Abstract
Background. Normalization of arterial pressure occurs in just a few patients with hypertensive chronic kidney disease undergoing kidney transplantation. Hypertension in kidney transplant recipients may be related to multiple factors. We aimed to assess whether hypertension in kidney-transplanted patients may be linked to reinnervation of renal arteries of the transplanted kidney.
Methods. We investigated renal arteries innervation from native and transplanted kidneys in three patients 5 months, 2 years and 11 years after transplantation, respectively. Four transplanted kidneys from non-hypertensive patients on immunosuppressive treatment without evidence of hypertensive arteriolar damage were used as controls.
Results. Evidence of nerve sprouting was observed as early as 5 months following transplantation, probably originated from ganglions of recipient patient located near the arterial anastomosis and was associated with mild hypertensive arteriolar damage. Regeneration of periadventitial nerves was already complete 2 years after transplantation. Nerve density tended to reach values observed in native kidney arteries and was associated with hypertension-related arteriolar lesions in transplanted kidneys. Control kidneys, albeit on an immunosuppressive regimen, presented only a modest regeneration of sympathetic nerves.
Conclusions. Our results suggest that the considerable increase in sympathetic nerves, as found in patients with severe arterial damage, may be correlated to hypertension rather than to immunosuppressive therapy, thus providing a morphological basis for hypertension recurrence despite renal denervation.
Keywords: hypertension, kidney transplantation, nerve sprouting, sympathetic renal innervation
INTRODUCTION
According to several studies, an exponential increase in sympathetic activity may be observed in chronic renal failure progression [1–3]. Persistent, often treatment-resistant hypertension is a well-known occurrence among patients affected by end-stage renal disease (ESRD) [4]. A recent study by our group of ESRD patients on haemodialysis has demonstrated a link between sympathetic hyperactivity and increased sympathetic nerves density within renal artery adventitia; a similar finding has been reported in hypertensive patients with histological evidence of arteriolar damage [5]. Successful kidney transplantation results in arterial pressure normalization only in a minority of cases, with 67–90% of post-transplant patients with persistent high blood pressure [6].
At present, there is no clear consensus regarding post-transplant hypertension pathophysiology, with both recipient- and donor-dependent factors appearing to be involved [6, 7]; recent clinical findings have drawn attention to the role of sympathetic hyperactivity [8]. Anatomical studies in animal models support the occurrence of nerve regeneration in kidney transplants [9]; there is, however, a lack of studies demonstrating nerve sprouting in arteries of human kidney transplants [10, 11]. The aim of the present study was to investigate the possible association between persistent post-transplant hypertension and reinnervation of renal arteries of the transplanted kidneys. To this end, we examined renal innervation in native kidneys and patient-matched kidney transplants in three patients; renal transplants were obtained 5 months, 2 years and 11 years after transplantation, respectively. A comparative assessment of patient-matched kidneys innervation was performed, with particular regard to grade and timing of nerve regeneration.
MATERIALS AND METHODS
Patient selection
Native and transplanted kidneys, with corresponding renal arteries and periadventitial tissue, from three kidney transplant recipients with post-transplant hypertension were selected for this study; clinical data of patients are reported in Table 1. All patients had received immunosuppressive treatment. Study patients underwent surgery for kidney transplantation in May 2014 (Patient 1), May 2012 (Patient 2) and April 2004 (Patient 3). Death occurred in November 2014 for Patient 1 (age 74 years; transplant-to-death interval = 5 months), in April 2014 for Patient 2 (age 54 years; transplant-to-death interval = 23 months) and in April 2015 for Patient 3 (age 62 years; transplant-to-death interval = 132 months).
Table 1.
Patients’ characteristics
| Parameters | Hypertensive patients with native and transplanted kidneys (n = 3 cases) | Transplanted kidneys from non-hypertensive patients (control group) (n = 4 cases) |
|---|---|---|
| Age, mean ± SD (years) | 72.4 ± 10.7 | 65.8 ± 16.4 |
| Sex (F/M) | 2/1 | 2/2 |
| History of hypertension (yes/no) | 3/0 | 3/1 |
| Grade of renal arterioles ≥4 | ||
| Native kidney | 3 | – |
| Transplanted kidney | 2 | 0 |
| Chronic kidney diseases (yes/no) | 3/0 | 4/0 |
| Coronary artery disease (yes/no) | 1/2 | 0/4 |
| Arrhythmias (yes/no) | 0/3 | 0/4 |
| Type 2 diabetes (yes/no) | 1/2 | 1/3 |
| Dyslipidaemia (yes/no) | 1/2 | 1/3 |
| Cause of death | ||
| Congestive heart failure | 2 | |
| Bronchopneumonia | 1 | |
| Histological changes of kidneys | ||
| Native kidney | ||
| Glomerulosclerosis with CKD | 2 | – |
| Polycystic kidney disease | 1 | – |
| Transplanted kidney | ||
| Glomerulosclerosis with CKD | 2 | 0 |
| Glomerulonephritis | 0 | 2 |
| Chronic rejection | 1 | 2 |
| Therapy | ||
| Anti-hypertensive drugs | 3 | 3 |
| Immunosuppressive drugs | ||
| Cyclosporine | 2 | 2 |
| Tacrolimus | 1 | 2 |
SD, standard deviation; F, female; M, male; CKD, chronic kidney disease.
Controls included four transplanted kidneys with no histological evidence of hypertensive arteriolar damage from non-hypertensive patients receiving immunosuppressive treatment.
All kidney transplants, surgical nephrectomies and autopsies were performed at the Tor Vergata University Hospital in Rome. Study procedures were approved by the Human Research Committee of the Tor Vergata University Hospital (authorization no. 54/14), and they were conducted in accordance with Italian regulations.
Histological protocol
Native and transplanted kidneys collected during the course of autopsies were immediately formalin-fixed and embedded in paraffin for histological evaluation. Kidneys were processed according to a previously reported protocol designed to evaluate nerve density [5]. Briefly, each artery was sampled along its entire length from origin to renal hilum, together with surrounding periadventitial tissue; for each artery, a minimum of six specimens was collected at average intervals of 5 mm, and serially cut into 5-μm-thick sections. For each specimen, two sections were stained with haematoxylin and eosin and Weigert stain, respectively, while additional sections were obtained for immunohistochemical studies.
In addition, for each patient a kidney sample featuring both cortex and medulla was collected to evaluate hypertension-related histological changes. Burke et al.'s scoring system [12] was used in order to evaluate hypertensive status, assessing 20 arterioles, 150–500 µm thick, per kidney sample. Median scores of 4 (i.e. concentric intimal thickening greater than the thickness of the media without concentric elastic duplication) or 5 (i.e. concentric intimal thickening greater than the thickness of the media with concentric elastic duplication in three or more vessels examined) were regarded as histological markers of hypertensive nephropathy.
Immunohistochemistry
Immunohistochemical stains for neurofilament protein (NFP; Ventana Medical Systems, Inc., Tucson, AZ, USA) and S100 (Ventana Medical Systems, Inc.) were used for nerve identification, in accordance with protocols from Sakakura et al. and van Amsterdam et al., respectively [13, 14]. Afferent nerves, efferent nerves and parasympathetic fibres were identified using anti-calcitonin gene-related peptide (CGRP) (Sigma-Aldrich, St Louis, MO, USA), anti-tyrosine hydroxylase (TH) (AB193083, Abcam, Bristol, UK) and anti-NOS antibodies (AB15203, Abcam), respectively. Further immunohistochemical staining was performed for growth-associated protein 43 (GAP43), an axonal growth cone-associated protein that is upregulated during nerve sprouting [15].
IScan Bioimagene scan and Bioimagene Image Viewer software were used for morphometric analysis of nerves within periadventitial tissue. For each section, number of nerves per unit area and size of nerve endings were assessed. For comparison purposes, measurements were taken within a concentric ring located at 0.5 and 2 mm distance from the beginning of adventitia; indeed, as also previously demonstrated [5], this area harbours the maximum density of nerves.
Statistical analysis
Data were analysed using the SPSS 16.0 (Statistical Package for the Social Sciences) software and expressed as the mean ± standard deviation. Data corresponding to the native and transplanted renal arteries were analysed using the Wilcoxon rank sum test. All P-values were determined by two-sided analysis, and P < 0.05 was considered statistically significant.
RESULTS
Hypertensive patients with native and transplanted kidneys
Patient 1 was a 74-year-old woman who died in November 2014 at the Tor Vergata University Hospital. She had a history of irreversible hypertension-related nephropathy, treated with dialysis for 5 years. Following kidney transplantation from a non-hypertensive donor in May 2014, she suffered from persistent hypertension, even though responsive to treatment. The patient, who was also undergoing continuous immunosuppressive treatment, developed a fatal cytomegalovirus infection in November 2014 and died. Autopsy showed pseudomembranous pancolitis, cardiac hypertrophy and acute terminal ventricular dilation. At histological examination, native kidney was characterized by evidence of hypertensive vascular damage with diffuse arteriolar wall thickening and significant internal elastic lamina duplication (score 5; Figure 1A) [12]. Renal parenchyma featured diffuse changes of chronic pyelonephritis with foci of tubular atrophy and widespread glomerulosclerosis. Histological evaluation of 5-month kidney transplant revealed interstitial chronic inflammation and focal glomerulosclerosis along with mild arteriolar damage (i.e. concentric intimal arteriolar thickening similar to the thickness of the tunica media; score 3) [12]. Native renal arteries exhibited evidence of fibromuscular dysplasia, with luminal stenosis <30%. In both arteries numerous nerves were observed in the adventitia, with 26- to 121-µm diameter nerves being more abundant in the area close to the tunica media (mean nerve density = 4.34 ± 0.21 nerves/mm2; Table 2, Figure 1B). Small nerves were concentrated in the innermost area of the adventitia, surrounded by larger nerves. Density and size of nerves were consistent with data of a previous study of kidneys in ESRD patients on haemodialysis [5]. Evaluation of transplant renal artery, distal to arterial anastomosis, revealed the presence of few stainable nerves in the innermost area of adventitial tissue (mean nerve density = 1.08 ± 0.16 nerves/mm2; Figure 1D). Such nerves were predominantly small (32–52 µm diameter), mainly consisting of regenerating axons, as demonstrated by GAP43 positivity [15]. Additional evidence of nerve sprouting was found in native ganglions sampled in proximity to arterial anastomosis (Figure 1G).
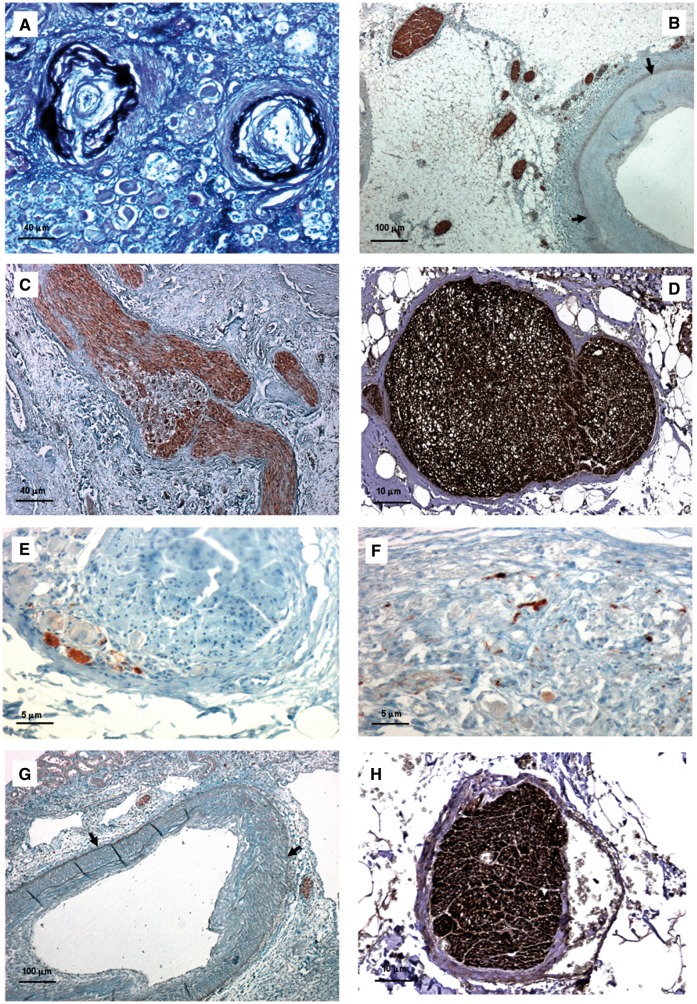
FIGURE 1.
Histologic features of native and transplanted kidneys in Patient 1. (A) Renal arterioles of native kidney featured concentric intimal thickening greater than thickness of media and concentric elastic duplication (score = 5; Weigert stain—bar = 40 μm). (B) In the native renal artery several nerves were observed within the adventitia, especially in the area close to the tunica media; nerve diameter ranged from 26 to 121 μm (NFP stain—bar = 100 μm). (C) Evidence of GAP43-positive nerve sprouting was noted in native ganglions close to arterial anastomoses (GAP43 stain—bar = 40 μm). (D–F) In renal nerves of native kidneys, TH-positive sympathetic efferent fibres (D) were more numerous than CGRP-positive sympathetic afferent fibres (E) and NOS-positive parasympathetic fibres (F) (D, bar = 10 μm; E, bar = 5 μm; F, bar = 5 μm). (G) Few GAP43-positive nerve axons could be observed in the renal artery from kidney transplant, distal to arterial anastomosis (GAP43 stain—bar = 100 μm). (H) In transplant renal artery, nerves consisted only of TH-positive sympathetic efferent fibres (TH stain—bar = 10 μm). (Adventitial–medial borders are highlighted by arrows.)
Table 2.
Renal nerve density in native and transplanted kidneys
| Hypertensive patients with native and transplanted kidneys |
Transplanted kidneys from non-hypertensive patients (control group) | |||
|---|---|---|---|---|
| Patient 1 (74-year- old woman) | Patient 2 (54-year- old man) | Patient 3 (62-year- old woman) | ||
| Interval between transplantation and explantation | 5 months | 2 years | 11 years | 10 months–7 years |
| Hypertensive arteriolar damage (grade, according to Burke et al. [12]) | ||||
| Native kidney | 5 | 4 | 5 | |
| Transplanted kidney | 3 | 5 | 4 | 3 (in all cases) |
| Native renal arteries | ||||
| Overall nerve density in the adventitial tissue (nerves × mm2) | 3.17 ± 0.16 | 2.94 ± 0.29 | 4.18 ± 0.24 | 0.85 ± 0.31 |
| Nerve density in the internal area of the adventitial tissue (nerves × mm2)a | 4.34 ± 0.21 | 3.81 ± 0.22 | 4.81 ± 0.18 | |
| Diameter of nerves, range (μm) | 26–131 | 19–128 | 28–320 | |
| Transplanted renal artery | ||||
| Overall nerve density in the adventitial tissue (nerves × mm2) | 0.75 ± 0.20 | 2.47± 0.22 | 3.89 ± 0.24 | |
| Nerve density in the internal area of the adventitial tissue (nerves × mm2)a | 1.08 ± 0.16 | 3.30 ± 0.21 | 4.22 ± 0.21 | 0.77 ± 0.30 |
| Diameter of nerves, range (μm) | 32–52 | 22–148 | 37–256 | 18–68 |
Within the first 0.5 mm from the beginning of adventitia.
Patient 2, a 54-year-old man affected by polycystic dominant kidney disease (PDKD) and hypertension on dialysis treatment, underwent kidney transplantation in 2012 from a non-hypertensive donor. Similar to Patient 1, persistent, treatment-responsive hypertension ensued following kidney transplantation. Allograft rejection occurred in April 2014, leading to explantation of both native kidney and transplant. Histological examination of native kidney highlighted features typical of a PDKD, in addition to evidence of chronic pyelonephritis in residual parenchyma and hypertensive arteriolar damage (Grade 4; Figure 2A) [12]. Numerous nerves were found in the periadventitial tissue of native renal arteries, mainly in the innermost area (mean nerve density = 3.81 ± 0.22 nerves/mm2; Figure 2B); nerve diameter was 57.1 ± 27.3 µm (range 19–128 µm). Kidney transplant exhibited histological features of both chronic T cell-mediated and antibody-mediated rejection (according to Banff classification) [16]. Transplant renal artery, distal to arterial anastomosis, exhibited mild luminal stenosis (<30%). Conversely, arterioles were markedly stenotic and showed evidence of severe arteriolar damage with duplication of internal elastic lamina, a hallmark of the hypertensive damage (score 5; Figure 2C) [12]. Evaluation of periadventitial tissue of transplant renal artery revealed complete regeneration of nerves, as supported by positive staining for NFP, S100 and GAP43 (Figure 2D). Nerves did not significantly differ in density and diameter from those observed in renal arteries of native kidney (P = 0.37) (Table 2).
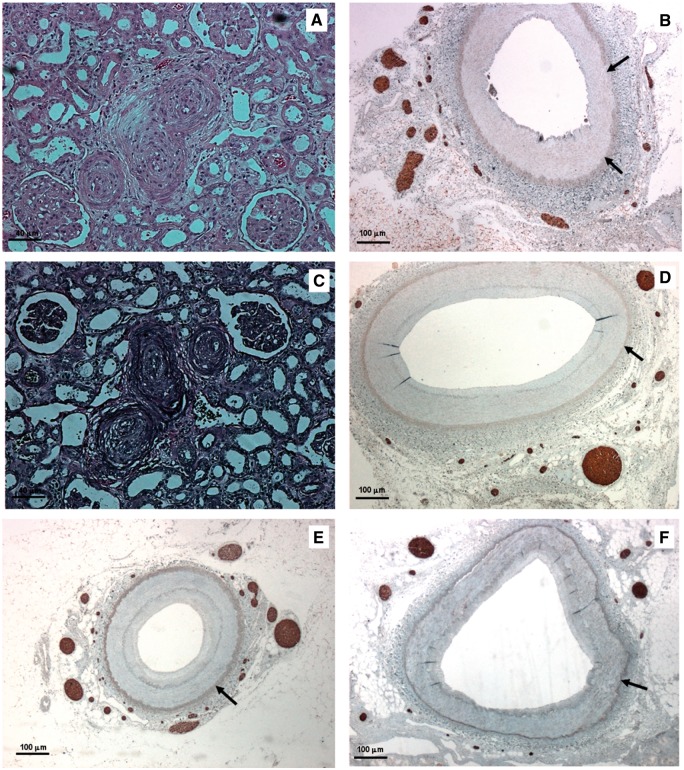
FIGURE 2.
Histologic findings of native and transplanted kidneys in Patient 2 (A–D) and Patient 3 (E, F). (A) Renal arterioles of native kidney with near occlusive concentric intimal thickening (score = 4) (haematoxylin and eosin—bar = 40 μm). (B) Native renal artery with numerous nerves within the adventitia (NFP stain—bar = 100 μm). (C) Renal arterioles of transplanted kidney with severe arteriolar damage and concentric elastic duplication (score = 5) (Weigert stain—bar = 40 μm). (D) Transplanted renal artery: complete regeneration of periadventitial nerves was observed with nerve density equalling values of native artery (GAP43 stain—bar = 100 μm). (E) Native renal artery with a significant density of nerves within the adventitia (NFP stain—bar = 100 μm). (F) Transplanted renal artery: complete regeneration of GAP43-positive nerves with nerve density similar to that measured in native kidneys (GAP43 stain—bar = 100 μm). (Adventitial-medial borders are highlighted by arrows.)
Patient 3 was a 62-year-old woman who underwent kidney transplantation for chronic hypertension-related chronic renal failure in 2004 from a non-hypertensive donor. Hypertension persisted following transplantation; she died in 2015 due to bilateral bronchopneumonia associated with cardiac hypertrophy. At histological examination, it was found that native kidney was affected by severe chronic pyelonephritis with diffuse hypertensive vascular damage (score 5) [12]. Kidney transplant was found to harbour glomerulosclerosis and chronic pyelonephritis; concentric thickening in arteriolar intima was found to be greater than thickness of media (score 4) [12]. Nerve density in transplanted kidney was similar to that measured in the native kidney (mean nerve density in the innermost area = 4.81 ± 0.18 versus 4.22 ± 0.21 nerves/mm2). All nerves stained positively for GAP43 (Figure 2F).
Immunohistochemical analysis of renal nerves in native kidneys revealed a higher density of TH-positive sympathetic efferent fibres compared with CGRP-positive sympathetic afferent fibres and eNOS-positive parasympathetic fibres. Data regarding density of parasympathetic fibers, however, should be considered with caution, as several studies seem to suggest that NOS might be involved in the afferent neuronal system rather than the parasympathetic system [17]. On the contrary, renal nerves in transplanted kidneys from both hypertensive and non-hypertensive patients consisted only of TH-positive sympathetic efferent fibres (Figures 1D–F).
Transplanted kidneys from non-hypertensive patients (control group)
Controls included kidney transplants from four patients undergoing surgical explantation of transplanted kidney, with intersurgical intervals ranging from 10 months to 7 years. Explantation was due to recurrence of primary chronic kidney disease (i.e. focal and segmental glomerulonephritis) in two patients, and chronic rejection in the remaining two patients. All control patients had been treated with immunosuppressive drugs for 1–7 years. Three out of four patients were also on effective anti-hypertensive treatment; one patient was free of hypertension.
Histological examination of control kidneys revealed absence of hypertensive arteriolar lesions (score ≤3). All renal arteries had stenosis <30% and showed signs of a fibromuscular dysplasia.
Average nerve density in renal adventitia was 0.35 ± 0.31/mm2. Nerve diameter ranged between 18 µm and 68 µm and did not significantly differ from those observed in renal arteries of other kidney transplants (P = 0.43; Table 2).
DISCUSSION
The results of the present study appear to support a correlation between persistent hypertension following kidney transplantation and periadventitial regeneration of nerves in kidney transplants arteries. Evidence of neural sprouting was demonstrated as early as 5 months after kidney transplantation, developing in association with clinical hypertension; in all likelihood, nerve sprouting stems from ganglions of receiving patients, close to arterial anastomoses. Importantly, neural regeneration appears to be limited to sympathetic efferent fibres only. Complete periadventitial nerve regeneration in hypertensive patients seems to be achieved within 24 months following transplantation; indeed, nerve density in kidney transplant arteries tends to reach levels observed in native arteries, being paralleled by a consensual worsening of hypertension-related lesions in distal arterioles.
Hypertension is frequently occurring among kidney transplant recipients, impairing allograft survival and increasing cardiovascular morbidity [7, 18, 19]. Risk factors for development of post-transplant hypertension include pre-transplant hypertension, old age of donor, recurrent or de novo glomerular disease, acute rejection, transplant renal artery stenosis, persistent post-transplant hyperparathyroidism and several pharmacologic therapies [6, 7]. Indeed, administration of immunosuppressive medications, such as corticosteroids and calcineurin inhibitors (CNIs, cyclosporine and/or tacrolimus), appears to increase the risk of post-transplant hypertension [7, 20, 21]. Previous studies have demonstrated that CNIs are a risk factor for post-transplant hypertension in cardiac transplant recipients and in patients with HLA-identical kidney transplants [21, 22]. We have demonstrated, however, that not all patients treated with immunosuppressive drugs develop severe renal hypertensive-related arteriolar damage (Table 1), probably because of effective pharmacological control of hypertension. It should be noted that significant increases in perirenal sympathetic innervation (i.e. reaching nerve density levels akin to those of native kidneys) could be observed only in patients developing significant arteriolar damage in kidney transplants (i.e. Patient 2 and Patient 3; Table 2). In Patient 1, no significant arteriolar changes were observed in kidney transplant, probably due to limited time interval between kidney transplantation and explantation; likewise, only mild regeneration of sympathetic nerves was noted, with nerve density being similar to that of controls (i.e. transplanted kidneys from non-hypertensive patients), despite ongoing immunosuppressive treatment. Accordingly, significant increases in sympathetic nerve density in association with severe arterial damage appear to be correlated with concurrent hypertension rather than with immunosuppressive therapy, as previously demonstrated in ESRD patients [5]. Nevertheless, no definitive answer may be provided to the question of whether the pronounced increase in perirenal nerves in transplanted kidneys is the cause of hypertension (rather than the consequence). It seems probable that nerve regeneration plays a role in maintenance of hypertension; further research is warranted in this regard.
Previous studies on animal models have documented in detail the occurrence of nerve sprouting after denervation [9, 23]. Morphological changes in nerve anatomy following Wallerian degeneration result in decreases in Nerve Growth Factor (NGF) concentration and subsequent synthesis and release of NGF by Schwann cells. NGF, acting as a molecular trigger for nerve repair, promotes nerve regeneration and sprouting in the anatomic site of previous innervation [24]. At present, there is a lack of studies addressing neural renal regeneration in humans. Previous studies from Gazdar and Dammin [10] and Norvell et al. [11] provided evidence of sympathetic renal reinnervation in human renal transplants; in these studies, however, no comparison between nerve density in kidney transplants and native kidneys was performed, nor was any correlation with hypertension investigated. Increased activity of the sympathetic system in kidney-transplanted patients was previously hypothesized, but only by means of physiological studies [6, 7]. To the best of our knowledge, our report is the first to provide morphological evidence of regeneration of sympathetic fibres in hypertensive transplanted patients.
GAP43-positive nerve density was quantified in order to measure nerve sprouting activity; GAP43 is a polypeptide that undergoes neuronal upregulation during axonal growth [15]. Following nerve injury and Wallerian degeneration, neurilemmal cell proliferation and axonal regeneration (i.e. nerve sprouting) ensue. A key event during axonal regeneration is the development of a growth cone. Meiri et al. [15] demonstrated that GAP43 is a component of neuronal growth cones, supporting the hypothesis that GAP43 expression is a prerequisite for axonal growth.
Kidney transplants with Wallerian degeneration of nerves may be regarded as a model of renal denervation; recent clinical studies demonstrated a short-term effect of renal denervation on blood pressure after a 1-year follow-up [25]. We believe that our findings might have a substantial clinical impact by providing the morphological basis of hypertension relapse in patients undergoing renal denervation. It seems reasonable that complete regeneration of sympathetic nerves might occur in these patients in a similar fashion to what was observed in our patients after transplantation. Further research appears justified in order to test this hypothesis.
Study limitation
The most important limitation of our study is the number of patients. Such limitation seems difficult to overcome, as the occurrence of double-kidney explants is very rare; accordingly, an autoptic study appears to be needed to allow for a comparative examination of both native and transplanted kidneys from the same patient. A further limitation of the present study is the limited number of assessed kidney transplant cases free of arteriolar damage. Finally, clinical evidence of sympathetic hyperactivity could not be provided in our patients, although the existence of sympathetic hyperactivity in ESRD patients has been confirmed by numerous studies [1, 2, 26, 27].
CONCLUSIONS
We believe that our results provide an anatomical framework for maintenance or recurrence of hypertension following kidney transplantation, providing valuable data in the quest to improve renal denervation strategies, although further research on a wider sample of patients is needed.
FUNDING
This work has been supported by Fondazione Roma grant 2015-2017 ‘Malattie Non trasmissibili Cronico-Degenerative (NCDs)’.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Converse RL Jr, Jacobsen TN, Toto RD. et al. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med 1992; 327: 1912–1918 [DOI] [PubMed] [Google Scholar]
- 2. Schlaich MP. Sympathetic activation in chronic kidney disease: out of the shadow. Hypertension 2011; 57: 683–685 [DOI] [PubMed] [Google Scholar]
- 3. Zoccali C, Mallamaci F, Parlongo S. et al. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation 2002; 105: 1354–1359 [DOI] [PubMed] [Google Scholar]
- 4. Agarwal R. Systolic hypertension in hemodialysis patients. Semin Dial 2003; 16: 208–213 [DOI] [PubMed] [Google Scholar]
- 5. Mauriello A, Rovella V, Anemona L. et al. Increased sympathetic renal innervation in hemodialysis patients is the anatomical substrate of sympathetic hyperactivity in end-stage renal disease. J Am Heart Assoc 2015; 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lakkis JI, Weir MR.. Treatment-resistant hypertension in the transplant recipient. Semin Nephrol 2014; 34: 560–570 [DOI] [PubMed] [Google Scholar]
- 7. Weir MR, Burgess ED, Cooper JE. et al. Assessment and management of hypertension in transplant patients. J Am Soc Nephrol 2015; 26: 1248–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schneider S, Promny D, Sinnecker D. et al. Impact of sympathetic renal denervation: a randomized study in patients after renal transplantation (ISAR-denerve). Nephrol Dial Transplant 2015; 30: 1928–1936 [DOI] [PubMed] [Google Scholar]
- 9. Mulder J, Hokfelt T, Knuepfer MM. et al. Renal sensory and sympathetic nerves reinnervate the kidney in a similar time-dependent fashion after renal denervation in rats. Am J Physiol Regul Integr Comp Physiol 2013; 304: R675–R682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gazdar AF, Dammin GJ.. Neural degeneration and regeneration in human renal transplants. N Engl J Med 1970; 283: 222–224 [DOI] [PubMed] [Google Scholar]
- 11. Norvell JE, Weitsen HA, Sheppek CG.. The intrinsic innervation of human renal homotransplants. Transplantation 1970; 9: 168–176 [PubMed] [Google Scholar]
- 12. Burke AP, Kolodgie FD, Farb A. et al. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation 2002; 105: 297–303 [DOI] [PubMed] [Google Scholar]
- 13. Sakakura K, Ladich E, Edelman ER. et al. Methodological standardization for the pre-clinical evaluation of renal sympathetic denervation. JACC Cardiovasc Interv 2014; 7: 1184–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Amsterdam WA, Blankestijn PJ, Goldschmeding R. et al. The morphological substrate for renal denervation: nerve distribution patterns and parasympathetic nerves. A post-mortem histological study. Ann Anat 2016; 204: 71–79 [DOI] [PubMed] [Google Scholar]
- 15. Meiri KF, Pfenninger KH, Willard MB.. Growth-associated protein, GAP-43, a polypeptide that is induced when neurons extend axons, is a component of growth cones and corresponds to pp46, a major polypeptide of a subcellular fraction enriched in growth cones. Proc Natl Acad Sci USA 1986; 83: 3537–3541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Racusen LC, Solez K, Colvin RB. et al. The Banff 97 working classification of renal allograft pathology. Kidney Int 1999; 55: 713–723 [DOI] [PubMed] [Google Scholar]
- 17. Aimi Y, Fujimura M, Vincent SR. et al. Localization of NADPH-diaphorase-containing neurons in sensory ganglia of the rat. J Comp Neurol 1991; 306: 382–392 [DOI] [PubMed] [Google Scholar]
- 18. Campistol JM, Romero R, Paul J. et al. Epidemiology of arterial hypertension in renal transplant patients: changes over the last decade. Nephrol Dial Transplant 2004; 19 (Suppl 3): iii62–iii66 [DOI] [PubMed] [Google Scholar]
- 19. Mange KC, Cizman B, Joffe M. et al. Arterial hypertension and renal allograft survival. JAMA 2000; 283: 633–638 [DOI] [PubMed] [Google Scholar]
- 20. Thomas B, Taber DJ, Srinivas TR.. Hypertension after kidney transplantation: a pathophysiologic approach. Curr Hypertens Rep 2013; 15: 458–469 [DOI] [PubMed] [Google Scholar]
- 21. Verghese PS, Dunn TB, Chinnakotla S. et al. Calcineurin inhibitors in HLA-identical living related donor kidney transplantation. Nephrol Dial Transplant 2014; 29: 209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Frist WH, Stinson EB, Oyer PE. et al. Long-term hemodynamic results after cardiac transplantation. J Thorac Cardiovasc Surg 1987; 94: 685–693 [PubMed] [Google Scholar]
- 23. Booth LC, Nishi EE, Yao ST. et al. Reinnervation of renal afferent and efferent nerves at 5.5 and 11 months after catheter-based radiofrequency renal denervation in sheep. Hypertension 2015; 65: 393–400 [DOI] [PubMed] [Google Scholar]
- 24. Burnett MG, Zager EL.. Pathophysiology of peripheral nerve injury: a brief review. Neurosurg Focus 2004; 16: E1. [DOI] [PubMed] [Google Scholar]
- 25. Bakris GL, Townsend RR, Flack JM. et al. 12-month blood pressure results of catheter-based renal artery denervation for resistant hypertension: the SYMPLICITY HTN-3 trial. J Am Coll Cardiol 2015; 65: 1314–1321 [DOI] [PubMed] [Google Scholar]
- 26. Augustyniak RA, Tuncel M, Zhang W. et al. Sympathetic overactivity as a cause of hypertension in chronic renal failure. J Hypertens 2002; 20: 3–9 [DOI] [PubMed] [Google Scholar]
- 27. Masuo K, Lambert GW, Esler MD. et al. The role of sympathetic nervous activity in renal injury and end-stage renal disease. Hypertens Res 2010; 33: 521–528 [DOI] [PubMed] [Google Scholar]