Why was the cohort set up?
The Stress in Pregnancy (SIP) Study cohort was set up in New York City in 2009 through funding made available by the National Institute of Mental Health (NIMH), to understand the extent to which an adverse environment in utero can alter fetal growth and development, with potential lifelong impacts on health and disease, based on the theoretical framework of the ‘Developmental Origins of Health and Diseases (DoHaD) Hypothesis’.1–6 Growing evidence7–9 suggests that not only the genome but also the epigenome, the heritable, quasi-stable yet dynamic control of gene expression, can be modulated by the environment, and plays a vital role in defining health and disease in growing offspring.10–12 Preclinical studies demonstrated that antenatal stress leads to dysregulated neurobehavioural functioning and problems with development, providing a solid platform for hypothesis testing in human studies. Human studies have also demonstrated that antenatal exposure to broadly defined stress (i.e. stressful life events and psychological problems) is linked to long-term neurobehavioural problems in offspring,13,14 such as autism,15,16 schizophrenia17–20 and attention deficit/hyperactivity disorder,21–23 as well as growth-related suboptimal reproductive outcomes such as intra-uterine growth restriction (IUGR)24–26 and obesity,27–29 through epigenetic mechanisms.
Human studies remain hampered by methodological restrictions, including: (i) the inability to randomize pregnant women in varying stressful condition and to systematically control for the level and timing of any exposure; (ii) stressful events (i.e. divorce, job loss) may not be independent of the mother’s genetic background, psychopathology and socioeconomic status (SES); and (iii) measures of psychosocial stress during pregnancy are generally broad, frequently measured by daily problems and upheavals30 or stressful life events from ordinary life.31,32 These measures often lack the negative valence for researchers to understand the nature of the relationship, and researchers may therefore be unable to elucidate whether a tipping point in the degree of stress exposure exists or the role of timing of exposure. To address this, in 2013 the SIP Study evolved by capitalizing on a serious and unfortunate natural disaster, Superstorm Sandy which devastated the New York City area, affecting a subset of the SIP cohort (n = 416). Our quasi-experiment has allowed the study to evaluate normative stress (resulting from everyday life events) in addition to traumatic stress (resulting from extreme and/or life-threatening events), allowing us to pinpoint both timing and duration, in addition to addressing key restrictions in prospective human data on antenatal stress and childhood development by randomly controlling for individual resources and susceptibility.
Post Sandy, we are in a unique position to evaluate stress exposure to disaster, and potential neurodevelopmental precursors to the emergence of developmental psychopathology later in childhood. Understanding GxE (Gene-Environment Interaction) effects, especially during sensitive periods of brain development, and conducting a thorough evaluation of neurodevelopment during early childhood, are at the core of the study objective. In sum, the study extends the evaluation of neurodevelopmental phenotypes of children and further investigates if and how the timing of exposure to stress (i.e. Superstorm Sandy) leads to different manifestations of neurobehavioural impairment, or moderates the effects of more normative stress on neurobehavioural impairment. The study, therefore, attempts to monitor neurobehavioural trajectories among offspring in early- and mid-childhood–critical periods of rapid left hemisphere development, which controls language, writing, logic, mathematical and gross motor skill development.
Table 1.
Demographic and perinatal characteristics among participating mothers
| Total sample | Subsample 1 Eligible sample who are actively in follow-up | Subsample 2 Those who selected for a case/control natural disaster study | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Parental characteristics | (N = 768) | (N = 576) | (N = 385) |
| Mother’s age | 27.43 (5.8) | 27.54 (5.9) | 27.69 (6.0) |
| Father’s age | 29.89 (7.5) | 29.84 (7.5) | 30.03 (7.4) |
| Parity | 2.93 (2.3) | 2.96 (2.2) | 2.84 (2.1) |
| Race | N (%) | N (%) | N (%) |
| White | 79 (10.3) | 65 (11.3) | 60 (14.4) |
| Black | 198 (25.8) | 136 (23.6) | 95 (22.8) |
| Hispanics | 399 (52.0) | 297 (51.6) | 209 (50.2) |
| Asian | 74 (9.6) | 66 (11.5) | 44 (10.6) |
| Others | 18 (2.3) | 12 (2.1) | 8 (1.9) |
| Marital status | N (%) | N (%) | N (%) |
| Married | 246 (32.1) | 191 (33.1) | 165 (39.7) |
| Common-law | 61 (8.0) | 39 (6.8) | 24 (5.8) |
| Single | 445 (58.0) | 334 (58.1) | 219 (52.6) |
| Widowed | 4 (0.5) | 3 (0.5) | 2 (0.5) |
| Divorced/separated | 11 (1.3) | 8 (1.4) | 6 (1.4) |
| Refused | 1 (0.1) | 1 (0.1) | |
| Education level | N (%) | N (%) | N (%) |
| Primary school | 30 (3.9) | 22 (3.8) | 14 (3.4) |
| Some high school | 133 (17.3) | 103 (17.9) | 56 (13.5) |
| High school of GEDa | 185 (24.1) | 133 (23.1) | 86 (20.7) |
| Some college | 202 (26.3) | 156 (27.1) | 119 (28.6) |
| AAb | 87 (11.3) | 58 (10.1) | 40 (9.6) |
| BAb | 83 (10.8) | 64 (11.1) | 61 (14.7 |
| Graduate/professionalc | 48 (6.3) | 40 (6.9) | 40 (9.6) |
| Superstorm Sandy timing | N (%) | N (%) | N (%) |
| Pre-Sandy | 422 (54.8) | 287 (49.8) | 163 (39.2) |
| Third trimester | 64 (8.3) | 48 (8.3) | 44 (10.6) |
| Second trimester | 53 (6.9) | 44 (7.6) | 39 (9.4) |
| First trimester | 159 (20.9) | 135 (23.4) | 113 (27.2) |
| Post-Sandy | 72 (9.4) | 62 (10.8) | 57 (13.7) |
| Pre-pregnancy weight mean (SD) (kg.) | 69.29 (40.9) | 68.73 (40.0) | 68.36 (40.5) |
Sample sizes in mothers and offspring differ because there are 43 siblings in the total sample, 38 siblings in the eligible and active sampl, and 31 siblings in the case/control natural disaster study.
General Educational Development (GED) tests are a group of four subject tests which, when passed, provide certification that the test taker has American high school-level academic skills.
An associate of arts degree (AA) is an undergraduate academic degree awarded by colleges and universities in the USA upon completion of a course of study lasting 2 years. It is considered to be a higher level of education than a high school diploma or GED but less than a Bachelor of Arts degree (BA) which is an undergraduate academic degree with 4 years of matriculation.
A graduate/professional degree includes higher level postgraduate degrees (Master’s, MD, JD, PhD etc).
Table 2.
Demographic and perinatal characteristics among participating children, N (%)
| Total sample | Subsample 1 Eligible sample who are actively in follow-up | Subsample 2 Those who selected for a case/control natural disaster study | |
|---|---|---|---|
| Child characteristics | (N = 811) | (N = 614) | (N = 416) |
| Gender | |||
| Male | 352 (43.4) | 312 (50.8) | 207 (49.8) |
| Female | 334 (41.3) | 302 (49.2) | 209 (50.2) |
| Unknown | 125 (15.3) | −- | −- |
| Birth outcomes | |||
| Birthweight (kg) | 3.20 (0.64) | 3.24 (0.58) | 3.24 (0.58) |
| Gestational age (week) | 38.79 (2.41) | 39.01 (2.02) | 38.87 (2.08) |
| 1-min Apgar score | 8.62 (1.08) | 8.69 (0.87) | 8.70 (0.86) |
| 5-min Apgar score | 8.87 (0.65) | 8.94 (0.30) | 8.91 (0.49) |
| Head circumference (cm) | 33.52 (2.76) | 33.72 (2.42) | 33.81 (2.43) |
| Body length (cm) | 49.94 (3.79) | 50.02 (3.71) | 50.15 (3.86) |
| Placenta weight (g) | 664.19 (181.19) | 660.48 (159.08) | 649.19 (147.74) |
| Number of siblings | |||
| None | 245 (30.2) | 171 (27.9) | 128 (30.8) |
| 1 | 169 (20.8) | 129 (21.0) | 88 (21.2) |
| 2 | 140 (17.3) | 114 (18.6) | 69 (16.6) |
| 3 | 102 (12.6) | 92 (15.0) | 63 (15.1) |
| 4+ | 143 (17.6) | 106 (17.2) | 67 (16.1) |
| Unknown | 12 (1.5) | 2 (0.3) | 1 (0.2) |
Sample sizes in mothers and offspring differ because there are 43 siblings in the total sample, 38 siblings in the eligible and active sample, and 31 siblings in the case/control natural disaster study.
Who is in the cohort?
Beginning in 2009 at the Mount Sinai Hospital, women in their second trimester receiving antenatal care in the OB/GYN Department were invited to participate in the SIP study. In 2012, the study expanded recruitment to women in the OB/GYN Department at New York Presbyterian-Queens. The cohort consists of an urban population encompassing a diverse range of ethnic/racial backgrounds and socioeconomic strata, with a majority of participants considered ethnic (Black, Hispanic and/or Asian) and financial minorities (low SES, living below the poverty line). Participating women are of high parity with an average of three previous pregnancies, ranging from 0 to 12. Inclusion criteria required absence of obstetric risk factors such as HIV positivity, significant congenital anomalies, neurological dysfunction, fetal chromosomal or fetal anomalies or inborn errors in metabolism, and plans to relocate out of the geographical area during the duration of the study. Written informed consent was obtained from all eligible women, for all study procedures; 763 women with 806 pregnancies (43 mothers enrolled with multiple pregnancies) were assessed for eligibility. Of those 763, 54 women (6.8%) did not meet inclusion criteria and were excluded from participation, thus 719 women consented to participate. Of these, 29 women (3.6%) were lost due to antenatal and perinatal death (15 miscarriage, 10 fetal demise, 4 stillbirth), resulting in a total of 690 mothers (722 neonates), including 10 sets of twins at birth. During the postpartum period three neonates died and five infants were removed from their mother’s care by Child Protective Services. Furthermore, 42 mothers relocated to a geographical area outside the study region and 58 mothers withdrew by 18 months postpartum. Thus, the final cohort includes 614 children, including nine sets of twins. Of those active 614 participants, unique to the SIP cohort is the subsample of 416 offspring comprising the natural disaster quasi-experiment, which includes women who experienced Superstorm Sandy during pregnancy (i.e. cases), and women who did not (i.e. controls).
Between 614 followed and 197 non-responders (including lost to follow-up, withdrawn and deceased in a total of 811 recruited into the study), there was no difference in maternal age (26.9 vs 27.5, P = 0.26), paternal age (30.4 vs 29.8, P = 0.71), parity (2.83 vs 2.96, P = 0.52), offspring gender, male (55.6% vs 51.0%, P = 0.46), marital status (P = 0.35) or maternal education (P = 0.25). However, there was a notable difference in race (P = 0.001, wherein a larger proportion of Asian but smaller proportion of Blacks responded). Between 416 invited and 198 not-invited participants in a total of 614 who were actively followed, there was no difference in maternal age (27.3 vs 27.7, P = 0.42), paternal age (28.8 vs 30.0, P = 0.21), parity (2.36 vs 2.17, P = 0.23) or offspring gender, male (52.6% vs 50.0%, P = 0.54). However, there was a marginal difference in: racial composition (P = 0.08) such that a larger proportion of Whites but smaller proportion of Blacks continued to participate; in marital status (P = 0.001), whereby more married and fewer single or common law-married participants continued to respond; and in maternal education (P < 0.001), where women with greater education responded more than those with less education.
How often have they been followed up?
Research staff regularly contacted participants during their second and third trimesters to collect biospecimens, fill out questionnaires and complete structured clinical interviews for psychopathology with a clinical research interviewer. A study hotline was established on the labour and delivery (L&D) floor, allowing the L&D nurses and residents to inform the study team immediately after the participant was admitted for delivery. Biospecimens were collected within 2 h and required information, including birth and obstetric outcomes, were extracted from medical charts during postpartum recovery. At 6 months postpartum, participants completed questionnaires about their infants and themselves, and again at 18 months during which mothers were invited to participate with their children in a follow-up study at 24 months postpartum. In the follow-up study, mothers and their children are invited to a child-friendly assessment centre at Queens College annually up to 48 months. Due to the nature of this cohort, which is composed of an urban population at high risk for perinatal complications and neonatal/infant mortality, mobility is high, resulting in loss of follow-up.
What has been measured?
All study data collection is presented in Tables 3 and 4. For information regarding candidate genes under investigation in the study, refer to Table 5.
Table 6.
Main Effects of Maternal GDM, Preeclampsia, and Obesity during Pregnancy on Global Methylation (%) in t he Placenta Tissues and Cord Blood in the Multivariable Model
| Global Methylation Placenta Tissue |
Global Methylation Umbilical Cord Blood |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Statistics |
Statistics |
|||||||||||
| Unadjusted Model |
Adjusted Model |
Unadjusted Model |
Adjusted Model |
|||||||||
| Maternal Characteristics | B | SE | P Value | B | SE | P Value | B | SE | P Value | B | SE | P Value |
| GDM | 4.34 | 1.25 | 0.001 | 4.47 | 1.22 | 0.003 | 3.45 | 3.27 | 0.30 | 3.72 | 3.44 | 0.29 |
| Preeclampsia | 2.90 | 1.51 | 0.06 | 3.02 | 1.48 | 0.05 | −0.29 | 3.96 | 0.94 | −0.68 | 4.17 | 0.87 |
| Obesity | −2.40 | 0.89 | 0.01 | −2.54 | 0.97 | 0.01 | 0.33 | 2.32 | 0.89 | 1.41 | 2.74 | 0.61 |
Abbreviations: GDM, gestational diabetes mellitus; SE, standard error.
In the unadjusted model, no confounders were included. In adjusted models, the effects of sex of the baby, mother's educational attainment at birth of her child, welfare status, marital status, and ethnicity were statistically controlled for. Maternal GDM, preeclampsia, and obesity during pregnancy were analysed in the same model simultaneously.
Table 3.
Data collection and examinations conducted during the antenatal period and at delivery
| Pregnant woman (maternal) | |
| Demographic/social | Ethnicity/race, marital status, education level, age, housing, occupation, employment |
| Lifestyle | Smoking, drinking, substance use |
| Normative stress | Perceived Stress Scale (PSS-14)33 |
| Depression symptoms (EPDS)34 | |
| State/Trait Anxiety (STAI)35 | |
| Pregnancy-specific anxiety (PRAQ-R)30 | |
| Stressful Life Events Interview (LEI)36 | |
| Psychopathology | Family history of psychological symptomatology (FHS)37 |
| History of psychiatric illness (SCID IV-R Axis I)38 | |
| Superstorm Sandy (traumatic stress) | Superstorm Sandy traumatic exposure (TEI)39 |
| Impact of Events Scale (IES)40 | |
| Posttraumatic Diagnostics Scale – (PDS general)41 | |
| Posttraumatic Diagnostic Scales – (PDS Superstorm Sandy specific) | |
| Posttraumatic Checklist (PCL-C)42 | |
| Social Adjustment Scale (SAS-SR)43 | |
| Obstetric outcomes | History of pregnancy/childbirth, obstetric complications |
| Medical record review | |
| Pre-pregnancy weight | |
| Blood pressure | |
| Weight gain | |
| Placenta weight | |
| Biospecimens | Urine at 2nd and 3rd trimesters |
| Blood (plasma, serum, PAX for RNA) | |
| Placenta biopsies | |
| Father of child (paternal) | |
| Psychological | Family history of psychological symptomatology (FHS)37 |
| Demographics | Age, race, employment |
| Newborn (offspring) | |
| Birth outcomes | Perinatal complications |
| Birthweight | |
| Head circumference | |
| Body length | |
| Apgar (at 1 min & 5 min) | |
| Biospecimens | Umbilical cord blood |
| Terminal meconium | |
Table 4.
Data collection after the birth of the child, timimg
| Mother’s assessment | |
|---|---|
| Demographics | |
| Marital status, education level, housing, occupation, employment | 24, 36, 48 months |
| Normative stress | |
| Depression symptoms (EPDS)34 | 6, 18, 24, 36, 48 months |
| Parenting Stress Index (PSI-4)44 | 6, 18, 24, 36, 48 months |
| Psychopathology and functional impairment | |
| Maternal Perinatal PTSD (PPQ)45 | 6 months (retrospective) |
| Perceived Stress Scale (PSS-14)33 | 6, 18, 24, 36, 48 months |
| Posttraumatic Stress Disorder Checklist (PCL-C)42 | 24, 36, 48 months |
| Mini International Neuropsychiatric Interview (MINI)46 | 24, 36, 48 months |
| Parental Bonding Instrument (PBI)47 | 24, 36, 48 months |
| Social functioning (SAS-SR)43 | 24, 36, 48 months |
| Social environment | |
| Social Support (MOS-SS)48 | 24, 36, 48 months |
| Home environment inventory (HOME)49 | 24, 36, 48 months |
| Physical, environmental exposures | |
| Antenatal Environmental Exposure | 24, 36, 48 months |
| Antenatal Auditory Exposure | 6 months (retrospective) |
| Antenatal Recreational Exercise50 | 6 months (retrospective) |
| Lifestyle | |
| Smoking, drinking, substance use | 24, 36, 48 months |
| Dietary behaviours51 | 24, 36, 48 months |
| Psychophysiology | |
| Skin conductance | 24, 36, 48 months |
| Respiratory sinus arrhythmia | 24, 36, 48 months |
| Heart rate and heart rate variability | 24, 36, 48 months |
| Observation | |
| Interaction between child and mother | 24, 36, 48 months |
| Biospecimens | |
| Buccal swab | 24, 36, 48 months |
| Saliva | 24, 36, 48 months |
| Hair | 24, 36, 48 months |
| Toenail clippings | 24, 36, 48 months |
| Child’s assessment | |
| Neurodevelopment | |
| Child temperament via maternal report (IBQ, ECBQ, CBQ)52–54 | 6, 18, 24, 36, 48 months |
| Brief Infant=Toddler Social & Emotional Assessment (B/ITSEA)55,56 | 24, 36, 48 months |
| Behavioral Assessment System for Children (BASC)57 | 24, 36, 48 months |
| Cognitive development (Bayley-III)58 | 24, 36 months |
| Receptive and expressive communication (Bayley-III)58 | 24, 36 months |
| Fine and gross motor (Bayley-III)58 | 24, 36 months |
| Social-emotional development (Bayley-III)58 | 24, 36 months |
| Verbal comprehension (WPPSI-IV)59 | 48 months |
| Visual spatial ability (WPPSI-IV)59 | 48 months |
| Working memory (WPPSI-IV)59 | 48 months |
| Physical development | |
| Height/weight | 24, 36, 48 months |
| Head circumference | 24, 36, 48 months |
| Medical record | 24, 36, 48 months |
| Psychophysiology | |
| Skin conductance | 24, 36, 48 months |
| Respiratory sinus arrhythmia | 24, 36, 48 months |
| Heart rate variability | 24, 36, 48 months |
| Observation | |
| Interaction between mother and child | 24, 36, 48 months |
| Psychopathology | |
| Preschool Age Psychiatric Assessment (PAPA)60 | 24, 36 months |
| Biospecimens | |
| Buccal swab | 24, 36, 48 months |
| Saliva | 24, 36, 48 months |
| Hair | 24, 36, 48 months |
| Toenail clippings | 24, 36, 48 months |
Table 5.
List of candidate genes that are under investigation in the study
| HPA system | 11β-HSD21 [NM_00196]2NR3C1 [NM_001204264] CRHR1 [NM_001145146] CRH2R [NM_001202481] CRHBP [NM_001882] POMC [NM_001035256] MAOA [NM_000240] MAOB [NM_000898] CFL1 [NM_005507] CREB1 [NM_004379] CREBBP [NM_004380] DYRK1A [NM_001396] 11β-HSB1 [NM_005525] NCOR1 [NM_006311] NCOR2 [NM_006312] UCN [NM_003353] UCN3 [NM_053049] AVP [NM_000490] AVPR1A [NM_000706] AVPR1B [NM_00707] NR3C1 [NM_000176] NR3C2 [NM_000901] NR4A1 [NM_002135] |
| Neurodevelopment | ZNF507 [NM_001206198] MECP2 [NM_004992] SRD5A3 [NM_024592] PON3 [NM_000940] CDKL5 [NM_0031592] KIRREL3 [NM_032531] FOXP1 [NM_001244808] ZNHIT6 [NM_017953] ADRA1A [NM_152547] ADRA1B [NM_000879] ADRA2A [NM_00681] DAOA [NM_172370] DBH [NM_000787] HTR1B [NM_000863] NRG1 [NM_013959] SNAP25 [NM_130811] TPH1 [NM_004179] TPH2 [NM_173353] OXT [NM_000915] OXTR [NM_000916] |
| Autonomic Nervous System (ANS) | REN [NM_000537] CYP11B2 [NM_000498] PHOX2B [NM_003924] SEMA3B [NM_004636] PAX6 [NM_000280] chAT [NM_020985] ABCB1 [NM_000927] DLGAP2 [NM_004745] IKBKG [NM_003639] NPY [NM_000905] |
| Dopamine System | DAT [NM_001044] DRD1 [NM_000794] DRD2 [NM_000795] DRD4 [NM_000797] PRL [NM_000948] TH [NM_199292] AMPD1 [NM_000036] ATP1A1 [NM_000701] SNCA [NM_000345] NR4A2 [NM_006186] COMT [NM_000754] SLC18A2 [NM_003054] |
| Serotonin System | HTR1A [NM_000524] HTR3A [NM_213621] SLC6A4 [NM_001045] BDNF [NM_170735] HTR2C [NM_001256760] HTR4 [NM_000870] HTR5A [NM_024012] |
| Inflammation | IFNG [NM_000619] IL10 [NM_000572] IL17A [NM_002190] IL1RB [NM_018725] IL-6 [NM_000600] IL1B [NM_000576] IL8 [NM_000584] LTA [NM_000595] TNF [NM_000594] TREM2 [NM_018965] |
Gene
Gene bank code
Data collection during the antenatal period
At recruitment (< 28 weeks of pregnancy) mothers complete a battery of self-report questionnaires to collect: demographic data; lifestyle antenatal risks; and a comprehensive assessment of normative stress covering perceived stress pregnancy-related anxieties,33 postnatal depression,34 state and trait anxiety and stressful life events.35 History of psychological problems38 in the mother and father of the baby is assessed via mother’s report. Furthermore, a trained research interviewer with a degree in clinical psychology assesses maternal psychopathology via clinical interview (Structured Clinical Interview for DSM-IV for Axis I diagnoses, or SCID-I).39 Maternal urine samples are collected twice, at the second and third trimesters, in polypropylene containers and aliquoted into 8 1-ml cryovials for storage. During the second trimester, maternal blood samples are collected by the attending nurse, including two 7.5-ml preservative-free heparin DNA tubes, one tube to extract serum and one PAX gene tube to extract RNA. Blood in serum tubes is centrifuged, and extracted serum and DNA tubes are aliquoted. PAX gene tubes are stored at room temperature for 2 h. All samples are stored at −80°C.
Data collection at delivery
At delivery, birth records including traditional indicators of pregnancy outcomes (i.e. birthweight, gestational age at birth, Apgar scores, head circumference, body length), obstetric information (e.g. preeclampsia, gestational diabetes, caesarean section) and neonatal problems (e.g. jaundice, macrosomia, shoulder dystocia, breech extraction, NICU admission) are collected via patient chart. Birth tissues (i.e, placenta, umbilical cord blood) and terminal meconium are collected following delivery by L&D staff on duty.
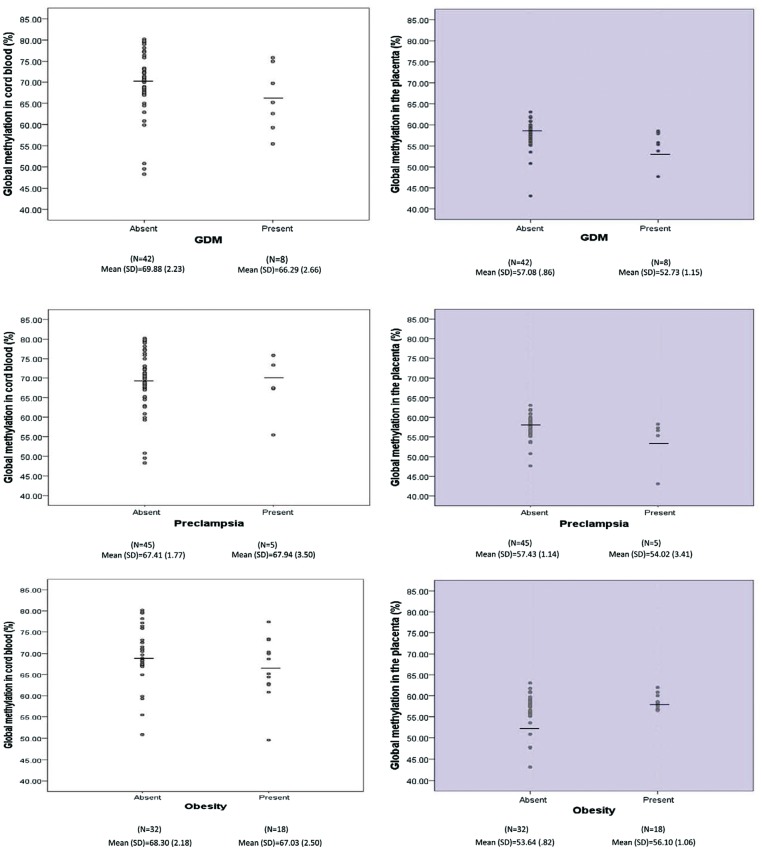
Figure 1.
The level of global methylation (%) in the cord blood and the placenta tissues by maternal gestational diabetes mellitus (GDM), pre-eclampsia, and obesity.61
Placenta tissues
Each placenta is first weighed and recorded by research staff. Then placenta biopsies, free of maternal decidua, are collected from each placenta quadrant midway between the cord insertion and the placental rim. The collected tissues are first placed into a liquid nitrogen tank for 24 h for ‘snap freezing’ and then stored at −80°C.
Umbilical cord blood
Before the placenta is delivered, a segment of the cord is cleansed with an alcohol wipe and a 10-ml syringe with a 19-gauge safety butterfly needle is used to withdraw blood. The blood in the syringe is emptied into two 7.5-ml preservative-free heparin DNA tubes, one tube to extract serum and one PAX gene tube to extract RNA. Blood in serum tubes is centrifuged, and extracted serum and DNA tubes are aliquoted. PAX gene tubes are stored at room temperature for 2 h. All samples are stored at −80°C.
Meconium
If the newborn passes transient meconium during labour, it is collected by the L&D nurse on duty. If the newborn did not pass meconium during the labour, their first soiled diapers are collected by postpartum nurses for meconium sampling. Study staff then combine meconium samples from collected diapers using a spatula into cryovials for storage at −80°C.
Data collection postpartum
Postpartum follow up (at 6, 18, 24, 36 and 48 months) includes routine collection of self-report data annually, including maternal depression and infant/child temperamental profiles [Infant Behavior Questionnaire Revised (IBQ-R)53 at 6 months and the Early Childhood Behavior Questionnaire (ECBQ)54 at 18 months]. At 24 months postpartum, follow-up data collection begins across different methods and informants, including: (i) mother’s report; (ii) child direct assessment; (iii) research staff observation; (iv) psychophysiological startle probe; and (v) biospecimen collection.
Mother’s report
Mother’s report provides the following information: demographic characteristics, normative stress (depressive symptoms and parenting stress), perinatal post-traumatic stress syndrome (PTSD), perceived stress, PTSD symptoms, parental bonding, and social functioning, social support, home environment, retrospective report of antenatal environmental exposures, exercise, lifestyle habits (e.g. smoking, marijuana use, drinking) and diet/nutrition. In addition, a comprehensive assessment is made of child behaviour and socio-emotional status with: (i) the Brief Infant-Toddler Social-Emotional Assessment (BITSEA)55 which examines four dimensions: externalizing behaviours, internalizing behaviours, dysregulation and competence; and the Behaviour Assessment System for Children, 2nd Edition, Parent Rating (BASC2-P)57 which measures positive (adaptive) and negative (clinical) dimensions of behaviour.
Clinical interview with mothers
Clinical interviews to assess mother’s psychopathology are conducted with mothers at annual visits (24, 36 and 48 months) using the MINI International Neuropsychiatric Assessment, a structured clinical interview to assess Axis-I symptomatology according to the DSM-IV. The Preschool Age Psychiatric Assessment (PAPA)60 is used to ascertain psychopathology in a selected sample of offspring ages 2-4 years, via mothers’ reports.
Direct clinical assessment
Concomitantly, offspring are assessed annually, beginning at 24 months, via the Bayley Scales of Infant and Toddler Development–Third Edition (Bayley-III),58 and subsequently via the Wechsler Preschool and Primary Scale of Intelligence–Fourth EditionTM (WPPSI-IV).59 The Bayley-III evaluates each child along five key domains (cognitive, language, motor, social-emotional and adaptive development). The WPPSI-IV provides primary index scales including the verbal comprehension index, working memory index and processing speed index.
Psychophysiological assessment using startle
Mother and child complete a psychophysiological assessment annually to collect skin conductance rate, respiratory sinus arrhythmia and heart rate in response to a startle paradigm using the Biopac integrated program via the MP150 system with Acknowledge software (Biopac Systems, Goleta, CA).
Growth
Offspring physical development including height, weight and head circumference is recorded at each visit.
Specimen collection
Maternal and child hair, toenails, saliva and buccal swabs are collected at each annual visit and stored in the study’s biorepository.
Data collection about the level of trauma and experience during Superstorm Sandy
Unique to this cohort is the collection of traumatic stress exposure to natural disaster (i.e. Superstorm Sandy), via a comprehensive battery of self-report questionnaires assessing storm-specific exposure, impact of the event, post-traumatic symptoms and impairment in social functioning.
Key findings and publications
Preliminary data from the SIP Study cohort demonstrates interesting findings across several disciplines, including genomics, microbiology, physiology and neuropsychology.
Global methylation in the placenta and umbilical cord blood differed between women with and without antenatal metabolic disorders [i.e. gestational diabetes (GDM), preeclampsia and obesity] and it was further associated with fetal/infant growth. Suggestive negative associations of global methylation level in the placenta with infant body length and head circumference were found. It is possible that the placenta tissue, but not umbilical cord blood, may be epigenetically programmed by maternal GDM, preeclampsia and obesity to carry out its own specific functions that influence fetal growth.61 We also found that mitochondrial DNA may help regulate stress in pregnancy. Specifically, the expression of the protein-coding mitochondrial-encoded gene MT-ND2 was positively associated with indices of stress in pregnancy.62 Furthermore, we have explored the impact of maternal stress in pregnancy in the offspring’s developing immune system. Increased levels of IL-1β, IL-4, IL-5, IL-6 and IL-8 were found to be associated with maternal stress in pregnancy, indicating that it may influence cytokine levels, in particular Th2-related cytokines. These findings may suggest that newborns to mothers with elevated levels of stress in pregnancy have a predisposition to immune-related disorders.63 Among microbiological outcomes, we have demonstrated that meconium contains diversified microbiota, suggesting that the initial colonization of gut flora may begin before birth. Meconium contains diversified microbiota irrespective of mode of delivery, and the fetal microbiome of newborns of mothers with diabetes is enriched for the same bacterial taxa as those found in the fecal microbiome of adult diabetics. In addition, we found that pregnancy-specific anxiety was the most robust predictor for overall meconium microbiota composition, with greater pregnancy-specific anxiety associated with lower levels of Enterococcacae.64
Outcomes in focus among preliminary analyses include neurobehavioural indices and psychophysiological measures of autonomic nervous system functioning. First, more adverse neurobehavioural outcomes in offspring of mothers exposed to Superstorm Sandy during pregnancy (i.e. cases, n = 269) than offspring of mothers who were unexposed (i.e. controls, n = 345) were observed. Specifically, at 6 months old, cases as compared with controls exhibited a more difficult temperament profile with greater negativity and lower emotion regulation (Y Nomura, unpublished results). Among physiological outcomes, an interaction was found between antenatal cannabis exposure and tobacco exposure and outcomes indicative of blunted autonomic responsivity as indicated by decreased electrodermal activity (EDA) amplitude responses to startle.65
Taken together, in utero exposure to stress, maternal endocrine disorders (e.g. GDM and preeclampsia) and infection appear to have negative impacts on optimal neurobehavioural development of young children. Particularly notable was the impact of objective stress (i.e. natural disaster exposure) during pregnancy on neurobehavioural indices.
What are the main strengths and weaknesses?
The breadth and depth of the data collected in the SIP study are a significant strength. Information collected in the SIP Study cohort includes: (i) a wide range of biospecimens across different times during pregnancy (second and third trimesters), at birth and in the postpartum period (6 months, 18 months, 24 months, 36 months and 48 months); (ii) various biospecimens from mothers and offspring during antenatal and perinatal periods (blood, urine, placenta tissues, umbilical cord blood and meconium) and at annual follow-up beginning at 2 years of age (saliva, hair and nail clippings); (iii) multidimensional clinical assessments including face-to-face interviews during pregnancy (maternal psychopathology) and at annual follow-up (child neurodevelopmental, neurocognitive and psychopathological assessments); (iv) comprehensive questionnaire data spanning several domains known to influence the developmental trajectory of the offspring (i.e. psychosocial stress, perinatal PTSD, psychosocial familial stress, attachment style, social support, coping and maternal psychopathology); (v) collection of psychophysiological data at multiple time points from both mother and child; and (vi) observational data on mother-child interactions.
In addition, the cohort is composed of an urban ethnic and financial minority population which is considered to be the most ‘high-risk’ for developing major childhood disorders, including ante- and perinatal birth problems, asthma, obesity and impaired neurobehavioural development. This population could potentially provide a unique insight into the most high-risk group in terms of several antenatal and postnatal risks for suboptimal offspring development. The most notable strength of our population is that a subgroup of our participants were exposed to Superstorm Sandy at different times in relation to their pregnancy (pre-, during and post-pregnancy) to a varying degree, providing us with the opportunity to conduct a natural experiment of psychosocial stress. The stress caused by Superstorm Sandy’s devastation occurred independently of SES, and psychological or genetic characteristics. Together with epi-/genetic measurements and early childhood behavioural data, and precisely timed stress exposure (by index to Superstorm Sandy), we are able to enhance our capability not only to elucidate the mechanisms of gene and environment interaction influencing the trajectory of neurobehavioural development, but also to examine the window of susceptibility to stress.
The study also has limitations. First, the sample size is relatively small in comparison with large-scale birth cohort studies. Overall, we consented 806 women. Of those, 751 were eligible for a participation. Of these eligible participants, 58 (7.7%) withdrew from the study during the 6 years following its inception. Furthermore, the mortality rate was fairly high; 32 participants out of the remaining 693 participants (4.6%) were lost by mortality during antenatal, perinatal and neonatal periods. In addition, as our participants are a metropolitan population, mobility is also fairly high: 42 women (6.4%) were lost to follow-up or moved out of state, and an additional 5 mothers lost their custody by CPS intervention, resulting in a total of 614 active participants. Thus the findings may be limited in generalizability.
Second, some of our data are collected by maternal self-report which is always subject to bias. Although we had mothers fill in extensive child temperament questionnaires at multiple times (6, 18, 24, 36 and 48 months of age), as mothers were our single informants, we need to be cautious about the potential bias resulting from her own psychological well-being. It is largely held that temperament is a relatively stable index and is less likely to fluctuate over time. Availability of the multiple measurements could indeed help evaluate the extent to which each mother’s report was affected by her psychological well-being. As the study also includes clinician-administered neurodevelopmental, neurobehavioural and neurocognitive assessments, we could further evaluate the predictive ability of temperament on suboptimal developmental problems later in life. We intend to capitalize on our extensive biorepository, to examine biomarkers to further validate other means of measurement (i.e. mother’s report, clinical interview and psychophysiology).
Can I get hold of the data? Where can I find out more?
Researchers interested in exploring the possibility of collaboration should contact the Principal Investigator, Professor Yoko Nomura [yoko.nomura@qc.cuny.edu].
SIP Study in a nutshell
The SIP Study is a multidisciplinary birth cohort study investigating the trajectories of neurodevelopmental derailments and abnormalities in a diverse urban sample in metropolitan New York.
Children were recruited from 806 baseline pregnancies of mothers who received antenatal care at either New York Presbyterian Queens or Mount Sinai Hospital from 2010 to 2015.
Follow-up includes 12 face-to-face examinations (6 months to 6 years); 690 mothers and 722 neonates were eligible at birth and 585 mothers and 614 children remain active at 4 years.
The dataset comprises phenotypic measures including neurodevelopmental, medical, paediatric, epi-/genetic and psychosocial indices in addition to developmental disorders of the offspring. Biological samples including cord blood, placenta samples, maternal blood, maternal urine, meconium, hair, saliva and buccal swabs are also collected.
Researchers interested in collaborations should contact the Principal Investigator, Professor Yoko Nomura.
Funding
This work is supported by grants K01-080062, K01-080062S and R01-102729 from the National Institutes of Mental Health (NIMH), and PSC-CUNY, Queens College Research Enhancement Grant to the PI, Nomura.
Conflict of interest: None declared.
Acknowledgements
We are grateful to the mothers and children who continue to participate in this study. First we acknowledge Dr Jia Chen as the Mount Sinai site PI who is in charge of epi-/genetic assays and Dr Jeffrey Halperin at Queens College, CUNY, as a coinvestigator of the study. We thank the co-investigators at the Icahn School of Medicine, including Drs Yasmin Hurd, Jeffrey Newcorn, Holly Loudon and Joanne Stone, and Dr Renee Goodwin at Queens College, CUNY, as well as our co-investigators at New York Presbyterian Queens, Drs Gary Eglinton and Daniel Skupski. We acknowledge Drs Vivette Glover at Imperial College, London, Patrick Shrout at NYU, Jacob Ham at Mount Sinai Beth Israel and Khushmand Rajendran at Borough of Manhattan Community College as our consultants in the second phase of the study. We thank our team of past and present research assistants at Queens College, City University of New York for their tireless work in recruiting and assessing participating mothers and their children. Special thanks to Ms Michelle Yoon at the Icahn School of Medicine for her instrumental role in establishing, supporting and expanding the study cohort, and to Ms Nancy Huynh, Jenny Ly, Katarzyna Zajac, Jenny Porter and Jessica Buthmann for helping to establish the follow-up phase of the SIP Study and assisting in the collection of clinical and psychophysiologcial data.
References
- 1. Barker DJP. Fetal origins of coronary heart disease. BMJ 1995;311:171-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barker DJP. In utero programming of chronic disease. Clin Sci 1998;95:115-28. [PubMed] [Google Scholar]
- 3. Barker DJP. Early growth and cardiovascular disease. Arch Dis Child 1999;80:305-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barker DJP. The developmental origins of chronic adult disease. Acta Paediatr 2004;93:26-33. [DOI] [PubMed] [Google Scholar]
- 5. Barker DJP, Osmond C, Winter PD, Margetts B, Simmonds SJ.. Weight in infancy and death from ischaemic heart disease. Lancet 1989;334:577-80. [DOI] [PubMed] [Google Scholar]
- 6. Langley-Evans SC. Metabolic programming in pregnancy: studies in animal models. Genes Nutr 2007;2:33-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diplas AI, Lambertini L, Lee M-J. et al. Differential expression of imprinted genes in normal and IUGR human placentas. Epigenetics 2009;4:235-40. [DOI] [PubMed] [Google Scholar]
- 8. Marsit CJ, Lambertini L, Maccani MA. et al. Placenta-imprinted gene expression association of infant neurobehaviour. J Pediatr 2012;160:854-60.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nomura Y, Lambertini L, Rialdi A. et al. Global methylation in the placenta and umbilical cord blood from pregnancies with maternal gestational diabetes, preeclampsia, and obesity. Reprod Sci 2013;21:131-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lambertini L, Lee M-J, Marsit JC, Che J.. Genomic imprinting in human placenta In: Zheng J. (ed). Recent Advances in Research on the Human Placenta. Rijeka, Croatia: InTech, 2012. [Google Scholar]
- 11. McEwen BS. Early life influences on life-long patterns of behaviour and health. Ment Retard Dev Disabils Res Rev 2003;9:149-54. [DOI] [PubMed] [Google Scholar]
- 12. Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci 2001;24:1161-92. [DOI] [PubMed] [Google Scholar]
- 13. Monk C, Fifer WP, Myers MM. et al. Effects of maternal breathing rate, psychiatric status, and cortisol on fetal heart rate. Dev Psychobiol 2010;53:221-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van den Bergh BRH, Mulder EJH, Mennes M, Glover V.. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanisms. A review. Neurosci Biobehav Rev 2005;29:237-58. [DOI] [PubMed] [Google Scholar]
- 15. Gregg JP, Lit L, Baron CA. et al. Gene expression changes in children with autism. Genomics 2008;91:22-29. [DOI] [PubMed] [Google Scholar]
- 16. Nakayama A, Masaki S, Aoki E.. [Genetics and epigenetics in autism]. Nihon Shinkei Seishin Yakurigaku Zasshi 2006;26:209-12. [PubMed] [Google Scholar]
- 17. Crespi B. Genomic imprinting in the development and evolution of psychotic spectrum conditions. Biol Rev 2008. doi: 10.1111/j.1469-185x.2008.00050.x. [DOI] [PubMed] [Google Scholar]
- 18. Gavin DP, Kartan S, Chase K, Jayaraman S, Sharma RP.. Histone deacetylase inhibitors and candidate gene expression: An in vivo and in vitro approach to studying chromatin remodeling in a clinical population. J Psychiatr Res 2009;43:870-76. [DOI] [PubMed] [Google Scholar]
- 19. Peedicayil J. The role of epigenetics in altered gene expression involved in GABAergic transmission in the cerebellum of schizophrenia patients. Am J Psychiatry 2009;166:493. [DOI] [PubMed] [Google Scholar]
- 20. Verdoux H. Perinatal risk factors for schizophrenia: How specific are they? Curr Psychiatry Rep 2004;6:162-67. [DOI] [PubMed] [Google Scholar]
- 21. DasBanerjee T, Middleton FA, Berger DF, Lombardo JP, Sagvolden T, Faraone SV.. A comparison of molecular alterations in environmental and genetic rat models of ADHD: A pilot study. Am J Med Genet 2008;147B:1554-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goos LM, Ezzatian P, Schachar R.. Parent-of-origin effects in attention-deficit hyperactivity disorder. Psychiatry Res 2007;149:1-9. [DOI] [PubMed] [Google Scholar]
- 23. Mill J, Petronis A.. Pre- and peri-natal environmental risks for attention-deficit hyperactivity disorder (ADHD): the potential role of epigenetic processes in mediating susceptibility. J Child PsycholPsychiatry 2008;49:1020-30. [DOI] [PubMed] [Google Scholar]
- 24. Guo L, Choufani S, Ferreira J. et al. Altered gene expression and methylation of the human chromosome 11 imprinted region in small for gestational age (SGA) placentae. Dev Biol 2008;320:79-91. [DOI] [PubMed] [Google Scholar]
- 25. Paoloni-Giacobino A. Epigenetics in reproductive medicine. Pediatr Res 2007;61(5 Part 2):51-57R. [DOI] [PubMed] [Google Scholar]
- 26. Sekita Y, Wagatsuma H, Nakamura K. et al. Role of retrotransposon-derived imprinted gene, Rtl1, in the feto-maternal interface of mouse placenta. Nat Genet 2008;40:243-48. [DOI] [PubMed] [Google Scholar]
- 27. Djiane J, Attig L.. Role of leptin during perinatal metabolic programming and obesity. J Physiol Pharmacol 2008;59(Suppl 1):55-63. [PubMed] [Google Scholar]
- 28. Nicolaïdis S. Antenatal imprinting of postnatal specific appetites and feeding behaviour. Metabolism 2008;57:S22-26. [DOI] [PubMed] [Google Scholar]
- 29. Vickers MH. Developmental programming and adult obesity: the role of leptin. Curr Opin Endocrinol Diabet Obes 2007;14:17-22. [DOI] [PubMed] [Google Scholar]
- 30. Huizink AC, Mulder EJ, Robles de Medina PG, Visser GH, Buitelaar JK.. Is pregnancy anxiety a distinctive syndrome? Early Hum Dev 2004;79:81-91. [DOI] [PubMed] [Google Scholar]
- 31. Hoffman S, Hatch MC.. Depressive symptomatology during pregnancy: evidence for an association with decreased fetal growth in pregnancies of lower social class women. Health Psychol 2000;19:535-43. [PubMed] [Google Scholar]
- 32. Li J, Robinson M, Malacova E, Jacoby P, Foster J, van Eekelen A.. Maternal life stress events in pregnancy link to children's school achievement at age 10 years. J Pediatr 2013;162:483-89. [DOI] [PubMed] [Google Scholar]
- 33. Cohen S, Kamarck T, Mermelstein R.. A global measure of perceived stress. J Health Soc Behav 1983;24:385-96. [PubMed] [Google Scholar]
- 34. Murray L, Carothers AD.. The validation of the Edinburgh Post-natal Depression Scale on a community sample. Br J Psychiatry 1990;157:288-90. [DOI] [PubMed] [Google Scholar]
- 35. Spielberger CD. State-trait Anxiety Inventory: A Comprehensive Bibliography. Palo Alto, CA: Consulting Psychologists Press, 1989. [Google Scholar]
- 36. Dohrenwend BS, Krasnoff L, Askenasy AR, Dohrenwend BP.. The psychiatric epidemiology research interview life events scale In: Goldgerg L, Breznitz S (eds). Handbook of Stress: Theoretical and Clinical Aspects. New York, NY: Free Press, 1982. [Google Scholar]
- 37. Weissman MM, Wickramaratne P, Adams P, Wolk S, Verdeli H, Olfson M.. Brief screening for family psychiatric history: the family history screen. Arch Gen Psychiatr 2000;57:675-82. [DOI] [PubMed] [Google Scholar]
- 38. First MB, Spitzer RL, Gibbins M, Williams JB.. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P W/PSY SCREEN). New York NY: Biometrics Research, New York State Psychiatric Institute, 2002. [Google Scholar]
- 39. Norris FH, Kaniasty KZ, Scheer DA.. Use of mental health services among victims of crime: frequency, correlates, and subsequent recovery. J Consult Clin Psychol 1990;58:538-47. [DOI] [PubMed] [Google Scholar]
- 40. Weiss DS, Marmar CR.. The Impact of Event Scale-Revised In: Wilson JP, Keane TM (eds). Assessing Psychological Trauma and PTSD. New York, NY: Guilford Press, 1997. [Google Scholar]
- 41. Fao E. Posttraumatic Diagnostic Scale Manual. Minneapolis, MN: National Computer Systems, 1996. [Google Scholar]
- 42. Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA.. Psychometric properties of the PTSD Checklist (PCL). Behav Res Ther 1996;34:669-73. [DOI] [PubMed] [Google Scholar]
- 43. Gameroff MJ, Wickramaratne P, Weissman MM.. Testing the Short and Screener versions of the Social Adjustment Scale-Self-report (SAS-SR). Int J Methods Psychiatr Res 2012;21:52-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abidin RR. The determinants of parenting behaviour. J Clin Child Psychol 1992;21:407-12. [Google Scholar]
- 45. Callahan JL, Hynan MT.. Identifying mothers at risk for postnatal emotional distress: further evidence for the validity of the perinatal posttraumatic stress disorder questionnaire. J Perinatol 2002;22:448-54. [DOI] [PubMed] [Google Scholar]
- 46. Sheehan DV, Lecrubier Y, Sheehan KH. et al. The Mini-International Neuropsychiatric Interview (M.I.N.I): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59(Suppl 20):22-33. [PubMed] [Google Scholar]
- 47. Cavedo L, Parker G.. Parental bonding instrument. Soc Psychiatry Psychiatr Epidemiol 1994;29:78-82. [PubMed] [Google Scholar]
- 48. Lopez ML, Cooper L.. Social Support Measures Review. Laytonsville, MD: National Center for Latino Child and Family Research, 2010. [Google Scholar]
- 49. Bradley RH, Caldwell BM.. The HOME Inventory and family demographics. Dev Psychol 1984;20:315. [Google Scholar]
- 50. Brantsæter AL, Owe KM, Haugen M, Alexander J, Meltzer HM, Longnecker MP.. Validation of self-reported recreational exercise in pregnant women in the Norwegian Mother and Child Cohort Study. Scand J Med Sci Sports 2010;20:e48-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fernandez S, Olendzki B, Rosal MC.. A dietary behaviours measure for use with low-income, Spanish-speaking Caribbean Latinos with type 2 diabetes: The Latino Dietary Behaviours Questionnaire (LDBQ). J Am Diabet Assoc 2011;111:589-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gartstein MA, Rothbart MK.. Studying infant temperament via the revised infant behaviour questionnaire. Infant Behav Dev 2003;26:64-86. [Google Scholar]
- 53. Putnam SP, Gartstein MA, Rothbart MK.. Measurement of fine-grained aspects of toddler temperament: the early childhood behaviour questionnaire. Infant Behav Dev 2006;29:386-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rothbart MK, Ahadi SA, Hershey KL, Fisher P.. Investigations of temperament at three to seven years: the Children's Behaviour Questionnaire. Child Dev 2001;72:1394-408. [DOI] [PubMed] [Google Scholar]
- 55. Briggs-Gowan MJ, Carter AS, Irwin JR, Wachtel K, Cicchetti DV.. The Brief Infant-Toddler Social and Emotional Assessment: screening for social-emotional problems and delays in competence. J Pediatr Psychol 2004;29:143-55. [DOI] [PubMed] [Google Scholar]
- 56. Carter AS, Briggs-Gowan MJ, Jones SM, Little TD.. The Infant-Toddler Social and Emotional Assessment (ITSEA): factor structure, reliability, and validity. J Abnorm Child Psychol 2003;31:495-514. [DOI] [PubMed] [Google Scholar]
- 57. Reynolds CR, Kamphaus RW.. Behaviour Assessment System for Children (BASC-2). 2nd edn New York, NY: Pearson, 2004. [Google Scholar]
- 58. Bayley N. Bayley Scales of Infant and Toddler Development: Bayley-III. San Antonio, TX: Harcourt Assessment, Psychological Corporation, 2006. [Google Scholar]
- 59. Wechsler D. Wechsler Preschool and Primary Scale of Intelligence-III (WPPSI-III). Sydney, NSW: Pearson, 2002. [Google Scholar]
- 60. Egger HL, Erkanli A, Keeler G, Potts E, Walter BK, Angold A.. Test-retest reliability of the Preschool Age Psychiatric Assessment (PAPA). J Am Acad Child Adolesc Psychiatry 2006;45:538-49. [DOI] [PubMed] [Google Scholar]
- 61. Nomura Y, Lambertini L, Rialdi A, Lee M, Mystal EY, Grabie M, Manaster I, Huynh N, Finik J, Davey M, Davey K, Ly J, Stone J, Loudon H, Eglinton G, Hurd Y, Newcorn JH, Chen J.. Global methylation in the placenta and umbilical cord blood from pregnancies with maternal gestational diabetes, preeclampsia, and obesity. Reprod Sci. 2014;21(1):131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lambertini L, Chen J, Nomura Y.. Mitochondrial gene expression profiles are associated with maternal psychosocial stress in pregnancy and infant temperament. PLoS One 2015;10:e0138929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Andersson NW, Li Q, Mills CW, Ly J, Nomura Y, Chen J.. Influence of antenatal maternal stress on umbilical cord blood cytokine levels. Arch Womens Ment Health 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hu J, Nomura Y, Bashir A. et al. Diversified microbiota of meconium is affected by maternal diabetes status. PLoS One 2013;8:e78257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Buthmann J, Finik J, Porter J, Nomura Y.. Maternal cannabis and tobacco use during pregnancy and offspring neurobehavioural dysregulation NIDA Marijuana and Cannabinoids: A Neuroscience Research Summit, 22 March 2016. Bethesda, MD: National Institute on Drug Abuse, 2016. [Google Scholar]