSA (European Food Safety Authority).. Dietary exposure to inorganic arsenic in the European population. EFSA J 2014;12:3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cottingham KL, Karimi R, Gruber JF, et al. Diet and toenail arsenic concentrations in a New Hampshire population with arsenic-containing water. Nutr J 2013;12:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Davis MA, Mackenzie TA, Cottingham KL, Gilbert-Diamond D, Punshon T, Karagas MR. Rice consumption and urinary arsenic concentrations in U.S. children. Environ Health Perspect 2012;120:1418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 update. A report from the American Heart Association. Circulation 2014;129:e28–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.