Abstract
Background: Autosomal-dominant polycystic kidney disease (ADPKD) is characterized by multitudes of expanding renal cysts associated with mononuclear interstitial infiltrates. Monocyte chemotactic protein-1 is produced in the kidneys and excreted in the urine (uMCP1) of these patients in increased amounts. In the TEMPO 3:4 trial, tolvaptan slowed the rate of increase in total kidney volume (TKV) and the rate of decline in estimated glomerular filtration rate (eGFR). In a sub-analysis, we determined whether tolvaptan administration for up to 3 years changed the urinary excretion of MCP-1 referenced to creatinine in 869 treated subjects compared with 438 placebo subjects.
Methods: Treatment group differences of uMCP1 at 0.75, 12, 24 and 36 months were evaluated by ANCOVA with factor of treatment and covariate baseline.
Results: At baseline, mean uMCP1 was 429 ± 224 pg/mg in the tolvaptan and 434 ± 233 pg/mg in the placebo groups, ∼4-fold greater than normal. Log uMCP1 associated positively with log TKV (r = 0.2645, P < 0.0001) and negatively with eGFR (r = −0.1555 P < 0.0001) and fasting urine osmolality (r = −0.1933, P < 0.0001). Tolvaptan reduced uMCP1 13.8 ± 4.4% (P < 0.0001) below placebo-treated subjects at 24 months and 14.4 ± 3.7% (P < 0.0001) at 36 months, and to the same extent in females and males. The effect of tolvaptan on uMCP1 excretion at 36 months extended across CKD Stage 1 (11.1 ± 6.4%, P = 0.0595), CKD 2 (13.9 ± 5.4%, P = 0.0050) and CKD 3 (21.4 ± 8.0%, P = 0.0020).
Conclusion: Tolvaptan, administered for 3 years to patients with ADPKD, caused a sustained reduction in the urinary excretion of MCP-1 relative to placebo.
Keywords: chemokine, chronic renal disease, disease progression, interstitial inflammation, renal biomarker
INTRODUCTION
Autosomal-dominant polycystic kidney disease (ADPKD) is a slowly progressive, irreversible disorder that leads to end-stage renal disease (ESRD) in the sixth decade in 50% of those carrying a mutated PKD1 gene [1]. Unrelenting cyst formation and growth causes local, direct damage to adjacent interstitial blood and lymphatic vessels and tubule obstruction to urine flow in association with interstitial inflammation and fibrosis, and declining renal function [2]. Hypertension, hematuria and renal pain signal renal parenchymal injury decades before changes in the glomerular filtration rate can be clinically detected.
A host of experimental evidence obtained in animals with various renal cystic disorders indicates that physiologically normal levels of arginine vasopressin (AVP) have a major role in causing cysts to form and to progressively enlarge [3–5]. In addition, acquired factors, e.g. increased dietary protein, potassium depletion, acidosis and interstitial inflammation, have the capacity to accelerate disease progression to variable degrees [6]. Damage to the parenchyma caused by cysts promotes the infiltration of macrophages that can be found in increased numbers about cysts and in non-cystic areas to a variable extent. Methylprednisolone, a broad spectrum anti-inflammatory steroid, ameliorates inflammation and preserves renal function in animal models of PKD [7]. Monocyte chemotactic protein-1 (MCP-1, CCL2) is expressed within most cystic kidneys; urinary excretion of MCP-1 is increased and associated with total kidney volume (TKV) in patients with ADPKD [8–11].
This chemokine is also elevated in the urine of patients with diabetic nephropathy, renal transplantation, glomerulonephritis and obstructive uropathy, all conditions noted for chronic renal injury, inflammation and fibrosis [12–15]. It is synthesized by macrophages and other types of cells including renal tubule epithelia [16, 17]. Some of the MCP-1 released in inflamed internal organs is excreted in urine. MCP-1 is a monomeric polypeptide with a molecular weight of ∼13 kDa; thus, MCP-1 synthesized in organs other than kidney can enter the blood to be filtered by glomeruli and possibly secreted into the urine by tubules [8, 9].
MCP-1 is also produced by renal cysts [8–11, 18] and can rise to extraordinarily high concentrations within cysts lacking tubule connections [8, 19]. MCP-1 entering the renal interstitium attracts macrophages that promote cyst growth, inflammation and fibrosis, all of which appear to contribute to the progressive reduction of GFR in end-stage cystic kidneys [20, 21]. Although urinary MCP-1 (uMCP1) is not a unique biomarker of ADPKD, the excretion of this chemokine may signify cyst injury to renal parenchyma beginning relatively early in the course of the disease [9].
AVP stimulates cyst formation and expansion in ADPKD, and accelerates progression to ESRD [3, 22, 23]. In a recent study the administration of tolvaptan, a specific vasopressin V2 receptor inhibitor, to ADPKD patients for up to 3 years (TEMPO 3:4 trial) slowed kidney enlargement and reduced the rate of GFR decline [24]. In this post hoc analysis of the TEMPO 3:4 study, we describe the effect of tolvaptan on the urinary excretion of MCP-1.
MATERIALS AND METHODS
Patients and design
TEMPO 3:4 was a prospective, blinded, randomized, controlled trial in adult patients with ADPKD, and TKV ≥750 mL and creatinine clearance ≥60 mL/min as estimated from serum creatinine (Cockcroft–Gault equation) [24]. MCP-1 was determined in the urine of 1307 subjects at baseline, the cohort for this evaluation. At baseline, overnight fasting, second-void (spot) urine samples (∼10–12 h of dehydration) were collected to determine the extent to which urine osmolality (Uosm, mOsm/kg H2O) was concentrated. Following baseline, spot urine samples prior to morning dosing for Week 3 and Months 12, 24 and 36 visits were obtained.
At the beginning of the study, all subjects were advised to drink enough fluid to prevent thirst. MCP-1, creatinine and osmolality were determined in bio-banked urine samples; MCP-1 was measured by ELISA (Quantikine MCP-1/CCL2 Immunoassay; Covance Central Laboratory Services, Minneapolis, MN) and referenced to urine creatinine (pg/mg creatinine) [8]. The intra-assay coefficient of variation ranged between 3.3 and 4.1%. The MCP-1 detection limit in urine was 70 pg/mL and was exceeded in all of the baseline samples. The estimated GFR (eGFR) was computed from IDMS-traceable, enzymatically measured serum creatinine (to three significant digits) using the CKD-EPI equation. Osmolality was determined from freezing point depression (Advanced Instruments Osmometer Model 2020). TKV and eGFR were determined as described previously. The Institutional Review Boards at each performance site approved the protocol and written, informed consent was obtained for all participants.
Statistical analysis
Baseline
Variables distributing normally were expressed as mean ± standard deviation; non-normal distributions were expressed with mean as anti-logarithm of mean of log-transformed data and standard deviation derived using the delta method on the log-transformed data, or medians and interquartile range. The significance of change from baseline was obtained by Wilcoxon Signed Ranked Test. The trend tests were performed by ANOVA.
Treatment effect
Differences in uMCP1 between tolvaptan and placebo treatments were derived from ANCOVA with factor of treatment and covariate baseline. Change from baseline at each time point was evaluated with the Wilcoxon Ranked Sign Test. Because subjects were lost from the study, sensitivity analysis was performed on a subgroup, all of whom had determinations of uMCP1 at baseline and 36 weeks. Following the baseline period, urine samples with MCP-1 concentrations <70 pg/mL were deleted from the analysis. In a sensitivity analysis 70 pg/mL was imputed to the deleted MCP-1 samples and the analysis repeated. Analyses were performed with SAS 9.3; the null hypothesis was rejected at P < 0.05.
RESULTS
General
TEMPO 3:4 is the largest prospective study of adult ADPKD patients in which uMCP1 has been evaluated as a biomarker of disease progression. Of 2122 screened, 1445 participants were randomly assigned to either tolvaptan (n = 961) or placebo (n = 484). For those with a measurement at the enrollment visit (n = 1307), uMCP1 was asymmetrically distributed (Figure 1). In order to achieve normality, individual uMCP1 data were log-transformed. The transformed mean of uMCP1 that adjusts the skewness of its distribution was well matched with the median (Table 1). The transformed mean and median values were also well matched between tolvaptan and placebo. Baseline gender, age, race, TKV, Uosm, serum creatinine concentration, eGFR, uMCP1, clinical signs, symptoms and medication use were closely matched between tolvaptan and placebo groups as shown in Table 2 and in the TEMPO 3:4 publication [24].
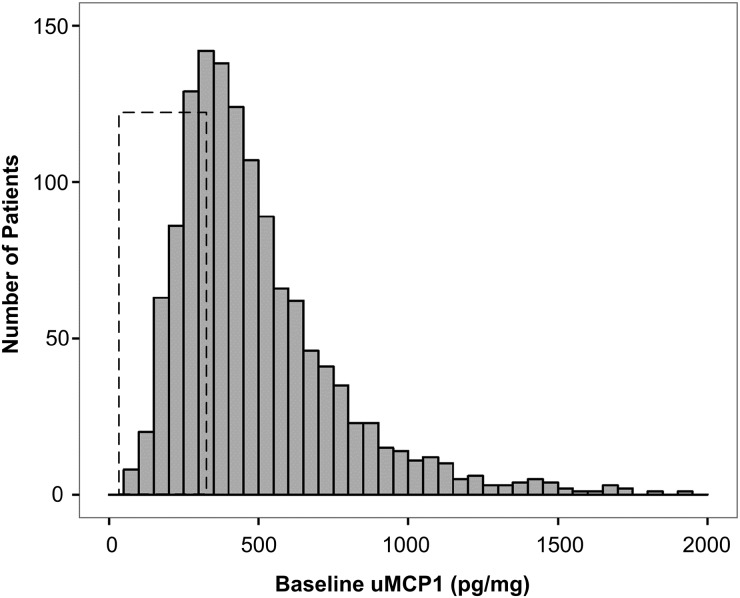
FIGURE 1.
Histogram of uMCP1 at baseline. Box shows range of uMCP1 values in 121 healthy controls 13 to 50.8 years old (range 35–262 pg/mg; mean uMCP1 97.8 ± 37.6 pg/mg) [8, 9, 12, 25, 26].
Table 1.
Baseline mean uMCP1 for tolvaptan and placebo treatment groups
| Treatment | N | Mean (SD) (pg/mg) | Meana (SD) (pg/mg) | Median (pg/mg) | IQR (pg/ mg) | Min (pg/ mg) | Max (pg/ mg) |
|---|---|---|---|---|---|---|---|
| Tolvaptan | 869 | 493 (288) | 429 (224) | 425 | 290 | 76 | 2576 |
| Placebo | 438 | 500 (281) | 434 (233) | 430 | 296 | 67 | 1829 |
aThe means were obtained as an anti-logarithm of the mean of log-transformed uMCP1 and the SD was determined by the delta method. SD, standard deviation; IQR, interquartile range.
Table 2.
Baseline descriptors and initial values for entire cohorta
| Characteristic | Tolvaptan (n = 869) | Placebo (n = 438) |
|---|---|---|
| Male, % | 53 | 53 |
| Age, years (mean, SD) | 38.3 (7.1) | 38.5 (7.2) |
| Race, % | ||
| White | 84 | 85 |
| Asian | 13 | 12 |
| Other | 3 | 3 |
| TKV, mL (median/IQR) | 1480 (969) | 1474 (943) |
| Uosm, mOsm/kg (mean/SD) | 508 (170) | 520 (175) |
| Serum creatinine, mg/dL (mean/SD) | 1.05 (0.30) | 1.05 (0.32) |
| eGFR, mL/min/1.73 m2 (mean/SD) | 81.6 (21.2) | 82.3 (23.2) |
aAdditional information can be found in reference [24]. SD, standard deviation; IQR, interquartile range.
Baseline features
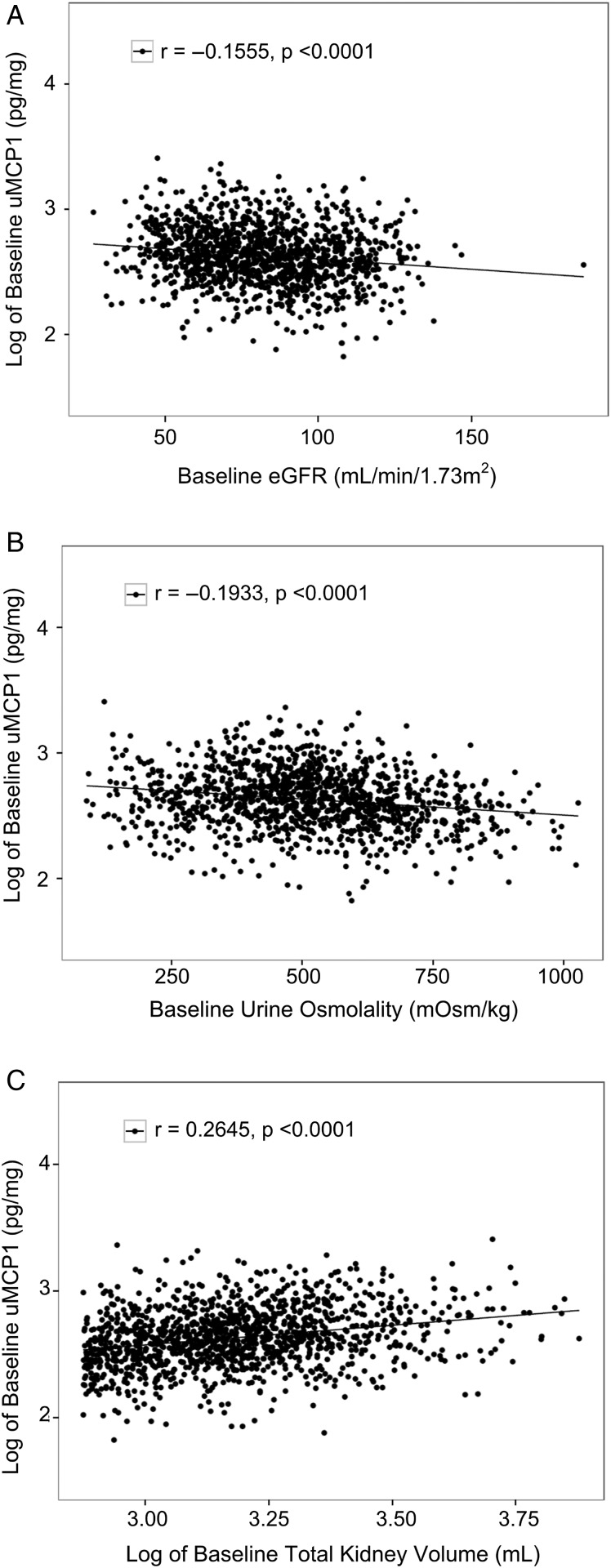
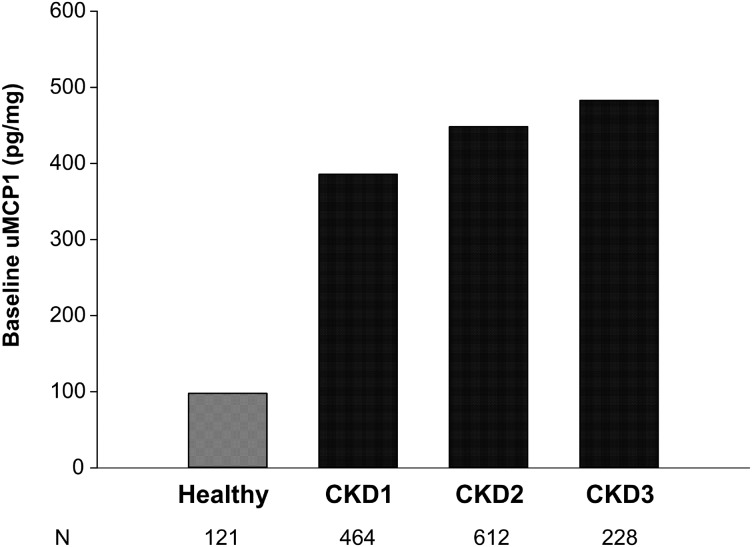
uMCP1 measured in healthy humans ranges from 36 to 262 pg/mg [8, 9, 12, 25, 26]; baseline values for the majority of the TEMPO 3:4 subjects were above the normal range (Table 1, Figure 1). Baseline uMCP1 was higher in females (493 ± 253 pg/mg) than in males (382 ± 195 pg/mg, P < 0.0001) as has been noted previously [8]. Mean baseline uMCP1 was higher in 658 subjects with a history of renal pain at baseline compared with 649 subjects without a history of renal pain (514 pg/mg ± 296 versus 476 pg/mg ± 273, respectively; P = 0.02). Baseline uMCP1 excretion associated negatively with Uosm (r = −0.1933; n = 1286; P < 0.0001) and eGFR (r = −0.1555; n = 1260; P < 0.0001) and positively with log TKV (r = 0.2645; n = 1307; P < 0.0001) (Figure 2). It is widely believed that the level of renal function estimated by eGFR reflects the severity of disease progression. We examined uMCP1 excretion in relation to the KDOQI/NKF/CKD stage of eGFR at baseline (Figure 3). uMCP1 appeared to be ∼4-fold greater in CKD Stage 1 than in the average of mean values gathered from healthy controls.
FIGURE 2.
Correlations of log baseline uMCP1 with markers of disease severity: (A) baseline eGFR; (B) baseline fasting Uosm; (C) log baseline TKV.
FIGURE 3.
Renal function status and uMCP1 at baseline. The healthy value is the mean from healthy persons in several published reports (see Figure 1 for details). CKD means were calculated with the delta method using anti-logarithm as the function. Excretion of uMCP1 in healthy subjects appeared lower than in ADPKD subjects across CKD Stages 1–3. Analysis by ANOVA revealed a trend for baseline uMCP1 to increase with increasing CKD stage (P < 0.0001).
Effect of tolvaptan on uMCP1 excretion
During the 3-week adjustment of tolvaptan dosage, uMCP1 excretion tended to rise in those receiving drug, but did not change in the placebo group (Figure 4). After 12 months of treatment, uMCP1 excretion fell below the baseline in the tolvaptan-treated subjects and remained similarly reduced for the remainder of the study. In placebo subjects, uMCP1 excretion decreased from baseline at 12 months but was elevated at 24 and 36 months. The effect of tolvaptan on uMCP1 excretion was in keeping with its effect to reduce the rate of increase in TKV and slow the rate of decline of eGFR reported previously [24] (Supplementary data, Figure S1).
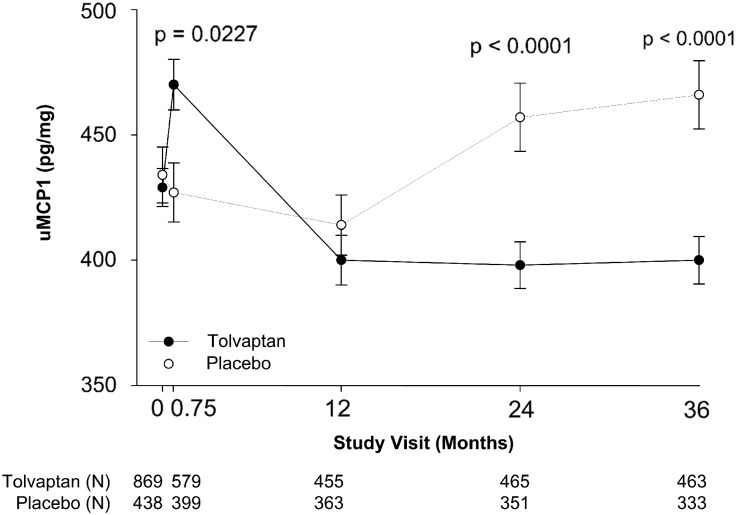
FIGURE 4.
Change in uMCP1 excretion caused by tolvaptan. Effect of tolvaptan on uMCP1 compared with placebo. Means and standard deviations were calculated with the delta method using anti-logarithm as the function. P-values in the figure were derived by ANCOVA with factor of treatment and covariate baseline. Tolvaptan group uMCP1 values fell below baseline at 12, 24 and 36 months (P < 0.0001); placebo group values fell below baseline at 12 months (P = 0.0119) and above baseline at 36 months (P = 0.0328) (Wilcoxon Signed Ranked Test). The number of subjects (N) in each treatment arm is specified below the graph for each study visit.
The effect of tolvaptan treatment on uMCP1 excretion was compared with placebo at each time point (Figure 4). Mean uMCP1 levels in tolvaptan-treated subjects were higher than placebo after 3 weeks of treatment. After 12 months, uMCP1 excretion was lower in the tolvaptan than in the placebo group, but did not reach statistical significance until 24 (13.8 ± 3.4%, P<0.0001) and 36 months (14.4 ± 3.7%, P<0.0001). Viewed against the baseline value, the tolvaptan effect on uMCP1 excretion appeared to begin between 3 weeks and 12 months of treatment and was sustained for 36 months.
Per protocol, physicians advised ADPKD patients to drink in advance of thirst to avoid the excretion of maximally concentrated urine and the secretion of high plasma levels of AVP [27]. Subjects in the placebo group appear to have substantially increased fluid intake as mean Uosm decreased from 509 mOsm/kg H2O at baseline to 460 mOsm/kg H2O by 3 weeks and to 448 and 440 mOsm/kg H2O at 24 and 36 months, respectively. After the 3-week titration phase, subjects treated with tolvaptan experienced a mean change in Uosm from 508 to 198 mOsm/kg H2O (P < 0.0001); mean Uosm remained below 240 mOsm/kg H2O for the remainder of the study.
During the first 12 months, there was an imbalance in the number of subjects who discontinued the trial relative to the final 24 months. A sensitivity analysis of subjects who completed the full 36 months (subjects with both baseline and 36 month measurements of uMCP1) showed a pattern of uMCP1 change similar to that seen in Figure 4 (Supplementary data, Figure S2). Tolvaptan suppressed uMCP1 excretion below placebo in this subgroup at 24 months (12.0 ± 3.9%, P = 0.0008) and 36 months (14.4 ± 3.7%, P < 0.0001).
While women excreted higher levels of MCP-1 at baseline (493 ± 253 pg/mg versus 382 ± 195 pg/mg, P < 0.0001), both males and females treated with tolvaptan reduced uMCP1 excretion below placebo at 24 months (males 12.3 ± 4.9%, P = 0.0063; females 14.3 ± 4.8%, P = 0.0012) and 36 months (males13.7 ± 5.1%, P = 0.0032; females 14.8 ± 5.4%, P = 0.0022).
The pronounced diuresis reduced uMCP1 concentrations below the 70 pg/mL limit of detection in 33.6% of the subjects taking tolvaptan, which was higher than in the placebo group (13.3%) who drank fluids ad lib itum. Samples with MCP-1 below the limit were not included in the primary analysis; such a deletion could potentially distort the effects of tolvaptan treatment on uMCP1. In a sensitivity analysis we imputed the lower limit of MCP-1 detection (70 pg/mL) for each deleted sample and reanalyzed. The replacement data revealed a pattern of uMCP1 excretion in response to tolvaptan (Supplementary data, Figure S3) similar to the original analysis in Figure 4. In this sensitivity analysis, tolvaptan suppressed uMCP1 excretion below placebo at 24 months (12.8 ± 3.0%, P < 0.0001) and 36 months (12.9 ± 3.3%, P < 0.0001).
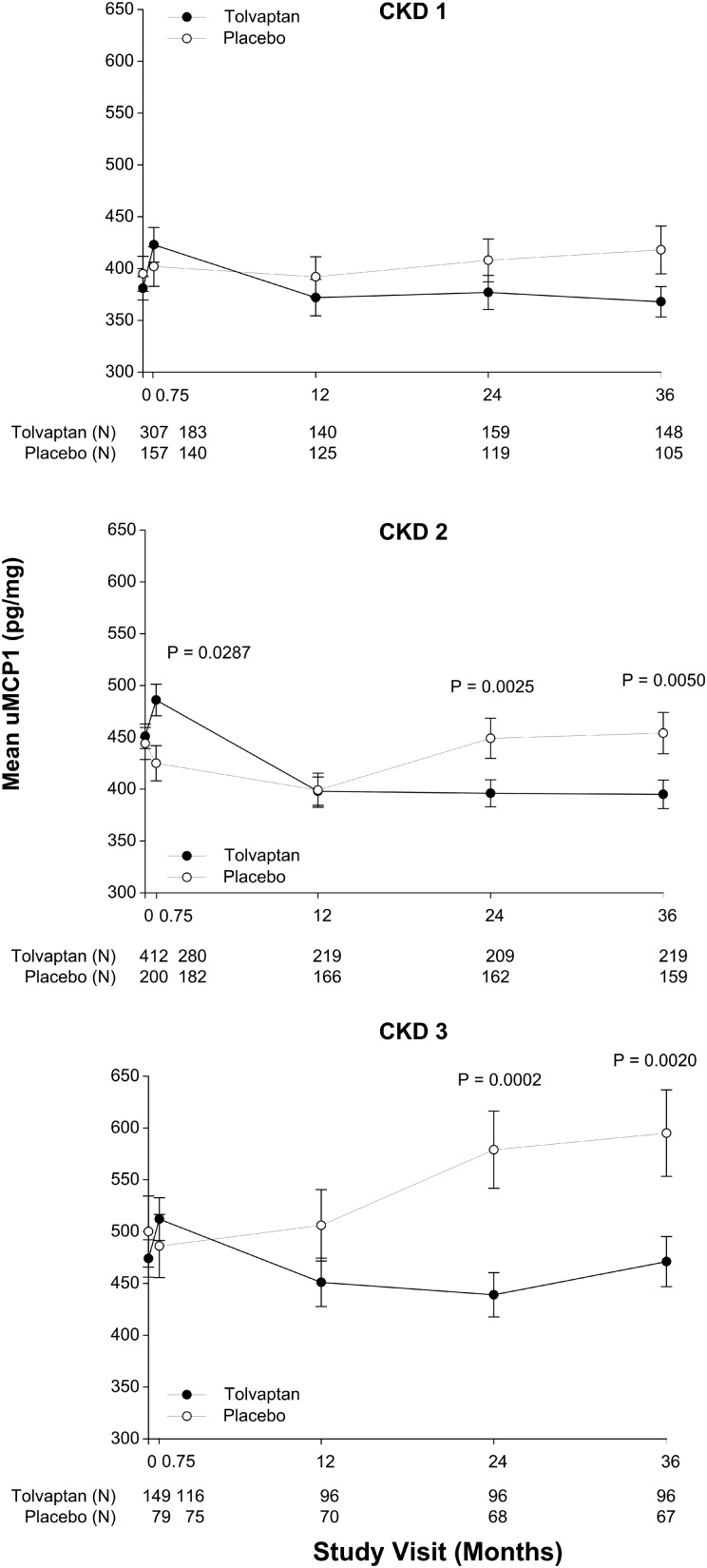
To determine whether the amount of residual renal function might alter the effect of tolvaptan on uMCP1 excretion we stratified baseline eGFRs of treated and placebo subjects across CKD Stages 1–3 determined at baseline (Figure 5). In CKD1, uMCP1 excretion after 36 months was lower than in placebo but fell short of reaching statistical significance; however, after 24 and 36 months, tolvaptan reduced uMCP1 excretion below placebo in CKD 2 (14.0 ± 5.1%, P = 0.0025 and 13.9 ± 5.4%, P = 0.0050, respectively) and CKD 3 (23.7 ± 7.2%, P = 0.0002 and 21.4 ± 8.0%, P = 0.0020, respectively).
FIGURE 5.
Effect of tolvaptan on uMCP1 excretion in different CKD stages. Mean difference ± standard error of the mean between treatment groups determined as in Figure 4. P-values indicate significance of differences between tolvaptan and placebo groups. The number of subjects (N) in each treatment arm is specified below the graph for each study visit.
DISCUSSION
With the prospect of targeted therapy on the horizon, nephrologists are seeking ways to monitor ADPKD progression before massive and irreversible damage is done to renal parenchyma. Injury-provoked biomarkers of disease progression expressed early in the disease would be useful in monitoring the effectiveness of new therapies. The current ‘gold standard’ biomarker of renal disease progression, GFR, is usually estimated from measurements of creatinine clearance or serum creatinine concentration.
Unfortunately, long standing progressive disorders cannot reliably announce through GFR when the number of filtering glomeruli have been significantly reduced because compensatory hyper-filtration of disease-free nephrons can keep the measured GFR within normal limits for decades [28]. Hyper-filtration obscures the impact of actual parenchymal loss until the compensated nephrons are destroyed as well—then GFR steadily declines.
TKV, the sum of thousands of individual cysts and residual parenchyma, provides the earliest visible and quantifiable evidence of serious injury in ADPKD. TKV predicts the future reduction of renal function and is considered by many in the field to be a strong surrogate endpoint of disease progression [29]. Cysts are currently the only biomarkers that consistently announce damage to the kidneys of individual patients before GFR declines and MCP-1 synthesis by them is a potential link to early renal injury.
uMCP1 was lowest in those with the smallest kidneys and highest Uosm and eGFR at baseline (Figure 2), in other words, subjects with the best renal function and preserved structure. uMCP1 seems to rise above normal limits in some ADPKD patients before the eGFR declines below Stage 1 [8, 9] (Figures 1 and 3). In preclinical studies, MCP-1 derived from cysts has been shown to recruit macrophages that promote the proliferation of mural epithelial cells, an effect that can be reversed by macrophage depletion [20, 21]. Most of the patients at baseline in the TEMPO 3:4 study excreted MCP-1 in amounts greater than normal (Figure 1). Given the capacity of MCP-1 to incite renal inflammation, it seems reasonable to suppose that an abnormal amount of MCP-1 in the urine is consistent with, but does not prove, an increase of inflammatory activity within polycystic kidneys.
Renal cysts intrude on adjacent capillaries, arterioles, venules, lymphatics and tubules during and following their formation; these injurious effects increase exponentially as the cysts grow more numerous and steadily enlarge [2, 30, 31]. Focal areas of interstitial inflammation develop relatively early in the course of the disease. As interstitial infiltration widens and filtering glomeruli are nullified, GFR appears to be compensated by residual normal glomeruli until the hyper-filtering nephrons are also impacted by cysts, causing the measured GFR to decline below normal limits. Evidently the persistent downhill course can be interrupted in ADPKD since tolvaptan slowed the increase in TKV, slowed the rate of eGFR decline and reduced the urinary excretion of MCP-1 in relation to placebo [24] (Figure 4). Tolvaptan reduced uMCP1 excretion below that of placebo in males and females and in subjects across CKD Stages 1–3 (Figure 5). This invites speculation that uMCP1 excretion might prove useful, together with TKV and GFR, in the evaluation of other potential therapeutic agents.
Tolvaptan caused a transient increase in uMCP1 excretion during the early dose adjustment period that was possibly related to the hemo- and hydrodynamic physiologic adjustments brought out by the blockade of AVP receptors [18, 32] (Figure 4). Within 12 months of tolvaptan treatment uMCP1 excretion decreased below the baseline, where it remained until the end of the study. In contrast, the excretion of uMCP1 in the placebo group fell below baseline during the first year, possibly secondary to increased fluid intake (Uosm fell from baseline of 520 mOsm/Kg to 462 mOsm/kg at 12 months), then rose progressively above baseline until the end of the study (Figure 4). Based on previous studies and the new data in Figures 2–5 it appears that MCP-1 excretion rises steadily as the disease progresses and that tolvaptan diminishes that increase.
The aggregate increase in individual cyst volume causes TKV to rise at an annual rate of ∼5.3% in adults with ADPKD [33]. We hypothesized that tolvaptan would reduce the formation of new cysts and the expansion of old ones by inhibiting mural proliferation of new cells that produce MCP-1. A robust effect size that reduced the rate of kidney growth by ∼50%, as noted in TEMPO 3:4 [24], could potentially lower the kidney growth rate 2–3% per year. Assuming that this reduction would also decrease the creation of additional epithelium capable of generating MCP-1, we supposed that it would require several months for changes in uMCP1 excretion to become detectable given the variability of uMCP1 excretion in ADPKD patients [8]. At the end of 3 years tolvaptan had reduced the rate of MCP-1 excretion ∼14% in comparison with placebo (Figure 4), i.e. ∼4–5% per year, which is of the same order of magnitude as our prediction.
The decrease of uMCP1 excretion within the first year of treatment is explained most economically by assuming that tolvaptan leads to a decrease in cyst formation and a decrease in MCP-1 synthesis within expanding renal cysts. Complicating the interpretation of drug effectiveness is the fact that most of the cysts large enough to be detected by magnetic resonance scans are closed, isolated sacs that impede MCP-1 synthesized within them from entering the final urine [19]. Chemokine levels within these closed sacs can be exceedingly high. Consequently, an effect of tolvaptan in closed cysts would be limited to decreasing the amount of chemokine that might leak through the walls. In contrast, new cysts remain connected to the renal drainage system for several years and a decrease in MCP-1 production in relatively young cysts would lead to reduced uMCP1 excretion. Vasopressin amplifies the production of proinflammatory mediators in traumatic brain injury [34, 35]. It remains to be determined whether tolvaptan can block a direct action of vasopressin on the production of chemokines by abnormal cyst epithelial cells.
As with any post hoc analysis, this study has limitations. (i) Plasma MCP-1 was not measured so we cannot determine the extent to which uMCP1 excretion and plasma MCP-1 may have changed in parallel. In a previous study [8] and in the Consortium for Radiologic Imaging Studies of PKD (CRISP) plasma MCP-1 levels in patients ranging in age from 15 to 45 years were not different from normal controls and bore no relationship to uMCP1 [29]. (ii) A cohort of healthy age- and gender-matched persons was not included, limiting comparisons to patients in tolvaptan and placebo groups. (iii) More tolvaptan-treated patients withdrew during the study than placebo-treated patients, primarily due to aquaretic symptoms [24]. In a sensitivity analysis (Supplementary data, Figure S2), tolvaptan administration revealed differences in uMCP1 excretion between tolvaptan-treated versus placebo patients that were similar to the full cohort. (iv) It has been suggested that drinking extra fluid may ameliorate ADPKD and many patients have adopted this as a routine. Extra fluid intake in the placebo group may have diminished differences in uMCP1 excretion between the placebo and tolvaptan treatment groups throughout the study. (v) The deletion of uMCP1 measurements that were below the detection limit in both tolvaptan and placebo treatment groups may have undermined the demonstration of an effect of tolvaptan to lower MCP-1 excretion even further. A sensitivity analysis (Supplementary data, Figure S3) showed that substituting the lower limit of the MCP-1 assay for the deleted samples did not uncover any disagreements with the original findings. (vi) Although uMCP1 is likely a biomarker of renal inflammation, its evaluation in this mature cohort of ADPKD subjects does not reveal the extent to which the excreted chemokine derives from cysts, from the secondary inflammation that is a feature of all chronic renal diseases that progress to the end-stage or from inflammation in other parts of the body. On the other hand, most of the reduction in uMCP1 excretion caused by AVPV2-receptor-selective tolvaptan was probably secondary to reduced renal production of the chemokine in distal cysts, reflecting a slowing of cyst formation and cyst expansion. (vii) This study cannot differentiate between an effect of tolvaptan on the production of MCP-1 in cysts bearing AVP V2 receptors and the effect of increased water intake to ameliorate the decline in eGFR as has been suggested in other types of progressive renal disease [36]. (viii) There are relatively few patients of African descent in this study. (ix) Finally, PKD genotype data were not available.
In conclusion, in this ADPKD cohort the renal excretion of MCP-1 was persistently reduced below placebo following the administration of tolvaptan over a time-frame similar to the slowing of renal growth and the rate of decline in eGFR reported previously [24]. The current study supports the further evaluation of uMCP1 as an efficacy biomarker in clinical trials including ADPKD patients.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the nurse coordinators for excellent assistance, the TEMPO 3:4 trial researchers and especially the patients who volunteered. The TEMPO 3:4 trial was funded by Otsuka Pharmaceuticals Co., Ltd Tokyo, Japan and Otsuka Pharmaceutical Development and Commercialization, Inc., Rockville, MD, USA.
CONFLICT OF INTEREST STATEMENT
A.B.C., O.D., R.T.G., E.H., R.D.P. and V.E.T. were members of the Steering Committee of the TEMPO 3:4 trial and are consultants for Otsuka Pharmaceutical Development and Commercialization, Inc., Rockville, MD, USA. J.J.G. is a consultant. J.B., W.Z., F.S.C., H.K. and J.O. are employed by this company. The results presented in this paper have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Grantham JJ. Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med 2008; 359: 1477–1485 [DOI] [PubMed] [Google Scholar]
- 2. Grantham JJ, Mulamalla S, Swenson-Fields KI. Why kidneys fail in autosomal dominant polycystic kidney disease. Nat Rev Nephrol 2011; 7: 556–566 [DOI] [PubMed] [Google Scholar]
- 3. Torres VE, Wang X, Qian Q et al. Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med 2004; 10: 363–364 [DOI] [PubMed] [Google Scholar]
- 4. Wang X, Gattone V II, Harris PC et al. Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat. J Am Soc Nephrol 2005; 16: 846–851 [DOI] [PubMed] [Google Scholar]
- 5. Wallace DP. Cyclic AMP-mediated cyst expansion. Biochim Biophys Acta 2011; 1812: 1291–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cowley BD Jr, Grantham JJ, Muessel MJ et al. Modification of disease progression in rats with inherited polycystic kidney disease. Am J Kidney Dis 1996; 27: 865–879 [DOI] [PubMed] [Google Scholar]
- 7. Gattone VH II, Cowley BD Jr, Barash BD et al. Methylprednisolone retards the progression of inherited polycystic kidney disease in rodents. Am J Kidney Dis 1995; 25: 302–313 [DOI] [PubMed] [Google Scholar]
- 8. Zheng D, Wolfe M, Cowley BD Jr et al. Urinary excretion of monocyte chemoattractant protein-1 in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2003; 14: 2588–2595 [DOI] [PubMed] [Google Scholar]
- 9. Azurmendi PJ, Fraga AR, Galan FM et al. Early renal and vascular changes in ADPKD patients with low-grade albumin excretion and normal renal function. Nephrol Dial Transplant 2009; 24: 2458–2463 [DOI] [PubMed] [Google Scholar]
- 10. Boertien WE, Meijer E, Gansevoort RT. Urinary biomarkers in autosomal dominant polycystic kidney disease: is there no prognostic value? Kidney Int 2012; 82: 361. [DOI] [PubMed] [Google Scholar]
- 11. Meijer E, Boertien WE, Nauta FL et al. Association of urinary biomarkers with disease severity in patients with autosomal dominant polycystic kidney disease: a cross-sectional analysis. Am J Kidney Dis 2010; 56: 883–895 [DOI] [PubMed] [Google Scholar]
- 12. Ye SD, Zheng M, Zhao LL et al. Intensive insulin therapy decreases urinary MCP-1 and ICAM-1 excretions in incipient diabetic nephropathy. Eur J Clin Invest 2009; 39: 980–985 [DOI] [PubMed] [Google Scholar]
- 13. Ho J, Wiebe C, Gibson IW et al. Elevated urinary CCL2: Cr at 6 months is associated with renal allograft interstitial fibrosis and inflammation at 24 months. Transplantation 2014; 98: 39–46 [DOI] [PubMed] [Google Scholar]
- 14. Tofik R, Ohlsson S, Bakoush O. Urinary concentration of monocyte chemoattractant protein-1 in idiopathic glomerulonephritis: a long-term follow-up study. PLoS One 2014; 9: e87857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chevalier RL. Chronic partial ureteral obstruction and the developing kidney. Pediatr Radiol 2008; 38(Suppl 1): S35–S40 [DOI] [PubMed] [Google Scholar]
- 16. Deshmane SL, Kremlev S, Amini S et al. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 2009; 29: 313–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang Y, Chen J, Chen L et al. Induction of monocyte chemoattractant protein-1 in proximal tubule cells by urinary protein. J Am Soc Nephrol 1997; 8: 1537–1545 [DOI] [PubMed] [Google Scholar]
- 18. Boertien WE, Meijer E, de Jong PE et al. Short-term renal hemodynamic effects of tolvaptan in subjects with autosomal dominant polycystic kidney disease at various stages of chronic kidney disease. Kidney Int 2013; 84: 1278–1286 [DOI] [PubMed] [Google Scholar]
- 19. Grantham JJ, Geiser JL, Evan AP. Cyst formation and growth in autosomal dominant polycystic kidney disease. Kidney Int 1987; 31: 1145–1152 [DOI] [PubMed] [Google Scholar]
- 20. Swenson-Fields KI, Vivian CJ, Salah SM et al. Macrophages promote polycystic kidney disease progression. Kidney Int 2013; 83: 855–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karihaloo A, Koraishy F, Huen SC et al. Macrophages promote cyst growth in polycystic kidney disease. J Am Soc Nephrol 2011; 22: 1809–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Belibi FA, Reif G, Wallace DP et al. Cyclic AMP promotes growth and secretion in human polycystic kidney epithelial cells. Kidney Int 2004; 66: 964–973 [DOI] [PubMed] [Google Scholar]
- 23. Aihara M, Fujiki H, Mizuguchi H et al. Tolvaptan delays the onset of end-stage renal disease in a polycystic kidney disease model by suppressing increases in kidney volume and renal injury. J Pharmacol Exp Ther 2014; 349: 258–267 [DOI] [PubMed] [Google Scholar]
- 24. Torres VE, Chapman AB, Devuyst O et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 2012; 367: 2407–2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Watson L, Midgley A, Pilkington C et al. Urinary monocyte chemoattractant protein 1 and alpha 1 acid glycoprotein as biomarkers of renal disease activity in juvenile-onset systemic lupus erythematosus. Lupus 2012; 21: 496–501 [DOI] [PubMed] [Google Scholar]
- 26. Tam FW, Sanders JS, George A et al. Urinary monocyte chemoattractant protein-1 (MCP-1) is a marker of active renal vasculitis. Nephrol Dial Transplant 2004; 19: 2761–2768 [DOI] [PubMed] [Google Scholar]
- 27. Torres VE, Bankir L, Grantham JJ. A case for water in the treatment of polycystic kidney disease. Clin J Am Soc Nephrol 2009; 4: 1140–1150 [DOI] [PubMed] [Google Scholar]
- 28. Hayslett JP, Kashgarian M, Epstein FH. Functional correlates of compensatory renal hypertrophy. J Clin Invest 1968; 47: 774–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chapman AB, Bost JE, Torres VE et al. Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2012; 7: 479–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fonseca JM, Bastos AP, Amaral AG et al. Renal cyst growth is the main determinant for hypertension and concentrating deficit in Pkd1-deficient mice. Kidney Int 2014; 85: 1137–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Galarreta CI, Grantham JJ, Forbes MS et al. Tubular obstruction leads to progressive proximal tubular injury and atubular glomeruli in polycystic kidney disease. Am J Pathol 2014; 184: 1957–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Irazabal MV, Torres VE, Hogan MC et al. Short-term effects of tolvaptan on renal function and volume in patients with autosomal dominant polycystic kidney disease. Kidney Int 2011; 80: 295–301 [DOI] [PubMed] [Google Scholar]
- 33. Grantham JJ, Torres VE, Chapman AB et al. Volume progression in polycystic kidney disease. N Engl J Med 2006; 354: 2122–2130 [DOI] [PubMed] [Google Scholar]
- 34. Szmydynger-Chodobska J, Fox LM, Lynch KM et al. Vasopressin amplifies the production of proinflammatory mediators in traumatic brain injury. J Neurotrauma 2010; 27: 1449–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Szmydynger-Chodobska J, Zink BJ, Chodobski A. Multiple sites of vasopressin synthesis in the injured brain. J Cereb Blood Flow Metab 2011; 31: 47–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang CJ, Grantham JJ, Wetmore JB. The medicinal use of water in renal disease. Kidney Int 2013; 84: 45–53 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.