Abstract
Background: The positive association between caesarean section (CS) and autism spectrum disorder (ASD) may be attributed to preterm delivery. However, due to lack of statistical power, no previous study thoroughly examined this association across gestational age. Moreover, most studies did not differentiate between emergency and planned CS.
Methods: Using population-based registries of four Nordic countries and Western Australia, our study population included 4 987 390 singletons surviving their first year of life, which included 671 646 CS deliveries and 31 073 ASD children. We used logistic regression to estimate odds ratios (OR) and their 95% confidence intervals (CI) for CS, adjusted for gestational age, site, maternal age and birth year. Stratified analyses were conducted by both gestational age subgroups and by week of gestation. We compared emergency versus planned CS to investigate their potential difference in the risk of ASD.
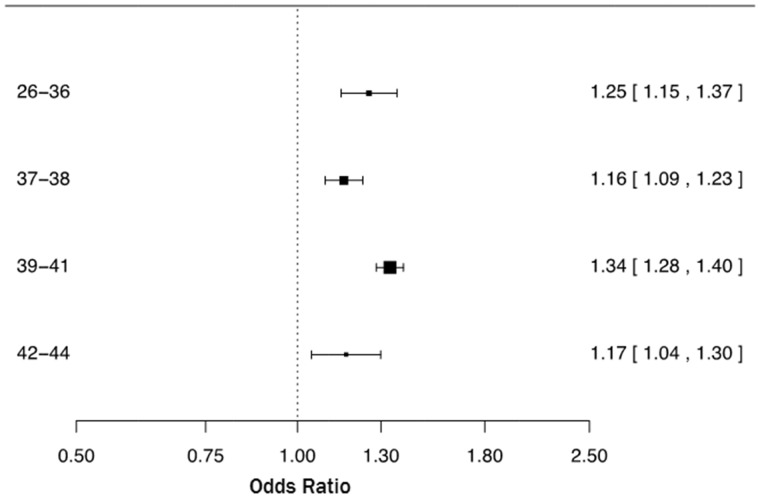
Results: Compared with vaginal delivery, the overall adjusted OR for ASD in CS delivery was 1.26 (95% CI 1.22–1.30). Stratified ORs were 1.25 (1.15–1.37), 1.16 (1.09–1.23), 1.34 (1.28–1.40) and 1.17 (1.04–1.30) for subgroups of gestational weeks 26–36, 37–38, 39–41 and 42–44, respectively. CS was significantly associated with risk of ASD for each week of gestation, from week 36 to 42, consistently across study sites (OR ranged 1.16–1.38). There was no statistically significant difference between emergency and planned CS in the risk of ASD.
Conclusion: Across the five countries, emergency or planned CS is consistently associated with a modest increased risk of ASD from gestational weeks 36 to 42 when compared with vaginal delivery.
Keywords: Autism, emergency caesarean section, planned caesarean section, gestational age, epidemiology, population based
Introduction
There has been a 20-fold increase in the diagnosis of autism spectrum disorder (ASD) since the 1980s1–5 and it is believed that both genetic and environmental risk factors are important contributors.6,7 Over a similar time period there has been an increasing trend in deliveries by caesarean section (CS). In some populations close to half of all births are delivered by CSs.8,9 In a US study, the population attributable fraction for ASD in relation to CS was estimated to be 7% for children born in 2000, higher than that for preterm birth or small for gestational age.10 It is therefore of major aetiological, clinical and public health interest to examine the relation between ASD and CS.
A positive association between CS and ASD has been shown in several earlier studies, but the results have varied in effect size.11–15 A recent meta-analysis reported that delivery by CS was associated with a 23% increased risk of ASD when compared with vaginal delivery.16 One possible explanation for the impact of CS on ASD is the fact that planned CS is normally scheduled weeks before the full 40-week gestation, to avoid spontaneous labour.17 It is also possible that the last few weeks before term are important for brain development.18 Thus deliveries before the full term might increase the risk of ASD, a hypothesis which is supported by previous studies of gestational age risk of ASD.3,4,10,19–21 Another aspect is the underlying indications leading to CS, which can vary by gestational age.22 Since the underlying maternal or fetal indications leading to emergency CS may be important independent risk factors of ASD, the magnitude of the independent risks of emergency CS for ASD may also vary by gestational age.22 We made a substantial search among the studies included in the most recent meta-analysis16 and among all the studies cited in our study, to find any studies that investigated the association of CS and ASD stratified by gestational age. Although many of the studies included gestational age14,23–29 as a confounder, only one study reported the adjusted OR of emergency CS and planned CS among non-preterm births, in the appendix.11
The rate of planned CS in singleton pregnancies has increased substantially in recent years.8,20,30–33 As maternal and clinician preferences may play a greater role in planned than in emergency CS,34 outcomes due to the CS procedure itself in planned CS deliveries at or near term may be less confounded by underlying medical indication in comparison with emergency (non-planned) CS at similar gestational ages. However, none of the mentioned studies had sufficient sample size to assess across gestational age the risk of ASD among children delivered by CS compared with children born after vaginal delivery, and most of them did not differentiate between emergency and planned CS.3,4,10,19–21
The aim of our study was to test the association between CS and ASD, overall and by type of CS, while taking gestational age into account and using the largest prospective, population-based sample of ASD to date. We hypothesized that the relation between CS and ASD may vary between gestational ages and between planned and emergency CS, where CS conducted near or at full term has lower risk than CS conducted preterm, and planned CS has a lower risk than emergency CS.
Methods
The study uses data from the International Collaboration for Autism Registry Epidemiology (iCARE).35 Access to these data is managed using the ViPAR software36 that enables the pooled analysis of prospectively measured multinational population-based data relating to ASD. Ethics committee approval, with waiver for informed consent, was obtained by each site.
Study population
The study population includes all singletons surviving their first year of living in: Norway and Sweden 1984–2004; Denmark 1997–2004; Finland 1987–2004; and the non-Aboriginal population in Western Australia (WA) 1984–99. Multiple births are highly correlated with ASD risk as well as with both preterm birth and CS, which could introduce bias.37 Excluding multiple births also reduces the risk of dependencies in the data which could affect the variance estimates. The final study sample size was not based on any statistical criterion but used all eligible national birth cohorts in the participating countries as made available by the ICARE collaboration, one of the largest available databases for population-based autism research.
ASD outcome, CS, gestational age and covariate information
Children were followed from birth to reported diagnosis of ASD or end of follow-up, which ever came first; through 2004 in WA, 2006 in Norway and 2009 in Denmark, Finland and Sweden. Denmark, Finland and Sweden provided ASD diagnoses from medical registries. ASD diagnoses from Norway and WA were derived from government-maintained service/benefits registries. Case identification, registry reporting procedures, details on the validity of case status and harmonization of diagnostic codes across sites have been described elsewhere.23 Paternal and maternal age, gestational age, sex, birth year and mode of birth delivery were obtained from birth or civil registries. For all countries in the study, throughout the births cohort in this study, mode of delivery was classified as vaginal or CS, with CS being further classified as planned, emergency or unspecified. A planned CS is a CS planned before the onset of labour. Emergency CS is performed either before the onset of labour (due to medical or pregnancy complications) or complications in labour. Availability of data on mode of delivery and type of CS (planned, emergency or unspecified) is presented in the online appendix (ST 1A, available as Supplementary data at IJE online).
The date of last menstrual period (LMP) is commonly used in Nordic countries to estimate gestation age, and LMP is corrected when there is a discrepancy with the ultrasound examination. Rules for estimating gestational age, however, often differ at maternity hospitals from national guidelines and the year when ultrasound examination was introduced varied between countries. In Denmark, ultrasound-based pregnancy dating was used to determine gestational age for 93% of those born in 1995 and 2000.38 In Sweden since 1990, early second-trimester ultrasound examination is routinely offered, and more than 95% of women accepted this offer; otherwise the date of LMP is used.24 In Finland, the LMP estimates are only corrected if they differ ± 7 days with the first- or second-trimester ultrasound examination.24 Ultrasound examination was introduced in 1967 and the 1980s in Norway and WA, respectively.25
Statistical analyses
Primary analyses
The primary analyses used a two-step approach. First, as commonly used in earlier studies, we estimated the risk of ASD when comparing births following any type of CS with births following vaginal delivery, which facilitates comparisons with previous findings. The odds ratio (OR) of ASD among births delivered by CS compared with vaginal delivery was obtained by fitting ordinary logistic regression models. We first estimated the OR for ASD overall, then in gestational age subgroups: weeks 26–36 (preterm), weeks 37–38 (early term), weeks 39–41 (term) and weeks 42–44 (post-term) and finally by week of gestation, except for weeks 26–30 for which data were too sparse for week-by-week analysis. All models included sex, site, birth year (1984–89,1990–94,1995–99 and 2000–04), categorized maternal age (< 25, 25–29, 30–34, 35–39 ≥ 40 years) and gestational age (by week or subgroup) as covariates, if not otherwise specified.
The medical indications for women undergoing either emergency or planned CS might be different. Thus, in the second-step analysis we also estimated OR of ASD among births delivered by emergency CS compared with planned CS (unspecified CS was excluded from this analysis). Due to lack of data on CS type at some sites at different time periods (ST 1A,) these analyses were restricted to birth cohorts 1997–2004 for Denmark, 1984–99 for WA, 1988–2004 for Norway and Sweden and 1990–2004 for Finland. For the analysis of emergency versus planned CS, we re-fitted the models adjusting for site, sex, birth year and categorized maternal age by week of gestation (weeks 26–30 as a single subgroup).
Site comparisons
Site-specific ORs of ASD among births delivered by CS compared with vaginal delivery were estimated by stratifying gestational age subgroup, and each week of gestation. Site heterogeneity and its influence on overall results were addressed by Cochran’s Q test and by verifying pooled results through the use of leave-one-out approach, i.e. OR was estimated by removing the indicated site being removed.
Subgroup and sensitivity analyses
ORs of ASD were estimated for CS in comparison with vaginal delivery in subgroups of gestational age (weeks 26–36, 37–38, 39–41 and 42–44) for male and female offspring, respectively. We repeated these analyses for autistic disorder (AD) cases only. Due to low frequency in some subgroups, analyses by week of gestation were not feasible.
As the likelihood of CS is much higher in women who have had a CS in a previous pregnancy, we repeated the primary analyses restricting to firstborn children only. Since information on paternal age was not available for Finnish births, the primary analyses did not include paternal age as a covariate. In a set of sensitivity analyses, having excluded Finland from the analysis dataset, we repeated the primary analyses by gestational age subgroups after adding paternal age (< 25, 25–29, 30–34, 35–39 and ≥ 40 years) to the models together with the other model terms.
A logistic regression model assumes that all subjects have been observed for the same length of time (i.e. equal length of follow-up or risk time) or that the length of follow-up does not affect the risk. If this assumption is violated, bias may be introduced. For this purpose, birth year was included in all models. We also performed a sensitivity analysis on data from Denmark, Finland and Sweden, as these countries had information about the date of ASD diagnosis. For the sensitivity analyses, comparing between CS and vaginal delivery, we estimated the hazard ratios (HR) of ASD across subgroups of gestational age based on the hazard ratio obtained by fitting stratified Cox regression with age at diagnosis as the underlying time scale, adjusting for site, sex and maternal age group. To adjust for calendar effects, we fitted the Cox regression with different strata for each birth-year group.26
All statistical tests were performed using the two-sided 5% level of significance and corresponding two-sided 95% CI’s. We did not adjust for multiplicity of statistical tests. It was not possible to identify siblings in the data and correct for possible sibling correlations in the analyses. Models’ goodness of fit was addressed by calculating the Pearson chi-square test and the Hosmer–Lemeshow test. All statistical analyses were performed using the R-software version 3.2.1.
Results
The study cohort included a total of 5 250 034 births. After sequentially excluding 99 072 who were born very early (< 26) or very late (> 44) weeks of gestational age, 146 619 multiple births and 16 957 who died before age of 1 year, 4 987 390 (95% of the total) remained in the final data for analysis (ST 1B). Sweden contributed 41% of the final data, Norway and Finland each contributed around 21% and Denmark and WA each contributed less than 10%. Of the final data, a total 4 315 744 (86.5%) children were delivered by vaginal birth, 243 749 (4.9%) by planned CS, 291 106 (5.9%) by emergency CS and 136 791 (2.7%) by unspecified CS. There were 31 073 (0.6%) children with ASD, of whom 10 418 children were diagnosed with AD (0.2% of total). Study cohort distributions of births, ASD and AD cases, sex, birth year, parental age and sites are shown in Table 1 by mode of delivery.
Table 1.
Study cohort characteristics by mode of delivery: vaginal or caesarean section
| Characteristic | Vaginal delivery Frequency (%) | Type of caesarean section |
All deliveries Frequency (%) | ||
|---|---|---|---|---|---|
| Planned Frequency (%) | Emergency Frequency (%) | Unspecified Frequency (%) | |||
| Number of singleton live births | 4 315 744 | 243 749 | 291 106 | 136 791 | 4 987 390 |
| ASD | 25 750 (0.6) | 1959 (0.8) | 2274 (0.8) | 1090 (0.8) | 31 073 (0.6) |
| AD | 8364 (0.2) | 726 (0.3) | 899 (0.3) | 329 (0.2) | 10 418 (0.2) |
| Male | 2 201 829 (51.0) | 126 614 (51.9) | 158 305 (54.4) | 72 432 (53.0) | 2 559 180 (51.3) |
| Gestational age group, weeks | |||||
| 26–36 | 156 667 (3.6) | 28 252 (11.6) | 35 106 (12.1) | 20 017 (14.6) | 240 042 (4.8) |
| 37–38 | 666 512 (15.4) | 108 434 (44.5) | 60 518 (20.8) | 44 648 (32.6) | 880 112 (17.6) |
| 39–41 | 3 176 324 (73.6) | 97 599 (40.0) | 163 326 (56.1) | 62 500 (45.7) | 3 499 749 (70.2) |
| 42–44 | 316 241 (7.3) | 9464 (3.9) | 32 156 (11.0) | 9626 (7.0) | 367 487 (7.4) |
| Site | |||||
| Norway | 924 475 (21.4) | 38 518 (15.8) | 65 691 (22.6) | 23 713 (17.3) | 1 052 397 (21.1) |
| Denmarka | 417 399 (9.7) | 26 129 (10.7) | 50 952 (17.5) | NA | 494 480 (9.9) |
| Finland | 891 684 (20.7) | 60 386 (24.8) | 62 981 (21.6) | 33 496 (24.5) | 1 048 547 (21.0) |
| Sweden | 1 802 958 (41.8) | 82 684 (33.9) | 81 561 (28.0) | 79 582 (58.2) | 2 046 785 (41.0) |
| Western Australiaa | 279 228 (6.5) | 36 032 (14.8) | 29 921 (10.3) | NA | 345 181 (6.9) |
| Birth year | |||||
| 1984–89 | 1 033 497 (23.9) | 14 419 (5.9) | 15 584 (5.4) | 108 611 (79.4) | 1 172 111 (23.5) |
| 1990–94 | 1 089 289 (25.2) | 66 235 (27.2) | 69 122 (23.7) | 18 673 (13.7) | 1 243 319 (24.9) |
| 1995–99 | 1 100 194 (25.5) | 78 929 (32.4) | 93 187 (32.0) | 3861 (2.8) | 1 276 171 (25.6) |
| 2000–04 | 1 092 764 (25.3) | 841 66 (34.5) | 113 213 (38.9) | 5646 (4.1) | 1 295 789 (26.0) |
| Maternal age group, years | |||||
| < 25 | 540 990 (12.5) | 21 474 (8.8) | 33 098 (11.4) | 11 878 (8.7) | 607 440 (12.2) |
| 25–29 | 1 374 489 (31.8) | 59 875 (24.6) | 84 384 (29.0) | 38 272 (28.0) | 1 557 020 (31.2) |
| 30–34 | 1 880 473 (43.6) | 107 865 (44.3) | 120 347 (41.3) | 63 821 (46.7) | 2 172 506 (43.6) |
| 35–39 | 4 725 66 (10.9) | 47 635 (19.5) | 45 682 (15.7) | 20 538 (15.0) | 586 421 (11.8) |
| ≥ 40 | 47 226 (1.1) | 6900 (2.8) | 7595 (2.6) | 2282 (1.7) | 64 003 (1.3) |
| Paternal age group, yearsb | |||||
| <25 | 167 094 (3.9) | 5 286 (2.2) | 13 209 (4.5) | 2780 (2.0) | 188 369 (3.8) |
| 25–29 | 703 734 (16.3) | 27 848 (11.4) | 49 584 (17) | 17 458 (12.8) | 798 624 (16.0) |
| 30–34 | 1 677 060 (38.9) | 81 806 (33.6) | 98 347 (33.8) | 53 507 (39.1) | 1 910 720 (38.3) |
| 35–39 | 697 362 (16.2) | 50 717 (20.8) | 48 436 (16.6) | 24 634 (18.0) | 821 149 (16.5) |
| ≥ 40 | 178 810 (4.1) | 17 706 (7.3) | 18 549 (6.4) | 4916 (3.6) | 219 981 (4.4) |
ASD, autistic spectrum disorder; AD, autistic disorder; NA, data not available.
aDenmark and Western Australia had no unspecified category for caesarean section.
bFinland had no information on paternal age. The sum of the frequencies does not add up to the total births.
The vast majority of the deliveries (96.3%) were in gestational weeks 36–42 (ST 2, available as Supplementary data at IJE online). The most frequently occurring gestational age at delivery was 40 weeks for vaginal birth (30.4%), 38 weeks for planned CS (34.6%), 39 weeks for emergency CS (21.2%) and 38 weeks for unspecified CS (24.4%). The absolute risk of ASD declined with increasing gestational age: the risk of ASD declined from 1.42% at gestational weeks 26–30 to 0.3% at gestational weeks 44 (ST 3, available as Supplementary data at IJE online). Overall, birth deliveries by CS had a higher risk to develop ASD than vaginal births, throughout gestational weeks 26–44.
Caesarean section versus vaginal delivery by gestational ages
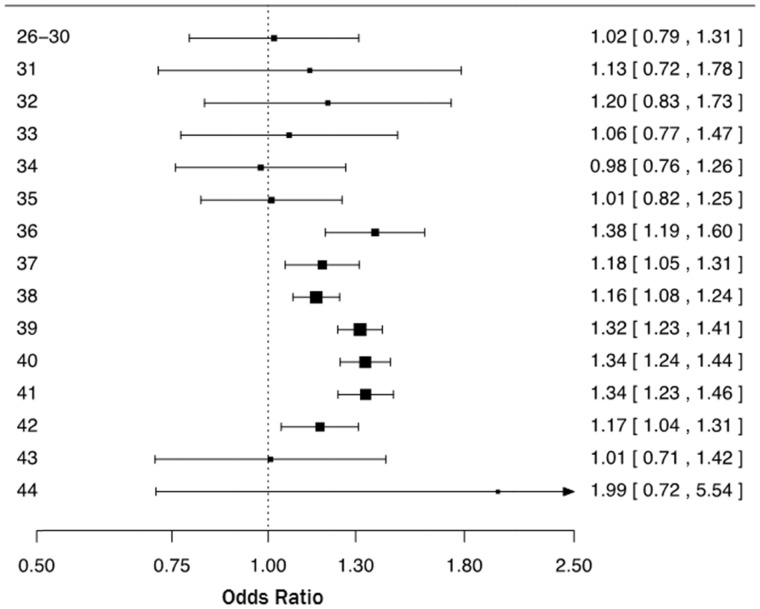
The upper part of Table 2 summarizes the results of the primary analysis. Compared with vaginal delivery, CS was associated with a statistically significant increased risk of ASD, with and without adjustment of potential confounders (site, birth year, sex and maternal age): crude OR = 1.33 (95% CI 1.29–1.37) and adjusted OR = 1.32 (95% CI 1.28–1.36). Further adjustment by including gestational age as a covariate resulted in OR = 1.26 (95% CI 1.22–1.30). As shown in Figure 1, the OR of ASD following CS was statistically significantly elevated across all gestational age subgroups (26–36, 37–38, 39–41 and 42–44 weeks of gestation). When the OR of ASD was estimated by week of gestation we found a statistically significant association between CS and ASD, starting from week 36 through week 42 (Figure 2).
Table 2.
Odds ratios (OR) and hazard ratios (HR) with two-sided 95% confidence intervals (CI) of autism spectrum disorder (ASD) among births delivered by caesarean section (CS) compared with vaginal delivery, considering different subgroups of birth data
| Analysis | Vaginal births (ASD %) | CS births (ASD %) | OR (95% CI) |
|---|---|---|---|
| Primary analysis: | |||
| Crude estimate without adjustment | 4 987 390 (0.6) | 31 073 (0.8) | 1.33 (1.29–1.37) |
| M1: without adjusting for gestational age | 4 987 390 (0.6) | 31 073 (0.8) | 1.32 (1.28–1.36) |
| M2: adjusting for gestational age | 4 987 390 (0.6) | 31 073 (0.8) | 1.26 (1.22–1.30) |
| Subgroup analysis: | |||
| M3: firstborn only | 960 021 (0.7) | 167 751 (0.8) | 1.26 (1.22–1.30) |
| M4: including paternal age | 3 423 186 (0.6) | 515 657 (0.8) | 1.27 (1.22–1.31) |
| M5: Cox regression (HR) | 3 111 057 (0.8) | 478 622 (1.0) | 1.27 (1.23–1.31) |
| M6: logistic regression, restricted date-of-diagnosis cohort | 3 111 057 (0.8) | 478 622 (1.0) | 1.25 (1.21–1.29) |
M1: logistic regression models included site (Denmark, Finland, Norway, Sweden, Western Australia), sex, birth year (1984–89, 1990–94, 1995–99, 2000–04) and maternal age (< 25, 25–29, 30–34, 35–39, ≥ 40) as covariates.
M2: M1 + gestational age in weeks group (26–36, 37–38, 39–41, 42–44).
M3: M2 but only included firstborn.
M4: M2 + including paternal age (< 25, 25–29, 30–34, 35–39, ≥ 40) as covariates, but excluded data from Finland due to missing information of paternal age.
M5: stratified Cox regression models using birth years (1984–89, 1990–94, 1995–99, 2000–04) as strata, and with covariates site (Denmark, Finland, Norway, Sweden), sex, birth year (1984–89, 1990–94, 1995–99, 2000–04), maternal age (< 25, 25–29, 30–34, 35–39, ≥ 40) and gestational age (26–36, 37–38, 39–41, 42–44).
M6: Denmark, Finland and Sweden were included and used the same set of variables as M5.
Figure 1.
Odds ratios and two-sided 95% confidence intervals of autism spectrum disorder following Caesarean section compared with vaginal delivery in gestational age subgroups (weeks 26–36, 37–38, 39–41, and 42–44). Each odds ratio was estimated from logistic regression adjusting for site (Denmark, Finland, Norway, Sweden, Western Australia), sex, birth year (1984–89, 1990-1994, 1995–99, and 2000-2004) and maternal age (<25, 25–29, 30–34, 35–39, ≥40).
Figure 2.
Odds ratios and two-sided 95% confidence intervals of autism spectrum disorder following Caesarean section compared with vaginal delivery by week of gestation (weeks 26–30 as one group). Each odds ratio was estimated from logistic regression adjusting for site (Denmark, Finland, Norway, Sweden, Western Australia), sex, birth year (1984–89, 1990–1994, 1995–99, and 2000–2004) and maternal age (<25, 25–29, 30–34, 35-39, ≥40).
Emergency versus planned CS
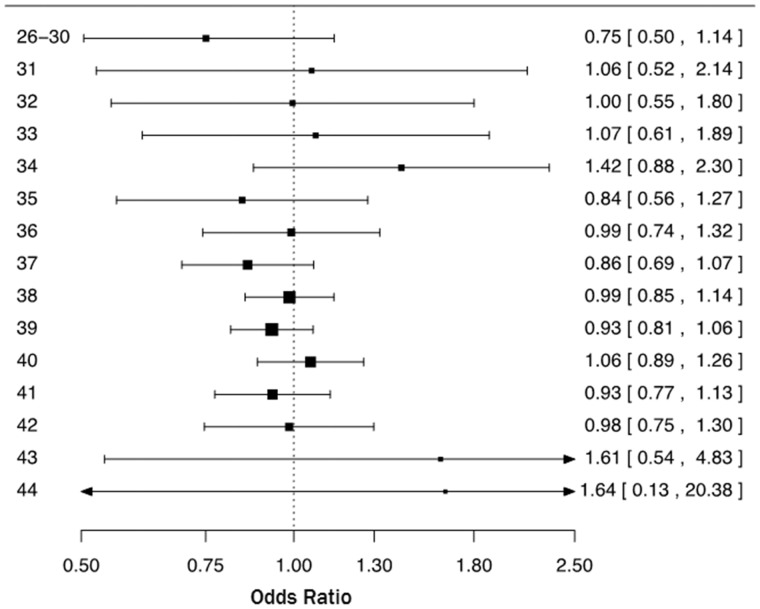
The risk of ASD was similar for emergency and planned CS, both overall (OR = 0.99, 95% CI 0.92–1.06) and by gestational week (Figure 3). Broadly similar OR patterns were seen for ASD when comparing emergency CS with vaginal delivery and planned CS with vaginal delivery across gestational age (SF 1a and b, respectively, available as Supplementary data at IJE online).
Figure 3.
Odds ratios and two-sided 95% confidence intervals of autism spectrum disorder following emergency Caesarean section compared with planned Caesarean section by week of gestation (weeks 26–30 as one group). Each odd ratio was estimated from logistic regression adjusting for site (Denmark, Finland, Norway, Sweden, Western Australia), sex, birth year (1984–89, 1990–1994, 1995–99, and 2000–2004) and maternal age (<25, 25–29, 30–34, 35–39, ≥40).
Site comparison: caesarean section versus vaginal delivery by gestational ages
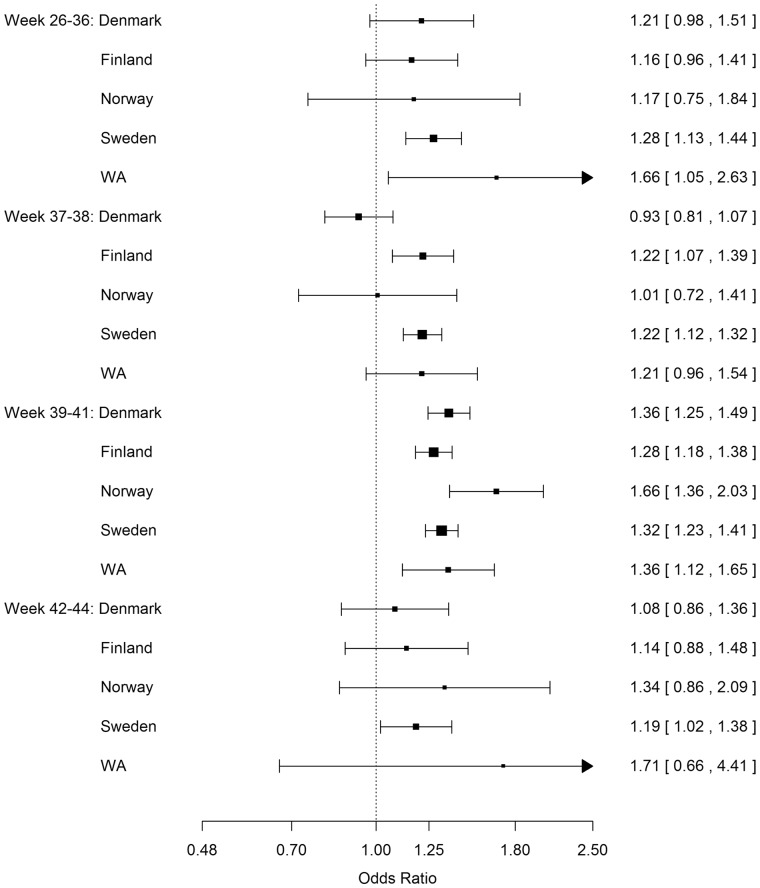
Between sites, there were broadly similar OR patterns for ASD in children delivered by CS compared with children delivered by vaginal delivery by gestational age group (Figure 4; and ST 4, available as Supplementary data at IJE online). In the 37–38 weeks gestational age group, only Denmark had an OR point estimate less than 1. In the 39–41 weeks gestational age group, all ORs were greater than 1. Although Norway had a somewhat higher OR point estimate in the gestational age group 39–41 weeks, the direction was consistent with other sites. In the per week of gestation analysis, there was a statistically significant higher risk of ASD in children born by CS at 36–42 weeks of gestation, which was consistent across sites (SF 2, available as Supplementary data at IJE online). The heterogeneity (Cochrane Q) test results indicated no site heterogeneity (all p-values > 0.9). We found no quantitative differences in the estimated ORs of ASD among children delivered by CS compared with vaginal delivery in the site influence analysis (SF 3, available as Supplementary data at IJE online).
Figure 4.
Odds ratios and two-sided 95% confidence intervals of autism spectrum disorder following Caesarean section compared with vaginal delivery in gestational age subgroups (weeks 26–36, 37–38, 39–41, and 42–44) for each site. WA: Western Australia. Each odds ratio was estimated from ordinary logistic regression adjusting for sex, birth year (1984–89, 1990-1994, 1995-99, and 2000–2004) and maternal age (<25, 25–29, 30–34, 35–39, ≥40).
Subgroup and sensitivity analyses
The OR of ASD among children delivered by CS compared with vaginal delivery was similar for boys (OR = 1.25, 95% CI 1.20–1.29) and girls (OR = 1.30, 95% CI 1.22–1.38). The gestational age group 39–41 weeks had the highest point estimate of OR for both sexes (SF 4 and ST 5, available as Supplementary data at IJE online).
The results of the sensitivity analyses for ASD are given in the lower part of Table 2. Restricting to firstborn children, the OR for ASD of CS versus vaginal delivery was estimated to be 1.22 (95% CI 1.15–1.30). Excluding Finland and adding paternal age as a covariate, the OR for ASD of CS versus vaginal delivery was 1.27 (95% CI 1.22–1.31). The OR estimate only differed by the fourth decimal when we excluded paternal age in this restricted cohort. Using Cox regression models, the HR for ASD of CS versus vaginal delivery was 1.27 (95% CI 1.23–1.31). Restricted to the same date-of-diagnosis cohort (Denmark, Finland and Sweden), the OR for ASD of CS versus vaginal delivery was 1.25 (95% CI 1.21–1.29).
The results of AD are summarized in ST 6–7 (available as Supplementary data at IJE online). Without adjustment for gestational age, the OR for AD of CS compared with vaginal delivery was 1.42 (95% CI 1.35–1.49), and after including gestational age (26–36, 37–38, 39–41 and 42–44 weeks) the OR was estimated as 1.34 (95% CI 1.27–1.41). The results of all the sensitivity analyses [firstborn only, including paternal age (Finland excluded) and Cox regression analysis] were similar and the estimated ORs and HRs ranged from 1.31 tot 1.35. CS was significantly associated with AD in all gestational age subgroups (26–36, 37–38, 39–41 and 42–44 weeks) (SF 5, available as Supplementary data at IJE online).
For all analyses (ASD, AD and subgroups), the goodness-of-fit analyses (Hosmer–Lemshow test) supported the assumption of data following a binomial distribution.
Discussion
To date, this is the largest population-based study of association between caesarean section and the risk of ASD. We included five million singletons in five countries (Norway, Sweden, Denmark, Finland and Western Australia) with more than 31 000 prospectively ascertained cases of ASD. Births delivered by CS had a moderately increased risk of ASD when compared with vaginal delivery, consistently from 36 weeks to 42 weeks of gestation. On average, a 26% higher risk of ASD was presented in all births from week 36 to 42, representing 95% of the total number of births or 90% of all CS births. There was no evidence for differences in ASD risk between emergency CS and planned CS, overall or by week of gestation. Similar overall risk patterns were observed for males and females and for AD specifically, and the patterns remained robust across a variety of sensitivity analyses.
Differently from previous studies,11–16,27,28,39–42 and made possible by the high statistical power, we were able to precisely estimate the association between CS and ASD in specific weeks of gestation, in subgroups of male and female offspring and for AD separately. Using a long study period, including sites with different type of diagnostic registries (medical registries versus government-maintained service/benefits registries), varying incidence of CS and ASD and differing medical practice (planned CS without medical indication is more common in WA than Nordic counties) strengthens the generalizability of our results.
Our results did not support our original hypotheses that CS conducted at or near full term has lower risk than CS conducted preterm, nor that emergency CS has a higher risk of ASD than planned CS. Although the precision of the risk estimates is lower for below 36 weeks of gestation compared with 36–42 weeks, the point estimates for ASD risk from CS below 36 weeks did not indicate any increased risk. This could mean that CS alone before 36 weeks does not add any additional risk for ASD, beyond the risks associated with the adverse medical indications leading to CS or with fetal prematurity. In contrast, CS may confer additional risk for ASD above risks associated with factors leading to CS between 36 and 42 weeks of gestation. Also, the risks from planned and emergency CS were very similar even during weeks 36–42 of gestation, indicating there might be induced risk from CS per se at or near term. A previous meta-analysis was carried out to examine the association between CS and ASD.16 When restricted to the 13 studies which had adjusted for other covariates (only nine of which were published after 199012–15,27,28,39–41 and only one of which39 had more cases than our smallest site), a pooled OR of 1.23 (95% CI 1.07–1.40) was obtained.16 The pooled estimate is similar in magnitude to our overall estimate but with considerably lower precision.
In addition to the ‘before term’ hypothesis, there are several other hypotheses regarding the potential relationship between delivery by CS and ASD. In one study of firstborn children, the risk of ASD was 52% higher in infants whose CS was performed under general anaesthesia than among those who were delivered vaginally or born through CS under regional anaesthesia.42 Neurotoxicity related to neonatal exposure to general anaesthesia might be a contributing factor. However, we found that the OR for ASD following emergency CS (more likely to be performed under general anaesthesia) was similar to the OR following planned CS. Exposure to skin microbiota due to the CS procedure29 and the beneficial effect of being born vaginally (enhancing the immune system)43–45 are other potential hypotheses. Last, there are indications that CS may increase risk of immunological diseases since the child is not exposed to the stress following a vaginal delivery,46 which normally would initiate positive changes in epigenetic expression.
Even though CS, planned or emergency, appears to be associated with a complex mix of factors also associated with ASD risk,47 we did not see a difference in risk of ASD from planned versus emergency CS, even in near-term and term deliveries. Similar to our results, a recent population-based study11 reported that both elective (planned) CS and emergency CS were associated with increased risk of ASD also when adjusting for selected confounding factors. By restricting the population to families with at least two siblings they were able to further adjust for familial confounding. When adjusting for familial confounding neither emergency CS nor elective CS was associated with ASD, indicating tha the association may not be directly causal and implying that unknown familial factors might explain the increased risk of both CS and ASD. However, bias from residual confounding may still remain, e.g. confounding by calendar time arising from changes in obstetric practice over time. In addition, mode of delivery in one pregnancy influences mode of delivery in a subsequent pregnancy, which cannot be controlled for in a sibling design. Assuming the unknown familial factors truly exist and that they are not being influenced by the aforementioned potential biases, our results indicate that the ORs associated with these familiar factors increase the risk of CS and ASD consistently across gestational weeks 36 to 42. A similar pattern also appeared consistently across study sites. Further work is needed to disentangle the contributions to ASD risk arising from the underlying indications versus the procedure itself, in both planned and emergency CS.
The major strength of this study is the large multinational sample size with prospective follow-up. With approximately 5 million live-born singletons and 31 073 ASD cases, our study is more than three times larger than the previous meta-analyses16,48 and almost twice the size of the recent Swedish study, thus providing higher statistical power to conduct more detailed subgroup analyses. For instance, in the gestational week-by-week analyses conducted by each site alone (SF 2), all 95% CIs included 1.0 (with the only exception at gestational week 44 for Norway) despite the consistent elevated ORs from gestational weeks 36 to 44 across sites. Our study also has limitations: the follow-up period from birth to reported diagnosis of ASD is too short for some of the birth years. For example, in Norway the follow-up was until 2006, but the last birth year included was 2004, i.e. the children were 2 years old and unlikely to be diagnosed with ASD. Since logistic regression is not able to directly adjust for the differences in length of follow-up for different individuals due to censoring, a bias may have been introduced. For this reason we fitted Cox regression as a complementary model. Even though we did not have information for all types of censoring, e.g. date of emigration or date of death of a child, this should not affect the results.
First, we only included children surviving their first year of life, removing the possibility of early censoring due to infant death, although the child death rates in the countries in the study are among the lowest in the world. On the other hand, the requirement of surviving the first year of life might potentially induce selection bias (i.e. collider stratification). However, ASD diagnosis before 1 year of age is extremely rare, so such bias should be small. Second, emigration out of the Nordic countries is very low. Third, bias could be introduced if participants are lost to follow-up due to reasons related to CS or gestational age. We see no reason to suspect differences in follow-up due to CS, although children born at early or late term could be at slightly higher risk of censoring due to death or comorbidity, hiding symptoms of autism. Nevertheless, the ORs from logistic regression agreed very closely with the hazard ratios from Cox regression. Also noteworthy is the varying reported ASD frequency between countries. For example, the prevalence of ASD is about one-fifth in Norway compared with the other countries. One possible explanation is that the diagnostic data from Norway were derived from government-maintained service/benefits registries, whereas other countries’ diagnostic data were from medical registries (with WA as an exception). Results from the leave-one-out site influence analysis indicated that the different type of diagnostic data did not influence the estimated ORs.
Our study also has similar limitations compared with previous studies, in that we did not have access to the underlying indication for CS nor sufficient information to conduct sibling designed analysis. Although several potential confounders, e.g. parental psychiatric information, maternal diabetes, pre-eclampsia and socioeconomic status, are available in each site, iCARE data do not contain such information. Also, iCARE was purposely designed to focus only on ASD and does not contain information on other psychological developments, such as attention-deficit/hyperactivity disorder (ADHD) or intellectual disability, which have also been reported to be associated with CS.15,49,50 As ultrasound dating of pregnancies was not universally used throughout the cohort, there may be some (but not) large inaccuracy in gestational age. However, the distribution of gestation periods at delivery (with the vast majority delivered at term) is reassuring in this regard. Due to the constraint of information available in the database, our classification of CS (planned and emergency) might be too simple. WHO recommended the 10-group classification (also known as Robson’s classification) as a global standard for assessing, monitoring and comparing CS rates internationally based on simple obstetric parameters (parity, previous CS, gestational age, onset of labour, fetal presentation and number of fetuses). However, this classification still does not explicitly record the underlying indication for doing CS nor differentiation of elective (maternal request in the absence of maternal or fetal indications) and emergency CS. Finally, we could not take into account the date of death or the date of immigration in the Cox regression analysis, since iCARE does not contain such information.
Conclusion
Across the five countries, birth delivery by CS, planned or emergency, is consistently associated with a modest increased risk of ASD from gestational weeks 36 to 42 when compared with vaginal delivery. These findings may have important implications, especially given the rise in CS around term for non-medical indications.
Supplementary Data
Supplementary data are available at IJE online
Funding
ICARE which supplied data for this work was funded by Autism Speaks grant numbers 6230, 6246, 6247, 6248, 6249, 6251 and 6295. Autism Speaks did not contribute to the design or conduct of the study, collection, management or analysis of the data or interpretation of the results. This study was also in part supported by the Beatrice and Samuel A. Seaver Foundation and by grants from the National Institutes of Health, grant HD073978 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of Environmental Health Sciences and the National Institute of Neurological Disorders and Stroke.
Key Messages
Caesarean section (CS) is associated with a modest increased risk of autism when compared with vaginal delivery.
Compared with vaginal delivery, CS is consistently associated with an increased risk of autism throughout all gestational weeks 36–42.
Thus, the observed risk during this gestational period cannot be attributed to effects of preterm birth for children delivered by CS.
There were no differences in the risk of autism between planned and emergency CS.
Supplementary Material
Acknowledgements
We thank Mr Francisco T. T. Lai for his assistance in the preparation of the manuscript for submission.
Conflict of interest: Authors of this study have had no involvements that might raise the question of bias in the work reported or in the conclusions, implications, or opinions stated.
References
- 1. Leonard H, Dixon G, Whitehouse AJO et al. Unpacking the complex nature of the autism epidemic. Res Autism Spectr Disord 2010;4:548–54. [Google Scholar]
- 2. Nassar N, Dixon G, Bourke J et al. Autism spectrum disorders in young children: effect of changes in diagnostic practices. Int J Epidemiol 2009;38:1245–54. [DOI] [PubMed] [Google Scholar]
- 3. Jensen CM, Steinhausen H-C, Lauritsen MB. Time trends over 16 years in incidence-rates of autism spectrum disorders across the lifespan based on nationwide Danish register data. J Autism Dev Disord 2014;44:1808–18. [DOI] [PubMed] [Google Scholar]
- 4. Taylor B, Jick H, Maclaughlin D. Prevalence and incidence rates of autism in the UK: time trend from 2004–2010 in children aged 8 years. BMJ Open 2013;3:e003219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lundstrom S, Reichenberg A, Anckarsater H, Lichtenstein P, Gillberg C. Autism phenotype versus registered diagnosis in Swedish children: prevalence trends over 10 years in general population samples. BMJ 2015;350:h1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A. The familial risk of autism. JAMA 2014;311:1770–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jordan B. Genes and non-mendelian diseases: dealing with complexity. Perspect Biol Med 2014;57:118–31. [DOI] [PubMed] [Google Scholar]
- 8. Vogel JP, Betrán AP, Vindevoghel N et al. Use of the Robson classification to assess caesarean section trends in 21 countries: a secondary analysis of two WHO multicountry surveys. Lancet Glob Health 2015;3:e260–70. [DOI] [PubMed] [Google Scholar]
- 9. Vieira GO, Fernandes LG, Oliveira NF de, Silva LR, Vieira TDO. Factors associated with cesarean delivery in public and private hospitals in a city of northeastern Brazil: a cross-sectional study. BMC Pregnancy Childbirth 2015;15:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schieve LA, Tian LH, Baio J et al. Population attributable fractions for three perinatal risk factors for autism spectrum disorders, 2002 and 2008. Autism and developmental disabilities monitoring network. Ann Epidemiol 2014;24:260–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Curran EA, Dalman C, Kearney PM et al. Association between obstetric mode of delivery and autism spectrum disorder: a population-based sibling design study. JAMA Psychiatry 2015;72:935–42. [DOI] [PubMed] [Google Scholar]
- 12. Glasson EJ, Bower C, Petterson B, Klerk N de, Chaney G, Hallmayer JF. Perinatal factors and the development of autism: a population study. Arch Gen Psychiatry 2004;61:618–27. [DOI] [PubMed] [Google Scholar]
- 13. Polo-Kantola P, Lampi KM, Hinkka-Yli-Salomäki S, Gissler M, Brown AS, Sourander A. Obstetric risk factors and autism spectrum disorders in Finland. J Pediatr 2014;164:358–65. [DOI] [PubMed] [Google Scholar]
- 14. Burstyn I, Sithole F, Zwaigenbaum L. Autism spectrum disorders, maternal characteristics and obstetric complications among singletons born in Alberta, Canada. Chronic Dis Can 2010;30:125–34. [PubMed] [Google Scholar]
- 15. Langridge AT, Glasson EJ, Nassar N et al. Maternal conditions and perinatal characteristics associated with autism spectrum disorder and intellectual disability. PLoS One 2013;8:e50963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Curran EA, O’Neill SM, Cryan JF et al. Research review: Birth by caesarean section and development of autism spectrum disorder and attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Child Psychol Psychiatry .015;56:500–08. [DOI] [PubMed] [Google Scholar]
- 17. American College of Obstetricians and Gynecologists. Committee Opinion No. 559: Cesarean delivery on maternal request. Obstet Gynecol 2013;121:904–07. [DOI] [PubMed] [Google Scholar]
- 18. MacKay DF, Smith GCS, Dobbie R, Cooper SA, Pell JP. Obstetric factors and different causes of special educational need: Retrospective cohort study of 407 503 schoolchildren. BJOG 2013;120:297–307. [DOI] [PubMed] [Google Scholar]
- 19. Leavey A, Zwaigenbaum L, Heavner K, Burstyn I. Gestational age at birth and risk of autism spectrum disorders in Alberta, Canada. J Pediatr 2013;162:361–68. [DOI] [PubMed] [Google Scholar]
- 20. Schendel D, Bhasin TK. Birth weight and gestational age characteristics of children with autism, including a comparison with other developmental disabilities. Pediatrics 2008;121:1155–64. [DOI] [PubMed] [Google Scholar]
- 21. Kolevzon A, Gross R, Reichenberg A. Prenatal and perinatal risk factors for autism: a review and integration of findings. Arch Pediatr Adolesc Med 2007;161:326–33. [DOI] [PubMed] [Google Scholar]
- 22. Hammond G, Langridge A, Leonard H et al. Changes in risk factors for preterm birth in Western Australia 1984–2006. BJOG 2013;120:1051–60. [DOI] [PubMed] [Google Scholar]
- 23. Sandin S, Schendel D, Magnusson P et al. Autism risk associated with parental age and with increasing difference in age between the parents. Mol Psychiatry 2015;April:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu X, Olsen J, Agerbo E et al. Birth weight, gestational age, fetal growth and childhood asthma hospitalization. Allergy Asthma Clin Immunol 2014;10:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blair E, Morich P, Stanley F. Why do aboriginal newborns weigh less? Gestational age at delivery: estimation, distribution and determinants. Aust N Z J Obstet Gynaecol 1994;34:158–63. [DOI] [PubMed] [Google Scholar]
- 26. Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol 1997;145:72–80. [DOI] [PubMed] [Google Scholar]
- 27. Hultman CM, Sparén P, Cnattingius S. Perinatal risk factors for infantile autism. Epidemiology 2002;13:417–23. [DOI] [PubMed] [Google Scholar]
- 28. Bilder D, Pinborough-Zimmerman J, Miller J, McMahon W. Prenatal, perinatal, and neonatal factors associated with autism spectrum disorders. Pediatrics 2009;123:1293300. [DOI] [PubMed] [Google Scholar]
- 29. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012;13:701–12. [DOI] [PubMed] [Google Scholar]
- 30. Einarsdóttir K, Ball S, Pereira G et al. Changes in caesarean delivery rates in Western Australia from 1995 to 2010 by gestational age at birth. Paediatr Perinat Epidemiol 2015;29:290–98. [DOI] [PubMed] [Google Scholar]
- 31. Osterman MJK, Martin JA. Changes in cesarean delivery rates by gestational age: United States, 1996–2011. NCHS Data Brief 2013;124:1–8. [PubMed] [Google Scholar]
- 32. Einarsdóttir K, Haggar F, Pereira G et al. Role of public and private funding in the rising caesarean section rate: a cohort study. BMJ Open 2013;3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O’Leary CM, Klerk N De, Keogh J et al. Trends in mode of delivery during 1984–2003: Can they be explained by pregnancy and delivery complications? BJOG 2007;114:855–64. [DOI] [PubMed] [Google Scholar]
- 34. Robson S, Campbell B, Pell G et al. Concordance of maternal and paternal decision-making and its effect on choice for vaginal birth after caesarean section. Aust N Z J Obstet Gynaecol 2015;55:257–61. [DOI] [PubMed] [Google Scholar]
- 35. Schendel DE, Bresnahan M, Carter KW et al. The International Collaboration for Autism Registry Epidemiology (iCARE): multinational registry-based investigations of autism risk factors and trends. J Autism Dev Disord 2013;43:2650–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carter KW, Francis RW, Carter KW et al. ViPAR: a software platform for the Virtual Pooling and Analysis of Research Data. Int J Epidemiol 2015, Oct 8. pii: dyv193. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ananth CV, Chauhan SP. Epidemiology of twinning in developed countries. Semin Perinatol 2012;36:156–61. [DOI] [PubMed] [Google Scholar]
- 38. Jørgensen FS. Epidemiological studies of obstetric ultrasound examinations in Denmark 1989–1990 versus 1994–1995. Acta Obstet Gynecol Scand 1999;78:305–09. [DOI] [PubMed] [Google Scholar]
- 39. Gregory SG, Anthopolos R, Osgood CE, Grotegut CA, Miranda ML. Association of autism with induced or augmented childbirth in North Carolina Birth Record (1990–1998) and Education Research (1997–2007) databases. JAMA Pediatr 2013;167:959–66. [DOI] [PubMed] [Google Scholar]
- 40. Maimburg RD, Vaeth M. Perinatal risk factors and infantile autism. Acta Psychiatr Scand 2006;114:257–64. [DOI] [PubMed] [Google Scholar]
- 41. Zhang X, Lv CC, Tian J et al. Prenatal and perinatal risk factors for autism in China. J Autism Dev Disord 2010;40:1311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chien L-N, Lin H-C, Shao Y-HJ, Chiou S-T, Chiou H-Y. Risk of autism associated with general anesthesia during cesarean delivery: a population-based birth-cohort analysis. J Autism Dev Disord 2015;45:932–42. [DOI] [PubMed] [Google Scholar]
- 43. Song SJ, Dominguez-Bello MG, Knight R. How delivery mode and feeding can shape the bacterial community in the infant gut. CMAJ 2013;185:373–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dominguez-Bello MG, Costello EK, Contreras M et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A 2010;107:11971–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Azad MB, Konya T, Maughan H et al. Gut microbiota of healthy Canadian infants: Profiles by mode of delivery and infant diet at 4 months. CMAJ 2013;18:385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Almgren M, Schlinzig T, Gomez-Cabrero D et al. Cesarean delivery and hematopoietic stem cell epigenetics in the newborn infant: Implications for future health? Am J Obstet Gynecol 2014;211:502.e1–502.e8. [DOI] [PubMed] [Google Scholar]
- 47. Nippita T, Lee Y, Patterson J et al. Variation in hospital caesarean section rates and obstetric outcomes among nulliparae at term: a population-based cohort study. BJOG 2015;122:702–11. [DOI] [PubMed] [Google Scholar]
- 48. Curran EA, Cryan JF, Kenny LC, Dinan TG, Kearney PM, Khashan AS. Obstetrical mode of delivery and childhood behavior and psychological development in a British cohort. J Autism Dev Disord 2015;46:603–14. [DOI] [PubMed] [Google Scholar]
- 49. Silva D, Colvin L, Hagemann E, Bower C. Environmental risk factors by gender associated with attention-deficit/hyperactivity disorder. Pediatrics 2014;133:e14–22. [DOI] [PubMed] [Google Scholar]
- 50. Curran EA, Khashan AS, Dalman C et al. Obstetric mode of delivery and attention-deficit/hyperactivity disorder: a sibling-matched study. Int J Epidemiol 2016;45:532–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.