Abstract
3,3'-Dichlorobiphenyl (PCB 11), a byproduct of pigment production, is increasingly detected in environmental samples. While more highly chlorinated PCB congeners are known developmental neurotoxicants, nothing is known about the potential developmental neurotoxicity of PCB 11. To address this critical data gap, we measured PCB 11 levels in human maternal plasma and quantified the effects of PCB 11 and its major metabolites on morphometric parameters of neuronal connectivity in cultured primary neurons. Mass spectrometry analyses of plasma from 241 pregnant women enrolled in the MARBLES study (University of California, Davis) detected PCB 11 in all samples at concentrations ranging from 0.005 to 1.717 ng/ml. Morphometric analyses of primary neuron-glia co-cultures dissociated from the neocortices or hippocampi of neonatal Sprague Dawley rats exposed to vehicle or concentrations ranging from 1 attamolar (aM) to 1 micromolar (µM) of PCB 11, OH-PCB 11, or PCB 11 sulfate indicated that PCB 11 and both metabolites significantly increased axonal and dendritic growth in cortical and hippocampal pyramidal neurons. PCB 11 significantly altered neuronal morphogenesis at concentrations as low as 1 femtomolar (fM), which is ∼0.22 ng/ml. These data suggest the potential for the developing human brain to be exposed to PCB 11, and demonstrate that environmentally relevant levels of PCB 11 alter axonal and dendritic growth in neuronal cell types critically involved in cognitive and higher-order behaviors. These findings identify PCB 11 as a potential environmental risk factor for adverse neurodevelopmental outcomes in humans.
Keywords: axonal growth, cell culture, dendritic growth, developmental neurotoxicity, exposure assessment, polychlorinated biphenyls
Polychlorinated biphenyls (PCBs) are highly lipophilic chemicals resistant to degradation that were synthesized for use in diverse industrial processes from the 1930s to 1970s. In 1979, PCB production was banned because of human health concerns. While initial research efforts focused on cancer outcomes, the developing brain has emerged as a primary endpoint of concern for PCBs. Multiple epidemiological studies have identified a negative association between developmental exposure to PCBs and measures of neuropsychological function in infancy or childhood, and this association has been confirmed in experimental animal models (reviewed in Carpenter, 2006; Korrick and Sagiv, 2008; Schantz et al., 2003; Winneke, 2011). More recently, PCBs have been proposed as environmental risk factors for neurodevelopmental disorders (NDD) (Landrigan et al., 2012; Stamou et al., 2013). In support of this hypothesis, associations between PCB exposures and increased risk of autism spectrum disorders (ASD) (Lyall et al., 2016; Rossignol et al., 2014) and attention deficit/hyperactivity disorder (ADHD) (reviewed in Eubig et al., 2010) have been reported. In addition, abnormalities in dendritic morphology, which are the most consistent pathologic correlate of behavioral deficits in heritable and environmentally triggered NDD (Copf, 2016; Garey, 2010; Penzes et al., 2011; Supekar et al., 2013), are observed in preclinical models of developmental PCB exposure, and mechanistic studies have linked PCB effects on dendritic arborization to activation of signaling pathways that map onto genes of susceptibility for NDD (reviewed in Stamou et al., 2013; and see Lesiak et al., 2014).
PCBs remain a current and significant risk to the developing human brain. The latest NHANES study confirmed widespread exposure to PCBs among women of childbearing age living in the United States (Thompson and Boekelheide, 2013), and recent studies detected PCBs in the indoor air of elementary schools in the United States at levels that exceed the EPA’s 2009 public health guidelines (Thomas et al., 2012). Legacy PCBs released from paint, caulking, and electrical transformers contribute to current human exposures; however, PCB congeners not found in commercial mixtures synthesized prior to the 1979 ban have recently been identified in the human chemosphere. In particular, PCB 11 has emerged as an ubiquitous contemporary PCB contaminant. PCB 11 has been documented worldwide in air (Basu et al., 2009; Choi et al., 2008; Du et al., 2009; Heo et al., 2014; Hu et al., 2008) and water (Du et al., 2008; King et al., 2002; Litten et al., 2002), and recent studies from California have detected this congener in commercial cow’s milk (Chen et al., 2017). PCB 11 is an unintentional byproduct of modern pigment manufacturing processes (Hu and Hornbuckle, 2010; Shang et al., 2014), with over 1.5 tons of PCB 11 produced in 2006 (Rodenburg et al., 2010). Of concern, PCB 11 was recently detected in serum from women and adolescent children living in rural Iowa and the greater Chicago area of the United States (Koh et al., 2015).
Despite the fact that PCB 11 is a pervasive environmental contaminant with documented human exposure, there are currently no published data regarding its potential developmental neurotoxicity. To address this critical data gap, we measured levels of PCB 11 in the blood of pregnant women and used these data to design in vitro studies as an initial assessment of the potential for environmentally relevant levels of this PCB congener to cause developmental neurotoxicity. Our data indicate that PCB 11 and two of its known biological metabolites, 4-OH-PCB 11 and 4-OSO3-PCB 11 (Hu et al., 2014), modulate morphometric parameters of neuronal connectivity in primary neurons at concentrations relevant to PCB 11 levels detected in maternal plasma of pregnant women enrolled in the Markers of Autism Risk in Babies – Learning Early Signs (MARBLES) study at UC Davis in California. These findings identify PCB 11 as a potential environmental risk factor for adverse neurodevelopmental outcomes in humans that warrants further investigations in animal models.
MATERIALS AND METHODS
Materials
PCB 11 (3,3′-dichloro-biphenyl), 4-OH-PCB 11 (3,3′-dichloro-biphenyl-4′-ol) and 4-OSO3-PCB 11 (3,3′-dichloro-4′-sulfooxy-biphenyl) were synthesized as previously described (Grimm et al., 2013; Lehmler and Robertson, 2001). All compounds were >99% pure as determined by 1H-NMR, 13C-NMR, and GC-MS (Supplementary Material). All PCB stock solutions were made in dry sterile dimethyl sulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO).
Human study population
All study protocols were approved by the UC Davis Institutional Review Committee on Human Research. Participation of human subjects did not occur until after informed consent was obtained. Human maternal plasma samples (n = 241) were obtained from the MARBLES study at University of California, Davis. Details describing this cohort were recently published (Hertz-Picciotto et al., 2017). In brief, women recruited into the MARBLES study lived within a 2.5 h drive of Sacramento, CA, were currently pregnant, and had a biological child diagnosed with ASD, which significantly increased their risk for having a second child with a NDD. The age of mothers in this study ranged from 18 to 50 years old with most mothers (>75%) over 30 years of age, including 7% over 40. The study cohort includes 22% Hispanic and another 24% non-White race subjects. All maternal blood samples were collected into sodium citrate Vacutainer tubes post-venipuncture. Whole blood samples were processed within 12 h of collection to separate plasma, which was stored at −80°C until thawed on ice for PCB 11 analysis.
Cell culture
Animals were treated humanely and with regard for the alleviation of suffering in strict accordance to protocols approved by the University of California, Davis Institutional Animal Care and Use Committee. Timed-pregnant Sprague Dawley rats were purchased from Charles River Laboratory (Hollister, California) and individually housed in clear plastic cages with corn cob bedding. Food and water were provided ad libitum. Temperatures were maintained at 22 ± 2°C throughout a 12-h light-dark cycle.
Primary cortical and hippocampal neuron-glia co-cultures from postnatal day 0 rat pups (sexes were combined) were prepared as previously described (Wayman et al., 2006). Dissociated cells were plated on glass coverslips (BellCo, Vineland, New Jersey) precoated with 500 µg/ml poly-l-lysine (Sigma-Aldrich), and maintained at 37°C in NeuralQ Basal Medium (MTI-GlobalStem, Gaithersburg, Maryland) supplemented with 2% GS21 (MTI-GlobalStem) and GlutaMAX (ThermoScientific, Waltham, Massachusetts) under 5% CO2. Neurons were plated at 83 000 cells/cm2 for analyses of dendritic morphology and cell viability, and at 33 000 cells/cm2 for quantification of axon lengths.
Morphometric analyses
For dendritic analyses, cultures were transfected at day in vitro (DIV) 6 with plasmid encoding microtubule-associated protein-2B-protein red fusion (MAP2B-FusRed) construct (provided by Dr. Gary Wayman, Washington State University, Pullman, Washington) using Lipofectamine-2000 (Invitrogen, Carlsbad, California) according to the manufacturer’s protocol. At DIV 7, cultures were exposed for 48 h to vehicle (DMSO; 1:1,000 dilution) or concentrations ranging from 1 attamolar (aM) to 1 micromolar (µM) of PCB 11, 4-OH-PCB 11, or 4-OSO3-PCB 11 diluted directly into culture media from 1000× stocks. Dendritic morphology was quantified from digital images of FusRed-positive neurons acquired via an unbiased automated imaging system (ImageXpress, Molecular Devices, Sunnyvale, California). ImageJ software (U.S. National Institutes of Health, Bethesda, Maryland, Version 1.49s) was used for Sholl analysis (Sholl, 1953) with rings set at 5 pixel increments from the soma (1 pixel = 0.65 micrometers); dendritic tips and primary dendrites were quantified manually.
For quantification of axon length, cultures were exposed to vehicle (DMSO; 1:1,000 dilution) or concentrations ranging from 1 aM to 1 µM of PCB 11, 4-OH-PCB 11 or 4-OSO3-PCB 11 for 48 h beginning 3 h after plating. Cultures were fixed with 4% paraformaldehyde and reacted with Tau-1 antibody (1:1000, Millipore, Billerica, Massachusetts). Axon lengths were quantified using ImageJ software with the NeuronJ plugin (Meijering et al., 2004). Hippocampal and cortical neurons have a single axon, identified as a Tau-1 immunopositive neurite whose length was at least 2.5 times the diameter of the cell body, and exceeded that of all other neurites from the same neuron (Dotti et al., 1988; Lein et al., 1992). Only neurons with a clearly defined axon were included in the analysis.
Cell viability
Cell viability was assessed by measuring lactate dehydrogenase (LDH) release (Mosmann, 1983) using the CytoTox-ONE Homogenous Membrane Integrity Assay (Promega, Madison, Wisconsin, USA) per the manufacturer’s directions. In addition, separate cultures were incubated with calcein-AM (0.25 μM, Invitrogen) and propidium iodide (1.25 μM, Sigma-Aldrich) to quantify the number of live and dead cells, respectively, by the same automated imaging system (ImageXpress). 0.2% Triton X-100 (Fisher Scientific, Pittsburgh, Pennsylvania) was used as a positive control for both assays.
Analysis of PCB 11
Detailed descriptions of methods used to extract and quantify PCB 11 are provided in the Supplementary Material. In brief, previously described methods were used to extract PCB 11 from human maternal plasma (Lin et al., 2013), and from tissue culture plates, cells, and medium (Yang et al., 2014). PCB 11 levels were quantified as described previously (Lin et al., 2013) using a Bruker Scion TQ triple quadruple mass spectrometer (Bruker, Fremont, California). 13C12-2,2′,3′,4,5-pentachloro-biphenyl (Cambridge Isotope Laboratories, Inc, Tewksbury, Massachusetts, USA) was used as a surrogate internal standard throughout the extraction and analytical procedures. Mirex was added after extraction to monitor any shifts in instrument performance during analysis. PCB 11 concentrations were calculated based on 7-point calibration curves.
Statistical analyses
Statistical analyses were performed using GraphPad Prism v6.07 (San Diego, California). Data were analyzed by nonparametric one-way ANOVA with significance set at P ≤ .05. Differences between groups were identified by post-hoc Dunnett’s test.
RESULTS
PCB 11 Is Detected in Human Maternal Plasma of California Women
To determine the potential for the developing human nervous system to be exposed to PCB 11, we used mass spectrometry to analyze PCB 11 content in plasma samples collected from women enrolled in the MARBLES study at UC Davis. MARBLES is a longitudinal study of pregnant women who have a biological child diagnosed with ASD, and thus are at increased risk for having a second child with NDD. PCB 11 was detected in all of the 241 samples that were analyzed. The mean concentration was 0.490 ng/mL, with concentrations ranging from a minimum of 0.005 ng/ml to a maximum of 1.717 ng/ml (Table 1).
Table 1.
PCB 11 Concentrations in Plasma Samples Collected from Pregnant Women in the UC Davis MARBLES Study
| Sample Type | Minimum | 25th Percentile | Meana | 75th Percentile | Maximum |
|---|---|---|---|---|---|
| Maternal | 0.005 ng/ml | 0.181 ng/ml | 0.490 ng/ml | 0.757 ng/ml | 1.717 ng/ml |
| Plasma | 0.02 nM | 0.81 nM | 2.20 nM | 3.39 nM | 7.70 nM |
Summary data of 241 unique samples collected during the 1st, 2nd, and 3rd trimester of pregnancy and at birth from 126 participants.
Arithmetic mean.
PCB 11 Alters Neuronal Morphogenesis in Cortical and Hippocampal Neurons
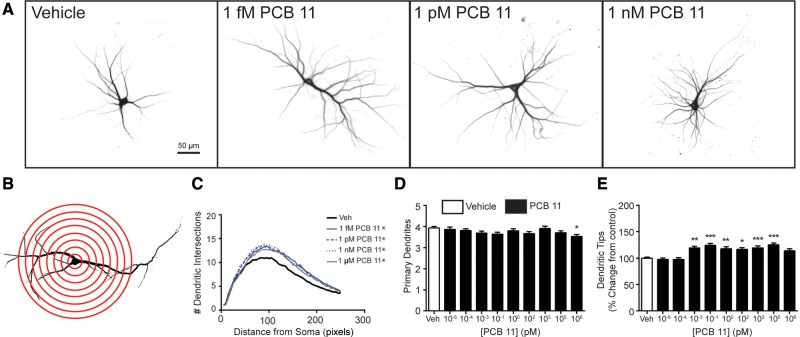
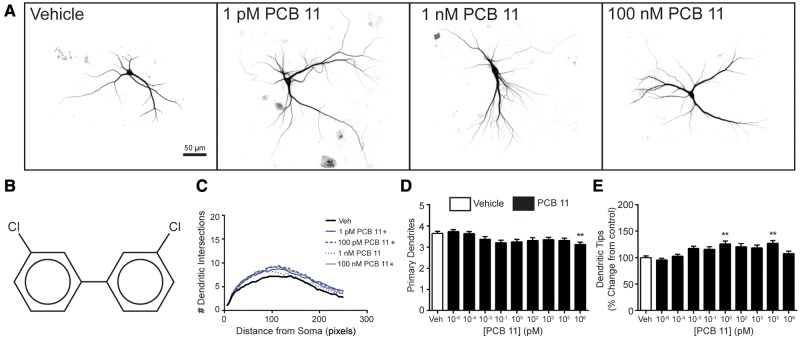
Higher chlorinated PCB congeners, specifically PCB 95 and PCB 136, have been shown to enhance dendritic growth in primary cortical and hippocampal neuron-glia co-cultures derived from neonatal rat brain (Wayman et al., 2012; Yang et al., 2009, 2014). Thus, we first investigated whether PCB 11 influences dendritic growth in these same in vitro models. Under the culture conditions used for these experiments, the dendritic arbor expands most rapidly between DIV 5 and 10 (Wayman et al., 2006); therefore, cultures were transfected with MAP2B-FusRed on DIV 6, then exposed from DIV 7-9 to vehicle (DMSO at 1:1000 dilution) or varying concentrations of PCB 11. Compared with the dendritic morphology of neurons in vehicle control cultures, cortical (Figure 1) and hippocampal (Figure 2) neurons exposed to PCB 11 had significantly more complex dendritic arbors, as determined by Sholl analyses and the number of dendritic tips per neuron. Interestingly, PCB 11 did not significantly change the number of primary dendrites per neuron at any concentration tested except 1 µM, suggesting that exposure to this PCB congener increased dendritic complexity by increasing branching. In cortical cell cultures, the dendrite promoting effects of PCB 11 were observed at concentrations as low as 1 femtomolar (fM) (Figure 1), while in hippocampal cell cultures, PCB 11-enhanced dendritic growth was observed at concentrations ≥1 pM (Figure 2). PCB 11 had no effect on dendritic growth in either neuronal cell type at concentrations ≤100 aM.
Figure 1.
PCB 11 enhances dendritic arborization in primary rat cortical neurons. (A) Representative photomicrographs of DIV 9 cortical neurons after 48 h exposure to vehicle (0.1% DMSO) or varying concentrations of PCB 11. (B) Diagram illustrating Sholl rings used to quantify dendritic arborization. (C) Sholl data. Dendritic arborization was also assessed by quantifying the following morphometric parameters per neuron: (D) number of primary dendrites and (E) number of dendritic tips, as determined by dividing the number of dendritic tips by the number of primary dendrites. Data presented as the mean ± SE (n > 70 neurons from 3 independent cultures). *P <.05, **P <.01, ***P <.001 relative to vehicle control. 1 Pixel = 0.65 micrometers.
Figure 2.
PCB 11 promotes dendritic growth in primary rat hippocampal neurons. (A) Representative photomicrographs of DIV 9 hippocampal neurons after 48 h exposure to vehicle (0.1% DMSO) or varying concentrations of PCB 11. (B) Chemical structure of PCB 11. Dendritic morphology was assessed using (C) Sholl analysis and by (D) quantifying the number of primary dendrites per neuron and (E) the number of dendritic tips per neuron. Data presented as the mean ± SE (n > 70 neurons from 3 independent cultures). *P < .05, **P <.01, ***P <.001 relative to vehicle control. 1 Pixel = 0.65 micrometers.
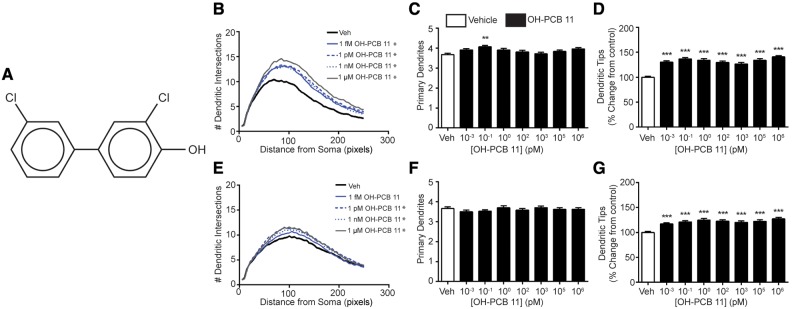
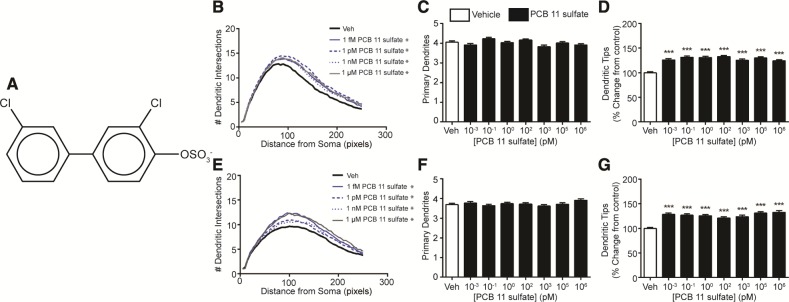
PCB 11 is metabolized within minutes upon entering the body, and the major metabolite formed is 4-OH-PCB 11 (Hu et al., 2013), referred to hereafter as OH-PCB 11. This hydroxylated metabolite is susceptible to further metabolism by sulfotransferases (Grimm et al., 2013) to form 4-OSO3-PCB 11, referred to hereafter as PCB 11 sulfate. Thus, we also tested whether OH-PCB 11 and PCB 11 sulfate influence dendritic growth. Exposure to the hydroxylated metabolite significantly increased dendritic arborization in cortical and hippocampal cells as determined by an upward shift of the Sholl plots (Figs. 3B and 3E) and an increased number of dendritic tips per neuron (Figs. 3D and 3G) relative to vehicle controls. The number of primary dendrites per neuron was not significantly altered in either cortical neurons (Figure 3C) or hippocampal neurons (Figure 3F) exposed to the hydroxylated metabolite, with the exception of cortical neurons exposed to OH-PCB 11 at 100 fM, which exhibited a significantly increased number of primary dendrites relative to vehicle controls. PCB 11 sulfate also significantly increased dendritic growth in cortical (Figs. 4B and 4D) and hippocampal (Figs. 4E and 4G) neurons, as determined by an upward shift in Sholl plots and an increased number of dendritic tips per neuron relative to vehicle controls. As observed for the parent compound and hydroxylated metabolite, PCB 11 sulfate had no significant effect on the number of primary dendrites in either cortical (Figure 4C) or hippocampal neurons (Figure 4F). Collectively, these data indicate that OH-PCB 11 and PCB 11 sulfate increased dendritic complexity by increasing dendritic branching.
Figure 3.
Hydroxylated PCB 11 increases dendritic growth in primary cortical and hippocampal neurons. (A) Chemical structure of 4′-OH-PCB 11. Dendritic morphology was assessed in DIV 9 cortical (B–D) and hippocampal (E–G) neurons after 48 h exposure to vehicle (0.1% DMSO) or varying concentrations of OH-PCB 11 using Sholl analysis (B, E) and by quantifying the number of primary dendrites per neuron (C, F) and the number of dendritic tips per neuron (D, G). Data presented as the mean ± SE (n > 70 neurons from 3 independent cultures). *P <.05, **P <.01, ***P <.001 relative to vehicle control. 1 Pixel = 0.65 micrometers.
Figure 4.
Sulfated PCB 11 promotes dendritic arborization in primary cortical and hippocampal neurons. (A) Chemical structure of 4′-OSO3-PCB 11. Dendritic morphology was assessed in DIV 9 cortical (B–D) and hippocampal (E–G) neurons after 48 h exposure to vehicle (0.1% DMSO) or varying concentrations of PCB 11 sulfate using Sholl analysis (B, E) and by quantifying the number of primary dendrites per neuron (C, F) and the number of dendritic tips per neuron (D, G). Data presented as the mean ± SE (n > 70 neurons from 3 independent cultures). *P <.05, **P <.01, ***P <.001 relative to vehicle control. 1 Pixel = 0.65 micrometers.
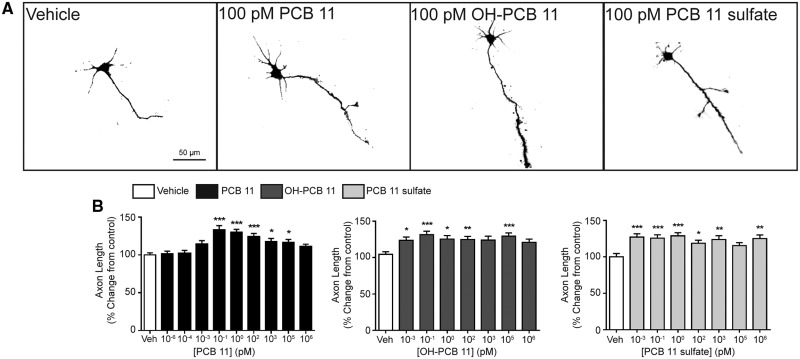
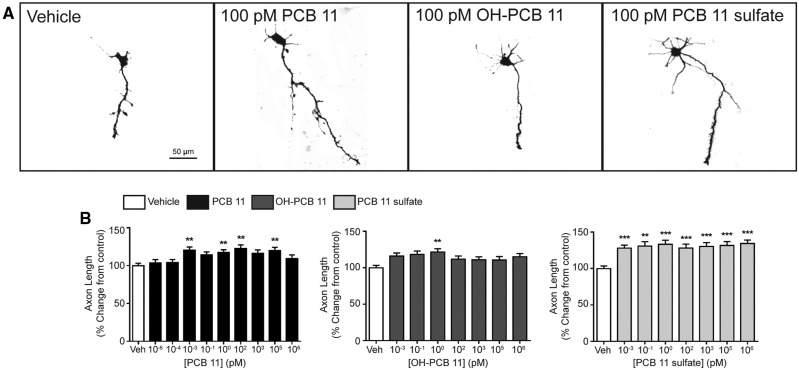
Axonal growth is another critical determinant of neuronal connectivity in the developing brain that is susceptible to perturbation by environmental contaminants (Barone et al., 2000; Chen et al., 2016; Coullery et al., 2016). Therefore, we tested whether PCB 11, OH-PCB 11 or PCB 11 sulfate influenced axon outgrowth in our primary neuron-glia co-culture model. In order to visualize the complete axonal plexus of individual neurons (Yang et al., 2014), dissociated cortical and hippocampal cells were plated at a lower cell density than was used for the dendritic outgrowth studies, and exposed to PCBs for 48 h beginning 3 h after plating. Exposure to PCB 11, OH-PCB 11, or PCB 11 sulfate significantly increased axonal growth in both cortical (Figure 5) and hippocampal (Figure 6) neurons. The PCB 11 parent compound increased axonal lengths by 15%–35% in cortical neurons exposed to PCB 11 at concentrations ranging from 100 fM to 100 nanomolar (nM) (Figure 5B). In hippocampal neurons, the effects of the parent PCB 11 compound were more variable across this same concentration range, but significantly increased axonal growth was observed at concentrations as low as 1 fM (Figure 6B). OH-PCB 11 significantly increased axonal growth in cortical neurons by 20%–35% at all concentrations tested, except 1 nM and 1 µM (Figure 5B), but only significantly increased axonal growth in hippocampal neurons at 1 pM (Figure 6B). With the exception of cortical cultures exposed to PCB 11 sulfate at 100 nM, the sulfated metabolite significantly increased axonal growth by 15%–30% in cortical neurons (Figure 5B), and by 25%–35% in hippocampal neurons (Figure 6B) at all concentrations tested.
Figure 5.
PCB 11 and its major metabolites increase axonal growth in primary cortical neurons. (A) Representative photomicrographs of DIV 2 cortical neurons after 48 h exposure to vehicle (0.1% DMSO) or varying concentrations of PCB 11, OH-PCB 11, or PCB 11 sulfate. (B) Quantification of axonal length as a percent of control (vehicle). Data presented as the mean ± SE (n = 60–90 neurons from 2 to 3 independent cultures). *P <.05, **P <.01, ***P <.001 relative to vehicle control.
Figure 6.
PCB 11 and its major metabolites promote axonal growth in primary hippocampal neurons. (A) Representative photomicrographs of DIV 2 hippocampal neurons after 48 h exposure to vehicle (0.1% DMSO) or varying concentrations of PCB 11, OH-PCB 11, or PCB 11 sulfate. (B) Quantification of axonal length as percent of control (vehicle). Data presented as the mean ± SE (n = 60–90 neurons from 2 to 3 independent cultures). *P <.05, **P <.01, ***P <.001 relative to vehicle control.
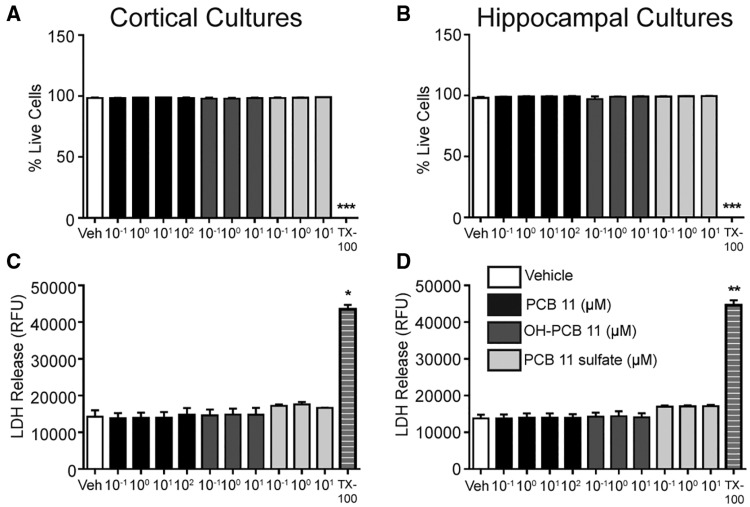
To determine whether the effects of PCB 11 and its metabolites on axonal and dendritic growth were secondary to cytotoxicity, cell viability was assessed in both cortical and hippocampal cultures exposed to these PCB congeners at 7 DIV for 48 h using 2 assays that measure different parameters of cell health: cellular uptake of calcein AM and propidium iodide (Vaughan et al., 1995) and LDH release (Lobner, 2000). Exposure to PCB 11 at concentrations ranging from 0.1 to 100 µM had no significant effect on either measure of cell viability in cortical or hippocampal cultures (Figure 7). Similarly, exposure to OH-PCB 11 or PCB 11 sulfate at concentrations ranging from 0.1 to 10 µM did not alter cell viability in either culture type (Figure 7).
Figure 7.
PCB 11 and its major metabolites do not decrease cell viability at concentrations that alter neuronal morphogenesis. Cell viability was assessed in primary cortical (A, C) and hippocampal (B, D) neurons at DIV 9 after 48 h exposure to vehicle (0.1% DMSO) or PCBs by quantifying cellular uptake of calcein AM and propidium iodide (A, B) or LDH release (C, D). Triton X-100 (TX-100, 0.2%) was used as a positive control for each assay. Data presented as the mean ± SE (n = 3 independent cultures). *P <.05, **P <.01, *** P <.001 relative to vehicle control. RFU, relative fluorescence unit.
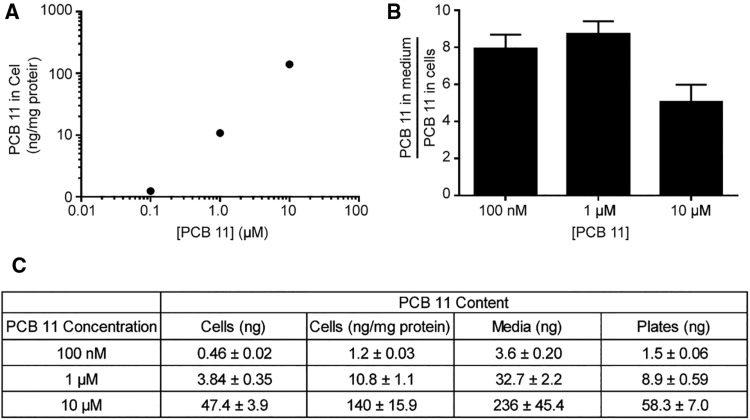
Quantification of PCB 11 in Primary Neuron-Glia Co-Cultures
PCB 11 was quantified in the culture medium and cell pellets after 48 h exposure to determine the actual concentration of the parent compound in cells. PCBs have been shown to adsorb to plastic surfaces (Pepe and Byrne, 1980; Yang et al., 2014), so we also measured levels of PCB 11 adsorbed to the plastic cell culture plates. The percentage of total PCB 11 recovered from the plate, media, and cellular fraction decreased as the initial concentration of PCB 11 added to the culture medium increased, with ∼25% recovered from cultures exposed to PCB 11 at an initial concentration of 100 nM in the culture medium; 20% in cultures exposed to 1 µM PCB 11; and 15% in cultures exposed to 10 µM PCB 11. Conversely, the amount bound to the tissue culture plastic increased with increasing concentrations of PCB 11 added to the culture medium (Figure 8). While the concentration of PCB 11 in the cell pellet increased with increasing concentrations added to the cell medium (Figs. 8A and 8C), the percentage of PCB 11 detected in the cell pellet was relatively constant across groups, ranging from 1.7 to 2.1. There were no significant differences in the ratio of PCB 11 in the medium to that in the cell pellet across initial concentrations in the medium (Figure 8B).
Figure 8.
Quantification of PCB 11 in cell cultures. PCB 11 was quantified in the culture media, cell pellets, and the plastic plates from primary cortical cell cultures at DIV 9 after 48 h exposure to varying concentrations of PCB 11. (A) Amount of PCB 11 in the cellular fraction after exposure to PCB 11 at varying concentrations in the cell medium. (B) Ratio of PCB 11 concentration in the medium to cell pellet. (C) PCB 11 content in the cell pellets, cell media and plates of cultures exposed to varying concentrations of PCB 11 in the medium. Data are presented as mean ± SE (n = 6 separate wells from 2 independent dissections).
DISCUSSION
The data from these studies are the first to suggest that PCB 11 poses a potential risk to the developing human brain. A key observation in support of this conclusion is that PCB 11 was detected in the plasma of pregnant women enrolled in the MARBLES cohort (Hertz-Picciotto et al., 2017). These data confirm and extend recent publications documenting the presence of PCB 11 in serum from mothers and their adolescent children living in rural and urban areas of Midwestern United States (Koh et al., 2015; Marek et al., 2013). In these earlier reports, PCB 11 was detected in ∼40% of study subjects; in contrast, the detection frequency of PCB 11 in plasma from 241 MARBLES subjects was 100%. The reason(s) for the more than 2-fold difference in detection frequency in MARBLES subjects versus the subjects in the midwestern cohorts are not known, but may reflect geographic differences between study cohorts, methodological differences in sample collection and/or analyses between studies, or differences in study populations. With respect to the latter, the women enrolled in the MARBLES study are at significantly increased risk for having a child with a NDD (Hertz-Picciotto et al., 2017). When considered in the context of recent epidemiological data indicating an association between PCB exposures and increased risk of ASD (Lyall et al., 2016; Rossignol et al., 2014) and ADHD (reviewed in Eubig et al., 2010), these data suggest the possibility that exposure of the developing brain to PCB 11 may increase susceptibility to a NDD.
Our findings also identify modulation of neuronal morphogenesis as a plausible biological mechanism by which PCB 11 may interfere with normal neurodevelopment. Specifically, we observed that PCB 11 and its major metabolites, OH-PCB 11 and PCB 11 sulfate, enhanced dendritic arborization and increased the rate of axonal growth in primary cortical and hippocampal neurons dissociated from the developing rat brain. Under the culture conditions used for these studies, significant morphogenic changes were observed at PCB concentrations that had no effect on cell viability, as assessed using two different measures, indicating that the morphogenic effects were not due to cytotoxic effects. While the parent compound and both metabolites had a significant effect on neuronal morphogenesis in both neuronal cell types at concentrations as low as 1 fM, the concentration-response relationship for specific morphogenic effects varied as a function of neuronal cell type, neurite type, and chemical structure. Cortical neurons were more sensitive than hippocampal neurons to the dendrite promoting effects of PCB 11 and the axon promoting effects of OH-PCB 11. However, hippocampal neurons were more sensitive than cortical neurons to the axon promoting effects of PCB 11. Within cortical neurons, dendrites were more sensitive than axons to PCB 11, yet within hippocampal neurons, the relationship was reversed with axons being more sensitive than dendrites to PCB 11. The latter relationship did not appear to be an inherent property of hippocampal axons since the dendrites of hippocampal neurons were more sensitive than their axons to OH-PCB 11. When all outcomes were considered in aggregate, PCB 11 sulfate appeared to be more potent than either the parent or hydroxylated metabolite in that it enhanced both dendritic and axonal growth in both cortical and hippocampal neurons at concentrations as low as 1 fM; in contrast, higher concentrations of PCB 11 or OH-PCB 11 were required to have an effect on a subset of these outcomes. These findings suggest that metabolism of PCB 11 is not solely a detoxification process.
Previous studies of higher chlorinated PCBs, such as PCB 95 and PCB 136, demonstrated that these PCB congeners similarly promote dendritic growth in these same in vitro model systems (Wayman et al., 2012; Yang et al., 2009, 2014). However, the dendrite-promoting activity of PCB 11 is more potent than that of PCB 95 and PCB 136, with the higher chlorinated PCBs eliciting increased dendritic arborization in primary cultures at low pM, but not low fM concentrations. The orders of magnitude difference in potency between PCB 11 and these higher chlorinated PCB congeners is not likely due to differences in cellular uptake since the media to cell ratio of PCB 11 in cultures exposed to this congener at 100 nM was ∼8; whereas, the media to cell ratio of PCB 136 in cultures exposed to PCB 136 at the same concentration was ∼14 (Yang et al., 2014). A caveat of this interpretation is that cellular uptake may not be linear at the much lower (fM) concentrations of PCB 11 observed to alter neurite outgrowth (DeVito et al., 1998; Kania-Korwel et al., 2008, 2012), but unfortunately we were not able to test this because the levels in the cultures exposed to fM concentrations were below the limit of detection. The morphogenic effects of PCB 11 also differed from those of PCB 95 and PCB 136 in that the latter had no effect on axonal growth. Collectively, these observations suggest that mechanism(s) underlying the morphogenic effects of PCB 11 differ from those that mediate the dendritic promoting activity of PCB 95 and PCB 136. Consistent with this hypothesis, the effects of PCB 95 and PCB 136 on dendritic arborization have been causally linked to binding of these congeners to the ryanodine receptor (Wayman et al., 2012; Yang et al., 2014); however, PCB 11 has recently been shown to not bind the ryanodine receptor (Holland et al., 2017). Testing the hypothesis that PCB 11 influences dendritic and axonal outgrowth via mechanism(s) independent of the ryanodine receptor is the focus of future studies.
A key question raised by our findings is whether the concentrations at which PCB 11 altered axonal and dendritic growth in vitro are relevant to levels that are present in the human gestational environment. In humans, it has been shown that PCBs, which are extremely lipophilic, are present in the developing fetus throughout in utero development (Lanting et al., 1998), and it is estimated that ∼30% of the PCB burden in maternal blood crosses the placenta into the fetus (Soechitram et al., 2004). Studies of PCB disposition in mice determined that pups exposed during gestation and lactation to PCB 95 in the maternal diet have detectable levels of PCB 95 in their serum and brain, and that PCB 95 levels in the brain are either equal to, or slightly greater than, levels in the blood (Kania-Korwel et al., 2012, 2015). While PCB 11 is a lower chlorinated congener than PCB 95, it is still a highly lipophilic molecule, and thus, its disposition in the body is likely similar to that of the higher chlorinated PCB congeners. As determined in this study, the lowest PCB 11 level detected in the plasma from pregnant women in the MARBLES study was ∼20 pM. Assuming a 30% transfer rate to the fetus, the resulting concentration in fetal blood would be ∼6 pM. If the concentration of PCB 11 in blood approximates the level of PCB 11 in the brain, then concentrations of PCB 11 in the developing brain would be well within the range that promoted significant dendritic and axonal growth in primary cortical and hippocampal neurons in vitro.
It should also be noted that the levels of PCB 11 detected in mothers in the Chicago area were strongly and significantly associated with levels of PCB 11 present in their adolescent children (Koh et al., 2015), suggesting that PCB 11 is also present in the brain during critical periods of postnatal neurodevelopment. While we did not measure levels of OH-PCB 11 or PCB 11 sulfate in the plasma of pregnant women enrolled in the MARBLES study, both the hydroxylated and sulfated forms of PCB 11 have been detected in human serum (Grimm et al., 2016; Marek et al., 2014; ZHu et al., 2013). Disposition studies in rodent models have shown that hydroxylated PCBs can cross the placenta and the blood brain barrier (Meerts et al., 2002), and that PCB 11 sulfate also crosses the blood brain barrier (Grimm et al., 2015). Collectively, these data suggest that not only the parent compound but also its major metabolites are likely present in the developing brain, potentially at levels shown to modulate neuronal morphogenesis in vitro.
The potential relevance to human health of in vitro data demonstrating that PCB 11 and its metabolites alter neuronal morphogenesis is suggested by experimental evidence that altered spatiotemporal patterns in axonal and dendritic growth can cause persistent changes in brain patterning and synaptic connectivity in rodent models (Berger-Sweeney and Hohmann, 1997; Cremer et al., 1997; Maier et al., 1999). Moreover, structural aberrations in axonal and dendritic number, length and branching are thought to contribute to clinical manifestations of diverse NDD in humans (Copf, 2016; Engle, 2010; Garey, 2010; Penzes et al., 2011; Robichaux and Cowan, 2014; Supekar et al., 2013). Thus, inherent imbalances in synaptic connectivity in children at risk for NDD likely provide the biological substrate for enhanced susceptibility to environmental triggers that converge on pathways that modulate axonal and dendritic growth during development (Stamou et al., 2013).
In conclusion, this study is the first to report the presence of PCB 11 in pregnant women, and to demonstrate that PCB 11 and 2 of its major metabolites promote dendritic and axonal growth in vitro in neuronal cell types critically involved in cognitive and higher-order behaviors. Since altered dendritic and axonal morphogenesis are associated with human NDD, our findings identify PCB 11 as a potential environmental risk factor for adverse neurodevelopmental outcomes in humans that warrants further investigation in preclinical models.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
FUNDING
This study was supported by the National Institute of Environmental Health [grant numbers R01 ES014901, R01 ES017425, P30 ES023513, P42 ES013661, P01 ES011269, and T32 ES007059 to S.S. and H.C.], the Eunice Kennedy Shriver National Institute of Child Health and Human Development [grant numbers U54 HD079125 and F32 HD088016 to K.P.K.]) and by the United States Environmental Protection Agency [grant number RD 83543201]. The contents of this study do not necessarily represent the official views of the NIEHS, NICHD, or USEPA; the NIEHS, NICHD and USEPA do not endorse the purchase of any commercial products or services mentioned in the publication. The sponsors were not involved in the study design, the collection, analysis, and interpretation of data, in the writing of the report or in the decision to submit the paper for publication.
Supplementary Material
REFERENCES
- Barone S. Jr, Das K. P., Lassiter T. L., White L. D. (2000). Vulnerable processes of nervous system development: a review of markers and methods. Neurotoxicology 21, 15–36. [PubMed] [Google Scholar]
- Basu I., Arnold K. A., Venier M., Hites R. A. (2009). Partial pressures of PCB-11 in air from several Great Lakes sites. Environ. Sci. Technol. 43, 6488–6492. [DOI] [PubMed] [Google Scholar]
- Berger-Sweeney J., Hohmann C. F. (1997). Behavioral consequences of abnormal cortical development: insights into developmental disabilities. Behav. Brain Res. 86, 121–142. [DOI] [PubMed] [Google Scholar]
- Carpenter D. O. (2006). Polychlorinated biphenyls (PCBs): routes of exposure and effects on human health. Rev. Environ. Health 21, 1–23. [DOI] [PubMed] [Google Scholar]
- Chen H., Streifel K. M., Singh V., Yang D., Mangini L., Wulff H., Lein P. J. (2016). BDE-47 and BDE-49 inhibit axonal growth in primary rat hippocampal neuron-glia co-cultures via ryanodine receptor-dependent mechanisms. Toxicol. Sci. 156, 375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Lin Y., Dang K., Puschner B. (2017). Quantification of polychlorinated biphenyls and polybrominated diphenyl ethers in commercial cows' milk from California by gas chromatography-triple quadruple mass spectrometry. PLoS One 12, e0170129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S. D., Baek S. Y., Chang Y. S., Wania F., Ikonomou M. G., Yoon Y. J., Park B. K., Hong S. (2008). Passive air sampling of polychlorinated biphenyls and organochlorine pesticides at the Korean Arctic and Antarctic research stations: implications for long-range transport and local pollution. Environ. Sci. Technol. 42, 7125–7131. [DOI] [PubMed] [Google Scholar]
- Copf T. (2016). Impairments in dendrite morphogenesis as etiology for neurodevelopmental disorders and implications for therapeutic treatments. Neurosci. Biobehav. Rev. 68, 946–978. [DOI] [PubMed] [Google Scholar]
- Coullery R. P., Ferrari M. E., Rosso S. B. (2016). Neuronal development and axon growth are altered by glyphosate through a WNT non-canonical signaling pathway. Neurotoxicology 52, 150–161. [DOI] [PubMed] [Google Scholar]
- Cremer H., Chazal G., Goridis C., Represa A. (1997). NCAM is essential for axonal growth and fasciculation in the hippocampus. Mol. Cell. Neurosci. 8, 323–335. [DOI] [PubMed] [Google Scholar]
- DeVito M. J., Ross D. G., Dupuy A. E. Jr., Ferrario J., McDaniel D., Birnbaum L. S. (1998). Dose-response relationships for disposition and hepatic sequestration of polyhalogenated dibenzo-p-dioxins, dibenzofurans, and biphenyls following subchronic treatment in mice. Toxicol. Sci. 46, 223–234. [DOI] [PubMed] [Google Scholar]
- Dotti C. G., Sullivan C. A., Banker G. A. (1988). The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 8, 1454–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du S., Belton T. J., Rodenburg L. A. (2008). Source apportionment of polychlorinated biphenyls in the tidal Delaware River. Environ. Sci. Technol. 42, 4044–4051. [DOI] [PubMed] [Google Scholar]
- Du S., Wall S. I., Cacia D., Rodenburg L. A. (2009). Passive air sampling for polychlorinated biphenyls in the Philadelphia metropolitan area. Environ. Sci. Technol. 43, 1287–1292. [DOI] [PubMed] [Google Scholar]
- Engle E. C. (2010). Human genetic disorders of axon guidance. Cold Spring Harb. Perspect. Biol. 2, a001784.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eubig P. A., Aguiar A., Schantz S. L. (2010). Lead and PCBs as risk factors for attention deficit/hyperactivity disorder. Environ. Health Perspect. 118, 1654–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey L. (2010). When cortical development goes wrong: schizophrenia as a neurodevelopmental disease of microcircuits. J. Anat. 217, 324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm F. A., He X., Teesch L. M., Lehmler H. J., Robertson L. W., Duffel M. W. (2015). Tissue distribution, metabolism, and excretion of 3,3'-dichloro-4'-sulfooxy-biphenyl in the rat. Environ. Sci. Technol. 49, 8087–8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm F. A., Lehmler H. J., He X., Robertson L. W., Duffel M. W. (2013). Sulfated metabolites of polychlorinated biphenyls are high-affinity ligands for the thyroid hormone transport protein transthyretin. Environ. Health Perspect. 121, 657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm F. A., Lehmler H. J., Koh W. X., DeWall J., Teesch L. M., Hornbuckle K. C., Thorne P. S., Robertson L. W., Duffel M. W. (2016). Identification of a sulfate metabolite of PCB 11 in human serum. Environ. Int. 98, 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J., Kim D., Lee G. (2014). Congener profiles and source-wise phase partitioning analysis of PCDDs/Fs and PCBs in Gyeonggi-do ambient air, South Korea. Int. J. Environ. Res. Public Health 11, 11065–11080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I., Bennett D., Walker C. K., Schmidt R. J., McKenzie O., Wise K., Giulivi C., Puschner B., Thomas J., LaSalle J., et al. (2017). Environmental contributions to, and biomarkers of, autism spectrum disorder: an introduction to the MARBLES study. Environ. Health Perspect. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland E. B., Feng W., Zheng J., Dong Y., Li X., Lehmler H. J., Pessah I. N. (2017). An extended structure-activity relationship of nondioxin-like PCBs evaluates and supports modeling predictions and identifies picomolar potency of PCB 202 towards ryanodine receptors. Toxicol. Sci. 155, 170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D., Hornbuckle K. C. (2010). Inadvertent polychlorinated biphenyls in commercial paint pigments. Environ. Sci. Technol. 44, 2822–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D., Martinez A., Hornbuckle K. C. (2008). Discovery of non-aroclor PCB (3,3'-dichlorobiphenyl) in Chicago air. Environ. Sci. Technol. 42, 7873–7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Adamcakova-Dodd A., Thorne P. S. (2014). The fate of inhaled (14)C-labeled PCB11 and its metabolites in vivo. Environ. Int. 63, 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Lehmler H. J., Adamcakova-Dodd A., Thorne P. S. (2013). Elimination of inhaled 3,3'-dichlorobiphenyl and the formation of the 4-hydroxylated metabolite. Environ. Sci. Technol. 47, 4743–4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I., Barnhart C. D., Lein P. J., Lehmler H. J. (2015). Effect of pregnancy on the disposition of 2,2',3,5',6-pentachlorobiphenyl (PCB 95) atropisomers and their hydroxylated metabolites in female mice. Chem. Res. Toxicol. 28, 1774–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I., Barnhart C. D., Stamou M., Truong K. M., El-Komy M. H., Lein P. J., Veng-Pedersen P., Lehmler H. J. (2012). 2,2',3,5',6-Pentachlorobiphenyl (PCB 95) and its hydroxylated metabolites are enantiomerically enriched in female mice. Environ. Sci. Technol. 46, 11393–11401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I., Hornbuckle K. C., Robertson L. W., Lehmler H. J. (2008). Dose-dependent enantiomeric enrichment of 2,2',3,3',6,6'-hexachlorobiphenyl in female mice. Environ. Toxicol. Chem. 27, 299–305. [DOI] [PubMed] [Google Scholar]
- King T. L., Yeats P., Hellou J., Niven S. (2002). Tracing the source of 3,3'-dichlorobiphenyl found in samples collected in and around Halifax Harbour. Mar. Pollut. Bull. 44, 590–596. [DOI] [PubMed] [Google Scholar]
- Koh W. X., Hornbuckle K. C., Thorne P. S. (2015). Human serum from urban and rural adolescents and their mothers shows exposure to polychlorinated biphenyls not found in commercial mixtures. Environ. Sci. Technol. 49, 8105–8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korrick S. A., Sagiv S. K. (2008). Polychlorinated biphenyls, organochlorine pesticides and neurodevelopment. Curr. Opin. Pediatr. 20, 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan P. J., Lambertini L., Birnbaum L. S. (2012). A research strategy to discover the environmental causes of autism and neurodevelopmental disabilities. Environ. Health Perspect. 120, a258–a260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanting C. I., Huisman M., Muskiet F. A., van der Paauw C. G., Essed C. E., Boersma E. R. (1998). Polychlorinated biphenyls in adipose tissue, liver, and brain from nine stillborns of varying gestational ages. Pediatr. Res. 44, 222–225. [DOI] [PubMed] [Google Scholar]
- Lehmler H. J., Robertson L. W. (2001). Synthesis of hydroxylated PCB metabolites with the Suzuki-coupling. Chemosphere 45, 1119–1127. [DOI] [PubMed] [Google Scholar]
- Lein P. J., Banker G. A., Higgins D. (1992). Laminin selectively enhances axonal growth and accelerates the development of polarity by hippocampal neurons in culture. Brain Res. Dev. Brain Res. 69, 191–197. [DOI] [PubMed] [Google Scholar]
- Lesiak A., Zhu M., Chen H., Appleyard S. M., Impey S., Lein P. J., Wayman G. A. (2014). The environmental neurotoxicant PCB 95 promotes synaptogenesis via ryanodine receptor-dependent miR132 upregulation. J. Neurosci. 34, 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. P., Pessah I. N., Puschner B. (2013). Simultaneous determination of polybrominated diphenyl ethers and polychlorinated biphenyls by gas chromatography-tandem mass spectrometry in human serum and plasma. Talanta 113, 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten S., Fowler B., Luszniak D. (2002). Identification of a novel PCB source through analysis of 209 PCB congeners by US EPA modified method 1668. Chemosphere 46, 1457–1459. [CrossRef]] [DOI] [PubMed] [Google Scholar]
- Lobner D. (2000). Comparison of the LDH and MTT assays for quantifying cell death: validity for neuronal apoptosis? J. Neurosci. Methods 96, 147–152. [DOI] [PubMed] [Google Scholar]
- Lyall K., Croen L. A., Sjodin A., Yoshida C. K., Zerbo O., Kharrazi M., Windham G. C. (2016). Polychlorinated biphenyl and organochlorine pesticide concentrations in maternal mid-pregnancy serum samples: association with autism spectrum disorder and intellectual disability. Environ. Health Perspect. 125, 474–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier D. L., Mani S., Donovan S. L., Soppet D., Tessarollo L., McCasland J. S., Meiri K. F. (1999). Disrupted cortical map and absence of cortical barrels in growth-associated protein (GAP)-43 knockout mice. Proc. Natl Acad. Sci. U. S. A. 96, 9397–9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek R. F., Thorne P. S., DeWall J., Hornbuckle K. C. (2014). Variability in PCB and OH-PCB serum levels in children and their mothers in urban and rural U.S. communities. Environ. Sci. Technol. 48, 13459–13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek R. F., Thorne P. S., Wang K., Dewall J., Hornbuckle K. C. (2013). PCBs and OH-PCBs in serum from children and mothers in urban and rural U.S. communities. Environ. Sci. Technol. 47, 3353–3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerts I. A., Assink Y., Cenijn P. H., Van Den Berg J. H., Weijers B. M., Bergman A., Koeman J. H., Brouwer A. (2002). Placental transfer of a hydroxylated polychlorinated biphenyl and effects on fetal and maternal thyroid hormone homeostasis in the rat. Toxicol. Sci. 68, 361–371. [DOI] [PubMed] [Google Scholar]
- Meijering E., Jacob M., Sarria J. C., Steiner P., Hirling H., Unser M. (2004). Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A 58, 167–176. [DOI] [PubMed] [Google Scholar]
- Mosmann T. (1983). Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65, 55–63. [DOI] [PubMed] [Google Scholar]
- Penzes P., Cahill M. E., Jones K. A., VanLeeuwen J. E., Woolfrey K. M. (2011). Dendritic spine pathology in neuropsychiatric disorders. Nat. Neurosci. 14, 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe M. G., Byrne J. J. (1980). Adhesion-binding of 2,2',4,4',5,5'-hexachlorobiphenyl to glass and plastic: a possible source of error for PCB analysis. Bull. Environ. Contam. Toxicol. 25, 936–940. [DOI] [PubMed] [Google Scholar]
- Robichaux M. A., Cowan C. W. (2014). Signaling mechanisms of axon guidance and early synaptogenesis. Curr. Top. Behav. Neurosci. 16, 19–48. [DOI] [PubMed] [Google Scholar]
- Rodenburg L. A., Guo J., Du S., Cavallo G. J. (2010). Evidence for unique and ubiquitous environmental sources of 3,3'-dichlorobiphenyl (PCB 11). Environ. Sci. Technol. 44, 2816–2821. [DOI] [PubMed] [Google Scholar]
- Rossignol D. A., Genuis S. J., Frye R. E. (2014). Environmental toxicants and autism spectrum disorders: a systematic review. Transl. Psychiatry 4, e360.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz S. L., Widholm J. J., Rice D. C. (2003). Effects of PCB exposure on neuropsychological function in children. Environ. Health Perspect. 111, 357–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang H., Li Y., Wang T., Wang P., Zhang H., Zhang Q., Jiang G. (2014). The presence of polychlorinated biphenyls in yellow pigment products in China with emphasis on 3,3'-dichlorobiphenyl (PCB 11). Chemosphere 98, 44–50. [DOI] [PubMed] [Google Scholar]
- Sholl D. A. (1953). Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat 87, 387–406. [PMC free article] [PubMed] [Google Scholar]
- Soechitram S. D., Athanasiadou M., Hovander L., Bergman A., Sauer P. J. (2004). Fetal exposure to PCBs and their hydroxylated metabolites in a Dutch cohort. Environ. Health Perspect. 112, 1208–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamou M., Streifel K. M., Goines P. E., Lein P. J. (2013). Neuronal connectivity as a convergent target of gene x environment interactions that confer risk for Autism Spectrum Disorders. Neurotoxicol. Teratol. 36, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K., Uddin L. Q., Khouzam A., Phillips J., Gaillard W. D., Kenworthy L. E., Yerys B. E., Vaidya C. J., Menon V. (2013). Brain hyperconnectivity in children with autism and its links to social deficits. Cell Rep. 5, 738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K., Xue J., Williams R., Jones P., Whitaker D. (2012). Polychlorinated Biphenyls (PCBs) in School Buildings: Sources, Environmental Levels and Exposures. United States Environmental Protection Agency. [Google Scholar]
- Thompson M. R., Boekelheide K. (2013). Multiple environmental chemical exposures to lead, mercury and polychlorinated biphenyls among childbearing-aged women (NHANES 1999–2004): Body burden and risk factors. Environ. Res. 121, 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan P. J., Pike C. J., Cotman C. W., Cunningham D. D. (1995). Thrombin receptor activation protects neurons and astrocytes from cell death produced by environmental insults. J. Neurosci. 15(7 Pt 2), 5389–5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman G. A., Impey S., Marks D., Saneyoshi T., Grant W. F., Derkach V., Soderling T. R. (2006). Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron 50, 897–909. [DOI] [PubMed] [Google Scholar]
- Wayman G. A., Yang D., Bose D. D., Lesiak A., Ledoux V., Bruun D., Pessah I. N., Lein P. J. (2012). PCB-95 promotes dendritic growth via ryanodine receptor-dependent mechanisms. Environ. Health Perspect. 120, 997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winneke G. (2011). Developmental aspects of environmental neurotoxicology: lessons from lead and polychlorinated biphenyls. J. Neurol. Sci. 308, 9–15. [DOI] [PubMed] [Google Scholar]
- Yang D., Kania-Korwel I., Ghogha A., Chen H., Stamou M., Bose D. D., Pessah I. N., Lehmler H. J., Lein P. J. (2014). PCB 136 atropselectively alters morphometric and functional parameters of neuronal connectivity in cultured rat hippocampal neurons via ryanodine receptor-dependent mechanisms. Toxicol. Sci. 138, 379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Kim K. H., Phimister A., Bachstetter A. D., Ward T. R., Stackman R. W., Mervis R. F., Wisniewski A. B., Klein S. L., Kodavanti P. R., et al. (2009). Developmental exposure to polychlorinated biphenyls interferes with experience-dependent dendritic plasticity and ryanodine receptor expression in weanling rats. Environ. Health Perspect. 117, 426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Mapuskar K. A., Marek R. F., Xu W., Lehmler H. J., Robertson L. W., Hornbuckle K. C., Spitz D. R., Aykin-Burns N. (2013). A new player in environmentally induced oxidative stress: polychlorinated biphenyl congener, 3,3'-dichlorobiphenyl (PCB11). Toxicol. Sci. 136, 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.