Abstract
Recent decades have seen a rapid increase in reported toxic effects of drugs and pollutants on mitochondria. Researchers have also documented many genetic differences leading to mitochondrial diseases, currently reported to affect ∼1 person in 4,300, creating a large number of potential gene-environment interactions in mitochondrial toxicity. We briefly review this history, and then highlight cutting-edge areas of mitochondrial research including the role of mitochondrial reactive oxygen species in signaling; increased understanding of fundamental biological processes involved in mitochondrial homeostasis (DNA maintenance and mutagenesis, mitochondrial stress response pathways, fusion and fission, autophagy and biogenesis, and exocytosis); systemic effects resulting from mitochondrial stresses in specific cell types; mitochondrial involvement in immune function; the growing evidence of long-term effects of mitochondrial toxicity; mitochondrial-epigenetic cross-talk; and newer approaches to test chemicals for mitochondrial toxicity. We also discuss the potential importance of hormetic effects of mitochondrial stressors. Finally, we comment on future areas of research we consider critical for mitochondrial toxicology, including increased integration of clinical, experimental laboratory, and epidemiological (human and wildlife) studies; improved understanding of biomarkers in the human population; and incorporation of other factors that affect mitochondria, such as diet, exercise, age, and nonchemical stressors.
Keywords: gene-environment interactions, mitochondrial homeostasis, mitochondrial disease, mitochondrial DNA, biomarker, mitohormesis
HISTORY OF MITOCHONDRIAL TOXICITY
Recognition of the ability of specific chemicals such as oligomycin (Chappell and Greville, 1961), 2,4-dinitrophenol (Gomez Puyou et al., 1964), pentachlorophenol (Buffa et al., 1959), or carbon monoxide (Villa et al., 1961) to poison mitochondria goes back more than 60 years. In subsequent decades, sporadic reports of chemicals affecting mitochondria continued to be published. The number of such reports, as well as the scope of mitochondrial impacts reported, began to expand in the 1990s, as described in the companion article by Dr Wallace (this issue reference). A series of groundbreaking reports by Drs. Will, Dykens, and colleagues beginning in 2007 (Dykens et al., 2007; Dykens and Will, 2007; Marroquin et al., 2007) raised awareness of how common drug-induced mitochondrial toxicities were. Since then, the potential importance of mitochondrial toxicity as a mode of toxicity for many chemicals including drugs, pollutants, and others has been highlighted by several reviews (Brunst et al., 2015; Meyer et al., 2013; Pereira et al., 2009; Sabri, 1998; Figure 1). Empirically, mitochondrial perturbations (most often, alterations in membrane potential) are one of the most common outcomes of in vitro toxicity screening efforts, including those of the National Toxicology Program (Attene-Ramos et al., 2013, 2015; Wills et al., 2015). We note, however, that chemicals which induce mitochondrial impairment can do so through multiple mechanisms (Chan et al., 2005; Dykens and Will, 2007; Wallace, 2015) including non-“traditional” targets, as highlighted below; that is, there is not just one “mechanism” of mitochondrial toxicity.
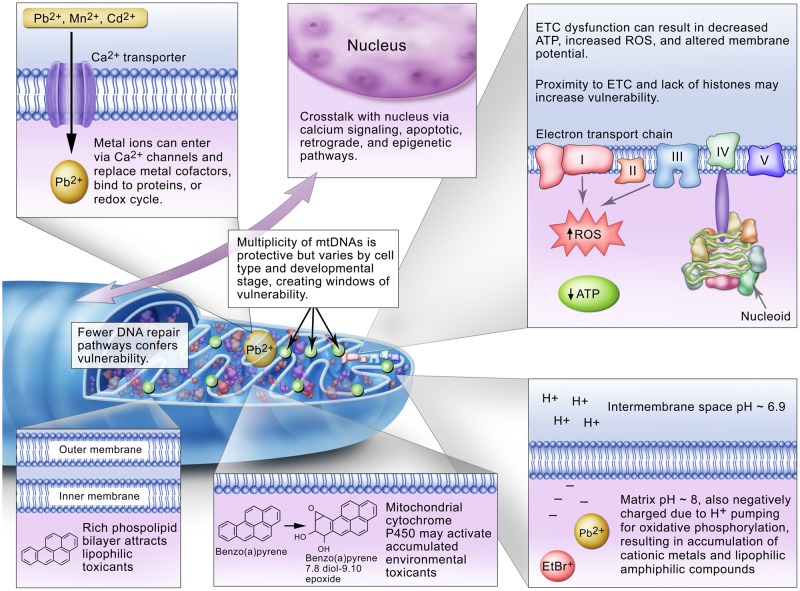
Figure 1.
Theoretical reasons for mitochondrial sensitivity to exposures to environmental chemicals. From Meyer et al. (2013).
In parallel with this growing understanding of mitochondrial chemical toxicity, awareness of the importance of mitochondria in disease has been growing rapidly. We now know that diseases caused by mutations in either the nuclear or mitochondrial genome, while each individually rare, collectively affect at least one person in 4,300 (Gorman et al., 2015). A much larger number of individuals is affected by diseases associated with, but not necessarily caused directly or entirely by mitochondrial dysfunction, including common diseases of aging such as Parkinson’s Disease, Alzheimer’s Disease, and cancers (Wallace, 2005). This raises the concerns that individuals suffering from mitochondrial diseases may be at greater risk from exposure to mitochondrial toxicants, or that such exposures may contribute to these diseases. This possibility was reviewed carefully in the context of idiosyncratic drug-induced liver injury, a major category of off-target drug effects, over 10 years ago (Boelsterli and Lim, 2007). In addition, clinical evidence for sensitivity of individuals with mitochondrial diseases to chemicals has emerged (Cohen, 2010), and work with mouse cells with mtDNA variants supports significant variability in response to mitotoxicants (Pereira et al., 2012). However, to our knowledge, there is little epidemiological literature addressing potential mitochondrial gene-environment interactions. This is unfortunate, because genetic contributions are typically modest, and environmental factors are likely quite important for many chronic diseases (Bookman et al., 2011; Rappaport, 2012). Therefore, as chronic diseases become more and more common in the United States and globally, the potential health significance of environmental exposures that perturb mitochondrial function will grow.
The rapid increase in interest in mitochondrial toxicity is reflected in the recent publication of a special issue of the journal Toxicology, entirely dedicated to mitochondrial toxicity (Meyer and Chan, 2017), as well as an updated and much-expanded version of the Drug-Induced Mitochondrial Dysfunction text edited by James Dykens and Yvonne Will, now re-titled Mitochondrial Dysfunction by Drugs and Environmental Toxicants (Dykens, in press), which includes chapters on organ-specific effects, mitochondrial toxicity assays, and clinical reports. Both contain a wealth of detailed information and extensive references.
CUTTING-EDGE AREAS OF RESEARCH
There are many areas in which our understanding of mitochondria is growing rapidly, including toxicology (Meyer and Chan, 2017) as well as basic biology. Following, we briefly describe some of the most toxicologically relevant areas of cutting-edge mitochondrial research.
The Paradox of Mitochondrial Reactive Oxygen Species (mtROS)
ROS are molecules containing oxygen that are more chemically reactive than molecular oxygen (O2), and which therefore have the potential to damage cellular macromolecules. We have long understood that in most cells, the majority of ROS are generated in mitochondria. The level of mtROS production depends on many factors, including the ease with which electrons flow through the electron transport chain (ETC). When the ETC is highly reduced (chemically), the likelihood of electrons moving to oxygen to form superoxide anion increases. In fact, early overestimates of the percentage of oxygen converted to superoxide anion in cells (∼2%, rather than the more accurate value of ∼0.1%) resulted from studies in which the ETC was inhibited with cyanide (Fridovich, 2004). Another factor influencing mtROS generation is calcium signaling, wherein calcium ions are passed from the endoplasmic reticulum (ER) to the mitochondria “quasi-synaptically,” that is, through very closely associated mitochondria-associated ER membranes (MAMs; Marchi et al., 2017). Calcium promotes ATP synthesis by stimulating ATP synthase and Krebs cycle enzymes (Rizzuto et al., 2000), and may therefore stimulate increased mitochondrial metabolic rate, oxygen consumption, and mtROS production. We note that while most data suggests that calcium accumulation in the mitochondria results in increased mtROS (e.g., Hansson et al., 2008), there are also reports of unchanged (Panov et al., 2007), or decreased (Starkov et al., 2002) mtROS production, potentially depending on the calcium load, metabolic state, and substrate availability of the mitochondria (Adam-Vizi and Starkov, 2010).
The early perspective on mtROS was that they were essentially damaging agents which could cause toxicity and pathology, but that this was the evolutionary price paid for the energetic efficiency of utilizing oxygen for energy production. It is now clear that mtROS also serve a variety of crucial roles. These include immune functions (addressed below), basic developmental processes (e.g., Coffman et al., 2009), but also adaptation to more pro-oxidant environments via the transcriptional upregulation of antioxidant and other defenses through redox-sensitive signaling pathways such as Nrf2/Keap1. Moreover, there is recent evidence that the intra-mitochondrial site of ROS formation, in addition to the amount and timing, may be important in determining destructive vs. signaling roles (Scialo et al., 2017), and that extra-mitochondrial ROS generation may be important as well (Di Meo et al., 2016). Critical ongoing research will permit increased mechanistic understanding of the beneficial roles of mtROS and the doses and timecourses over which they occur, and how environmental exposures may alter these processes (Blajszczak and Bonini, 2017). This knowledge will in turn allow a better understanding of the beneficial and deleterious effects of mtROS.
Increased Understanding of Mitochondrial Homeostasis
Mitochondria possess many of the same defense systems present elsewhere in the cell, including proteases, lipases, antioxidant enzymes and molecules, chaperones, and DNA repair enzymes, all of which are current topics of research. In addition, mitochondria, like some other organelles, can be recycled via autophagy including mitophagy, in which specifically damaged mitochondria are targeted for lysosomal degradation. However, they also possess both unique vulnerabilities in that they lack some defense mechanisms, and unique attributes that can make them more robust to stressor challenge. For example, mitochondria have multiple copies of their own genome, but lack nucleotide excision repair, a pathway responsible for the repair of DNA damage resulting from a wide range of very important environmental exposures including polycyclic aromatic hydrocarbons, aflatoxins, cisplatin, and high-energy ultraviolet radiation. On the other hand, the multiplicity of mitochondrial genomes, combined with the ability to remove and replace mitochondria and mtDNA via mitochondrial fusion, fission, autophagy, and biogenesis, allows them to tolerate and slowly remove otherwise irreparably damaged DNA (Bess et al., 2012). This may be why it has been challenging to detect mitochondrial DNA (mtDNA) mutagenesis in laboratory models after chemical exposure (Valente et al., 2016), despite reasons to expect such mutagenesis (Meyer and Chan, 2017). Genetic deficiencies in these processes, however, may complicate these responses; for example, there are a large number of mutations affecting the mtDNA replication machinery, and these can result in sensitivity to stressors (Chan, 2017). Mitochondrial fusion and fission are increasingly understood to be a key part of the mitochondrial stress response: mitochondrial morphology changes in a nonmonotonic fashion in response to mitochondrial stress (Meyer et al., 2017), and there is evidence that the genetic loss of such processes (which occurs in the human population) greatly increases sensitivity to some chemicals (Luz et al., 2017). Emerging pathways that are distinct from classical mitophagy, including mitochondria-derived vesicles and micro-mitophagy, also serve to recycle damaged mitochondrial components (Bohovych and Khalimonchuk, 2016; Meyer et al., 2017). Finally, mitochondria or mitochondrial contents may be removed by cellular export processes including exocytosis but potentially also other, newly identified mechanisms, at least from some cell types. This may serve to remove damaged molecules (Davis et al., 2014; Melentijevic et al., 2017), to activate the immune system (addressed in the next paragraph), or to signal to the nucleus. Indeed, a key area of rapid growth in mitochondrial homeostasis research is elucidation of the multiple signaling mechanisms employed by mitochondria to signal to the nucleus, including biosynthetic intermediates, nucleotides, peptides, cardiolipoin, mtROS, the mitochondrial unfolded protein response (mtUPR), energetic deficit detected by a reduced AMP:ATP ratio and/or reduced membrane potential, calcium release, dynamics and signaling in MAMs, and more (Bohovych and Khalimonchuk, 2016; Giorgi et al., 2015; Quiros et al., 2016).
Mitochondrial Role in the Immune Response
The relationship between mitochondria and the immune system is a rapidly growing field. It is now clear that mitochondria can act as crucial regulators of innate immune responses through distinct mechanisms. One way is through the release of damage-associated molecular patterns (mtDAMPs), which include mtDNA, ATP, cardiolipin, and formyl peptides. Due to the bacterial ancestry of mitochondria, mtDAMPs are recognized by the same set of innate immune receptors involved in the detection of bacterial infections, triggering inflammatory responses such as chemotaxis of innate immune cells and cytokine production (Rongvaux, 2017). Mitochondria are additionally involved in other important innate immune processes, including mtROS-mediated activation of multiple immune responses (Pinegin et al., 2017; West, 2017); neutrophil extracellular traps, a recently discovered phenomenon in which extruded cellular contents including DNA are used to snare pathogens (Douda et al., 2015); and possibly also by direct production of their own ROS to accelerate pathogen destruction in phagolysosomes (Pinegin et al., 2017). However, while mitochondrial-induced immunity is essential for an effective antimicrobial defense, damaged mitochondria can also lead to abnormal activation of the innate immune system resulting in auto-inflammatory or autoimmune diseases (Rongvaux, 2017). Thus, mitochondrial damage caused by environmental exposures may be involved in the development and/or progression of auto-inflammatory or autoimmune diseases, as well as diseases resulting from altered levels of mtROS. The potential for several types of mitotoxicants to affect immune function, including heavy metals, pesticides, herbicides, cigarette and cookstove smoke, airborne particulate matter, was recently reviewed (West, 2017). For example, the Complex I inhibitor rotenone causes mtROS generation, release of mtDAMPs including mtDNA, and inflammatory responses, leading to the hypothesis that such responses may comprise a portion of the environmental contribution to some neurodegenerative diseases (West, 2017). There is a clear need for studies elucidating the mechanisms and chemical-specific contexts in which mitochondrial dysfunction contribute to the development of drug or pollutant-related diseases.
Cell Nonautonomous and Systemic Effects
The effects of mitochondrial dysfunction are often confusingly cell type-specific, as is the case for the majority of known mitochondrial diseases, in which a mutation results in a pathology in only one or a few tissues. For example, inherited optic nerve degeneration can be caused by mutations in mitochondrial genes in the cases of Leber’s Hereditary Optic Neuropathy (arising primarily from mutations in mtDNA-encoded ETC components) and Autosomal Dominant Optic Atrophy (arising from a mutation in the nuclear-encoded OPA1 outer membrane fusion protein), yet other high energy use and post-mitotic cell types seem unaffected by these mutations (Bagli et al., 2017). On the other hand, mitochondrial effects can be confusingly systemic, as in the case of exercise, which induces mitochondrial biogenesis in the brain and liver as well as muscle (Gusdon et al., 2017; Little et al., 2011). Furthermore, a long-term endurance exercise regimen even rescued the systemic early aging and premature death phenotype in mtDNA mutator mice which bear a proofreading-deficient mtDNA polymerase, again by inducing mitochondrial biogenesis in all tissues studied: skeletal muscle, lungs, heart, ovary, and liver (Safdar et al., 2011). Systemic mitochondrial biosynthesis was accompanied by remarkable protection from early pigment loss in fur (graying), cardiac pathology, sarcopenia, splenomegaly, and decline in motor performance. Similarly, work in model organisms as simple as Caenorhabditis elegans resulted in the identification of cell-non-autonomous effects of mitochondrial stress: genetic knockdown of complex IV in neurons triggered a mtUPR in intestinal cells (Durieux et al., 2011); conversely, rotenone-mediated Complex I inhibition in intestinal cells was neuroprotective (Chikka et al., 2016). Further characterization of the mechanisms by which these cell-non-autonomous effects are mediated is an important area of current research.
Long-Term Effects of Mitochondrial Perturbation and Mitochondrial-Epigenetic Cross-Talk
There are increasing reports of long-term effects of mitochondrial toxicity. For example, in utero as well as postnatal exposure to low-level arsenic (which exerts part of its toxicity in mitochondria) in mice fed a high-fat “Western” diet resulted in persistent alterations to energetics in young adults (Ditzel et al., 2016). Similarly, in utero exposure to nucleoside reverse transcriptase inhibitors (NRTIs), which among other effects interfere with mtDNA polymerase function, resulted in persistent mitochondrial defects (Divi et al., 2010). Maternal high-fat and high-sugar diet led to three generations of altered mitochondria and metabolic dysfunction in mice (Saben et al., 2016). The preceding examples fit into the Developmental Origins of Health and Disease paradigm, suggesting the possibility that mitochondrial function is particularly susceptible to persistent alteration, perhaps due to the dynamic changes that may establish mitochondrial parameters early in development (Meyer et al., 2013). However, there are also cases of persistent mitochondrial effects resulting from adult exposures, such as the progressive and cumulative cardiotoxicity of the chemotherapy drug doxorubicin (Carvalho et al., 2010). The possibility that such exposures could lead to very long-term effects, including in future generations, is of great concern. Thus, there is a need to understand how common these outcomes are, how much timing of exposure matters, and the mechanism(s) by which they occur. In some cases, such as NRTI-induced damage, the long-term effects might be driven at least in part by what are essentially teratogenic effects (altered tissue formation). In other cases, however, it is possible that persistent effects are driven by epigenetic reprogramming. For example, Ferreira et al. (2017) identified changes in nuclear DNA methylation associated with the doxorubicin-mediated cardiomyopathy. There are also reports of altered cytosine methylation in mtDNA associated with pollutant exposure in people (Byun et al., 2013), and the potential importance of epigenetic regulation of the mitochondrial genome is itself a new area of research. However, in general, it remains unclear whether the chemical exposures directly affect mitochondria, which subsequently triggers alterations in epigenetic patterns, or whether the causative event is a toxicant-induced change to epigenetic patterning that subsequently affects mitochondria via altered transcription. Possible mechanisms for both have been recently reviewed (Weinhouse, 2017).
Advances in Testing Chemical Mitotoxicity
Because chemicals can induce mitochondrial impairment through multiple mechanisms, a variety of methods are required to evaluate mitotoxicity. Classic methods include measurement of the content or activities of mitochondrial enzymes; ATP levels; membrane potential; oxygen consumption; reduction potential; metabolite production; and morphological analysis (Dykens, in press). Recent advances include improved understanding of limitations of classic methods; higher-sensitivity, higher-specificity, and or higher-throughput versions of those methods; and novel methods. Will and Dykens recently published a review highlighting what has been learned in the context of preclinical testing of drugs for mitochondrial toxicity (Will and Dykens, 2014).
Important limitations of classic methods include specificity, throughput, and extrapolability of in vitro to in vivo conditions. For example, dye reduction assays such as the MTT assay, while extensively employed, are nonspecific because of reduction capacity present in other cellular compartments (Berridge and Tan, 1993). Measurement of the activity of isolated ETC complexes may not reflect in vivo mitochondrial dysfunction, as normal function depends on the integrity of many processes (Brand and Nicholls, 2011); this has been addressed by the development of technologies to measure mitochondrial function in intact cells, tissues, and even small organisms (discussed below). Some of these technologies have been adapted to medium- and high-throughput platforms, including mitochondrial respiration (Beeson et al., 2010; Hynes et al., 2016), microscopic analysis of mitochondrial morphology (i.e., mass, size, and number per cell), membrane potential, ROS production (Billis et al., 2014; Iannetti et al., 2016), mitophagy (Esteban-Martinez et al., 2017; Sauvat et al., 2017), and more (Wills, 2017).
As most of the above-mentioned methods were optimized for in vitro studies, it is important to highlight that one of the major concerns related to most cell culture studies is the use of tumor-derived cell lines and high glucose media (Marroquin et al., 2007). These cells can be highly resistant to mitochondrial toxicants, as they are typically capable of relying on glycolysis for ATP. Strategies to circumvent this problem include the use of primary cell cultures (Wills et al., 2015), low-glucose media (Andreux et al., 2014; Mot et al., 2016), or glucose-free medium supplemented with galactose (Dott et al., 2014; Marroquin et al., 2007). However, while these approaches have been successful in some cases, false negative results have also been reported (Swiss et al., 2013; Wills et al., 2015), and combined approaches have been developed to reduce these (Eakins et al., 2016).
A limitation of in vitro assays is the challenge of extrapolating to chronic, developmental, or latent/later-life effects of mitotoxicant exposures. To address this, in vivo assays are used. Recently, these have included medium-throughput analysis of small alternative animal models; in particular, the nematode C. elegans and zebrafish (Danio rerio) are among the most promising. Both have been used in medium-throughput assays (Andreux et al., 2014; Bestman et al., 2015; Jayasundara et al., 2015; Luz et al., 2015; Maurer et al., 2018), and both have been successfully used in studies that identified late-life consequences of mitochondrial dysfunction (Gonzalez-Hunt et al., 2014; Luz et al., 2017; Pinho et al., 2013). However, the complexity of whole organisms can sometimes limit interpretation and inferences about mechanisms of toxicity (Brand and Nicholls, 2011). This issue has been addressed by combining in vitro with in vivo assays (Andreux et al., 2014) and by systematic analysis of different energy pathways in vivo (Luz et al., 2016). Another important recent advance has been the inclusion of age and genetic deficiency as variables that may affect mitotoxicity (Boelsterli and Hsiao, 2008; Luz et al., 2017; Pereira et al., 2012).
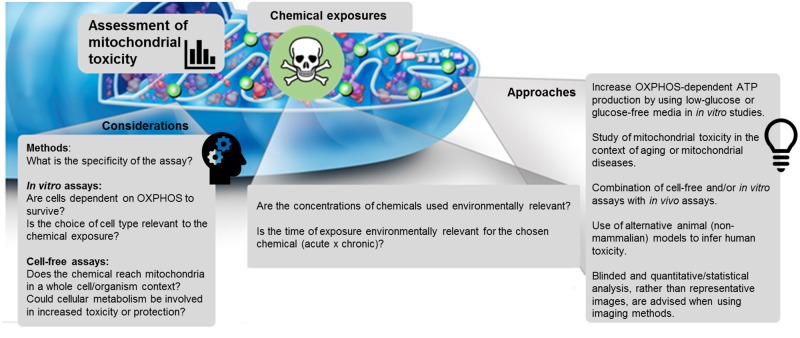
In conclusion, no one approach is ideal; therefore, one must carefully consider the strengths and limitations of each (Figure 2).
Figure 2.
Contemporary approaches and important considerations when assessing mitochondrial toxicity. Topics presented in this figure include, but are not limited to, those discussed within the section Advances in Testing Chemical Mitotoxicity.
MITOHORMESIS
The definition of “hormesis” varies among researchers, but the term is generally used to describe a biphasic dose response in which low-level exposure to a stressor results in beneficial outcomes, in contrast to higher-level exposures which are deleterious. The concept of hormesis has recently received more attention in toxicology (Calabrese and Baldwin, 2003), but is controversial in a number of ways (Kaiser, 2003), including the kinds of stressors that are likely to have hormetic effects. Interestingly, evidence for hormesis is long standing and widely accepted in mitochondrial biology. For example, exercise increases generation of ROS (Powers et al., 2010), but also improves a very wide range of health-related parameters, from mitochondrial function to disease incidence. Exercise and many other environmental factors that affect mitochondria (dietary restriction, genetic manipulation, dietary phytochemicals, and low-level ROS) are generally accepted as having “mitohormetic” effects (Ristow, 2014; Yun and Finkel, 2014). Strong evidence corroborates that these are low-“dose” effects: for instance, mild genetic knockdown of mitochondrial ETC components increases lifespan in C. elegans, while stronger knockdown decreases lifespan (Rea et al., 2007 b). Although there are some exceptions (Chikka et al., 2016; De Haes et al., 2014; Schmeisser et al., 2013), published examples of mitohormesis are derived largely from studies in which the mitochondrial stressors were not chemical in nature. Therefore, it is important to further test whether chemical stressors behave in a qualitatively similar fashion, or whether there are differences. For example, some pollutant exposures may be more persistent (e.g., in the case of a metal) than the stress caused by exercise.
Another important question is whether any trade-offs are associated with mitohormesis. For example, a stressor that results in chronic maintenance of highly-networked mitochondria might result, on an average, in improved mitochondrial function at the population level due to increased mitochondrial efficiency (Meyer et al., 2017). However, this could come at a cost of decreased mitophagy, which would theoretically increase the likelihood of mtDNA mutation accumulation—a rare event that would likely only be evident over time and in a few individuals, and therefore challenging to identify experimentally. Another possible trade-off is energetics; mild stress increases both mitochondrial degradation and biogenesis (Meyer et al., 2017), presumably resulting in maintenance of a pool of relatively “young” and highly functional mitochondria. However, this would require increased energetic resources, potentially at the cost of other organismal functions. This possibility is consistent with the fact that in some of the studies demonstrating mitohormesis in C. elegans, reductions in somatic growth and reproduction are also reported (e.g., Rea et al., 2007a; Zuryn et al., 2010).
FUTURE DIRECTIONS
There is still a great deal to be learned in laboratory studies of mitochondrial toxicity, the fundamental biology of mitochondria and the mitochondrial stress response, and how they overlap in gene-environment interactions. We have addressed many of these above (“Cutting-edge areas of research”). However, it is also critical that we expand our efforts to test the real-world relevance of mitochondrial toxicity in clinical and population studies. Efforts have been made to alert clinicians to the potential for mitochondrial toxicity, but these have been focused in large part on drugs (Cohen, 2010). Pollutant-induced effects have begun to be examined both in wildlife (Jayasundara, 2017) and human population studies (Brunst et al., 2015; Zhong et al., 2017). However, there are several factors that complicate our ability to bridge laboratory and population studies. Three particularly critical limitations are uncertainty about what the best biomarkers are; the inability to access target tissues and lack of clarity about whether accessible biomarkers in people are reflective of target tissues; and the potential for many variables to affect mitochondrial function.
Epidemiological studies typically rely on samples that can be obtained relatively easily and noninvasively, such as urine or blood. To date, researchers have often measured alterations in mtDNA copy number and damage, cardiolipin, oxygen consumption, metabolites, and more. However, efforts to identify biomarkers of mitochondrial dysfunction are ongoing (Shaughnessy et al., 2010). We propose that advances in this area may be made in part by taking advantage of the growing understanding of the immune response to mitochondrial damage: important biomarkers may often be those that we know are common and meaningful precisely because our immune systems have evolved to respond to them.
Access to target tissues can be challenging both for logistical reasons, and because the target tissue is not always known. White blood cells, which unlike red blood cells contain mitochondria, are often studied. In some cases, researchers examine these cells because they may in fact represent a target tissue, or may cause oxidative damage to other tissues due to toxicant-induced dysfunction (Zhong et al., 2017). In other cases, however, an implicit assumption is made that the measured biomarker is representative of, or at least reflective of, mitochondrial health in the tissue(s) affected. This assumption should be made carefully, because most mitotoxic exposures, like most mitochondrial diseases, affect some tissues more than others (Dykens, in press). Nonetheless, there is evidence that this assumption is accurate in at least some cases. For example, in the disease Friedreich’s Ataxia, although neurons of the spinal cord appear to be the most functionally affected cell type, high levels of mtDNA damage were detected in white blood cells of patients (Haugen et al., 2004). A key area for future work is developing better tools for assessing mitochondrial (dys)function in target tissues in vivo, and basing assessment of proxy tissue effects on our growing fundamental understanding of how and when systemic effects that result from mitochondrial stress in specific cells occur (discussed above). Determining the pathways by which cell autonomous and nonautonomous mitochondrial signals are carried will be a major advance in this area: attractive possibilities include secreted protein signals such as neurotrophic factors and exosome delivery of signaling cargo (Arenaccio and Federico, 2017). We hope that future work in laboratory as well as wild animals include measurement both in target tissues and in blood and urine to establish these critical links. It may also be useful to combine detection of biomarkers of mitochondrial damage with biomarkers of mitochondrial adaptation or mitohormesis.
Finally, there is evidence that multiple variables beyond pollutant exposure and (epi)genetic background can affect mitochondrial “health.” These include disease, diet, exercise, age, and other sources of stress (Kim et al., 1996; Payne and Chinnery, 2015). These may interact, and of course in many cases involve potential multiple factors in each category for a given person (e.g., multiple chemical exposures, multiple genetic vulnerabilities, etc.). In addition, there may well be additional variables that remain unidentified, suggesting that a systems-type approach (e.g., “mitochondriomics”: Brunst et al., 2015) would be best.
Despite these difficulties, progress in understanding mitochondrial toxicities has accelerated greatly in recent years, and we are confident that this trend will continue as future research efforts reduce these difficulties. Combining the complementary strengths of laboratory and epidemiological studies (Adami et al., 2011) will be critical to improving our ability to prevent and mitigate mitotoxic exposures and inform therapeutic efforts.
FUNDING
This work was supported by the NIH (R01ES028218, P42ES010356, F32ES027306, and 1R21ES026743) and the DoD (GW150184).
REFERENCES
- Adam-Vizi V., Starkov A. A. (2010). Calcium and mitochondrial reactive oxygen species generation: how to read the facts. J. Alzheimers Dis. 20, S413–S426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adami H. O., Berry C. L., Breckenridge C. B., Smith L. L., Swenberg J. A., Trichopoulos D., Weiss N. S., Pastoor T. P. (2011). Toxicology and epidemiology: improving the science with a framework for combining toxicological and epidemiological evidence to establish causal inference. Toxicol. Sci. 122, 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreux P. A., Mouchiroud L., Wang X., Jovaisaite V., Mottis A., Bichet S., Moullan N., Houtkooper R. H., Auwerx J. (2014). A method to identify and validate mitochondrial modulators using mammalian cells and the worm C. elegans. Sci. Rep. 4, 5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenaccio C., Federico M. (2017). The multifaceted functions of exosomes in health and disease: an overview. Adv. Exp. Med. Biol. 998, 3–19. [DOI] [PubMed] [Google Scholar]
- Attene-Ramos M. S., Huang R., Michael S., Witt K. L., Richard A., Tice R. R., Simeonov A., Austin C. P., Xia M. (2015). Profiling of the Tox21 chemical collection for mitochondrial function to identify compounds that acutely decrease mitochondrial membrane potential. Environ. Health Perspect. 123, 49–56. Research Support, N.I.H., Extramural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attene-Ramos M. S., Huang R., Sakamuru S., Witt K. L., Beeson G. C., Shou L., Schnellmann R. G., Beeson C. C., Tice R. R., Austin C. P., et al. (2013). Systematic study of mitochondrial toxicity of environmental chemicals using quantitative high throughput screening. Chem. Res. Toxicol. 26, 1323–1332. Research Support, N.I.H., Extramural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagli E., Zikou A. K., Agnantis N., Kitsos G. (2017). Mitochondrial membrane dynamics and inherited optic neuropathies. In Vivo 31, 511–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson C. C., Beeson G. C., Schnellmann R. G. (2010). A high-throughput respirometric assay for mitochondrial biogenesis and toxicity. Anal. Biochem. 404, 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. V., Tan A. S. (1993). Characterization of the cellular reduction of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch. Biochem. Biophys. 303, 474–482. [DOI] [PubMed] [Google Scholar]
- Bess A. S., Crocker T. L., Ryde I. T., Meyer J. N. (2012). Mitochondrial dynamics and autophagy aid in removal of persistent mitochondrial DNA damage in Caenorhabditis elegans. Nucleic Acids Research doi: 10.1093/nar/gks532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestman J. E., Stackley K. D., Rahn J. J., Williamson T. J., Chan S. S. (2015). The cellular and molecular progression of mitochondrial dysfunction induced by 2,4-dinitrophenol in developing zebrafish embryos. Differentiation 89, 51–69. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billis P., Will Y., Nadanaciva S. (2014). High-content imaging assays for identifying compounds that generate superoxide and impair mitochondrial membrane potential in adherent eukaryotic cells. Curr. Protoc. Toxicol. 59, 25 1 1–14. [DOI] [PubMed] [Google Scholar]
- Blajszczak C., Bonini M. G. (2017). Mitochondria targeting by environmental stressors: implications for redox cellular signaling. Toxicology. 391, 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelsterli U. A., Hsiao C. J. (2008). The heterozygous Sod2(+/-) mouse: modeling the mitochondrial role in drug toxicity. Drug Discov. Today 13, 982–988. [DOI] [PubMed] [Google Scholar]
- Boelsterli U. A., Lim P. L. (2007). Mitochondrial abnormalities–a link to idiosyncratic drug hepatotoxicity? Toxicol. Appl. Pharmacol. 220, 92–107. [DOI] [PubMed] [Google Scholar]
- Bohovych I., Khalimonchuk O. (2016). Sending out an SOS: mitochondria as a signaling hub. Front. Cell Dev. Biol. 4, 109.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookman E. B., McAllister K., Gillanders E., Wanke K., Balshaw D., Rutter J., Reedy J., Shaughnessy D., Agurs-Collins T., Paltoo D., et al. (2011). Gene-environment interplay in common complex diseases: forging an integrative model-recommendations from an NIH workshop. Genetic Epidemiol. 35, 217–225. Consensus Development Conference, NIH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M. D., Nicholls D. G. (2011). Assessing mitochondrial dysfunction in cells. Biochem. J. 435, 297–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunst K. J., Baccarelli A. A., Wright R. J. (2015). Integrating mitochondriomics in children's environmental health. J. Appl. Toxicol. 35, 976–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffa P., Azzone G. F., Carafoli E., Muscatelo U. (1959). [The mitochondrial biochemical lesion in pentachlorophenol intoxication]. Boll. Soc. Ital. Biol. Sper. 35, 1816–1820. [PubMed] [Google Scholar]
- Byun H. M., Panni T., Motta V., Hou L., Nordio F., Apostoli P., Bertazzi P. A., Baccarelli A. A. (2013). Effects of airborne pollutants on mitochondrial DNA methylation. Part. Fibre Toxicol. 10, 18.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese E. J., Baldwin L. A. (2003). Hormesis: the dose-response revolution. Annu. Rev. Pharmacol. Toxicol. 43, 175–197. [DOI] [PubMed] [Google Scholar]
- Carvalho R. A., Sousa R. P., Cadete V. J., Lopaschuk G. D., Palmeira C. M., Bjork J. A., Wallace K. B. (2010). Metabolic remodeling associated with subchronic doxorubicin cardiomyopathy. Toxicology 270, 92–98. [DOI] [PubMed] [Google Scholar]
- Chan K., Truong D., Shangari N., O'Brien P. J. (2005). Drug-induced mitochondrial toxicity. Expert Opin. Drug Metab. Toxicol. 1, 655–669. [DOI] [PubMed] [Google Scholar]
- Chan S. S. L. (2017). Inherited mitochondrial genomic instability and chemical exposures. Toxicology. 391, 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell J. B., Greville G. D. (1961). Effects of oligomycin on respiration and swelling of isolated liver mitochondria. Nature 190, 502–504. [DOI] [PubMed] [Google Scholar]
- Chikka M. R., Anbalagan C., Dvorak K., Dombeck K., Prahlad V. (2016). The mitochondria-regulated immune pathway activated in the C. elegans intestine is neuroprotective. Cell Rep. 16, 2399–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman J. A., Coluccio A., Planchart A., Robertson A. J. (2009). Oral-aboral axis specification in the sea urchin embryo III. Role of mitochondrial redox signaling via H2O2. Dev. Biol. 330, 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B. H. (2010). Pharmacologic effects on mitochondrial function. Dev. Disab. Res. Rev. 16, 189–199. Review. [DOI] [PubMed] [Google Scholar]
- Davis C. H., Kim K. Y., Bushong E. A., Mills E. A., Boassa D., Shih T., Kinebuchi M., Phan S., Zhou Y., Bihlmeyer N. A., et al. (2014). Transcellular degradation of axonal mitochondria. Proc. Natl. Acad. Sci. U S A. 111, 9633–9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haes W., Frooninckx L., Van Assche R., Smolders A., Depuydt G., Billen J., Braeckman B. P., Schoofs L., Temmerman L. (2014). Metformin promotes lifespan through mitohormesis via the peroxiredoxin PRDX-2. Proc. Natl. Acad. Sci. U S A. 111, E2501–E2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Meo S., Reed T. T., Venditti P., Victor V. M. (2016). Role of ROS and RNS sources in physiological and pathological conditions. Oxid. Med. Cell Longev. 2016, 1245049.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditzel E. J., Nguyen T., Parker P., Camenisch T. D. (2016). Effects of arsenite exposure during fetal development on energy metabolism and susceptibility to diet-induced fatty liver disease in male mice. Environ. Health Perspect. 124, 201–209. Research Support, N.I.H., Extramural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divi R. L., Einem T. L., Fletcher S. L., Shockley M. E., Kuo M. M., St Claire M. C., Cook A., Nagashima K., Harbaugh S. W., Harbaugh J. W., et al. (2010). Progressive mitochondrial compromise in brains and livers of primates exposed in utero to nucleoside reverse transcriptase inhibitors (NRTIs). Toxicol. Sci. 118, 191–201. Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dott W., Mistry P., Wright J., Cain K., Herbert K. E. (2014). Modulation of mitochondrial bioenergetics in a skeletal muscle cell line model of mitochondrial toxicity. Redox. Biol. 2, 224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douda D. N., Khan M. A., Grasemann H., Palaniyar N. (2015). SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc. Natl. Acad. Sci. U S A. 112, 2817–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux J., Wolff S., Dillin A. (2011). The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell 144, 79–91. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykens J. A., Marroquin L. D., Will Y. (2007). Strategies to reduce late-stage drug attrition due to mitochondrial toxicity. Expert Rev. Mol. Diagn. 7, 161–175. [DOI] [PubMed] [Google Scholar]
- Dykens J. A., Will Y. (2007). The significance of mitochondrial toxicity testing in drug development. Drug Discov. Today 12, 777–785. [DOI] [PubMed] [Google Scholar]
- Eakins J., Bauch C., Woodhouse H., Park B., Bevan S., Dilworth C., Walker P. (2016). A combined in vitro approach to improve the prediction of mitochondrial toxicants. Toxicol. In Vitro 34, 161–170. [DOI] [PubMed] [Google Scholar]
- Esteban-Martinez L., Villarejo-Zori B., Boya P. (2017). Cytofluorometric assessment of mitophagic flux in mammalian cells and tissues. Methods Enzymol. 588, 209–217. [DOI] [PubMed] [Google Scholar]
- Ferreira A., Cunha-Oliveira T., Simoes R. F., Carvalho F. S., Burgeiro A., Nordgren K., Wallace K. B., Oliveira P. J. (2017). Altered mitochondrial epigenetics associated with subchronic doxorubicin cardiotoxicity. Toxicology 390, 63–73. [DOI] [PubMed] [Google Scholar]
- Fridovich I. (2004). Mitochondria: are they the seat of senescence? Aging Cell 3, 13–16. [DOI] [PubMed] [Google Scholar]
- Giorgi C., Missiroli S., Patergnani S., Duszynski J., Wieckowski M. R., Pinton P. (2015). Mitochondria-associated membranes: composition, molecular mechanisms, and physiopathological implications. Antioxid. Redox. Signal. 22, 995–1019. [DOI] [PubMed] [Google Scholar]
- Gomez Puyou A., Feder W., Tuena M., Pena Diaz A. (1964). The effect of triamcinolone and 2, 4-dinitrophenol on the adenosinetriphosphatase activity and the P32 Atp exchange reaction of fresh liver mitochondria. Arch. Biochem. Biophys. 106, 455–460. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Hunt C. P., Leung M. C., Bodhicharla R. K., McKeever M. G., Arrant A. E., Margillo K. M., Ryde I. T., Cyr D. D., Kosmaczewski S. G., Hammarlund M., et al. (2014). Exposure to mitochondrial genotoxins and dopaminergic neurodegeneration in Caenorhabditis elegans. PLoS One 9, e114459. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman G. S., Schaefer A. M., Ng Y., Gomez N., Blakely E. L., Alston C. L., Feeney C., Horvath R., Yu-Wai-Man P., Chinnery P. F., et al. (2015). Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann. Neurol. 77, 753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusdon A. M., Callio J., Distefano G., O'Doherty R. M., Goodpaster B. H., Coen P. M., Chu C. T. (2017). Exercise increases mitochondrial complex I activity and DRP1 expression in the brains of aged mice. Exp. Gerontol. 90, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson M. J., Mansson R., Morota S., Uchino H., Kallur T., Sumi T., Ishii N., Shimazu M., Keep M. F., Jegorov A., et al. (2008). Calcium-induced generation of reactive oxygen species in brain mitochondria is mediated by permeability transition. Free Radic. Biol. Med. 45, 284–294. [DOI] [PubMed] [Google Scholar]
- Haugen A. C., Kelley R., Collins J. B., Tucker C. J., Deng C., Afshari C. A., Brown J. M., Ideker T., Van Houten B. (2004). Integrating phenotypic and expression profiles to map arsenic-response networks. Genome Biol. 5, R95.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes J., Carey C., Will Y. (2016). Fluorescence-based microplate assays for in vitro assessment of mitochondrial toxicity, metabolic perturbation, and cellular oxygenation. Curr. Protoc. Toxicol. 70, 2 16 1–2 16 30. [DOI] [PubMed] [Google Scholar]
- Iannetti E. F., Smeitink J. A., Beyrath J., Willems P. H., Koopman W. J. (2016). Multiplexed high-content analysis of mitochondrial morphofunction using live-cell microscopy. Nat. Protoc. 11, 1693–1710. [DOI] [PubMed] [Google Scholar]
- Jayasundara N. (2017). Ecological significance of mitochondrial toxicants. Toxicology. 391, 64–74. [DOI] [PubMed] [Google Scholar]
- Jayasundara N., Kozal J. S., Arnold M. C., Chan S. S. L., Di Giulio R. T., Zhang J. (2015). High-throughput tissue bioenergetics analysis reveals identical metabolic allometric scaling for teleost hearts and whole organisms. PLoS One 10, e0137710. Research Support, N.I.H., Extramural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser J. (2003). Hormesis. Sipping from a poisoned chalice. Science 302, 376–379. [DOI] [PubMed] [Google Scholar]
- Kim J. D., McCarter R. J., Yu B. P. (1996). Influence of age, exercise, and dietary restriction on oxidative stress in rats. Aging (Milano) 8, 123–129. [DOI] [PubMed] [Google Scholar]
- Little J. P., Safdar A., Benton C. R., Wright D. C. (2011). Skeletal muscle and beyond: the role of exercise as a mediator of systemic mitochondrial biogenesis. Appl. Physiol. Nutr. Metab. 36, 598–607. [DOI] [PubMed] [Google Scholar]
- Luz A. L., Godebo T. R., Smith L. L., Leuthner T. C., Maurer L. L., Meyer J. N. (2017). Deficiencies in mitochondrial dynamics sensitize Caenorhabditis elegans to arsenite and other mitochondrial toxicants by reducing mitochondrial adaptability. Toxicology. 387, 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luz A. L., Lagido C., Hirschey M. D., Meyer J. N. (2016). In vivo determination of mitochondrial function using luciferase-expressing Caenorhabditis elegans: contribution of oxidative phosphorylation, glycolysis, and fatty acid oxidation to toxicant-induced dysfunction. Curr. Protoc. Toxicol. 69, 25 8 1–25 8 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luz A. L., Smith L. L., Rooney J. P., Meyer J. N. (2015). Seahorse extracellular flux-based analysis of cellular respiration in Caenorhabditis elegans. Curr. Protoc. Toxicol. 66, 25.7.1–25.7.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi S., Bittremieux M., Missiroli S., Morganti C., Patergnani S., Sbano L., Rimessi A., Kerkhofs M., Parys J. B., Bultynck G. (2017). Endoplasmic reticulum-mitochondria communication through Ca(2+) signaling: the importance of mitochondria-associated membranes (MAMs). Adv. Exp. Med. Biol. 997, 49–67. [DOI] [PubMed] [Google Scholar]
- Marroquin L. D., Hynes J., Dykens J. A., Jamieson J. D., Will Y. (2007). Circumventing the crabtree effect: replacing media glucose with galactose increases susceptibility of HepG2 cells to mitochondrial toxicants. Toxicol. Sci. 97, 539–547. [DOI] [PubMed] [Google Scholar]
- Maurer L. L., Luz A. L., Meyer J. N. (2018). Detection of mitochondrial toxicity of environmental pollutants using Caenorhabditis elegans In Drug-Induced Mitochondrial Dysfunction (Dykens J. A., Will Y., Eds.). pp. 655–689. New York: Wiley. [Google Scholar]
- Melentijevic I., Toth M. L., Arnold M. L., Guasp R. J., Harinath G., Nguyen K. C., Taub D., Parker J. A., Neri C., Gabel C. V., et al. (2017). C. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress. Nature 542, 367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J. N., Chan S. S. L. (2017). Sources, mechanisms, and consequences of chemical-induced mitochondrial toxicity. Toxicology 391, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J. N., Leung M. C., Rooney J. P., Sendoel A., Hengartner M. O., Kisby G. E., Bess A. S. (2013). Mitochondria as a target of environmental toxicants. Toxicol. Sci. 134, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J. N., Leuthner T. C., Luz A. L. (2017). Mitochondrial fusion, fission, and mitochondrial toxicity. Toxicology. 391, 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mot A. I., Liddell J. R., White A. R., Crouch P. J. (2016). Circumventing the crabtree effect: a method to induce lactate consumption and increase oxidative phosphorylation in cell culture. Int. J. Biochem. Cell Biol. 79, 128–138. [DOI] [PubMed] [Google Scholar]
- Panov A., Dikalov S., Shalbuyeva N., Hemendinger R., Greenamyre J. T., Rosenfeld J. (2007). Species- and tissue-specific relationships between mitochondrial permeability transition and generation of ROS in brain and liver mitochondria of rats and mice. Am. J. Physiol. Cell Physiol. 292, C708–C718. [DOI] [PubMed] [Google Scholar]
- Payne B. A., Chinnery P. F. (2015). Mitochondrial dysfunction in aging: much progress but many unresolved questions. Biochim. Biophys. Acta 1847, 1347–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira C. V., Moreira A. C., Pereira S. P., Machado N. G., Carvalho F. S., Sardao V. A., Oliveira P. J. (2009). Investigating drug-induced mitochondrial toxicity: a biosensor to increase drug safety? Curr. Drug Saf. 4, 34–54. Research Support, Non-U.S. Gov't Review. [DOI] [PubMed] [Google Scholar]
- Pereira C. V., Oliveira P. J., Will Y., Nadanaciva S. (2012). Mitochondrial bioenergetics and drug-induced toxicity in a panel of mouse embryonic fibroblasts with mitochondrial DNA single nucleotide polymorphisms. Toxicol. Appl. Pharmacol. 264, 167–181. [DOI] [PubMed] [Google Scholar]
- Pinegin B., Vorobjeva N., Pashenkov M., Chernyak B. (2017). The role of mitochondrial ROS in antibacterial immunity. J. Cell Physiol. doi: 10.1002/jcp.26117 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Pinho B. R., Santos M. M., Fonseca-Silva A., Valentao P., Andrade P. B., Oliveira J. M. A. (2013). How mitochondrial dysfunction affects zebrafish development and cardiovascular function: an in vivo model for testing mitochondria-targeted drugs. Br. J. Pharmacol. 169, 1072–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers S. K., Duarte J., Kavazis A. N., Talbert E. E. (2010). Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp. Physiol. 95, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiros P. M., Mottis A., Auwerx J. (2016). Mitonuclear communication in homeostasis and stress. Nat. Rev. Mol. Cell Biol. 17, 213–226. [DOI] [PubMed] [Google Scholar]
- Rappaport S. M. (2012). Discovering environmental causes of disease. J. Epidemiol. Commun. Health 66, 99–102. Research Support, N.I.H., Extramural. [DOI] [PubMed] [Google Scholar]
- Rea S. L., Ventura N., Johnson T. E. (2007a). Relationship between mitochondrial electron transport chain dysfunction, development, and life extension in Caenorhabditis elegans. PLoS Biol. 5, 2312–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea S. L., Ventura N., Johnson T. E. (2007b). Relationship between mitochondrial electron transport chain dysfunction, development, and life extension in Caenorhabditis elegans. PLoS Biol. 5, e259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow M. (2014). Unraveling the truth about antioxidants: mitohormesis explains ROS-induced health benefits. Nat. Med. 20, 709–711. [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Bernardi P., Pozzan T. (2000). Mitochondria as all-round players of the calcium game. J. Physiol. Lond. 529, 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongvaux A. (2017). Innate immunity and tolerance toward mitochondria. Mitochondrion doi: 10.1016/j.mito.2017.10.007 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Saben J. L., Boudoures A. L., Asghar Z., Thompson A., Drury A., Zhang W., Chi M., Cusumano A., Scheaffer S., Moley K. H. (2016). Maternal metabolic syndrome programs mitochondrial dysfunction via germline changes across three generations. Cell Rep. 16, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri M. I. (1998). Toxin induced mitochondrial dysfunction and neurodegeneration In Mitochondrial DNA Mutations in Aging, Disease, and Cancer (Singh K. K., Ed.), pp. 297–317. New York: Springer-Verlag. [Google Scholar]
- Safdar A., Bourgeois J. M., Ogborn D. I., Little J. P., Hettinga B. P., Akhtar M., Thompson J. E., Melov S., Mocellin N. J., Kujoth G. C., et al. (2011). Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc. Natl. Acad. Sci. U S A. 108, 4135–4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvat A., Zhou H., Leduc M., Gomes-da-Silva L. C., Bezu L., Muller K., Forveille S., Liu P., Zhao L., Kroemer G., et al. (2017). Automated analysis of fluorescence colocalization: application to mitophagy. Methods Enzymol. 588, 219–230. [DOI] [PubMed] [Google Scholar]
- Schmeisser S., Schmeisser K., Weimer S., Groth M., Priebe S., Fazius E., Kuhlow D., Pick D., Einax J. W., Guthke R., et al. (2013). Mitochondrial hormesis links low-dose arsenite exposure to lifespan extension. Aging Cell 12, 508–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scialo F., Fernandez-Ayala D. J., Sanz A. (2017). Role of mitochondrial reverse electron transport in ROS signaling: potential roles in health and disease. Front. Physiol. 8, 428.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaughnessy D. T., Worth L., Lawler C. P., McAllister K. A., Longley M. J., Copeland W. C. (2010). Meeting report: identification of biomarkers for early detection of mitochondrial dysfunction. Mitochondrion 10, 579–581. [DOI] [PubMed] [Google Scholar]
- Starkov A. A., Polster B. M., Fiskum G. (2002). Regulation of hydrogen peroxide production by brain mitochondria by calcium and Bax. J. Neurochem. 83, 220–228. [DOI] [PubMed] [Google Scholar]
- Swiss R., Niles A., Cali J. J., Nadanaciva S., Will Y. (2013). Validation of a HTS-amenable assay to detect drug-induced mitochondrial toxicity in the absence and presence of cell death. Toxicol. In Vitro 27, 1789–1797. [DOI] [PubMed] [Google Scholar]
- Valente W. J., Ericson N. G., Long A. S., White P. A., Marchetti F., Bielas J. H. (2016). Mitochondrial DNA exhibits resistance to induced point and deletion mutations. Nucleic Acids Res. 44, 8513–8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa T., Andri L., Brasca F. (1961). [Enzymatic research at the mitochondrial level in experimental carbon monoxide poisoning. Behavior of the cytochrome oxidases, aldolases and glutamic-oxalacetic and glutamic-pyruvic transaminases in the hepatic and renal mitochondria]. Folia Med. (Napoli) 44, 486–495. [PubMed] [Google Scholar]
- Wallace D. C. (2005). A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annual Review of Genetics 39, 359–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace K. B. (2015). Multiple targets for drug-induced mitochondrial toxicity. Curr. Med. Chem. 22, 2488–2492. [DOI] [PubMed] [Google Scholar]
- Weinhouse C. (2017). Mitochondrial-epigenetic crosstalk in environmental toxicology. Toxicology. 391, 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West A. P. (2017). Mitochondrial dysfunction as a trigger of innate immune responses and inflammation. Toxicology. 391, 54–63. [DOI] [PubMed] [Google Scholar]
- Will Y., Dykens J. (2014). Mitochondrial toxicity assessment in industry–a decade of technology development and insight. Expert Opin. Drug Metab. Toxicol. 10, 1061–1067. [DOI] [PubMed] [Google Scholar]
- Will Y., Dykens J.A. (2018). Mitochondrial Dysfunction by Drug and Environmental Toxicants. New York: John Wiley & Sons Limited. [Google Scholar]
- Wills L. P. (2017). The use of high-throughput screening techniques to evaluate mitochondrial toxicity. Toxicology. 391, 34–41. [DOI] [PubMed] [Google Scholar]
- Wills L. P., Beeson G. C., Hoover D. B., Schnellmann R. G., Beeson C. C. (2015). Assessment of toxcast phase II for mitochondrial liabilities using a high-throughput respirometric assay. Toxicol. Sci. 146, 226–234. Research Support, Non-U.S. Gov't. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun J., Finkel T. (2014). Mitohormesis. Cell Metab. 19, 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J., Karlsson O., Wang G., Li J., Guo Y., Lin X., Zemplenyi M., Sanchez-Guerra M., Trevisi L., Urch B., et al. (2017). B vitamins attenuate the epigenetic effects of ambient fine particles in a pilot human intervention trial. Proc. Natl. Acad. Sci. U S A. 114, 3503–3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuryn S., Kuang J., Tuck A., Ebert P. R. (2010). Mitochondrial dysfunction in Caenorhabditis elegans causes metabolic restructuring, but this is not linked to longevity. Mech. Ageing Dev. 131, 554–561. [DOI] [PubMed] [Google Scholar]