Abstract
Background. Incident hemodialysis patients may experience rapid weight loss in the first few months of starting dialysis. However, trends in weight changes over time and their associations with survival have not yet been characterized in this population.
Methods. In a large contemporary US cohort of 58 106 patients who initiated hemodialysis during 1 January 2007–31 December 2011 and survived the first year of dialysis, we observed trends in weight changes during the first year of treatment and then examined the association of post-dialysis weight changes with all-cause mortality.
Results. Patients' post-dialysis weights rapidly decreased and reached a nadir at the 5th month of dialysis with an average decline of 2% from baseline, whereas obese patients (body mass index ≥30 kg/m2) did not reach a nadir and lost ∼3.8% of their weight by the 12th month. Compared with the reference group (−2 to 2% changes in weight), the death hazard ratios (HRs) of patients with −6 to −2% and greater than or equal to −6% weight loss during the first 5 months were 1.08 (95% confidence interval, 1.02–1.14) and 1.14 (1.07–1.22), respectively. Moreover, the death HRs with 2–6% and ≥6% weight gain during the 5th to 12th months were 0.91 (0.85–0.97) and 0.92 (0.86–0.99), respectively.
Conclusions. In patients who survive the first year of hemodialysis, a decline in post-dialysis weight is observed and reaches a nadir at the 5th month. An incrementally larger weight loss during the first 12 months is associated with higher death risk, whereas weight gain is associated with greater survival during the 5th to 12th month but not in the first 5 months of dialysis therapy.
Keywords: body mass index, body weight, hemodialysis, mortality
INTRODUCTION
Obesity is believed to be an important risk factor for cardiovascular disease, chronic kidney disease and mortality in the general population [1–3]. However, it has been found to have paradoxical associations with outcomes in severely ill patients such as those with congestive heart failure, chronic obstructive pulmonary disease and rheumatoid arthritis [4–6]. This phenomenon has been referred to as the ‘obesity paradox’ or ‘reverse epidemiology’, where obesity has actually been associated with better survival [7–11]. This inverse relationship between obesity and outcomes is particularly strong and consistent among patients with end-stage renal disease (ESRD) [12].
In particular, many observational studies among patients undergoing maintenance hemodialysis (MHD) have shown that a higher baseline body mass index (BMI) at dialysis initiation, not a lower BMI, is associated with better outcomes [13–18]. However, most literature on obesity in ESRD has examined the association of baseline BMI levels ascertained at a single point in time with future outcomes, which offer limited insight into how changes in weight over time impact outcomes in dialysis patients. Recent large observational studies on changes in weight over time on dialysis have also indicated that weight gain, mostly in the first 6 months after starting dialysis, is associated with better survival, whereas weight loss harbors a higher death risk [19–23]. Trends in weight change after starting dialysis have not yet been characterized in these studies. However, it should be noted that patients' weight is a fluctuating parameter, which is not static but dynamic over time [24]. Given frequent observations in clinical practice that patients' weight tends to rapidly decrease in the first few months of starting hemodialysis, and then gradually increase or stabilize during treatment, it could be speculated that the associations of changes in weight with mortality may differ according to the time period in which this occurs. For example, weight changes in the early phases of dialysis may have a differential association with outcomes when compared with changes in later phases of dialysis. However, this hypothesis has never been tested.
Therefore, we undertook this study to characterize trends in post-dialysis dry weight changes during the first year of dialysis initiation and to also evaluate the association of weight changes with mortality in incident hemodialysis patients. Given that the baseline BMI may affect the trajectory of weight changes over time, we also examined the potential contribution of baseline BMI to this weight change–mortality relationship. We hypothesized that the inverse association between changes in weight and mortality in MHD patients are consistently observed independent of baseline BMI and may be even stronger in the early months of weight loss.
MATERIALS AND METHODS
Study population
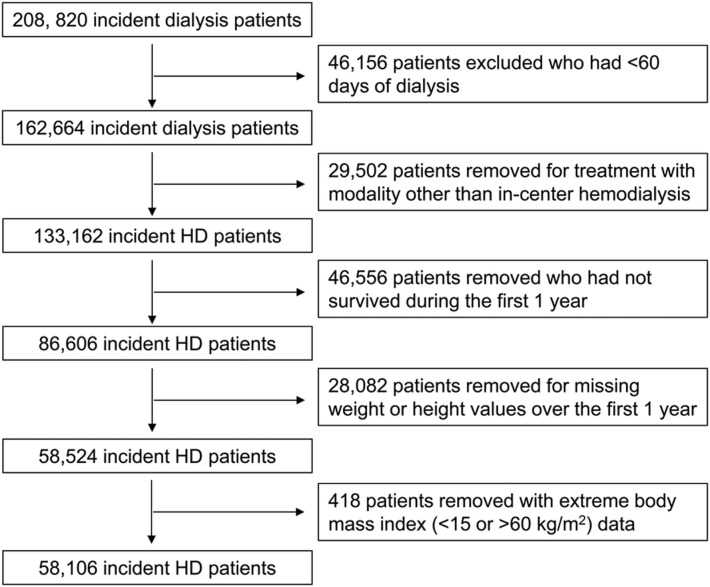
We extracted and examined data from all patients with ESRD who underwent MHD treatments between January 2007 and December 2011 within one of the outpatient facilities of a large dialysis organization. The creation of this patient cohort has been described previously [25]. Patients were included provided that they were 18 years or older and received dialysis therapy for at least 60 days. We additionally restricted our analysis to patients who were treated with only in-center hemodialysis over the entire duration of follow-up and who survived during the first year of dialysis initiation. We further excluded those patients with missing BMI data and those with baseline BMI data <15 or >60 kg/m2. Therefore, the final study population consisted of 58 106 patients (Figure 1). The study was approved by the University of California Irvine. Given the large sample size, anonymity of the patients studied and nonintrusive nature of the research, the requirement for written consent was waived.
FIGURE 1.
Flow chart of participants in the cohort.
Demographic, clinical and laboratory measures
Data from dialysis facility electronic medical records were used to determine demographics and comorbidities. Each patient's weight is measured and recorded at the beginning and the end of every dialysis treatment. BMI was calculated from post-dialysis dry body weight in kilograms divided by height in meters squared. To minimize measurement variability, patients' weights for each month interval from the date of dialysis therapy initiation were averaged into one-single monthly value. In conjunction with body weights, ultrafiltration and post-dialysis systolic blood pressure (SBP) were also ascertained as the mean of all available values over successive monthly intervals from the date of dialysis initiation. The patients' first month of dialysis was considered the baseline month. Accordingly, each patient had up to 13 recorded body weight, ultrafiltration and post-dialysis SBP measurements corresponding to thrice-weekly MHD treatments. All repeated laboratory parameters were averaged for each patient for each month and used in all models. Blood samples were drawn using standardized techniques in all dialysis clinics and were transported to a central laboratory in Deland, Florida, typically within 24 h. All laboratory values were measured using automated and standardized methods.
Outcome ascertainment
The study outcome of interest was all-cause mortality, and patients were followed over a 4-year period from the first day of the second year of starting hemodialysis therapy. Patients were censored for loss to follow-up, discontinuation of dialysis therapy, kidney transplantation or transfer to a nonaffiliated dialysis clinic.
Statistical analyses
We examined the trend of changes in weight during the first year of dialysis using linear regression models with unstructured variance to account for intrasubject correlations in repeated measurements. For sensitivity analyses, we also examined the trajectories of change in monthly averaged ultrafiltration and post-dialysis SBP during the first year following dialysis initiation to further address the potential role of volume factors for change in body weight. To evaluate its relationship with baseline BMI, we divided BMI into four a priori selected categories: underweight (<20 kg/m2), normal weight (20 to <25 kg/m2), overweight (25 to <30 kg/m2) and obese status (≥30 kg/m2). These increments were consistent with those selected in our previous study [19]. Although underweight BMI has been usually defined as <18.5 kg/m2 based on the World Health Organization guidelines, in an attempt to minimize the possible statistical error which can be caused by lower sample size in this group (n = 1442; 2.5% of entire cohort), we considered underweight patients as those included in the lowest BMI category (<20 kg/m2) in this study [26]. Based on results from the trend analyses, we decided to divide the first year weight change observational period into two different intervals, between 1–5 months and 5–12 months. We calculated changes in weight during each interval according to the difference between consecutive post-dialysis weight values, expressed as a percentage [interval 1 weight change: 100× ((month 5 post-dialysis weight (kg) − month 1 post-dialysis weight (kg))/ month 1 post-dialysis weight (kg)) %; interval 2 weight change: 100× ((month 12 post-dialysis weight (kg) − month 5 post-dialysis weight (kg))/ month 5 post-dialysis weight (kg)) %]. Additionally, we divided the percent weight change in each interval into five ordinal categories (less than or equal to −6%, −6 to less than or equal to −2%, −2 to 2%, 2 to <6%, and ≥6%). Categories were selected based on identifying the middle quintile range (−2 to 2%, reference group) weight change during the first year of dialysis and adding a 4% weight change increment in each direction. For subsequent analyses, the effect of weight changes was nested within BMI categories, creating a 20-category ‘BMI-weight changes’ variable. Normal weight (BMI of 20 to <25 kg/m2) and minimal weight change (−2 to 2% of weight change) were designated as the reference groups. In sensitivity analysis, associations of weight changes were additionally examined across strata of ultrafiltration (<1.5, 1.5 to <2.5, 2.5 to <3.5 and ≥3.5 kg) and baseline serum albumin concentrations (<3.2, 3.2 to <3.5, 3.5 to <3.8 and ≥3.8 g/dL).
Cox proportional hazard regression models were performed to study the associations of weight changes during two time intervals over the first year of dialysis with all-cause mortality and to assess the effect of baseline BMI on these associations. Weight change percentage exposures were evaluated both as continuous and categorical predictors. For each analysis, three models were constructed based on the level of multivariate adjustment: (i) minimally adjusted models, which included the weight change percentage exposure interval of interest and weight of first month in each treatment period (month 1 or month 5 in intervals 1 or 2, respectively); (ii) case-mix adjusted models, which adjusted for baseline characteristics of age, sex, race/ethnicity (white, African American, Hispanic, Asian or other), primary insurance (Medicare, Medicaid or other), initial vascular access type (central venous catheter, arteriovenous fistula, arteriovenous graft or other), 10 comorbid conditions (diabetes mellitus, hypertension, atherosclerotic heart disease, congestive heart failure, other cardiovascular disease, cerebrovascular disease, dyslipidemia, HIV, chronic obstructive pulmonary disease and malignancy), alcohol dependence, substance abuse, dialysis ultrafiltration and dialysis dose as indicated by single-pool Kt/V; and (iii) case-mix plus malnutrition-inflammation-cachexia syndrome models, which included all covariates in the case-mix model plus 10 laboratory variables that bear associations with clinical outcomes in hemodialysis patients: white blood cells, lymphocytes, serum albumin, creatinine, bicarbonate, calcium, phosphorus, hemoglobin, total iron binding capacity and normalized protein catabolic rate. Both baseline month 1 and month 5 (or month 5 and month 12) laboratory values were included in the respective models. Mortality associations are expressed as a hazard ratio (HR) and 95% confidence interval (CI).
Data were summarized using proportions, means (±standard deviation, SD) or median (interquartile range) as appropriate, and were compared using ANOVA, Kruskal–Wallis and χ2 tests, respectively. Patients were excluded from an analysis if they had missing covariate data (<1% for all tests). All statistical analyses were implemented using Stata, version 13.1 (Stata Corp LP).
RESULTS
Patient characteristics
The baseline demographics, clinical and laboratory characteristics of the patients stratified by categories of baseline BMI are summarized in Table 1. The mean age of patients was 61.8 years (range, 18–109 years), 44% were females and 68% were diabetic. The mean starting post-dialysis weight and baseline BMI were 81.6 ± 21.9 kg and 28.7 ± 7.1 kg/m², respectively. Patients with a higher baseline BMI tended to be younger and diabetic, had a lower achieved dialysis adequacy, but had higher ultrafiltration. Patients in the lowest BMI group tended to be Asian and had a lower creatinine.
Table 1.
Patient characteristics stratified by baseline body mass index categories
| Characteristics | Total | Body mass index (kg/m2) |
|||
|---|---|---|---|---|---|
| <20 | 20 to <25 | 25 to <30 | ≥30 | ||
| (n = 58 106) | (n = 3772) | (n = 16 414) | (n = 17 385) | (n = 20 535) | |
| Age (years) | 61.8 ± 14.8 | 62.5 ± 17.3 | 63.6 ± 15.9 | 62.7 ± 14.5 | 59.4 ± 13.2 |
| Gender: % women | 43.8 | 49.4 | 37.6 | 38.8 | 51.8 |
| Race (%) | |||||
| White | 42.1 | 40.0 | 40.2 | 41.5 | 44.4 |
| Black | 34.1 | 35.5 | 31.5 | 32.5 | 37.4 |
| Hispanic | 16.3 | 13.5 | 18.4 | 18.4 | 13.3 |
| Asian | 3.5 | 7.2 | 5.7 | 3.3 | 1.2 |
| Other | 4.0 | 3.9 | 4.2 | 4.2 | 3.7 |
| Primary insurance (%) | |||||
| Medicare | 52.1 | 55.8 | 53.7 | 52.3 | 49.9 |
| Medicaid | 7.5 | 9.3 | 8.3 | 7.4 | 6.6 |
| Other | 40.4 | 34.9 | 38.0 | 40.3 | 43.4 |
| Initial vascular access type (%) | |||||
| Central venous catheter | 73.9 | 76.8 | 74.9 | 73.3 | 73.1 |
| Arteriovenous fistula | 17.2 | 14.1 | 16.6 | 18.1 | 17.4 |
| Arteriovenous graft | 4.7 | 4.7 | 4.6 | 4.6 | 4.9 |
| Other and unknown | 4.2 | 4.4 | 3.9 | 4.0 | 4.6 |
| Comorbidities (%) | |||||
| Diabetes | 68.3 | 48.7 | 60.7 | 68.7 | 77.6 |
| Hypertension | 53.1 | 58.9 | 56.3 | 53.3 | 49.4 |
| Congestive heart failure | 44.5 | 36.7 | 39.6 | 43.6 | 50.7 |
| Atherosclerotic heart disease | 16.6 | 16.1 | 16.6 | 16.6 | 16.7 |
| Other cardiovascular disease | 16.3 | 16.8 | 16.4 | 16.4 | 16.2 |
| Cerebrovascular disease | 1.8 | 1.9 | 1.9 | 1.8 | 1.7 |
| Dyslipidemia | 29.8 | 28.0 | 28.5 | 30.3 | 30.9 |
| HIV | 0.4 | 0.8 | 0.7 | 0.4 | 0.3 |
| Chronic obstructive pulmonary disease | 4.9 | 5.4 | 4.1 | 4.8 | 5.5 |
| History of malignancy | 2.0 | 2.2 | 2.2 | 2.0 | 1.7 |
| Alcohol dependence (%) | 0.2 | 0.5 | 0.3 | 0.2 | 0.1 |
| Substance abuse (%) | 0.2 | 0.6 | 0.3 | 0.2 | 0.1 |
| Body mass index (kg/m2) | 28.7 ± 7.1 | 18.6 ± 1.1 | 22.8 ± 1.4 | 27.3 ± 1.5 | 36.4 ± 5.6 |
| Post-dialysis weight at 1 month (kg) | 81.6 ± 21.9 | 53.4 ± 7.6 | 65.3 ± 9.1 | 78.5 ± 10.6 | 102.6 ± 19.4 |
| Post-dialysis weight at 5 months (kg) | 79.7 ± 20.9 | 53.8 ± 8.3 | 64.7 ± 9.7 | 76.7 ± 11.2 | 98.9 ± 19.1 |
| Post-dialysis weight at 12 months (kg) | 79.9 ± 21.0 | 54.8 ± 9.0 | 65.5 ± 10.4 | 77.1 ± 12.2 | 98.5 ± 19.7 |
| Dialysis dose: single-pool Kt/V | 1.4 ± 0.4 | 1.6 ± 0.4 | 1.5 ± 0.4 | 1.4 ± 0.4 | 1.3 ± 0.4 |
| Dialysis ultrafiltration (kg) | 1.9 ± 0.9 | 1.5 ± 0.8 | 1.7 ± 0.8 | 1.8 ± 0.9 | 2.1 ± 0.9 |
| Normalized protein catabolic rate (g/kg/day) | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.2 |
| Laboratory parameters | |||||
| Hemoglobin (g/dL) | 10.3 ± 1.2 | 10.3 ± 1.3 | 10.3 ± 1.3 | 10.3 ± 1.2 | 10.3 ± 1.2 |
| White blood cells (× 103/µL) | 8.0 ± 3.0 | 7.8 ± 3.2 | 7.8 ± 3.1 | 7.9 ± 2.9 | 8.3 ± 2.9 |
| Lymphocyte (% of white blood cells) | 19.5 ± 7.8 | 19.1 ± 8.1 | 19.4 ± 8.0 | 19.5 ± 7.7 | 19.6 ± 7.6 |
| Albumin (g/dL) | 3.5 ± 0.5 | 3.4 ± 0.5 | 3.4 ± 0.5 | 3.5 ± 0.5 | 3.5 ± 0.5 |
| Calcium (mg/dL) | 8.5 ± 0.7 | 8.5 ± 0.8 | 8.5 ± 0.7 | 8.5 ± 0.7 | 8.6 ± 0.7 |
| Phosphorus (mg/dL) | 4.7 ± 1.3 | 4.6 ± 1.3 | 4.7 ± 1.3 | 4.7 ± 1.2 | 4.8 ± 1.2 |
| Creatinine (mg/dL) | 6.0 ± 2.4 | 5.6 ± 2.2 | 6.0 ± 2.3 | 6.1 ± 2.4 | 6.0 ± 2.4 |
| Bicarbonate (mEq/L) | 23.6 ± 3.3 | 23.3 ± 3.5 | 23.5 ± 3.3 | 23.5 ± 3.2 | 23.7 ± 3.2 |
| Total iron binding capacity (mg/dL) | 228.7 ± 51.3 | 214.1 ± 51.6 | 222.1 ± 49.6 | 228.6 ± 50.6 | 236.8 ± 51.7 |
Data are presented as the mean ± standard deviation or percentages.
Trends in weight change during the first year of dialysis
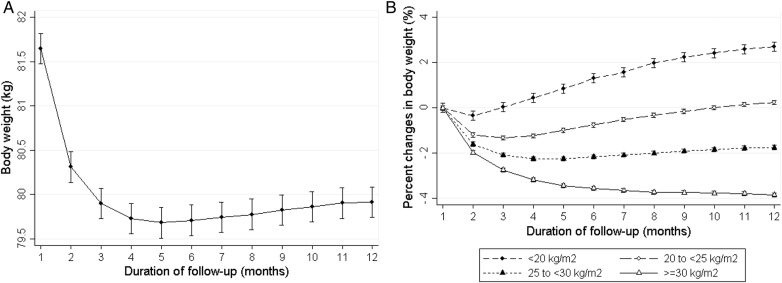
After initiating dialysis, patients' weight on average rapidly decreased and reached a nadir of weight at the 5th month of dialysis with an average weight drop of 2% from baseline; thereafter patient weights gradually increased up to the 12th month (Figure 2A). However, trends differed across groups of baseline BMI groups. Obese patients (BMI ≥30 kg/m2) did not reach a nadir and lost ∼3.8% of their weight by the 12th month (Figure 2B). In sensitivity analyses, when extending the observation period up to the first 2 years of dialysis in 33 209 patients who survived over 2 years of therapy, trends in weight change were observed to be similar (Supplementary data, Figure S1). In addition, we also examined the trends of change in monthly averaged ultrafiltration and post-dialysis SBP during the first year following dialysis initiation to further address the potential role of volume factors for change in body weight. We found that their trends did not mirror the body weight trajectory over time; ultrafiltration rapidly increased up to the 5th month of dialysis and then gradually increased without a nadir, whereas post-dialysis SBP sharply decreased up to the 5th month of dialysis and continued to decrease by the 12th month of dialysis. Unlike body weight, the trends were also consistent across all baseline BMI categories (Supplementary data, Figure S2).
FIGURE 2.
The trajectory of body weight changes over the first 1-year period of dialysis in an unadjusted model. (A) Change in weight expressed as kilograms in entire cohort. (B) Change in weight expressed as percentage stratified by four body mass index categories.
Body weight changes and all-cause mortality
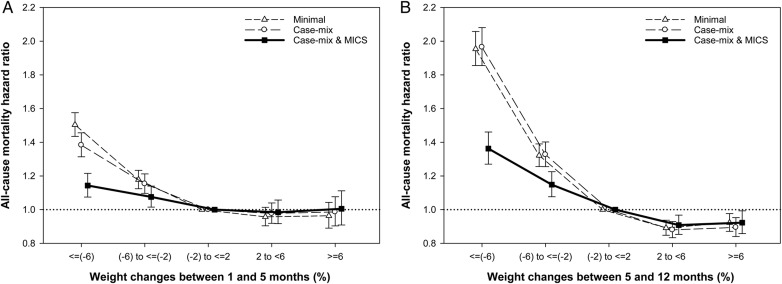
During the mean follow-up of 17.3 months, 12 913 deaths occurred with a crude mortality rate of 154 deaths per 1000 patient-years (95% CI, 151–157). To account for weight changes in the first year of dialysis and assessment of all-cause mortality, we performed Cox regression analyses separately according to two different treatment periods: changes in weight during the 1st to 5th months versus 5th to 12th months of dialysis initiation (Table 2; Figure 3). Given the body weight changes occurring between the 1st and 5th months, compared with the reference group (−2 to 2% of weight change), the association between weight changes of −6 to −2% and greater than or equal to −6% with mortality were: HR 1.08 (95% CI, 1.02–1.14; P= 0.013) and HR 1.14 (95% CI, 1.07–1.22; P < 0.001), respectively. Notably, the observed associations of weight changes with mortality were even stronger when changes in weight between the 5th to 12th months were considered. Each 4% increase of body weight between the 5th and 12th months was associated with an HR of 0.92 (95% CI, 0.90–0.93; P < 0.001), whereas this same degree of body weight change was associated with an HR of 0.96 (95% CI, 0.95–0.98; P < 0.001) over first 5 months. Moreover, patients with 2–6% and ≥6% of weight gains were significantly associated with 9% and 8% lower risks of death, respectively.
Table 2.
Association of percent changes in body weight with all-cause mortality
| Minimal |
Case-mix |
Case-mix and MICS |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| (n = 58 106) |
(n = 51 052) |
(n = 38 828) |
|||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Between 1 and 5 months | |||||||||
| Continuous model | |||||||||
| (per 4% increase) | 0.89 | (0.88–0.90) | <0.001 | 0.91 | (0.90–0.92) | <0.001 | 0.96 | (0.95–0.98) | <0.001 |
| Categorical model | |||||||||
| ≤(−6%) | 1.50 | (1.44–1.58) | <0.001 | 1.38 | (1.31–1.46) | <0.001 | 1.14 | (1.07–1.22) | <0.001 |
| (−6%) to ≤(−2%) | 1.18 | (1.12–1.23) | <0.001 | 1.15 | (1.10–1.21) | <0.001 | 1.08 | (1.02–1.14) | 0.013 |
| (−2%) to 2% | 1.0 | 1.0 | 1.0 | ||||||
| 2% to <6% | 0.96 | (0.90–1.01) | 0.135 | 0.98 | (0.92–1.04) | 0.462 | 0.98 | (0.92–1.06) | 0.661 |
| ≥6% | 0.96 | (0.89–1.04) | 0.357 | 0.99 | (0.90–1.08) | 0.756 | 1.01 | (0.91–1.11) | 0.922 |
| Between 5 and 12 months | |||||||||
| Continuous model | |||||||||
| (per 4% increase) | 0.85 | (0.84–0.86) | <0.001 | 0.84 | (0.83–0.85) | <0.001 | 0.92 | (0.90–0.93) | <0.001 |
| Categorical model | |||||||||
| ≤(−6%) | 1.94 | (1.89–2.06) | <0.001 | 1.96 | (1.86–2.08) | <0.001 | 1.36 | (1.27–1.46) | <0.001 |
| (−6%) to ≤(−2%) | 1.32 | (1.26–1.39) | <0.001 | 1.33 | (1.26–1.40) | <0.001 | 1.15 | (1.08–1.22) | <0.001 |
| (−2%) to 2% | 1.0 | 1.0 | 1.0 | ||||||
| 2% to <6% | 0.89 | (0.85–0.94) | <0.001 | 0.88 | (0.83–0.93) | <0.001 | 0.91 | (0.85–0.97) | 0.003 |
| ≥6% | 0.92 | (0.87–0.98) | 0.005 | 0.89 | (0.84–0.85) | <0.001 | 0.92 | (0.86–0.99) | 0.031 |
Adjustments in minimally adjusted models: mortality data and weight of first month in each treatment period; case-mix adjusted models: minimally adjusted model plus baseline characteristics of age, sex, race/ethnicity (white, African American, Hispanic, Asian or other), primary insurance (Medicare, Medicaid or other), vascular access type (central venous catheter, arteriovenous fistula, arteriovenous graft or other), 10 comorbid conditions (diabetes mellitus, hypertension, atherosclerotic heart disease, congestive heart failure, other cardiovascular disease, cerebrovascular disease, dyslipidemia, HIV, chronic obstructive pulmonary disease and malignancy), alcohol dependence, substance abuse, dialysis ultrafiltration and dialysis dose as indicated by single-pool Kt/V; case-mix plus malnutrition-inflammation-cachexia syndrome (MICS) models: case-mix adjusted model plus 10 laboratory variables including white blood cells, lymphocytes, serum albumin, creatinine, bicarbonate, calcium, phosphorus, hemoglobin, total iron binding capacity and normalized protein catabolic rate. HR, hazard ratio; CI, confidence interval.
FIGURE 3.
Association of percent changes in body weight with all-cause mortality (hazard ratios and 95% confidence interval error bars). (A) Weight changes between 1 and 5 months. (B) Weight changes between 5 and 12 months. MICS, malnutrition-inflammation-cachexia syndrome. Models adjusted for case–mix covariates and markers of malnutrition and inflammation (see text for covariate list).
In addition, when we considered changes in body weight as an absolute amount (kg) as opposed to a proportion of overall weight, associations between weight change and mortality were similar and consistent, indicating that incrementally greater declines in post-dialysis dry weight during the first 12 months of dialysis therapy were associated with higher death risk, whereas weight gain was associated with greater survival during the 5th to 12th months, but not in the first 5 months of dialysis therapy (Supplementary data, Table S1).
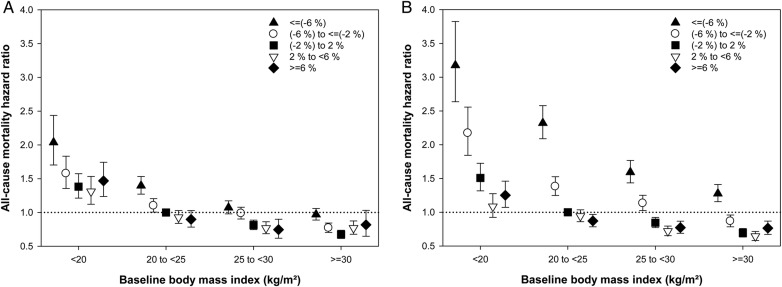
Mortality risk for combinations of weight change with baseline body mass index
As we found that the trends in weight change differ according to baseline BMI, we additionally examined for potential effect modification of baseline BMI on the weight–mortality relationship. Mortality risks for combinations of weight changes with baseline BMI are shown in Table 3 and Figure 4. Similar inverse relationships between percent changes in weight and mortality were observed in all stratified BMI groups; patients undergoing a weight loss in both intervals had a higher death risk, whereas weight gain was associated with better survival, except in patients with extreme weight gain (≥6%). Moreover, this relationship was particularly linear and strong among patients with normal BMI (20–25 kg/m2) at the time of dialysis initiation. When considering kilogram changes in weight, similar associations were found, suggesting that the association between weight changes and mortality might be influenced by baseline BMI, but the trend of an inverse relationship between the two is likely to be consistent across all BMI strata (Supplementary data, Figure S3). Finally, to address possible confounding effects between weight changes and ultrafiltration and malnutrition-inflammation, we performed additional sensitivity analyses based on a priori defined mean ultrafiltration (<1.5, 1.5 to <2.5, 2.5 to <3.5, and ≥3.5 kg) and baseline serum albumin concentrations (<3.2, 3.2 to <3.5, 3.5 to <3.8, and ≥3.8 g/dL) in each time period. These findings also showed that association between incrementally higher losses in post-dialysis weight and poorer survival was strong and consistent across all ultrafiltration and albumin groups (Supplementary data, Figures S4 and S5).
Table 3.
Association of percent changes in body weight with all-cause mortality by baseline BMI groups
| BMI | Weight changes | Minimal |
Case-mix |
Case-mix and MICS |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n = 58 106) |
(n = 51 052) |
(n = 38 828) |
||||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | ||
| Between 1 and 5 months | ||||||||||
| <20 | ≤(−6%) | 1.98 | (1.68–2.33) | <0.001 | 2.04 | (1.70–2.44) | <0.001 | 1.52 | (1.24–1.87) | <0.001 |
| (−6%) to ≤(−2%) | 1.57 | (1.37–1.81) | <0.001 | 1.58 | (1.36–1.83) | <0.001 | 1.37 | (1.16–1.63) | <0.001 | |
| (−2%) to 2% | 1.25 | (1.11–1.42) | <0.001 | 1.38 | (1.21–1.57) | <0.001 | 1.25 | (1.07–1.45) | 0.004 | |
| 2% to <6% | 1.13 | (0.98–1.31) | 0.089 | 1.31 | (1.12–1.53) | 0.001 | 1.16 | (0.97–1.39) | 0.108 | |
| ≥6% | 1.32 | (1.14–1.55) | <0.001 | 1.47 | (1.24–1.74) | <0.001 | 1.41 | (1.15–1.72) | 0.001 | |
| 20 to <25 | ≤(−6%) | 1.54 | (1.41–1.67) | <0.001 | 1.40 | (1.27–1.53) | <0.001 | 1.14 | (1.02–1.27) | 0.020 |
| (−6%) to ≤(−2%) | 1.13 | (1.04–1.23) | 0.005 | 1.10 | (1.01–1.20) | 0.038 | 0.99 | (0.89–1.10) | 0.836 | |
| (−2%) to 2% | 1.0 | 1.0 | 1.0 | |||||||
| 2% to <6% | 0.92 | (0.84–1.02) | 0.106 | 0.93 | (0.84–1.03) | 0.152 | 0.90 | (0.80–1.02) | 0.091 | |
| ≥6% | 0.85 | (0.75–0.96) | 0.010 | 0.90 | (0.78–1.03) | 0.117 | 0.92 | (0.79–1.07) | 0.298 | |
| 25 to <30 | ≤(−6%) | 1.20 | (1.10–1.30) | <0.001 | 1.07 | (0.98–1.17) | 0.136 | 0.92 | (0.83–1.02) | 0.131 |
| (−6%) to ≤(−2%) | 1.03 | (0.95–1.12) | 0.443 | 0.99 | (0.90–1.08) | 0.760 | 0.98 | (0.88–1.08) | 0.647 | |
| (−2%) to 2% | 0.83 | (0.76–0.89) | <0.001 | 0.81 | (0.75–0.89) | <0.001 | 0.83 | (0.76–0.92) | <0.001 | |
| 2% to <6% | 0.77 | (0.69–0.86) | <0.001 | 0.77 | (0.69–0.86) | <0.001 | 0.83 | (0.73–0.94) | 0.005 | |
| ≥6% | 0.72 | (0.61–0.85) | <0.001 | 0.75 | (0.62–0.90) | 0.002 | 0.81 | (0.65–1.00) | 0.052 | |
| ≥30 | ≤(−6%) | 1.05 | (0.97–1.13) | 0.221 | 0.97 | (0.89–1.06) | 0.489 | 0.85 | (0.75–0.92) | <0.001 |
| (−6%) to ≤(−2%) | 0.78 | (0.72–0.84) | <0.001 | 0.77 | (0.70–0.84) | <0.001 | 0.75 | (0.67–0.83) | <0.001 | |
| (−2%) to 2% | 0.68 | (0.62–0.73) | <0.001 | 0.67 | (0.62–0.74) | <0.001 | 0.71 | (0.64–0.78) | <0.001 | |
| 2% to <6% | 0.72 | (0.64–0.81) | <0.001 | 0.77 | (0.68–0.87) | <0.001 | 0.84 | (0.72–0.97) | 0.015 | |
| ≥6% | 0.85 | (0.70–1.04) | 0.126 | 0.82 | (0.65–1.03) | 0.089 | 0.81 | (0.62–1.06) | 0.127 | |
| Between 5 and 12 months | ||||||||||
| <20 | ≤(−6%) | 3.04 | (2.58–3.58) | <0.001 | 3.18 | (2.64–3.82) | <0.001 | 1.67 | (1.33–2.10) | <0.001 |
| (−6%) to ≤(−2%) | 1.89 | (1.63–2.20) | <0.001 | 2.17 | (1.84–2.56) | <0.001 | 1.66 | (1.37–2.02) | <0.001 | |
| (−2%) to 2% | 1.37 | (1.22–1.55) | <0.001 | 1.51 | (1.32–1.73) | <0.001 | 1.39 | (1.19–1.62) | <0.001 | |
| 2% to <6% | 0.97 | (0.83–1.12) | 0.654 | 1.09 | (0.92–1.27) | 0.315 | 1.05 | (0.87–1.26) | 0.629 | |
| ≥6% | 1.23 | (1.06–1.41) | 0.005 | 1.25 | (1.07–1.46) | 0.004 | 1.16 | (0.97–1.38) | 0.103 | |
| 20 to <25 | ≤(−6%) | 2.38 | (2.07–2.51) | <0.001 | 2.32 | (2.09–2.58) | <0.001 | 1.49 | (1.31–1.68) | <0.001 |
| (−6%) to ≤(−2%) | 1.36 | (1.24–1.50) | <0.001 | 1.38 | (1.25–1.53) | <0.001 | 1.13 | (1.01–1.28) | 0.035 | |
| (−2%) to 2% | 1.0 | 1.0 | 1.0 | |||||||
| 2% to <6% | 0.93 | (0.85–1.02) | 0.106 | 0.94 | (0.86–1.04) | 0.223 | 0.94 | (0.85–1.05) | 0.277 | |
| ≥6% | 0.88 | (0.80–0.97) | 0.012 | 0.87 | (0.78–0.97) | 0.010 | 0.89 | (0.79–1.00) | 0.059 | |
| 25 to <30 | ≤(−6%) | 1.70 | (1.55–1.87) | <0.001 | 1.59 | (1.44–1.77) | <0.001 | 1.19 | (1.05–1.34) | 0.005 |
| (−6%) to ≤(−2%) | 1.19 | (1.09–1.31) | <0.001 | 1.13 | (1.02–1.25) | 0.015 | 1.04 | (0.92–1.16) | 0.548 | |
| (−2%) to 2% | 0.87 | (0.80–0.94) | <0.001 | 0.84 | (0.77–0.92) | <0.001 | 0.88 | (0.80–0.98) | 0.018 | |
| 2% to <6% | 0.75 | (0.69–0.82) | <0.001 | 0.72 | (0.65–0.80) | <0.001 | 0.77 | (0.69–0.87) | <0.001 | |
| ≥6% | 0.81 | (0.73–0.90) | <0.001 | 0.77 | (0.69–0.87) | <0.001 | 0.85 | (0.75–0.98) | 0.020 | |
| ≥30 | ≤(−6%) | 1.30 | (1.10–1.42) | <0.001 | 1.28 | (1.16–1.41) | <0.001 | 0.95 | (0.84–1.06) | 0.357 |
| (−6%) to ≤(−2%) | 0.90 | (0.83–0.99) | 0.028 | 0.87 | (0.78–0.96) | 0.005 | 0.80 | (0.71–0.90) | <0.001 | |
| (−2%) to 2% | 0.71 | (0.65–0.77) | <0.001 | 0.69 | (0.63–0.76) | <0.001 | 0.71 | (0.64–0.79) | <0.001 | |
| 2% to <6% | 0.69 | (0.63–0.76) | <0.001 | 0.64 | (0.58–0.72) | <0.001 | 0.69 | (0.61–0.78) | <0.001 | |
| ≥6% | 0.78 | (0.70–0.87) | <0.001 | 0.76 | (0.67–0.87) | <0.001 | 0.76 | (0.65–0.88) | <0.001 | |
Adjustments in minimally adjusted models: mortality data and weight of first month in each treatment period; case-mix adjusted models: minimally adjusted model plus baseline characteristics of age, sex, race/ethnicity (white, African American, Hispanic, Asian or other), primary insurance (Medicare, Medicaid or other), vascular access type (central venous catheter, arteriovenous fistula, arteriovenous graft or other), 10 comorbid conditions (diabetes mellitus, hypertension, atherosclerotic heart disease, congestive heart failure, other cardiovascular disease, cerebrovascular disease, dyslipidemia, HIV, chronic obstructive pulmonary disease and malignancy), alcohol dependence, substance abuse, dialysis ultrafiltration and dialysis dose as indicated by single-pool Kt/V; case-mix plus malnutrition-inflammation-cachexia syndrome (MICS) models: case-mix adjusted model plus 10 laboratory variables including white blood cells, lymphocytes, serum albumin, creatinine, bicarbonate, calcium, phosphorus, hemoglobin, total iron binding capacity and normalized protein catabolic rate. BMI, body mass index; HR, hazard ratio; CI, confidence interval.
FIGURE 4.
All-cause mortality hazard ratios by percent changes in weight and baseline body mass index. (A) Weight changes between 1 and 5 months. (B) Weight changes between 5 and 12 months. Models adjusted for case-mix covariates and markers of malnutrition and inflammation (see text for covariate list).
DISCUSSION
In a large contemporary cohort of 58 106 patients treated with thrice-weekly MHD for up to 5 years, and who survived the first year of hemodialysis, we showed that patients undergo rapid weight losses in the first 5 months after starting dialysis, but that these trends differ across different baseline BMI strata. Moreover, incrementally greater declines in post-dialysis dry weight during the first 12 months of dialysis therapy were associated with a higher death risk, whereas weight gains were associated with greater survival during the 5th to 12th months, but not in the first 5 months of dialysis therapy. The linear inverse relationships between changes in weight and mortality were consistent across all baseline BMI strata.
It has been clinically observed that hemodialysis patients tend to undergo rapid weight loss in the first few months of starting dialysis, but this observation has not yet been characterized in large observational studies. The robust analyses of our study using linear regression models clearly showed that patients who survived the first year of dialysis reached a nadir of weight at the 5th month of dialysis with an average decline of 2% from baseline. When extending the observation period up to the first 2 years of dialysis, this trend was consistent and robust, supporting that body weight is a fluctuating parameter in patients undergoing MHD. Moreover, obese patients (BMI ≥30 kg/m2) did not reach a nadir and lost ∼3.8% of their weight by the 12th month, suggesting that the trajectory of weight change during the first year of therapy may differ by baseline BMI.
A few prior studies have examined the association of weight change over time on dialysis with mortality outcomes, but have focused solely on the first few months after dialysis initiation [19–23]. Kalantar-Zadeh et al. [19] explored the impact of changes in weight by examining the regression slope of changes in weight over time on all-cause and cardiovascular mortality in a 2-year non-concurrent cohort of 54 535 MHD patients in the USA and found that progressively worsening weight loss was significantly associated with poorer survival and higher cardiovascular mortality. In addition, Chazot et al. [20] conducted a prospective observational study of 5592 incident hemodialysis patients in Southern Europe between January 2000 and September 2005 and also showed that patients with a decrease in weight (less than −5.8% in 1 year) had significantly lower survival compared with patients whose body weight remained stable during the first year of dialysis. Similarly, several recent studies on weight changes among patients treated with MHD have consistently described that short-term weight gains and losses were associated with lower and higher mortality risk, respectively, in the first 6 months of dialysis [21–23]. Of note, none of the aforementioned studies described the patients' weight change trajectory over time, or took into consideration weight change–mortality association analyses.
To date, only one other study has examined the association of weight trajectories in the first year of dialysis with survival. In a study of 363 patients who initiated hemodialysis in France and survived the first year of therapy, the authors reported that body weight decreased by 6.5% during the first 8 weeks, but the initial weight change was not associated with patient survival [27]. In contrast, in our large study, patients reached a nadir of weight at the 5th month of dialysis with an average decline of 2% from baseline, and weight loss in this period was also significantly associated with a higher mortality. Notwithstanding this difference, both studies corroborate that there may be a favorable impact of weight gain after an initial phase of weight changes on patient survival. The rapid loss of weight in the post-dialysis period is likely due to ultrafiltration and dry weight achievement; this is presumably more accentuated in patients with more volume overload in the pre-renal replacement therapy period. Hence the association of initial rapid weight loss with mortality could reflect an underlying more severe congestive heart failure (something that may not be captured by the binomial congestive heart failure diagnosis used for adjustment). The weight change occurring later is more likely to reflect changes in nutritional status. These two different mechanisms may also explain why the associations with mortality are quantitatively different in the two time periods, being more robust in the later one.
Another notable finding in our study is the impact of baseline BMI on the association of change in weight with mortality. This is of particular interest because it could be speculated that weight changes in obese and lean patients may be precipitated by different factors and thereby may have different associations with mortality. Interestingly, one recent study of 6797 European prevalent hemodialysis patients recruited and followed prospectively for over 3 years addressed this issue [23]. They evaluated the association of weight changes in the first 6 months of dialysis with mortality and showed that weight loss during the first 6 months of dialysis was associated with higher mortality independent of baseline BMI, while a 6-month dry weight gain was associated with lower mortality in all BMI groups except among obese patients (BMI >30 kg/m2), in whom no benefit was observed. Similarly, in our study, weight gain was significantly associated with greater survival during the 5th to 12th months, but not in the first 5 months of dialysis therapy (Table 2). On the other hand, compared with patients with normal BMI (20–25 kg/m2) and minimal changes in weight (−2 to 2% of weight) as reference, obese patients (BMI ≥30 kg/m2) with weight gains of 2–6% and ≥6% during the 5th to 12th months had 31 and 24% lower risks of death, respectively (Table 3). This finding suggests that weight gain may indeed be protective and affords survival advantages in obese MHD patients. Most likely, the more subdued benefit associated with weight gain in patients with baseline obesity may not be readily apparent; again it is more crucial to understand the dynamics of weight changes over time and their impact on patient survival across the different time periods.
Despite the strengths of our study including a large sample size of >50 000 MHD patients, a relatively long follow-up for up to 5 years and serial monthly weight measurements that enabled linear mixed regression models to account for the trajectory of weight changes over time, several potential limitations should be mentioned. First, observed changes in weight may have been unintentional, and our study cannot differentiate between intentional versus spontaneous weight loss or gain. Moreover, it is unclear what the exact reason(s) for the observed weight change might be; these could include changes in hydration status and body composition (i.e. water, lean body mass and fat mass) over time, or a combination of them. Second, we cannot definitely determine whether weight loss contributes directly to patient death or may be confounded by the poorer health status of patients [28]. However, given the recent observational study showing the linear inverse association of BMI and patient survival using a quasi-experimental marginal structural model design, we might postulate that the obesity paradox in ESRD is unlikely to be due to residual confounding alone and has biologic plausibility [29]. However, interventional studies are warranted to examine this hypothesis. Third, residual confounding may still be a limitation as we did not have complete data on intercurrent illnesses, residual renal function and inflammatory markers such as serum C-reactive protein. Additional confounding factors such as acute illnesses, hospitalization and infection were not captured in this database. Therefore, we cannot assume that all measured covariates are sufficient to adjust for all biases; nonetheless, it can be addressed at least in part by vigorous adjustment for measured covariates such as demographic, clinical and laboratory parameters. Finally, it should be mentioned that our study examined US patients who survived the first year of dialysis, which may raise concerns about selection bias. Thus, the results of our study should be interpreted in light of some potential survival bias and the differences in obesity prevalence trends between the USA and other regions such as Europe and Asia.
In conclusion, among patients treated with MHD who survived the first year of dialysis, post-dialysis dry weight change over time reaches a nadir at the 5th month after dialysis initiation. Incrementally larger drops in post-dialysis dry weight over the first year of dialysis therapy are associated with higher death risk, whereas weight gain is associated with greater survival only during the 5th to 12th months, but not in the first 5 months of therapy. Moreover, this detrimental or protective association of weight loss or gain with patient survival is consistent across all baseline BMI categories. These findings may have important clinical implications in dialysis patient care. The interesting results of this study warrant further investigation through interventional trials in MHD patients.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
Supplementary Material
ACKNOWLEDGEMENTS
We thank DaVita Clinical Research® (DCR) for providing the clinical data for this research. Portions of this report were presented at the American Society of Nephrology Kidney Week Conference, November 2015, in San Diego, CA. The work is supported by K.K.-Z.'s NIH (NIDDK) grants K24-DK09141, R01-DK078106 and R01-DK095668, and philanthropic grants from Mr Harold Simmons, Mr Louis Chang, Joseph Lee and AVEO, Inc., C.P.K.'s NIH (NIDDK) grants R01-DK096920 and U01-DK102163, and C.M.R.'s NIH (NIDDK) grant K23-DK102903. ES is supported by a career development award from the Office of Research and Development of the Department of Veterans Affairs (IK2-CX001266-01).
CONFLICT OF INTEREST STATEMENT
K.K.-Z. has received honoraria and/or support from Abbott, Abbvie, Alexion, Amgen, American Society of Nephrology, Astra-Zeneca, AVEO, Chugai, DaVita, Fresenius, Genetech, Haymarket Media, Hospira, Kabi, Keryx, National Institutes of Health, National Kidney Foundation, Relypsa, Resverlogix, Sanofi, Shire, Vifor and ZS-Pharma. C.P.K. has received honoraria from Sanofi-Aventis, Relypsa and ZS Pharma.
REFERENCES
- 1. US Renal Data System: Annual Data Report. US Department of Public Health and Human Services, Public Health Service. Bethesda, MD: National Institutes of Health, 2014 [Google Scholar]
- 2. Chertow GM, Hsu CY, Johansen KL. The enlarging body of evidence: obesity and chronic kidney disease. J Am Soc Nephrol 2006; 7: 1501–1502 [DOI] [PubMed] [Google Scholar]
- 3. Prospective Studies Collaboration, Whitlock G, Lewington S. et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009; 373: 1083–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Curtis JP, Selter JG, Wang Y. et al. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med 2005; 165: 55–61 [DOI] [PubMed] [Google Scholar]
- 5. Gunen H, Hacievliyagil SS, Kosar F. et al. Factors affecting survival of hospitalised patients with COPD. Eur Respir J 2005; 26: 234–241 [DOI] [PubMed] [Google Scholar]
- 6. Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: role of comorbidity and systemic inflammation. Arch Intern Med 2005; 165: 1624–1629 [DOI] [PubMed] [Google Scholar]
- 7. Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol 2009; 53: 1925–1932 [DOI] [PubMed] [Google Scholar]
- 8. Lavie CJ, Milani RV, Artham SM. et al. The obesity paradox, weight loss, and coronary disease. Am J Med 2009; 122: 1106–1114 [DOI] [PubMed] [Google Scholar]
- 9. Kalantar-Zadeh K, Abbott KC, Salahudeen AK. et al. Survival advantages of obesity in dialysis patients. Am J Clin Nutr 2005; 81: 543–554 [DOI] [PubMed] [Google Scholar]
- 10. Abbott KC, Glanton CW, Trespalacios FC. et al. Body mass index, dialysis modality, and survival: analysis of the United States Renal Data System Dialysis Morbidity and Mortality Wave II Study. Kidney Int 2004; 65: 597–605 [DOI] [PubMed] [Google Scholar]
- 11. Kalantar-Zadeh K, Rhee CM, Amin AN. To legitimize the contentious obesity paradox. Mayo Clin Proc 2014; 89: 1033–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park J, Ahmadi SF, Streja E. et al. Obesity paradox in end-stage kidney disease patients. Prog Cardiovasc Dis 2014; 56: 415–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fleischmann E, Teal N, Dudley J. et al. Influence of excess weight on mortality and hospital stay in 1346 hemodialysis patients. Kidney Int 1999; 55: 1560–1567 [DOI] [PubMed] [Google Scholar]
- 14. Kopple JD, Zhu X, Lew NL. et al. Body weight-for-height relationships predict mortality in maintenance hemodialysis patients. Kidney Int 1999; 56: 1136–1148 [DOI] [PubMed] [Google Scholar]
- 15. Leavey SF, McCullough K, Hecking E. et al. Body mass index and mortality in ‘healthier’ as compared with ‘sicker’ haemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 2001; 16: 2386–2394 [DOI] [PubMed] [Google Scholar]
- 16. Port FK, Ashby VB, Dhingra RK. et al. Dialysis dose and body mass index are strongly associated with survival in hemodialysis patients. J Am Soc Nephrol 2002; 13: 1061–1066 [DOI] [PubMed] [Google Scholar]
- 17. Johansen KL, Young B, Kaysen GA. et al. Association of body size with outcomes among patients beginning dialysis. Am J Clin Nutr 2004; 80: 324–332 [DOI] [PubMed] [Google Scholar]
- 18. Park J, Jin DC, Molnar MZ. et al. Mortality predictability of body size and muscle mass surrogates in Asian vs white and African American hemodialysis patients. Mayo Clin Proc 2013; 88: 479–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kalantar-Zadeh K, Kopple JD, Kilpatrick RD. et al. Association of morbid obesity and weight change over time with cardiovascular survival in hemodialysis population. Am J Kidney Dis 2005; 46: 489–500 [DOI] [PubMed] [Google Scholar]
- 20. Chazot C, Gassia J-P, Di Benedetto A. et al. Is there any survival advantage of obesity in Southern European haemodialysis patients? Nephrol Dial Transplant 2009; 24: 2871–2876 [DOI] [PubMed] [Google Scholar]
- 21. Kalantar-Zadeh K, Streja E, Kovesdy CP. et al. The obesity paradox andmortality associated with surrogates of body size andmusclemass in patients receiving hemodialysis. Mayo Clin Proc 2010; 85: 991–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kalantar-Zadeh K, Streja E, Molnar MZ. et al. Mortality prediction by surrogates of body composition: an examination of the obesity paradox in hemodialysis patients using composite ranking score analysis. Am J Epidemiol 2012; 175: 793–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cabezas-Rodriguez I, Carrero JJ, Zoccali C. et al. Influence of body mass index on the association of weight changes with mortality in hemodialysis patients. Clin J AmSoc Nephrol 2013; 8: 1725–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bellizzi V, Cioffi MF, Cianciaruso B. Body weight is a fluctuating parameter in hemodialysis patients. Kidney Int 2000; 58: 900. [DOI] [PubMed] [Google Scholar]
- 25. Kuttykrishnan S, Kalantar-Zadeh K, Arah OA. et al. Predictors of treatment with dialysis modalities in observational studies for comparative effectiveness research. Nephrol Dial Transplant 2015; 30: 1208–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser2000; 894: i–xii 1–253 [PubMed] [Google Scholar]
- 27. Chazot C, Deleaval P, Bernollin AL. et al. Target weight gain during the first year of hemodialysis therapy is associated with patient survival. Nephron Clin Pract 2014; 126: 128–134 [DOI] [PubMed] [Google Scholar]
- 28. Flegal KM, Graubard BI, Williamson DF. et al. Reverse causation and illness-related weight loss in observational studies of body weight and mortality. Am J Epidemiol 2011; 173: 1–9 [DOI] [PubMed] [Google Scholar]
- 29. Doshi M, Streja E, Rhee CM. et al. Examining the robustness of the obesity paradox in maintenance hemodialysis patients: a marginal structural model analysis. Nephrol Dial Transplant 2016; 31: 1310–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.