Abstract
Background
Recent studies have implicated the FTO gene in child and adult obesity. A longer duration of exclusive breastfeeding (EXBF) has been shown to reduce body mass index (BMI) and the risk of being overweight in the general population and among FTO gene carriers. However, it remains unclear whether the preventive effect of EXBF could be explained by its impact on early life growth development, e.g. ages at adiposity peak (AP) and adiposity rebound (AR) and BMI velocities in the first years of life, which are major determinants of overweight and obesity later in life.
Methods
We studied 5590 children from the British Avon Longitudinal Study of Parents and Children (ALSPAC) cohort and modelled their longitudinal BMI profiles with mixed effects models from birth to 16 years of age, as well as their ages at AP, AR and BMI velocities in relation to the FTO gene variant and EXBF.
Results
A longer duration of EXBF (i.e. at least 5 months) has substantial impact on BMI growth trajectories among children carrying the FTO adverse variant by modulating the age at AP, age at AR and BMI velocities. EXBF acts antagonistically to the FTO rs9939609 risk allele and by the age of 15, the predicted reduction in BMI after 5 months of EXBF is 0.56 kg/m2 [95% confidence interval (CI) 0.11–1.01; P = 0.003] and 1.14 kg/m2 (95% CI 0.67–1.62; P < 0.0001) in boys and girls, respectively.
Conclusions
EXBF influences early life growth development and thus plays a critical role in preventing the risks of overweight and obesity even when those are exacerbated by genetic factors.
Keywords: Breastfeeding, FTO gene, BMI, overweight, early child development, birth cohort
Introduction
Obesity is a major global public health problem. The World Health Organization (WHO) estimated in 2014 that there were at least 41 million overweight children under the age of 5 years and one billion overweight adults globally.1 Rates of obesity and overweight are rising in nearly all countries around the globe.2 Treating childhood obesity is a major public health challenge.3 The gestational and early postnatal periods have both been identified as critical developmental windows for future obesity risk. It is known that obesity in childhood is highly predictive of obesity in adulthood.4 Early postnatal weight gain is another critical period for determining later overweight and obesity.5 Previous research has clearly established that the susceptibility to obesity results from a combination of genetic, behavioural and environmental factors.
Recent studies have supported the role of the (FTO) gene in increasing body mass index (BMI) in young adults,6–9 in particular through genome-wide association studies (GWAS) of BMI and adiposity.6,8,10 Risk alleles of the FTO gene can be very common in the general population, e.g. the minor (A) allele of the most reported single nucleotide polymorphism (SNP), rs9939609, has a frequency of 46% in the Caucasian Hapmap population so that 70% of this population is carrier. In meta-analyses, the addition of each minor (A) allele at the SNP rs9939609 within the first intron of FTO has been shown to be consistently associated with a higher BMI of up to 0.39 kg/m2 and a 1.20-fold increase in obesity.7 Recently, we have shown that known adult genetic determinants of BMI (including FTO variants and other important variants identified through GWAS) have observable effects on growth also in early childhood.11 The biological mechanisms underlying this association with FTO are not completely understood, but evidence suggest that this locus could affect fuel utilization through appetite, or the control of macronutrient intake, or the sensing of amino acids and nutrients, and could also have epigenetic implications as a DNA-RNA demethylase-modifying enzyme or an epitranscriptomic marker.12–14 A recent study reported the causal role of a risk-conferring FTO variant (rs1421085) at intron 1 of FTO on adiposity, which is believed to act by disrupting ARID5B-mediated repression of IRX3 and IRX5, thereby influencing mitochondrial respiration and adipocyte browning.15
A major challenge for public health and community health research is to identify interventions that could prevent the risk of obesity early in life. Observational studies have suggested that certain levels of physical activity and dietary intakes could alter changes in BMI and in other body composition and/or adiposity phenotypes due to FTO variants,16–21 although these results remain highly controversial.22–24 On the other hand, breastfeeding could potentially be an effective target for intervention programmes, but its role has not been well studied. Some studies have shown that a longer duration of breastfeeding was associated with a decrease in the risk of being overweight later in life.25–27 A recent study from the Western Australian Pregnancy (RAINE) cohort showed that early infant feeding can affect BMI trajectories through a possible change in the timing of adiposity rebound (AR).28 Dedoussis et al.29 indicated that in two moderately sized paediatric cohorts from Greece, breastfeeding might exert a modifying effect on the relationship between variants at the FTO locus and indices of adiposity; however, these findings were not replicated in the British ALSPAC study. More recently, it was reported in the RAINE cohort that a longer duration of exclusive breastfeeding (EXBF) could attenuate the increase in BMI among carriers of the risk allele of the FTO SNP rs9939609.30 However, what remains unclear is whether breastfeeding and FTO gene variants could affect child BMI growth through an indirect effect on early life events such as the timing of the adiposity peak (AP) and the adiposity rebound (AR), as alluded to in recent studies.29,30 If that is case, that would suggest that early interventions that target longer duration of breastfeeding (i.e. at least 5 months) might delay the timing of AP and AR and hence prevent the risk of overweight/obesity later in life. The goal of this paper is therefore to better understand the role of EXBF on early life development (i.e. age at AP and AR and the BMI velocities around these developmental windows) and its subsequent impact on BMI growth trajectories up to adolescence in carriers and non-carriers of the FTO gene variant.
The study protocol was initially approved by the institutional review boards of the Brigham and Women’s Hospital and the Harvard School of Public Health. Participants provided implied consent by virtue of voluntarily returning their questionnaires. Additional approval was obtained from Mount Sinai Hospital in Toronto.
Materials and Methods
Avon Longitudinal Study of Parents and Children (ALSPAC)
This is a prospective birth cohort study described in full elsewhere.31,32 Pregnant women resident in one of three Bristol-based health districts with an expected delivery date between 1 April 1991 and 31 December 1992 were invited to participate. Follow-up has included parent- and child-completed questionnaires, links to routine health care data, and clinic attendance. A subset of 5590 children was used for analysis in this study. Ethical approval for the study was obtained from the ALSPAC Law and Ethics Committee and the local research ethics committees. Please note that the study website contains details of all the data that are available through a fully searchable data dictionary.33
Assessment of BMI
Birth length (crown-heel) was measured by ALSPAC staff who visited newborns soon after birth (median 1 day, range 1–14 days), using a Harpenden Neonatometer (Holtain Ltd). Birthweight was extracted from medical records. From birth to 5 years, length and weight measurements were extracted from health visitor records, which form part of standard child care in the UK. In this cohort we had up to four measurements taken on average at 6 weeks and 10, 21 and 48 months of age. For a random 10% of the cohort, we also have length/height measurements from eight research clinic visits, held between the ages of 4 months and 5 years. From age 7 years upwards, all children were invited to approximately annual clinics. In addition, parent-reported child height and weight were also available from the questionnaires. BMI was derived from height and weight measurements (mean eight measures per person with a total of 45 534) and calculated as the weight (in kg) divided by the square of height (in cm).34
Assessment of breastfeeding
Information pertaining to early infant feeding was collected. Mothers recorded the age at which breastfeeding was stopped (in months), and the age at which milk other than breast milk was introduced (in months). This information was determined from the mother’s diary of early feeding milestones, as well as from an interview with the study nurse at the 6-month child follow-up and survey questions at the 15-months child follow-up. The duration of exclusive breastfeeding (EXBF) was defined for mothers who breastfed their baby as the time from birth until they started feeding their baby with other milk (non-breast milk) or any solid. In our longitudinal models, we evaluated the effect of 5 months of EXBF vs no breastfeeding on BMI trajectories. A duration of 5 months was chosen because it is close to the 6 months duration recommended by WHO,35 and we had sufficient observations to estimate the predicted BMI curves for this duration. However, in the descriptive analyses below, a period of 3 months of EXBF vs no breastfeeding was used so as to have enough observations to represent the distribution of BMI with respect to EXBF, gender and the FTO genotypes.
Other variables
Several known confounding variables were added to our analysis, including mother’s pre-pregnancy BMI (MomBMI), gestational age (GA) (in months) and social, education and smoking status. The mother’s BMI was obtained from the ‘About Yourself’ questionnaire administered to the mother at 12 weeks of gestation and calculated from weight and height measures. The gestational age was recorded in a variety of ways using both last menstrual period, paediatric assessment, obstetric assessment and ultrasound assessment. The mother’s and father’s social status and mother’s education status were obtained from the ‘Your Pregnancy’ questionnaire administered to the mother at 32 weeks of gestation and coded in six categories: Professional occupations; Managerial and technical occupations; Skilled non-manual occupations; Skilled manual occupations; Partly skilled occupations; Non-skilled occupations. The mother’s education corresponds to the highest degree of education qualification and was coded as: Certificate of Secondary Education (CSE)/none; Vocational; O level; A level; Degree; Missing. The mother’s smoking status was obtained from the ‘Having a Baby’ questionnaire administered to the mother at 18 weeks of gestation and was coded as: Never; Yes during pregnancy; No during pregnancy. The distribution of these variables is given in Table 1. The variables social status, education level and smoking status were analysed as categorical variables and the variables gestational age and mother’s BMI were analysed as continuous variables. Since mother’s and father’s social status and mother’s education and smoking status were not found associated with the child’s longitudinal BMI, these variables were dropped from our analysis.
Table 1.
Summary statistics for subject-level variables and number of BMI measurements by age
| Subject-level variables |
Time-varying variable |
||||||
|---|---|---|---|---|---|---|---|
| Boys (N = 2849) |
Girls (N = 2741) |
# of BMI measurements by age |
|||||
| N | % | N | % | Age, years | Boys | Girls | |
| SNP Genotypes | |||||||
| TT | 1059 | 37.2 | 1011 | 36.9 | 0 | 2531 | 2461 |
| TA | 1360 | 47.7 | 1275 | 46.5 | 1 | 686 | 658 |
| AA | 430 | 15.1 | 455 | 16.6 | 2 | 628 | 604 |
| EXBF | 3 | 630 | 607 | ||||
| 0 | 1261 | 44.3 | 1101 | 40.2 | 4 | 398 | 383 |
| 1 | 195 | 6.8 | 237 | 8.7 | 5 | 2091 | 1967 |
| 2 | 381 | 13.4 | 315 | 11.5 | 6 | 514 | 470 |
| 3 | 682 | 23.9 | 719 | 26.2 | 7 | 2298 | 2184 |
| 4 | 287 | 10.1 | 304 | 11.1 | 8 | 1864 | 1833 |
| 5+ | 43 | 1.5 | 65 | 2.4 | 9 | 1669 | 1692 |
| Mother’s education | 10 | 2446 | 2412 | ||||
| CSE/none | 364 | 13.3 | 367 | 12.9 | 11 | 1904 | 1904 |
| Vocational | 232 | 8.5 | 259 | 9.1 | 12 | 1761 | 1843 |
| O level | 981 | 35.9 | 1045 | 36.8 | 13 | 1752 | 1710 |
| A level | 704 | 25.8 | 726 | 25.5 | 14 | 302 | 315 |
| Degree | 451 | 16.5 | 446 | 15.7 | 15 | 1377 | 1467 |
| Mother’s social status | 16 | 66 | 97 | ||||
| Foreman | 172 | 7.2 | 168 | 6.8 | |||
| Manager | 813 | 34.2 | 856 | 34.5 | |||
| Supervisor | 1015 | 42.7 | 1078 | 43.4 | |||
| Leading | 138 | 5.8 | 154 | 6.2 | |||
| Self-employed | 210 | 8.8 | 200 | 8.0 | |||
| None | 32 | 1.3 | 29 | 1.2 | |||
| Father’s social status | |||||||
| Foreman | 355 | 13.3 | 342 | 13.2 | |||
| Manager | 968 | 36.3 | 941 | 36.3 | |||
| Supervisor | 311 | 11.7 | 301 | 11.6 | |||
| Leading | 743 | 27.8 | 720 | 27.8 | |||
| Self-employed | 229 | 8.6 | 220 | 8.5 | |||
| None | 63 | 2.4 | 67 | 2.6 | |||
| Mother’s smoking status | |||||||
| Never | 1513 | 55.8 | 1536 | 54.5 | |||
| Yes during pregnancy | 510 | 18.8 | 528 | 18.7 | |||
| No during pregnancy | 687 | 25.4 | 754 | 26.7 | |||
| Continuous variables | Mean | SD | Mean | SD | |||
| EXBF by month > 0 | 2.75 | 0.99 | 2.79 | 1.05 | |||
| EXBF by month | 1.54 | 1.56 | 1.67 | 1.59 | |||
| Gestational age (in weeks) | 39.44 | 1.81 | 39.6 | 1.59 | |||
| Mother’s pre-pregnancy BMI | 22.95 | 3.79 | 22.89 | 3.78 | |||
Missing data
A total of 7325 Caucasian children with genotype data on the FTO gene were available for this study. Among those, 1100 children did not have breastfeeding information, 189 were missing BMI measurements and 446 did not have information on their mother’s BMI and social status, leaving a total of 5590 children (2849 boys and 2741 girls) for the analysis.
Statistical analysis
A mixed effects model approach (Supplementary material A.1, available as Supplementary data at IJE online) with cubic spline basis (Supplementary material A.2, available as Supplementary data at IJE online) was used to fit the longitudinal BMI data from the ALSPAC cohort from birth to 16 years of age in boys and girls separately.36 Let denote the longitudinal BMI measurements for the ith individual with ni measurements. A child i’s BMI at time j can be expressed in the linear mixed effects model framework as:
| (1) |
where is the cubic spline function of age (Supplementary material A.2) that is used to catch the nonlinear BMI growth curve, represents the variables of interest including MomBMI, GA, FTO and EXBF, and refers to the interaction terms of spline function of age and the variables of interest that measure time-varying effects of on BMI. The β’s are fixed effects and is a vector of random effects including random intercept and random slopes of age and age-quadratic term. Variable selection was used to select the best model (Supplementary material A.3 and A.4, available as Supplementary data at IJE online); and hypothesis testing of the FTO-by-time, EXBF-by-time and FTO/EXBF-by-time effects was performed by using the general linear hypothesis (GLH) approach (Supplementary material A.3). We calculated the predicted child-specific BMI trajectories by combining the estimated fixed effects, i.e. the population average shared by all individuals, with the child-specific predicted random effects up to age 15 years. In the next step, the age at AP and age at AR for each child were obtained by Newton-Raphson maximization method to the individual-specific BMI trajectory curve. BMI velocities (rate and direction of change in BMI) were calculated from birth to AP, from AP to AR, and from AR to 15 years of age (Supplementary material A.5, available as Supplementary data at IJE online). Finally, linear models were used to estimate the variation of AP, AR and BMI velocities with FTO and EXBF.
The statistical software R version 2.13.2 was used for the analyses where mixed models were fitted with the R library ‘nlme’ and linear model were analysed by ‘lm’ function.
Results
Observed BMI trajectories and the risk of being overweight
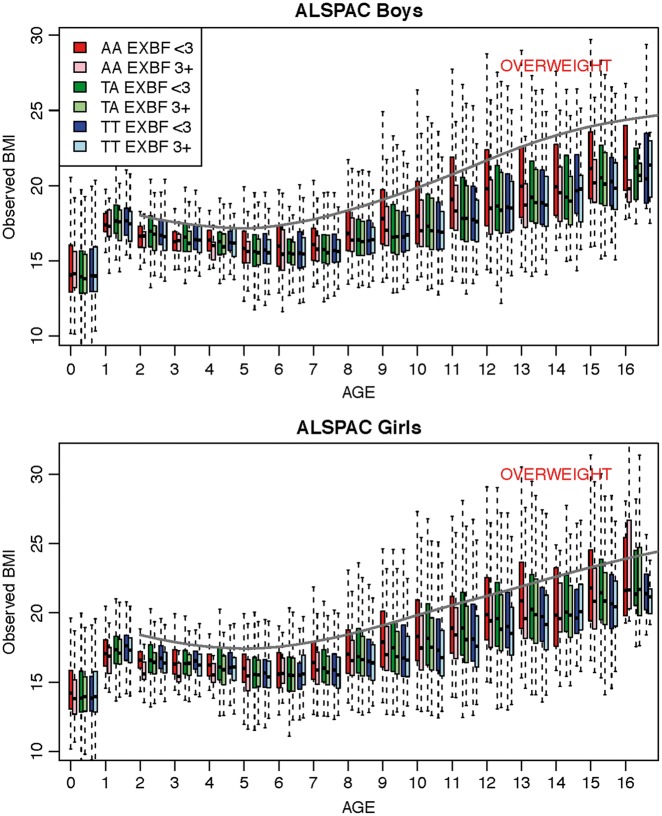
The observed BMI distribution at various child ages is depicted in Figure 1. We notice that among boys aged 9 years and older, only those with the AA risk genotype and with an EXBF of less than 3 months have an upper quartile BMI value crossing the overweight line. A duration of EXBF of 3 months or more will put their BMI trajectory below the overweight line for all genotype categories. The same pattern is observed among girls except that they cross the overweight line at an earlier age (i.e. 7 years for either the AA or AT SNP genotype). A duration of EXBF of 3 months or more will put their BMI trajectory below the overweight line for all genotype categories. The definition of overweight is based on the 85% quantile of BMI.37 At age 15, 25.4% of the boys and 31.4% of the girls with the rs9939609 AA genotype are overweight if they had less than 3 months EXBF, whereas these percentages drop to 14.1% and 22.7%, respectively, if they had 3 months or more EXBF (Supplementary material B, Table 4, available as Supplementary data at IJE online). We also compare the 85% BMI percentile values observed to the Centers for Disease Control and Prevention (CDC) overweight cutoff values (Supplementary material B, Table 5). Whereas the ALSPAC 85% BMI percentiles are much higher in the AA genotype group with less than 3 months of EXBF than the CDC overweight cutoff, the difference is much smaller when children had received 3 months or more of EXBF.
Figure 1.
Box plots of BMI measurements from birth to age 16 years for boys and girls (where age was rounded to the closest integer). Interquartiles range (IQR) is represented by a box and the extreme values (+/- 1.5 IQR) by the whiskers. The three FTO genotypes AA, TA and TT and two EXBF categories (< 3 months and ≥ 3 months) are ordered at each age as indicated in the top legend (from left to right, first box is for AA EXBF <3, second box for AA EXBF 3+, etc…). The grey plain line in each plot corresponds to the overweight age-specific cutoff value as published from the Centers for Disease Control and Prevention (CDC)37.
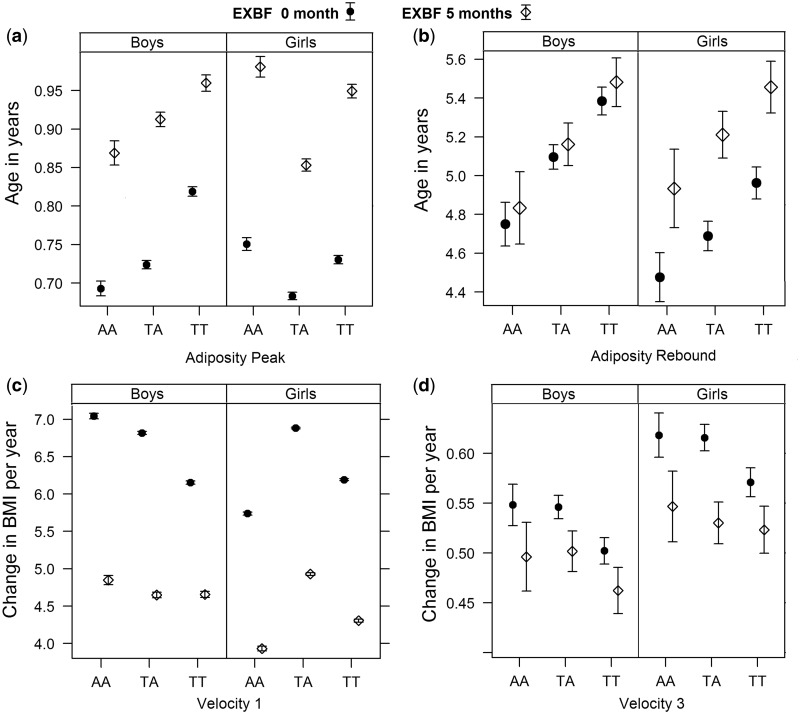
Predicted effect of the FTO SNP rs9939609 on BMI trajectories
Figure 2 and Supplementary Table 6 (available as Supplementary data at IJE online) show the estimated age at AP, age at AR and the BMI velocities before AP (velocity 1) and after AR (velocity 3) with respect to the SNP genotypes and EXBF. The overall effect of the SNP A allele is to decrease the time to AP from 0.05 year [95% confidence interval (CI) 0.04–0.06; P < 0.0001] to 0.13 year (95% CI 0.11–0.14; P < 0.0001) except in girls with AA genotype (Figure 2a), and this effect depends on whether children carry one or two copies of the A allele and on their gender. It shortens the time to AR from 0.27 year (95% CI 0.16–0.39; P < 0.0001) to 0.63 year (95% CI 0.50–0.77; P < 0.0001) (Figure 2b). Further, SNP A allele increases substantially the BMI velocity before AP from 0.66 (95% CI 0.63–0.70; P < 0.0001) to 0.89 (95% CI 0.85–0.94; P < 0.0001), except in girls AA (Figure 2c); and it increases the velocity after AR from 0.04 (95% CI 0.03–0.06; P < 0.0001) to 0.05 (95% CI 0.02–0.07; P = 0.0005) (Figure 2d). In addition, the SNP A allele reduces the duration between AP and AR and decreases the BMI velocity between AP and AR (velocity 2) (Supplementary Table 6).
Figure 2.
Effect of the three FTO genotypes and 5 months of EXBF on age at adiposity peak (AP) and adiposity rebound (AR), and on BMI velocity before AP (velocity 1) and BMI velocity after AR (velocity 3).
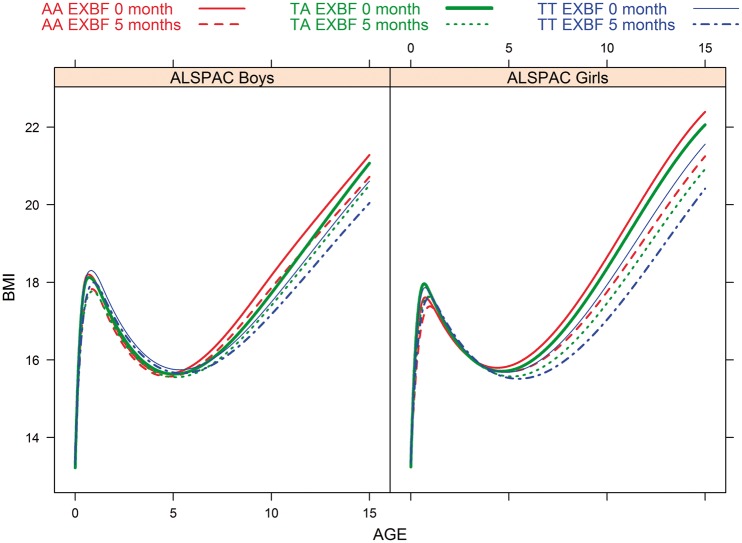
The overall BMI growth curves estimated from model (1) from birth to age 16 years by SNP genotypes, duration of EXBF (0 or 5 months) and gender are displayed in Figure 3. The long-term BMI patterns show that the genotype-specific curves separate after 6 years of age (i.e. after AR) in boys and girls, with a difference that increases slightly over time. The longitudinal profiles confirm that the AA genotype predisposes to higher BMI during childhood whereas AT children have an intermediate trajectory (i.e. between AA and TT genotype profiles).
Figure 3.
Estimated BMI trajectory from birth to 16 years of age by FTO genotype and duration of EXBF.
The effect size of rs9939609 SNP on the overall BMI trajectories among boys and girls is summarized in Supplementary Table 7 (available as Supplementary data at IJE online). At age 15 years, carrying one copy of the A allele increases BMI by 0.46 (95% CI 0.16–0.77; P = 0.003) in boys and 0.50 in girls (95% CI 0.17–0.83; P = 0.003). Carrying two copies of the A alleles increases BMI by 0.67 (95% CI 0.25–1.10; P = 0.003) and 0.83 (95% CI 0.39–1.28; P = 0.002) in boys and girls, respectively. The increase in BMI given by the AA genotype becomes substantial from 6 and 7 years of age and up to 15 years in girls and boys, whereas carrying only one copy of the A allele has a strong effect on BMI starting at 7 and 10 years of age in girls and boys, respectively.
Role of early life growth development on BMI variability at age 15 years
Table 2 shows that a large part of the BMI variability at 15 years can be explained by the age at AR (83% and 73% in boys and girls, respectively) and by the BMI velocity after AR (i.e. velocity 3) (81% and 67% in boys and girls, respectively). Age at AR remains the most important independent predictor of BMI at age 15 even after adjustment for age at AP and the BMI velocities. Supplementary Table 8 (available as Supplementary data at IJE online) depicts the change in BMI associated with the FTO SNP genotypes after adjusting for age at AR. It clearly shows substantial decrease in the FTO SNP effect size when the model is adjusted for age at AR, and confirms that age at AR is a major determinant of BMI in adolescence.
Table 2.
Adjusted R-squares for predicted BMI at age 15 years (linear regression models adjusted for gestational age, mother’s BMI, FTO genotypes and duration of exclusive breastfeeding)
| Boys | Girls | |
|---|---|---|
| Age at AP | 0.66 | 0.44 |
| Age at AR | 0.83 | 0.73 |
| Age at AP-AR | 0.82 | 0.72 |
| Velocity 1 | 0.52 | 0.29 |
| Velocity 2 | 0.66 | 0.52 |
| Velocity 3 | 0.81 | 0.67 |
Protective effect of EXBF on FTO-induced early life growth development and BMI growth trajectories
EXBF exerts its effect in the early years of child development, as early as the periods around age at AP and at AR (Figure 2 and Supplementary Table 6). EXBF compensates completely the decrease in age at AP due to the SNP A allele among boys and girls and age at AR among girls only (Figure 2a and 2b). The effect is such that children with the AT and AA genotypes receiving 5 months of EXBF have ages at AP and AR (for girls only) much higher than children with the TT genotype who did not receive any EXBF. A longer duration of EXBF also has substantial impact on decreasing BMI velocity before AP and after AR, and offsets largely the effect of the FTO SNP A allele (Figure 2c, d). EXBF also decreases the velocity between AP and AR and the duration between these two time points (Supplementary Table 6). Noteworthy, the interaction between EXBF and the FTO SNP genotype is associated with age at AP (P < 0.0001) and BMI velocity before AP (P < 0.0001) in boys and girls (Supplementary Table 6).
The long-term effect of EXBF and the FTO SNP genotypes on BMI growth is depicted in Figure 3. Among boys and girls carrying one or two copies of the SNP A allele, 5 months of EXBF will line up their longitudinal BMI trajectories to those with the TT genotype who did not receive any EXBF. However, the EXBF and FTO SNP genotype interaction was not detected on the overall longitudinal BMI. The effect of 5 months of EXBF on BMI reduction for the three SNP genotype categories is given in Supplementary Table 7 (columns 4 and 8). A substantial reduction in BMI starts as early as 6 years of age in girls (0.28 kg/m2, 95% CI 0.04–0.52; P = 0.024) and 10 years of age in boys (0.32 kg/m2, 95% CI 0.02–0.62; P = 0.037). EXBF acts antagonistically to the rs9939609 SNP A allele and compensates almost completely the A allele effect among boys and girls. By the age of 15, the predicted reduction in BMI after 5 months of EXBF is 0.56 kg/m2 (95% CI 0.11–1.01; P = 0.003) and 1.14 kg/m2 (95% CI 0.67–1.62; P < 0.0001) in boys and girls, respectively.
Discussion
Principal findings
Our study gives further insights into the role of EXBF in children’s early life growth development. Our main result shows that a long duration of EXBF (i.e. at least 5 months) has substantial preventive effect on BMI growth trajectories among a genetically susceptible group of children carrying an adverse genetic variant in the FTO gene. EXBF exerts its effect even on very early growth development such as ages at AP and AR, as well as on the BMI velocities in infancy and childhood. This result suggests that early interventions that target a longer duration of breastfeeding (i.e. at least 5 months) might delay the timing of AP and AR and hence prevent the risk of overweight/obesity later in life, in particular in a group of children with high genetic predisposition.
Comparisons with other studies
There is now growing evidence demonstrating the benefits of breastfeeding on early life growth development. Several studies showed that infants fully breastfed (i.e. breastfed for at least 4 months) gained less weight compared with mixed- or formula-fed children38,39 or with non-fully breastfed infants40–42 during the first 12 months of life, but grow equally in length.38 In these studies, the protective effect of breastfeeding on the risk of becoming overweight is explained by a decrease in weight velocity in early infancy, which could be one of the first steps driving the pathway to obesity development. A study from Chivers et al.28 from the RAINE study also reported that stopping breastfeeding at less than 4 months was associated with earlier age at AR and higher BMI at this age, and that the beneficial role of breastfeeding on BMI was maintained up to the age of 14 years. More recently, it was also reported in the RAINE cohort that a longer duration of exclusive breastfeeding (EXBF) could attenuate the increase in BMI among carriers of the risk allele of the FTO SNP rs9939609.30
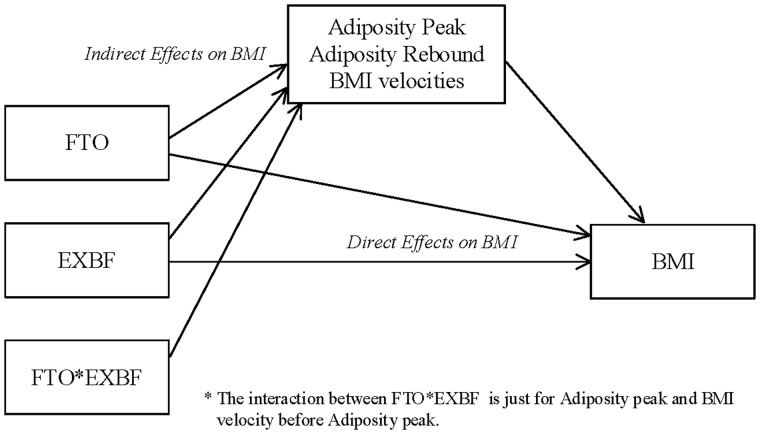
Our present study confirms these previous findings from the RAINE study and extends beyond. By investigating BMI trajectories among a group of children carrying a genetic variant in the FTO gene, which is known to predispose to overweight/obesity in childhood and adulthood, our study gives some insights into the mechanisms underlying the preventive role of EXBF. In a very large cohort of 5590 children from the ALSPAC study, we show that the critical role of EXBF could be to modulate the timing of AP and AR and the BMI velocities in infancy and childhood, which in turn are major determinants of overweight and obesity in adolescence and adulthood. Children carrying of the FTO SNP A allele have an earlier age at AP, earlier age at AR and higher BMI velocities in the few months after birth, but 5 months of EXBF offset completely the SNP adverse effect (Figure 2). The long-term effect of EXBF on BMI trajectories is more substantial in girls than boys, which could reflect a stronger effect of EXBF on the age at AR and on the BMI velocity after AR in girls. Finally, we found a gene by environment interaction between EXBF and the FTO SNP determining the age at AP and BMI velocity before AP, but not the BMI trajectories. These results demonstrate the complex interplay between the FTO SNP, EXBF and BMI during child development. This complex association could be mediated by the SNP and EXBF effects on markers of development, such as the age at AP, the age at AR and the BMI velocities in early life, which are all strong predictors of BMI and overweight/obesity in adolescence and adulthood (Figure 4). The role of EXBF could therefore be to regulate important markers of early child development and attenuate the effect of abnormal conditions such as adverse genetic factors on the risk of overweight/obesity later in life.
Figure 4.
Mediation plot explaining the possible direct and indirect relationships of EXBF and FTO on age at AP and AR, BMI velocities and longitudinal BMI.
Potential mechanisms
The biological role of breastfeeding remains elusive, but there is translational evidence that links milk signalling with FTO-activated transcription of the milk recipient. There is currently indirect evidence supporting the view that hypomethylation of specific cytosine phosphate guanine (CpG) sites of the FTO gene enhance FTO expression. As pointed out by Mlenik et al.,43 milk could also alter DNA methylation and function like an ‘epigenetic transfection system’ via transfer of milk-derived exosomes. More particularly, milk could act via miRNA-29b/miRNA-21/DNMT signalling that promotes CpG demethylation at intron 1 of FTO resulting in increased expression of FTO, which functions as a critical amplifier of the transcriptional machinery for postnatal growth.43 Further evidence is needed to confirm this hypothesis.
Study strengths and limitations
Further studies are needed to better understand how genes and environment regulate the different epochs of a child’s development. Our study focuses specifically on the role of EXBF, but it is likely that other factors such as physical activity and nutrition also impact on BMI trajectories in children. Besides, many other genetic variants have also been associated with BMI and/or obesity in large GWAS and would be worth considering in a similar framework, for example using a more general genetic risk score. A better assessment of the impact of the duration of EXBF as well as exclusivity of breastfeeding vs mixed bottle/breastfeeding is also needed. On the other hand, our study provides robust evidence for the role of EXBF in children carrying an adverse allele in the FTO gene, in a large cohort of 5590 children. These results on the role of EXBF in FTO-induced BMI trajectories replicate our previous findings from the RAINE study,30 but to our knowledge its mediation effect on early life development events has never been shown before.
Conclusions and policy implications
This study shows that the critical role of exclusive breastfeeding could be to modulate the timing of the adiposity peak, the adiposity rebound and the BMI velocities in infancy and childhood, which in turn are major determinants of overweight and obesity in adolescence and adulthood. A major issue when defining prevention programsme against the risk of overweight/obesity is to know when to intervene. This study suggests that early intervention through the promotion of exclusive breastfeeding for at least 5 months could have major influence on child BMI growth trajectories, by modulating markers of early life development.
Supplementary Data
Supplementary data are available at IJE online.
Funding
This work was supported by the ALVA foundation, Toronto, and by the Canadian Institute for Health Research (CIHR) training grant in Genetic Epidemiology and Statistical Genetics (STAGE fellowship GET-101831).
Key Messages
A longer duration of exclusive breastfeeding (i.e. at least 5 months) has been shown to attenuate the increase in BMI among carriers of the risk allele of the FTO SNP rs9939609, but the mechanisms underlying this association are not well known.
Our study shows that the critical role of exclusive breastfeeding could be to modulate the timing of the adiposity peak, the adiposity rebound and the BMI velocities in infancy and childhood, which in turn are major determinants of overweight and obesity in adolescence and adulthood.
A major issue when defining prevention programmes against the risk of overweight/obesity is to know when to intervene. This study suggests that early intervention through the promotion of exclusive breastfeeding for at least 5 months could have major influence on child BMI growth trajectories, by modulating markers of early life development.
Supplementary Material
Acknowledgements
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. The UK Medical Research Council and the Wellcome Trust (grant ref: 102215/2/13/2) and the University of Bristol provide core support for ALSPAC.
Conflict of interest: None declared.
References
- 1. World Health Organization. Obesity and overweight fact sheet, June 2016 http://www.who.int/mediacentre/factsheets/fs311/en/ [4 September 2016, date last accessed].
- 2. de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr 2010;92:1257–64. [DOI] [PubMed] [Google Scholar]
- 3. Whitlock EP, Williams SB, Gold R. et al. Screening and interventions for childhood overweight: a summary of evidence for the US Preventive Services Task Force. Pediatrics 2005;116:e125–44. [DOI] [PubMed] [Google Scholar]
- 4. Whitaker RC, Wright JA, Pepe MS. et al. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med 1997;337:869–73. [DOI] [PubMed] [Google Scholar]
- 5. Sachdev HS, Fall CH, Osmond C. et al. Anthropometric indicators of body composition in young adults: relation to size at birth and serial measurements of body mass index in childhood in the New Delhi birth cohort. Am J Clin Nutr 2005;8:456–66. [DOI] [PubMed] [Google Scholar]
- 6. Speliotes EK, Willer CJ, Berndt SI. et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 2010;4):937–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frayling TM, Timpson NJ, Weedon MN. et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007;316:889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hinney A, Nguyen TT, Scherag A. et al. Genome wide association (GWA) study for early onset extreme obesity supports the role of fat mass and obesity associated gene (FTO) variants. PLoS One 2007;2:e1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cecil JE, Tavendale R, Watt P. et al. An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med 2008;359:2558–66. [DOI] [PubMed] [Google Scholar]
- 10. Dina C, Meyre D, Gallina S. et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet 2007;39:724–26. [DOI] [PubMed] [Google Scholar]
- 11. Warrington NM, Howe LD, Wu YY. et al. Association of a body mass index genetic risk score with growth throughout childhood and adolescence. PLoS One 2013;8:e79547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gulati P, Yeo GS. The biology of FTO: from nucleic acid demethylase to amino acid sensor. Diabetologia 2013;56:2113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao X, Yang Y, Sun BF. et al. FTO and obesity: mechanisms of association. Curr Diab Rep 2014;14:486. [DOI] [PubMed] [Google Scholar]
- 14. Livingstone KM, Celis-Morales C, Lara J. et al. Associations between FTO genotype and total energy and macronutrient intake in adults: a systematic review and meta-analysis. Obes Rev 2015;16:666–78. [DOI] [PubMed] [Google Scholar]
- 15. Claussnitzer M, Dankel SN, Kim KH. et al. FTO Obesity variant circuitry and adipocyte browning in humans. N Engl J Med 2015;373:895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andreasen CH, Stender-Petersen KL, Mogensen MS. et al. Low physical activity accentuates the effect of the FTO rs9939609 polymorphism on body fat accumulation. Diabetes 2008;57:95–101. [DOI] [PubMed] [Google Scholar]
- 17. Li S, Zhao JH, Luan J. et al. Physical activity attenuates the genetic predisposition to obesity in 20,000 men and women from EPIC-Norfolk prospective population study. PLoS Med 2010;7:1000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lappalainen T, Lindstrom J, Paananen J. et al. Association of the fat mass and obesity-associated (FTO) gene variant (rs9939609) with dietary intake in the Finnish Diabetes Prevention Study. Br J Nutr 2012;108:1859–65. [DOI] [PubMed] [Google Scholar]
- 19. Schum J, Blumenstock G, Weber K. et al. Variants of the FTO gene in obese children and their impact on body composition and metabolism before and after lifestyle intervention. Exp Clin Endocrinol Diabetes 2012;120:128–31. [DOI] [PubMed] [Google Scholar]
- 20. Zhang X, Qi Q, Zhang C. et al. FTO genotype and 2-year change in body composition and fat distribution in response to weight-loss diets: the POUNDS LOST Trial. Diabetes 2012;61:3005–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park SL, Cheng I, Pendergrass SA. et al. Association of the FTO obesity risk variant rs8050136 with percentage of energy intake from fat in multiple racial/ethnic populations: the PAGE study. Am J Epidemiol 2013;178:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lappalainen TJ, Tolppanen AM, Kolehmainen M. et al. The common variant in the FTO gene did not modify the effect of lifestyle changes on body weight: the Finnish Diabetes Prevention Study. Obesity (Silver Spring) 2009;17:832–36. [DOI] [PubMed] [Google Scholar]
- 23. Ahmad T, Lee IM, Pare G. et al. Lifestyle interaction with fat mass and obesity-associated (FTO) genotype and risk of obesity in apparently healthy U.S. women. Diabetes Care 2011;34:675–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moleres A, Rendo-Urteaga T, Zulet MA. et al. Obesity susceptibility loci on body mass index and weight loss in Spanish adolescents after a lifestyle intervention. J Pediatr 2012;161:466–70 e2. [DOI] [PubMed] [Google Scholar]
- 25. Gillman MW, Rifas-Shiman SL, Camargo CA Jr. et al. Risk of overweight among adolescents who were breastfed as infants. JAMA 2001;285:2461–67. [DOI] [PubMed] [Google Scholar]
- 26. Hediger ML, Overpeck MD, Kuczmarski RJ. et al. Association between infant breastfeeding and overweight in young children. JAMA 2001;285:2453–60. [DOI] [PubMed] [Google Scholar]
- 27. Harder T, Bergmann R, Kallischnigg G. et al. Duration of breastfeeding and risk of overweight: a meta-analysis. Am J Epidemiol 2005;162:397–403. [DOI] [PubMed] [Google Scholar]
- 28. Chivers P, Hands B, Parker H. et al. Body mass index, adiposity rebound and early feeding in a longitudinal cohort (Raine Study). Int J Obes (Lond) 2010;34:1169–76. [DOI] [PubMed] [Google Scholar]
- 29. Dedoussis GV, Yannakoulia M, Timpson NJ. et al. Does a short breastfeeding period protect from FTO-induced adiposity in children? Int J Pediatr Obes 2011;6:e326–35. [DOI] [PubMed] [Google Scholar]
- 30. Abarin T, Yan Wu Y, Warrington N. et al. The impact of breastfeeding on FTO-related BMI growth trajectories: an application to the Raine pregnancy cohort study. Int J Epidemiol 2012;41:1650–60. [DOI] [PubMed] [Google Scholar]
- 31. Boyd A, Golding J, Macleod J. et al. Cohort Profile: The ‘children of the 90s’–the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 2013;42:111–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fraser A, Macdonald-Wallis C, Tilling K. et al. Cohort Profile: The Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol 2013;42:97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.University of Bristol, 2016. Avon Longitudinal Study of Parents and Children. http://www.bristol.ac.uk/alspac/researchers/access/ [4 September 2016, date last accessed].
- 34. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000;320:1240–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding (Review). Cochrane Database Syst Rev 2012;8:CD003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York, NY; Springer, 2009. [Google Scholar]
- 37. Centers for Disease Control and Prevention. Body Mass Index: BMI for Children and Teens, May 2015. www.cdc.gov [21 March 2017, date last accessed].
- 38. Rzehak P, Sausenthaler S, Koletzko S. et al. Period-specific growth, overweight and modification by breastfeeding in the GINI and LISA birth cohorts up to age 6 years. Eur J Epidemiol 2009;24:449–67. [DOI] [PubMed] [Google Scholar]
- 39. Gubbels JS, Thijs C, Stafleu A. et al. Association of breast-feeding and feeding on demand with child weight status up to 4 years. Int J Pediatr Obes 2011;6:e515–22. [DOI] [PubMed] [Google Scholar]
- 40. Kalies H, Heinrich J, Borte N. et al. The effect of breastfeeding on weight gain in infants: results of a birth cohort study. Eur J Med Res 2005;10:36–42. [PubMed] [Google Scholar]
- 41. Gunnarsdottir I, Schack-Nielsen L, Michaelsen KF. et al. Infant weight gain, duration of exclusive breast-feeding and childhood BMI – two similar follow-up cohorts. Public Health Nutr 2010;13:201–07. [DOI] [PubMed] [Google Scholar]
- 42. de Hoog ML, van Eijsden M, Stronks K. et al. The role of infant feeding practices in the explanation for ethnic differences in infant growth: the Amsterdam Born Children and their Development study. Br J Nutr 2011; 106:1592–601. [DOI] [PubMed] [Google Scholar]
- 43. Melnik BC. Milk: an epigenetic amplifier of FTO-mediated transcription? Implications for Western diseases. J Transl Med 2015;13:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.