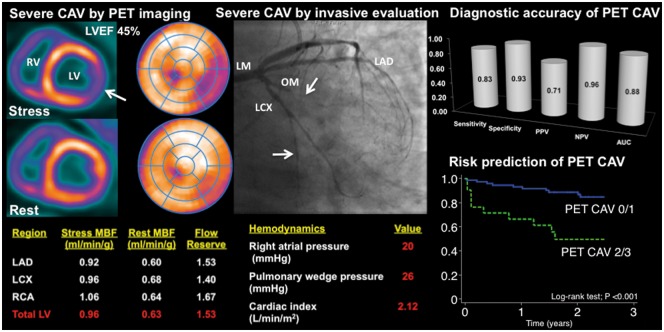
Abstract
Aims
Cardiac allograft vasculopathy (CAV) is a leading cause of death in orthotopic heart transplant (OHT) survivors. Effective non-invasive screening methods are needed. Our aim was to investigate the added diagnostic and prognostic value of myocardial blood flow (MBF) to standard myocardial perfusion imaging (MPI) with positron emission tomography (PET) for CAV detection.
Methods and results
We studied 94 OHT recipients (prognostic cohort), including 66 who underwent invasive coronary angiography and PET within 1 year (diagnostic cohort). The ISHLT classification was used as standard definition for CAV. Positron emission tomography evaluation included semiquantitative MPI, quantitative MBF (mL/min/g), and left ventricular ejection fraction (LVEF). A PET CAV severity score (on a scale of 0–3) was modelled on the ISHLT criteria. Patients were followed for a median of 2.3 years for the occurrence of major adverse events (death, re-transplantation, acute coronary syndrome, and hospitalization for heart failure). Sensitivity, specificity, positive, and negative predictive value of semiquantitative PET perfusion alone for detecting moderate-severe CAV were 83% [52–98], 82% [69–91], 50% [27–73], and 96% [85–99], respectively {receiver operating characteristic (ROC area: 0.82 [0.70–0.95])}. These values improved to 83% [52–98], 93% [82–98], 71% [42–92], and 96% [97–99], respectively, when LVEF and stress MBF were added (ROC area: 0.88 [0.76–0.99]; P = 0.01). There were 20 major adverse events during follow-up. The annualized event rate was 5%, 9%, and 25% in patients with normal, mildly, and moderate-to-severely abnormal PET CAV grading (P < 0.001), respectively.
Conclusion
Multiparametric cardiac PET evaluation including quantification of MBF provides improved detection and gradation of CAV severity over standard myocardial perfusion assessment and is predictive of major adverse events.
Keywords: Cardiac allograft vasculopathy , PET , Myocardial blood flow
Introduction
Cardiac allograft vasculopathy (CAV) remains as one of the leading causes of death in long-term orthotopic heart transplant (OHT) survivors and the principal cause of re-transplantation after 1 year.1 From a prognostic viewpoint, angiographically obstructive CAV and allograft dysfunction are independently associated with a higher risk of death and re-transplantation.2,3 Consequently, the International Society for Heart and Lung Transplantation (ISHLT) developed a CAV nomenclature with strong prognostic value based on information provided by invasive coronary angiography (ICA) in combination with markers of cardiac allograft function.4
Annual surveillance with ICA to assess the presence and severity of CAV is the current standard of care. However, ICA is a costly, invasive procedure with a risk for complications. The complexity of annual surveillance with ICA and the contrast dye that is required to perform the procedure is compounded by the fact that renal dysfunction is highly prevalent in the OHT population.1 Non-invasive imaging modalities such as dobutamine stress echocardiography (DSE) and single photon emission computed tomography (SPECT) are relatively insensitive for detection of CAV.5,6 Therefore, novel effective non-invasive screening methods are needed.
Myocardial perfusion imaging (MPI) with positron emission tomography (PET) is one of the most accurate non-invasive imaging approaches for the diagnosis and risk stratification of coronary artery disease in non-transplant populations and may have value in OHT patients.7 Our objective was to test the hypothesis that a multiparametric PET grading system that included quantitative myocardial blood flow (MBF), in addition to the standard regional MPI evaluation, would provide a more accurate diagnosis of CAV when compared to each individual technique, and thus, enhance risk stratification for major adverse events.
Methods
Study cohort and design
We retrospectively studied 94 OHT recipients referred for CAV evaluation with 13N-ammonia (13NH3) PET at Brigham and Women’s Hospital between January 2011 and August 2015. We included consecutive patients with at least one ICA before and/or after PET. We excluded one patient who underwent combined heart and lung transplantation, and patients who had Rb-82 PET (n = 8; prior to 2011) and/or received dobutamine for stress (n = 1).
The study cohort was sub-grouped as follows: (i) Diagnostic cohort (n = 66): Since rapid progression of CAV can occur within 1 year of span, for the diagnostic comparison analysis we only included patients who either (a) had routine ICA before (median 374 days [IQR 357–385]) and after PET (median 364 days [IQR 356–379]) without evidence of CAV progression between pre- and post-PET ICA (n = 41), or (b) had ICA performed within 30 days of PET (n = 25; median 13 days [IQR 5–18]). (ii) Prognostic cohort (n = 94): All patients in the study who had PET and at least one ICA at any time were included in the prognostic analysis. Cardiac PET imaging was performed as part of the annual screening evaluation. Our current CAV surveillance protocol includes vasodilator stress 13NH3 PET alternating with ICA every other year for most patients ≥ 5 years post-OHT followed at our institution. Additionally, PET is performed in patients who cannot undergo ICA due to renal dysfunction, access issues or other physicians’ concerns. In a subset of patients, ICA may be performed soon after PET if there is a significant abnormality indicating flow-limiting stenosis. The Partners Human Research Committee approved this study, including a waiver of consent.
Positron emission tomography imaging
All subjects were imaged using a whole body PET/CT system (Discovery RX or STE LightSpeed 64, GE Healthcare, Milwaukee, WI, USA). Starting with the bolus administration of 13NH3 (∼740 MBq) list-mode 2D PET imaging was obtained over 20 min. Then, a standard intravenous infusion of regadenoson (n = 90) or adenosine (n = 4) was given. At peak stress, a second dose of 13NH3 was injected and images were recorded in the same manner.
Positron emission tomography imaging analysis
For semi-quantitative assessment of regional MPI, 17-segment visual interpretation of MPI was performed in a blind fashion by experienced operators using a standard five-point scoring system. Left ventricular ejection fraction (LVEF) was calculated from gated MPI at rest and during stress with commercially available software (Corridor4DM; Ann Arbor, MI, USA). Myocardial perfusion imaging was considered abnormal when the summed stress score was ≥ 2. Therefore, our proposed PET score assigns the same weight to fixed and reversible MPI defects.
Myocardial blood flow quantification
Global and regional (on a vascular territory basis) MBF (mL/min/g) was quantified from the dynamic rest and stress datasets using commercially available software (Corridor4DM; Ann Arbor, MI, USA).
Coronary angiography
All patients underwent selective ICA using standard clinical techniques, with two or more projections obtained per vessel distribution, and angles of projection optimized for cardiac position. The presence and degree of coronary stenosis of the major epicardial coronary arteries were graded independently by semi-quantitative visual analysis of experienced operators on an ordinal scale and applied to the ISHLT CAV classification4 in blinded fashion.
Right heart catheterization
Right heart catheterization was performed in all patients using standard clinical techniques. Mean right atrial pressure, mean pulmonary capillary wedge pressure, and cardiac index by the Fick method were recorded.
Echocardiography
A standard clinical scanning protocol was implemented in all subjects using a Siemens or Philips ultrasound machine. Analysis was focused on LVEF assessment using the biplane method.
Cardiac allograft vasculopathy adjudication
The ISHLT classification was used as the reference for CAV (see Supplementary material online, Table S1).4 ISHLT CAV severity was determined by integrating (i) ICA findings, (ii) echocardiographic LVEF, and (iii) presence of restrictive haemodynamics from right heart catheterization in blinded fashion. The ICA closest to PET was used for the final adjudication.
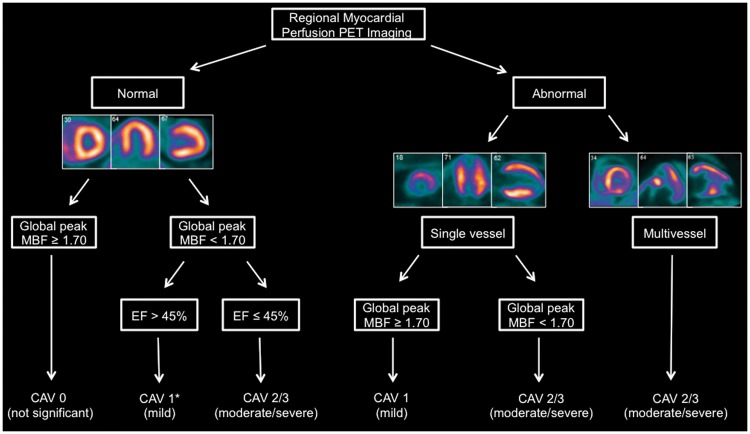
Non-invasive diagnostic algorithm for cardiac allograft vasculopathy
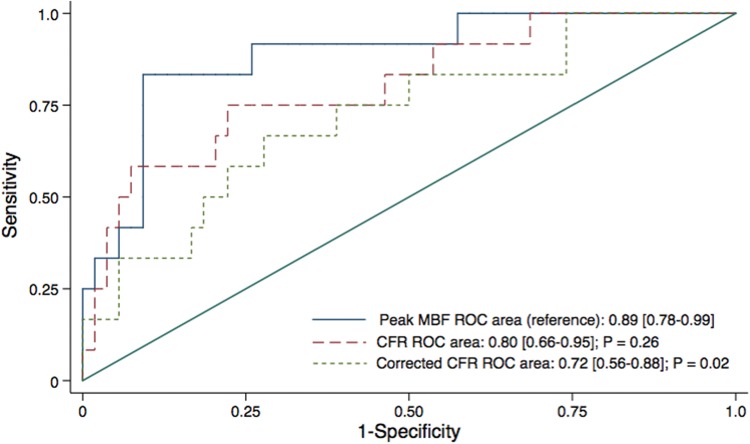
The use of coronary flow reserve (CFR) is potentially more limited in transplant than in non-transplant cohorts due to the more variable but generally higher resting MBF that presumably results from the higher heart rate in the denervated heart. Consequently, peak MBF may provide an advantage in these patients. In preliminary analyses, the area under the receiver operating characteristic (ROC) curve for peak MBF was superior compared to CFR and rate-pressure-product corrected-CFR for the diagnosis of ISHLT CAV 2/3 (Figure 1). A peak MBF < 1.70 had the best trade-off between sensitivity and specificity and also proven to be superior over CFR <1.70 and CFR < 2.0 for the detection of significant CAV (see Supplementary material online, Figure S1). Consequently, global peak MBF <1.70 was considered abnormal for the purposes of our study.
Figure 1.
Comparison of the area under receiver operating characteristic (ROC) curve analysis of global myocardial blood flow (MBF), coronary flow reserve (CFR), and corrected CFR for the detection of ISHLT cardiac allograft vasculopathy 2/3. P-values are against peak MBF ROC curve.
Subsequently, we developed a diagnostic algorithm for the evaluation of CAV severity (Figure 2) based on a PET grading system that was modelled on the ISHLT criteria that included the following components: (i) semi-quantitative MPI assessment, which was used to ascertain the presence, location and severity of angiographically obstructive CAV, (ii) global peak MBF was used as a marker of the severity of focal but especially diffuse CAV, and (iii) PET LVEF was used as a marker of overall allograft function. For the purposes of our algorithm, abnormal MPI corresponded to either a reversible and/or fixed relative perfusion deficit, and the highest LVEF value derived from the rest/stress protocol was included into the algorithm.
Figure 2.
Proposed diagnostic algorithm for the non-invasive evaluation of cardiac allograft vasculopathy (CAV) using a positron emission tomography grading system that integrates myocardial perfusion imaging with absolute flow quantification. False negative cases for CAV 2/3 most likely to occur within branch marked with asterisk (*).
Outcomes
Patients were retrospectively followed for the occurrence of major adverse events, including death, re-transplantation, and admission for acute coronary syndrome (ACS) or heart failure (HF). Time-to-first event was analysed. Ascertainment of clinical events was determined by blinded expert adjudication of the longitudinal medical record and Partners Healthcare Research Patient Data Registry. The date of the last consultation was used to determine follow-up. There were no patients lost to follow-up.
Statistical analysis
We used the Fisher’s exact or the χ2 tests and one-way ANOVA for categorical and continuous variables, respectively. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and the area under the ROC curve were computed for MPI, global peak MBF, and PET CAV score (index test) using ISHLT CAV 2/3 as the reference standard according to the Standards for Reporting Diagnostic Accuracy guidelines (see Supplementary material online, Figure S2). Simple correlations were assessed using Pearson’s correlation coefficient. The Kaplan–Meier event-free survival curves for major events were compared using the log-rank test across dichotomous categories of PET CAV (0/1 vs. 2/3). Cox proportional-hazards models were used to examine univariate and multivariable associations with events. Data were censored at the time of the first event or last visit. All statistical tests were two-tailed, and a P-value <0.05 was considered statistically significant. We analysed the data using STATA (version 13.1).
Results
Baseline, haemodynamic, and imaging characteristics of the 94 OHT patients included in the study are presented in Tables 1and2. Most patients (94%) underwent PET ≥ 5 years post-OHT. Cardiac allograft vasculopathy (any degree) and reduced glomerular filtration rate (GFR) (< 60 mL/min per 1.73 m2) were present in 47% and 64% of patients, respectively. ISHLT CAV 2/3 was present in 15% of patients. Myocardial perfusion imaging was abnormal in 30% (reversible MPI defects in 20 and fixed defects in 8 patients). Global peak MBF was impaired in 40%. In general, imaging and haemodynamic characteristics were comparable between patients with ISHLT CAV 0 and 1, but were significantly different compared to patients with ISHLT CAV 2/3 (Table 2).
Table 1.
Baseline characteristics of heart transplant patients
| Baseline characteristic | All patients (n = 94) |
|---|---|
| Age at PET (years) | 56 ± 16 |
| Males | 74 (79) |
| Time between OHT and PET (years) | 11.8 ± 5.7 |
| <5 years since OHT | 6 (6) |
| 5–9 years since OHT | 35 (37) |
| ≥10 years since OHT | 53 (56) |
| Hypertension | 69 (73) |
| Diabetes | 28 (30) |
| Ischaemic cardiomyopathy prior to OHT | 32 (34) |
| Coronary revascularization prior to PET | 1 (1) |
| Prednisone | 79 (84) |
| Mycophenolate | 39 (41) |
| Tacrolimus | 19 (20) |
| Azathioprine | 14 (15) |
| Cyclosporine | 27 (29) |
| Sirolimus | 3 (3) |
| Beta-blockers | 27 (29) |
| Calcium channel blockers | 55 (60) |
| Angiotensin converting enzyme inhibitors | 27 (29) |
| Diuretics | 25 (27) |
| Aspirin | 55 (60) |
| Statins | 73 (79) |
Data are expressed as mean ± standard deviation or as number (percentage).
Table 2.
Baseline imaging and haemodynamic parameters according to ISHLT cardiac allograft vasculopathy grading
| Baseline characteristics | All patients (n = 94) | ISHLT CAV 0 (n = 50) | ISHLT CAV 1 (n = 30) | ISHLT CAV 2/3 (n = 14) | P-value |
|---|---|---|---|---|---|
| LVEF (%) | 61 ± 7 | 62 ± 5 | 61 ± 4 | 53 ± 13 | <0.001 |
| LVEF ≤ 45% | 2 (2) | 0 | 0 | 2 (14) | 0.003 |
| Abnormal MPI PET | 28 (30) | 8 (16) | 9 (30) | 11 (79) | <0.001 |
| Rest MBF (mL/min/g) | 0.97 ± 0.27 | 0.99 ± 0.30 | 0.97 ± 0.25 | 0.87 ± 0.18 | 0.32 |
| Peak MBF (mL/min/g) | 1.83 ± 0.50 | 1.97 ± 0.48 | 1.83 ± 0.42 | 1.28 ± 0.32 | <0.001 |
| CFR (unitless) | 1.94 ± 0.51 | 2.06 ± 0.53 | 1.94 ± 0.42 | 1.50 ± 0.38 | <0.001 |
| Peak MBF < 1.70 mL/min/g | 38 (40) | 14 (28) | 11 (37) | 13 (93) | <0.001 |
| Right atrial pressure (mmHg) | 7.6 ± 4.1 | 7.1 ± 3.3 | 6.8 ± 3.4 | 10.9 ± 6.1 | 0.003 |
| Pulmonary wedge pressure (mmHg) | 12.7 ± 4.7 | 12.1 ± 3.8 | 11.8 ± 3.6 | 16.4 ± 7.6 | 0.005 |
| Cardiac index (L/min/m2) | 3.02 ± 0.68 | 3.13 ± 0.79 | 3.00 ± 0.53 | 2.66 ± 0.39 | 0.090 |
| Restrictive filling pressures | 2 (2) | 0 | 0 | 2 (14) | 0.003 |
| Haemoglobin (g/dL) | 12.9 ± 1.6 | 13.0 ± 1.7 | 12.9 ± 1.5 | 12.5 ± 1.7 | 0.68 |
| Creatinine (mg/dL) | 1.52 ± 0.75 | 1.42 ± 0.55 | 1.53 ± 0.55 | 1.86 ± 1.43 | 0.15 |
| GFR ≥ 60 mL/min/1.73m2 | 34 (36) | 24 (48) | 6 (20) | 4 (29) | |
| GFR 30–59 mL/min/1.73m2 | 52 (55) | 23 (46) | 22 (73) | 7 (50) | 0.038 |
| GFR < 30 mL/min/1.73m2 | 8 (9) | 3 (6) | 2 (7) | 3 (21) |
Data are expressed as mean ± standard deviation or as number (percentage).
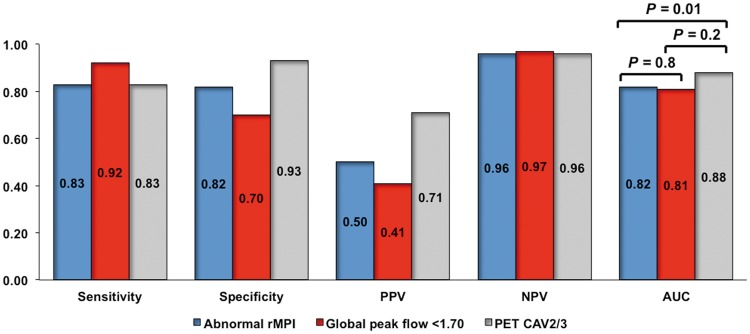
Diagnostic accuracy analysis
The agreement of PET CAV 2/3 with ISHLT CAV 2/3 was substantial (κ = 0.71 [0.47–0.95]; Supplementary material online, Table S2). Figure 3 summarizes the diagnostic accuracy analyses of the combined vs. individual components of PET for the diagnosis of ISHLT CAV 2/3. Global peak MBF < 1.70 had the highest sensitivity and NPV, whereas the PET CAV score 2/3 displayed the highest specificity and PPV. Overall, the area under the curve (AUC) for the diagnosis of ISHLT CAV 2/3 was superior for PET CAV 2/3, compared with global peak MBF <1.70 or MPI. Representative cases of discrepancies between ISHLT and PET CAV grading are illustrated in Supplementary material online, Figure S3. The effect of different estimates of disease prevalence on the PPV and NPV of each test is shown in Supplementary material online, Table S3.
Figure 3.
Diagnostic accuracy analysis of the combined vs. individual components of multiparametric positron emission tomography for the detection of ISHLT cardiac allograft vasculopathy 2/3.
We also explored the relationship of peak MBF with the individual components of the ISHLT CAV nomenclature. Peak MBF showed a modest, but significant relationship with angiographic CAV stenosis (r = –0.47; P < 0.001; Supplementary material online, Figure S4), mean right atrial pressure (r = –0.58; P < 0.001), and mean pulmonary capillary wedge pressure (r = –0.51; P < 0.001).
Outcomes analysis
Patients were followed for a median time of 2.3 years [1.6–2.8], and 20 events were recorded as follows: 12 deaths, 2 re-transplantations, 1 ACS, and 5 hospital admissions for HF. Death from CAV progression was confirmed in five cases (see Supplementary material online, Table S4). In the univariate analysis (Table 3), patients experiencing events were more likely to have reduced GFR, anaemia, elevated filling pressures, lower LVEF, IHSLT CAV 2/3, abnormal MPI, reduced peak MBF, and higher PET CAV grading.
Table 3.
Univariate associations with major adverse events of heart transplant patients
| Prognostic variables | No events (n = 74) | Events (n = 20) | Hazard ratio [95% CI] | P-value |
|---|---|---|---|---|
| Creatinine (mg/dL) | 1.42 ± 0.49 | 1.86 ± 1.28 | 2.03 [1.32–3.11] | 0.001 |
| Haemoglobin (g/dL) | 13.1 ± 1.6 | 12.2 ± 1.7 | 1.37 [1.04–1.81] | 0.024 |
| ISHLT classification | ||||
| Right atrial pressure (mmHg) | 6.9 ± 3.4 | 10.3 ± 5.1 | 1.20 [1.09–1.31] | <0.001 |
| Pulmonary wedge pressure (mmHg) | 11.7 ± 3.8 | 16.1 ± 6.1 | 1.21 [1.11–1.32] | <0.001 |
| Restrictive filling pressures | 0 | 2 (10) | 25.9 [4.9–136] | <0.001 |
| LVEF (%) | 62 ± 5 | 54 ± 11 | 1.09 [1.05–1.13] | <0.001 |
| LVEF ≤ 45% | 0 | 2 (10) | 14.1 [3.07–65] | 0.001 |
| ISHLT CAV | 44 (59) | 6 (30) | Reference | |
| ISHLT CAV 1 | 27 (37) | 3 (15) | 0.84 [0.21–3.36] | 0.8 |
| ISHLT CAV 2/3 | 3 (4) | 11 (55) | 11.5 [4.08–32] | <0.001 |
| PET classification | ||||
| Abnormal MPI | 18 (24) | 10 (50) | 3.11 [1.26–7.66] | 0.014 |
| Peak MBF (mL/min/g) | 1.91 ± 0.46 | 1.51 ± 0.49 | 6.55 [2.12–20] | 0.001 |
| Peak MBF < 1.70 mL/min/g | 24 (32) | 14 (70) | 4.05 [1.55–10] | 0.004 |
| CFR (unitless) | 2.03 ± 0.49 | 1.61 ± 0.44 | 6.24 [2.03–19] | 0.001 |
| PET CAV 0 | 42 (57) | 5 (25) | Reference | |
| PET CAV 1 | 21 (28) | 5 (25) | 1.92 [0.56–6.65] | 0.30 |
| PET CAV 2/3 | 11 (15) | 10 (50) | 5.93 [2.02–17.4] | 0.001 |
Only variables with Cox hazard ratios achieving P-value <0.05 are shown. Data are expressed as mean ± standard deviation or as number (percentage).
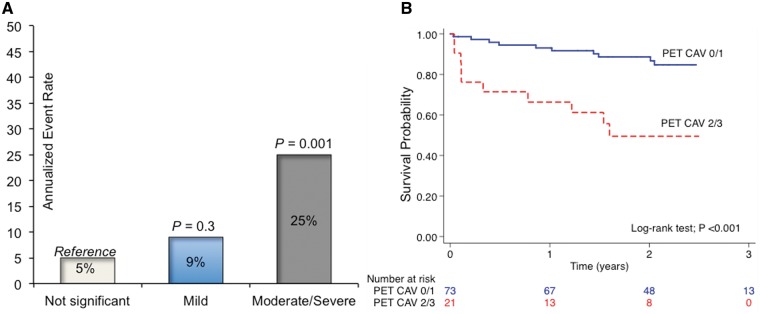
There was a stepwise increase in the annualized event rate with increasing PET CAV grading (Figure 4A). The Kaplan–Meier curves in Figure 4B further illustrate the reduced event-free survival of patients with CAV 2/3 according to PET. In a multivariable analysis PET CAV 2/3 (HR 3.5 [1.36–9.0]; P = 0.009), when used in lieu of ICA, remained as a significant predictor of events after adjusting for filling pressures and LVEF (see Supplementary material online, Table S5).
Figure 4.
Annualized event rates (A) and event-free survival curves (B) of major adverse events according to positron emission tomography cardiac allograft vasculopathy severity.
Take home figure.
Multiparametric myocardial perfusion positron emission tomography imaging score including absolute flow quantification is a versatile and powerful tool for the diagnosis and risk stratification of individuals with suspected cardiac allograft vasculopathy (CAV). In this example, moderate amount of ischaemia is seen in the inferolateral wall (arrow), suggestive of obstructive CAV in the left circumflex (LCX) territory. Importantly, there is a severe and diffuse reduction in myocardial blood flow (MBF) during peak hyperaemia, here reflecting the integrated effects of large and small vessel abnormalities, consistent with severe diffuse CAV, which agrees with findings of invasive left and right heart catheterization. LM, left main; LAD, left anterior descending; OM, obtuse marginal; LV, left ventricle; RV, right ventricle.
Discussion
Cardiac allograft vasculopathy remains one of the leading causes of morbidity and mortality among long-term survivors of OHT. Although non-invasive screening of CAV would be desirable, annual ICA remains the current clinical standard of care for the detection of obstructive CAV, especially within the first 5 years after OHT.4 In the present study, we developed a multiparametric non-invasive PET score (derived from a practical algorithm) that integrates MPI, absolute MBF quantification, and allograft function, with the aim of addressing both the focal and diffuse nature of this disease. We found high diagnostic accuracy of the comprehensive PET score for detecting and excluding moderate/severe CAV as assessed by the current ISHLT classification. Importantly, our multiparametric PET CAV score also provided significant risk stratification.
To date, only a handful of studies have employed the ISHLT-recommended criteria for the detection of moderate to severe CAV, a subgroup of patients with high morbidity and mortality in both adult and paediatric populations.3,8 Using this new reference, Wenning et al. reported moderate sensitivity (64%) and good specificity (84%) for MPI with SPECT in 161 OHT recipients,5 whereas Chirakarnjanakorn et al.6 found poor sensitivity (28%) and very high specificity (98%) for DSE (PPV 71%, NPV 89%) in 310 OHT patients. In comparison, we observed that our proposed PET CAV grading system was associated with higher sensitivity and NPV, and similar specificity compared to the published experience with SPECT and DSE. This seemingly superior diagnostic accuracy was the result of a trade-off between the high sensitivity derived from absolute MBF quantification and the specificity imposed by MPI, which, in combination with flow quantification improved significantly the overall diagnostic accuracy.
One unique advantage of PET is its ability to quantify regional and global MBF, a sensitive marker more closely associated with the pathobiology of CAV that includes intimal proliferation in the epicardial coronary arteries and medial thickening in the small intramyocardial coronary vessels, resulting in focal epicardial coronary stenoses, diffuse distal vessel tapering, and obstructive microvasculopathy.9 In this respect, except for one patient, all OHT recipients with significant CAV had evidence of globally reduced peak MBF indicating impaired vasodilator capacity of the coronary resistive vessels, and nearly all coronary vessels with angiographic stenosis ≥70% had regionally reduced peak MBF. Beyond angiographic stenosis, we also observed a significant association between peak MBF and markers of graft function as all subjects with significant LV systolic dysfunction and/or restrictive physiology had impaired peak MBF, highlighting the potential clinical value of this quantitative marker for detecting diffuse CAV. In this sense, another strength of our proposed protocol is the inclusion of PET-derived LVEF into our diagnostic algorithm. In certain cases, CAV can manifest with angiographically silent findings that may eventually progress to the development of restrictive physiology and allograft dysfunction. Therefore, the combination of severely impaired peak MBF and LV dysfunction, independent of the MPI results, should enhance the clinical suspicion for the presence of underlying severe CAV (see Supplementary material online, Figure S3A), especially after excluding other possibilities that can manifest in a similar way (e.g. late rejection).
There are additional caveats and limitations to consider when comparing anatomical vs. functional testing. Some coronary lesions may have non-obstructive angiographic appearance but indeed exhibit significant inducible ischaemia on PET (see Supplementary material online, Figure S3B), which likely represents functionally, but not anatomically, significant CAV. In addition, presence of myocardial scar on PET imaging in the absence of significant angiographic coronary stenosis may be indicative of CAV affecting preferentially the smaller intramural coronary vessels that are beyond the spatial resolution of coronary angiography (see Supplementary material online, Figure S3C). In other cases, inducible myocardial ischaemia can be extensive and global peak MBF markedly reduced suggestive of multivessel disease, and yet coronary angiography may be normal, which is diagnostic of severe microvascular involvement (see Supplementary material online, Figure S3D).
Importantly, our multiparametric PET CAV score also provided significant risk stratification. Positron emission tomography cardiac allograft vasculopathy was established as a significant predictor for future events, even after adjusting for standard clinical parameters such as LVEF and filling pressures. Indeed, we found an important stepwise increase in risk with worsening PET CAV score. In this respect, our findings extend the results of prior CAV studies demonstrating an association between abnormal myocardial perfusion on SPECT10 or impaired flow on PET7 and outcomes, by introducing a practical diagnostic algorithm with significant prognostic value that incorporates not only MPI, but more importantly absolute flow quantification into a multiparametric PET score, thereby integrating the broader spectrum of the pathobiology of CAV.
Despite significant advances in cardiovascular imaging, to this date, ICA remains the accepted clinical standard for CAV diagnosis given its broad availability and prognostic significance. Yet, the test is invasive, and, carries a small but significant risk, including contrast-induced nephropathy. The latter becomes a greater concern in the post-OHT population, as prevalence of renal dysfunction is very high, affecting 52% and 68% of OHT survivors after 5 and 10 years, respectively.1 Therefore, accurate non-invasive modalities for the diagnosis and risk-stratification of CAV are needed in these high-risk patients, and our proposed multiparametric PET approach emerges as a potential diagnostic alternative.
Finally, for the purpose of establishing the presence of CAV, the proposed PET score assigns identical weight to fixed and reversible regional MPI abnormalities. Consequently, a stress-only protocol would be reasonable for diagnosing CAV. However, PET scans are also used to guide subsequent management once a diagnosis is made. Therefore, in the absence of regional MPI defects during stress, a stress-only protocol would be reasonable for making diagnostic/prognostic assessments and management recommendations in this population. In patients with stress MPI defects, however, a resting study would generally be necessary to define the presence and magnitude of ischaemia/scar, which may have important clinical implications for management (e.g. revascularization).
Study limitations
Our study has several limitations that deserve discussion. While the study cohort represents a consecutive series of patients undergoing CAV screening, this is a retrospective single centre study and carries the inherent limitations of this study design. The time from OHT to PET was >10 years in 56% of patients, and as a result the prevalence of CAV was high in our cohort, which may especially affect the predictive value of our test, as well as the generalizability of our findings to patients early after transplantation. The ISHLT definition of CAV used in this study includes presence of focal stenosis on ICA. Consequently, the value of the PET CAV score (especially quantitative MBF) for early detection of CAV that is not yet angiographically manifested as focal disease cannot be assessed. The relatively small study cohort and limited number of events precluded additional adjustments in our multivariable modelling to further ascertain the prognostic interaction between the PET CAV score and the results of ICA and other important predictors. Finally, referral bias, the problem of multiple comparisons, and absence of a proper validation cohort are important limitations that will need to be addressed in future studies.
Conclusion
We have demonstrated that a multiparametric non-invasive score integrating the focal and diffuse aspects of the pathobiology of CAV obtained with PET imaging provides an effective non-invasive screening tool. This score is practical, shows high accuracy for the diagnosis of high-risk CAV, and provides risk stratification for major adverse events. While ICA is likely to remain an important diagnostic and prognostic tool for the evaluation of OHT survivors, our findings offer an alternative to potentially avoid annual catheterizations for the evaluation of CAV. Further studies with a prospective design are necessary to validate our preliminary findings in different cohorts and to determine the cost-effectiveness of this non-invasive screening strategy.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
We would like to thank all the staff of the PET centre at Brigham and Women’s Hospital for their technical support.
Funding
National Institutes of Health (1T32HL094301 and 1T32HL076136), in part.
Conflict of interest: none declared.
References
- 1. Lund LH, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, Dobbels F, Goldfarb SB, Levvey BJ, Meiser B, Yusen RD, Stehlik J.. The registry of the International Society for Heart and Lung Transplantation: thirty-first official adult heart transplant report-2014; focus theme: retransplantation. J Heart Lung Transplant 2014;33:996–1008. [DOI] [PubMed] [Google Scholar]
- 2. Costanzo MR, Naftel DC, Pritzker MR, Heilman JK 3rd, Boehmer JP, Brozena SC, Dec GW, Ventura HO, Kirklin JK, Bourge RC, Miller LW.. Heart transplant coronary artery disease detected by coronary angiography: a multiinstitutional study of preoperative donor and recipient risk factors. Cardiac Transplant Research Database. J Heart Lung Transplant 1998;17:744–753. [PubMed] [Google Scholar]
- 3. Kindel SJ, Law YM, Chin C, Burch M, Kirklin JK, Naftel DC, Pruitt E, Carboni MP, Arens A, Atz AM, Dreyer WJ, Mahle WT, Pahl E.. Improved detection of cardiac allograft vasculopathy: a multi-institutional analysis of functional parameters in pediatric heart transplant recipients. J Am Coll Cardiol 2015;66:547–557. [DOI] [PubMed] [Google Scholar]
- 4. Mehra MR, Crespo-Leiro MG, Dipchand A, Ensminger SM, Hiemann NE, Kobashigawa JA, Madsen J, Parameshwar J, Starling RC, Uber PA.. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy. J Heart Lung Transplant 2010;29:717–727. [DOI] [PubMed] [Google Scholar]
- 5. Wenning C, Stypmann J, Papavassilis P, Sindermann J, Schober O, Hoffmeier A, Scheld HH, Stegger L, Schafers M.. Left ventricular dilation and functional impairment assessed by gated SPECT are indicators of cardiac allograft vasculopathy in heart transplant recipients. J Heart Lung Transplant 2012;31:719–728. [DOI] [PubMed] [Google Scholar]
- 6. Chirakarnjanakorn S, Starling RC, Popovic ZB, Griffin BP, Desai MY.. Dobutamine stress echocardiography during follow-up surveillance in heart transplant patients: diagnostic accuracy and predictors of outcomes. J Heart Lung Transplant 2015;34:710–717. [DOI] [PubMed] [Google Scholar]
- 7. Mc Ardle BA, Davies RA, Chen L, Small GR, Ruddy TD, Dwivedi G, Yam Y, Haddad H, Mielniczuk LM, Stadnick E, Hessian R, Guo A, Beanlands RS, deKemp RA, Chow BJ.. Prognostic value of rubidium-82 positron emission tomography in patients after heart transplant. Circ Cardiovasc Imaging 2014;7:930–937. [DOI] [PubMed] [Google Scholar]
- 8. Prada-Delgado O, Estévez-Loureiro R, Paniagua-Martín MJ, López-Sainz A, Crespo-Leiro MG.. Prevalence and prognostic value of cardiac allograft vasculopathy 1 year after heart transplantation according to the ISHLT recommended nomenclature. J Heart Lung Transplant 2012;31:332–333. [DOI] [PubMed] [Google Scholar]
- 9. Arbustini E, Roberts WC.. Morphological observations in the epicardial coronary arteries and their surroundings late after cardiac transplantation (allograft vascular disease). Am J Cardiol 1996;78:814–820.http://dx.doi.org/10.1016/S0002-9149(96)00427-4 [DOI] [PubMed] [Google Scholar]
- 10. Manrique A, Bernard M, Hitzel A, Bubenheim M, Tron C, Agostini D, Cribier A, Vera P, Bessou JP, Redonnet M.. Diagnostic and prognostic value of myocardial perfusion gated SPECT in orthotopic heart transplant recipients. J Nucl Cardiol 2010;17:197–206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.