Abstract
Background: Chronic kidney disease (CKD) is a significant risk factor for cognitive impairment. Previous studies have examined differences in cognitive impairment between persons with and without CKD using multiple cognitive outcomes, but few have done this for an extensive battery of cognitive tests. We relate early-stage CKD to two indices of impairment for 22 measures of cognitive ability.
Methods: The study was community-based and cross-sectional with 898 individuals free from dementia and end-stage renal disease. Estimated glomerular filtration rate (eGFR) was calculated using the chronic kidney disease epidemiology collaboration equation and classified as <60 or ≥60 mL/min/1.73 m2, based on consensus definitions of Stage 3 or greater CKD. The eGFR classifications were related to modest [≥1 standard deviation (SD) below the mean] and severe (≥1.5 SD below the mean) impairment on each measure using logistic regression analyses adjusting for potential risk factors.
Results: A total of 146 individuals (16.3%) had eGFR <60 mL/min/1.73 m2 (mean 51.6 ± 10.1 mL/min/1.73 m2). These participants had significantly greater risk for modestly impaired abilities in the scanning and tracking and visual-spatial organization/memory (VSOM) domains after accounting for comorbidity-related risk factors [odds ratios (ORs) between 1.68 and 2.16], as well as greater risk for severely impaired functioning in the language domain (OR = 2.65).
Conclusions: Participants with eGFR <60 mL/min/1.73 m2 were at higher risk for cognitive impairment than those with eGFR ≥60 mL/min/1.73 m2 on the majority of cognitive abilities, specifically those within the VSOM, Language, and scanning and tracking domains. Targeted screening for cognitive deficits in kidney disease patients early in their disease course may be warranted.
Keywords: cardiovascular disease, chronic kidney disease, CKD-EPI, cognitive impairment, cognitive norms
INTRODUCTION
Chronic kidney disease (CKD) has been identified as a significant risk factor for lowered cognitive performance [1–3]. These deficits appear to be more pronounced in patients with end-stage renal disease (ESRD) [4–7]. This is a salient public health issue, as patient burden in the treatment of CKD is heavy and preventing the development of treatment-related cognitive deficit is important.
Many earlier studies of kidney disease and cognition utilized one or a few measures of different cognitive abilities. However, it has since been recognized that individuals with kidney disease have intact function for some cognitive abilities and significant impairment in others [1–3]. Abilities at particularly high risk for impairment are generally thought to be those that rely on higher-order cognitive domains, including executive functioning and abstract reasoning [2, 6]. To date, few studies have utilized multiple cognitive outcome measures in state-of-the-art neurocognitive test batteries to gain a clearer picture of affected abilities and the domains that they comprise.
Schneider et al. [4] recently provided clinically relevant data with regard to performance on tests of multiple cognitive abilities in ESRD utilizing a healthy control group. Though valuable information was provided in this study, there has been some debate regarding the suitability of a healthy control group in studies of cognition and CKD. We have previously suggested that it may lead to biased comparisons, as differences in common CKD-related comorbidities (e.g. hypertension, cardiac diseases) may themselves account for lower cognitive performance [8]. Persons with early-stage CKD would make a better reference group in future examinations of ESRD, as they have kidney disease of a less serious nature but potentially similar comorbidities.
To further evaluate the validity of this recommendation, it would be important to know the level of modest and more severe cognitive impairment in persons with early-stage CKD and to have these data for as many individual measures of cognitive ability as possible. One shortcoming of previous studies of early-stage CKD from a clinical perspective is that information is not given with respect to individual test scores, e.g. Elias et al. [9]; rather, scores from individual tests are combined to make domain (composite) scores. While this approach is ideal from a theoretical standpoint of cognitive deficit, having raw scores from individual tests available would also provide nephrologists with reference data to which they could compare the performance of their own patients.
In the present study, we report findings for 22 well-established tests of cognitive ability using data from the community-based Maine Syracuse Longitudinal Study (MSLS) [10]. For each of these 22 measures, we express results in terms of (i) risk for modest cognitive impairment, defined by Schneider et al. [4] as performance 1.0 standard deviation (SD) below the mean and (ii) risk for severe cognitive impairment, a more stringent criterion, defined as performance 1.5 SD below the mean in the literature [10]. We hypothesize that individuals with CKD will show deficit in cognitive performance that meets criteria for modest and severe impairment (compared with a non-CKD reference group), and that the number of tests for which impairment is seen will be attenuated with adjustment for demographic variables, risk factors and CKD-related comorbidities [1–4].
MATERIALS AND METHODS
Study design
Data for the present cross-sectional study were obtained from the sixth wave of the MSLS, a community-based study of cardiovascular risk factors (CVD-RF) and cognitive function [11, 12]. Participants were recruited from the Central New York State area. There were no requirements for participation at baseline other than the absence of diagnosed psychiatric disorder, non-institutionalization and absence of alcoholism. Between 2001 and 2006 (wave six), data necessary to examine a broad range of cognitive measures and to estimate glomerular filtration rate (eGFR) were obtained for the first time.
Beginning with 1040 study participants, individuals were excluded based on (i) missing data necessary to calculate eGFR (n = 77); (ii) dementia (n = 9); (iii) active dialysis treatment (n = 4); (iv) under 40 years of age (n = 29) and (v) missing cognitive data (n = 23). The final sample consisted of 898 participants, 752 with eGFR ≥60 mL/min/1.73 m2 (non-CKD reference group) and 146 with eGFR <60 mL/min/1.73 m2 (CKD group).
The exclusion of dementia cases was based on our interest in characterizing relationships between CKD and cognition in persons who vary in cognitive function but do not suffer from major impairment. Clinical diagnoses of dementia were determined from cognitive data and medical records using the National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria [13] and confirmed using the ICD-10 guidelines [14]. Detailed steps of our diagnostic process can be found in Supplementary data, Appendix 1. The age exclusion was employed due to a significant imbalance in age between the two renal function groups when persons <40 years of age were included.
Procedures
One week prior to neuropsychological testing, participants in the present study completed the Center for Epidemiological Studies Depression Scale (CES-D) [15]. A blood sample was drawn the morning after a fast from midnight. This was followed by a light breakfast, medical history, multiple automated blood pressure (BP) measurements (GE Dinamap 100DPC-120XEN) and neuropsychological assessment. All assay methods have been described previously [9, 11, 12]. Coefficients of variation for these procedures were <5.0%. We used the four variable (serum creatinine, age, sex and ethnicity) Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation to calculate eGFR, which has been found to perform better than the Modification of Diet in Renal Disease (MDRD) equation, with less bias and greater accuracy [16, 17].
Determinations of the following variables were performed as previously described [11, 12, 18]: high-sensitivity C-reactive protein (CRP), serum vitamin B12, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, glucose and standard ApoE genotyping. Medical records indicating a diagnosis of anemia were required for identification of anemic participants. Hemoglobin levels and complete blood count were not utilized in the present study, as we did not anticipate an examination of kidney function as a CVD-RF at the time of data collection.
Mean systolic BP (SBP) and diastolic BP (DBP) were based on 15 BP measurements (five sitting, five standing and five reclining). Hypertension was defined as SBP ≥140 mmHg and/or DBP ≥90 mmHg. Additional covariates that were used or considered in various analyses included ethnicity (black versus other), diabetes mellitus (DM), body mass index (BMI; kg/m2), self-reported number of cigarettes smoked per week, self-reported ounces of alcohol consumed per week, triglyceride levels, the presence of CVD (self-reported and confirmed by medical records) and stroke. DM was defined by a fasting blood glucose level of ≥126 mg/dL, treatment with insulin or oral anti-diabetic agents. CVD was defined as the presence of: myocardial infarction (4.3%), coronary artery disease (8.1%), heart failure (2.5%), angina pectoris (6.0%) and/or transient ischemic attack (3.7%) [19, 20]. Stroke (based on self-report or medical records and confirmed by record review) was defined as a focal neurological deficit of acute onset persisting for >24 h.
The protocol for this investigation was approved by the University of Maine Institutional Review Board. We adhered to the Declaration of Helsinki and obtained informed consent for data collection from all study participants.
Neurocognitive tests
The cognitive outcomes utilized were raw scores from 22 neurocognitive tests, each of which is described in Table 1. In previous MSLS studies, several of these measures have been combined to produce four composite scores that each describe a domain of cognitive functioning [9]. These domains are verbal episodic memory, visual-spatial organization/memory (VSOM), scanning and tracking, and working memory. Verbal episodic memory is required for learning and remembering oral and written material. Problem solving with respect to visual-spatial relationships requires the use of VSOM. Scanning and tracking requires attention and concentration, placing demands on organization and planning. Working memory requires holding information in short-term memory while manipulating it to perform a task. [21–25].
Table 1.
Neurocognitive tests used to assess the function of cognitive abilities and the domains that define them
| Cognitive domains and the neurocognitive tests that define them | Cognitive ability measured |
|---|---|
| Verbal episodic memory | |
| Logical Memory: Immediate Recalla Abbr. Logic Mem IR |
Immediate verbal memory |
| Logical Memory: Delayed Recalla Abbr. Logic Mem DR |
Delayed verbal memory |
| Hopkins Verbal Learning Task Abbr. Hopkins VLT |
Verbal learning and memory |
| Visual-spatial organization/memory | |
| Visual Reproduction: Immediate Recalla Abbr. Vis Repro IR |
Immediate recall, visual memory and visual-spatial problem solving |
| Visual Reproduction: Delayed Recalla Abbr. Vis Repro DR |
Delayed recall, visual memory and visual-spatial problem solving |
| Matrix Reasoningb | Abstract reasoning and pattern recognition |
| Hooper Visual Organization Test Abbr. Hooper VOT |
Visual-spatial organization and demands on executive functioning |
| Object Assemblyc | Speed of visual-spatial organization |
| Block Designc | Visual-spatial perception, organization and construction |
| Scanning and tracking | |
| Trail Making Ad | Visual scanning and tracking; concentration and attention |
| Trail Making Bd | Trail A plus demands on executive functioning |
| Digit Symbol Substitutionc Abbr. Digit Symbol |
Psychomotor performance |
| Symbol Searchb | Visual processing speed |
| Working memory | |
| Digit Span: Forwardc Abbr. Digit Span F |
Attention and concentration |
| Digit Span: Backwardc Abbr. Digit Span B |
Attention, concentration and working memory |
| Letter-Number Sequenceb Abbr. Letter Number |
Information processing while holding information in memory |
| Abstract reasoning | |
| Similaritiesc | Verbal intelligence and abstract reasoning |
| Executive functioning | |
| CLOX 1e | The ability to plan, organize and shift response set for the purpose of problem solving |
| CLOX 2e | Copying skills |
| Controlled Oral Word Association Test Abbr. COWA |
Verbal fluency and executive functioning |
| General mental state | |
| Mini-Mental State Examination Abbr. MMSE |
Orientation, registration and attention |
| Language | |
| Boston Naming Test Abbr. Boston Naming |
Ability to name objects; early loss seen in dementia |
aOrigin: Wechsler Memory Scale-Revised.
bOrigin: Wechsler Adult Intelligence Scale III.
cOrigin: Wechsler Adult Intelligence Scale.
dOrigin: Halstead–Reitan Neuropsychological Test Battery.
eCLOX is the name of a clock drawing task.
In order to examine a wider range of abilities in the present study, neurocognitive tests assessing the following additional domains were also included: executive function, abstract reasoning, language and general mental state. Executive function describes the regulation of cognitive processes including: reasoning, task flexibility, problem solving, planning and execution [26]. Abstract reasoning is utilized when analyzing information and solving complex problems [9]. Language abilities are utilized in the recall of simple object names. General mental state relies on several functions incorporated in other domains, including: attention and calculation, recall, orientation and language [27].
Raw test scores for Trail Making Tests A and B were normalized (log to the base 10) to achieve distributional assumptions. The MMSE, CLOX 1 and CLOX 2 were also significantly skewed, but could not be normalized with transformation and were eliminated from analyses utilizing categorical regression and χ2. All other neurocognitive tests met assumptions for normality.
Statistical analyses
Mean (SD) cognitive scores were calculated for study participants with and without CKD, for each of the measures in our neurocognitive test battery. The percentage of participants who performed at least 1.0 SD and 1.5 SD below the mean were also calculated and compared. In a normal distribution, 1 SD would fall in the 16th percentile and 1.5 SD in the 7th percentile of cognitive functioning.
Risk of modest and severe cognitive impairment on each of the 22 neurocognitive tests was calculated using logistic regression [28]. Three sets of covariate controls, which were independent predictors of new-onset CKD and cognitive functioning identified in previous studies [9, 19, 29], were used in each analysis. Models were introduced in a serial stepwise order:
Unadjusted model: CKD only;
Demographic-adjusted model: CKD + age + sex + education + race;
Comorbidity-adjusted model: CKD + age + sex + education + race + DM + SBP + BMI + cigarettes/week + HDL + CVD + stroke + CRP.
To be included in these models, covariates had to be relevant to the literature on CKD and cognitive function or significantly related the predictor and outcome measures. All statistical analyses were performed with IBM SPSS Statistics for Windows, Version 22.0.
RESULTS
Participant characteristics
Table 2 displays the demographic and health characteristics of study participants. Compared with the non-CKD reference group, participants with CKD were significantly older, had higher levels of CRP, higher SBP, consumed more ounces of alcohol per week and had a higher prevalence of folate deficiency, DM and CVD. Mean eGFR in the CKD group was 51.6 ± 10.1 mL/min/1.73 m2 and 96% of these participants had Stage 3 CKD.
Table 2.
Demographic and health characteristics of study participants
| Variable | No CKD: eGFR ≥60 mL/min/ 1.73 m2 (n = 752) |
CKD: eGFR <60 mL/min/ 1.73 m2 (n = 146) |
P-value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (years) | 61.6 | 11.3 | 70.8 | 11.1 | <0.001 |
| Education (years) | 14.6 | 2.7 | 14.2 | 2.7 | 0.082 |
| CRP (mg/L) | 3.9 | 4.2 | 5.2 | 5.8 | 0.002 |
| Total cholesterol (mg/dL) | 202.1 | 40.4 | 198.7 | 44.3 | 0.368 |
| HDL cholesterol (mg/dL) | 53.7 | 15.6 | 52.0 | 14.5 | 0.217 |
| LDL cholesterol (mg/dL) | 120.9 | 33.6 | 117.0 | 34.8 | 0.208 |
| S BP (mmHg) | 130.7 | 21.2 | 136.9 | 22.6 | 0.001 |
| D BP (mmHg) | 70.9 | 9.9 | 69.9 | 10.4 | 0.306 |
| Cigarettes per week | 8.8 | 34.4 | 7.0 | 28.7 | 0.551 |
| BMI (kg/m2) | 29.4 | 5.9 | 29.6 | 6.4 | 0.760 |
| Alcoholic (oz/week) | 1.6 | 3.1 | 1.0 | 1.9 | 0.027 |
| Creatinine (mg/dL) | 0.9 | 0.2 | 1.3 | 0.5 | <0.001 |
| CKD-EPI eGFR (mL/min/1.73 m2) | 82.5 | 14.6 | 51.6 | 10.1 | <0.001 |
| Percentage | Percentage | ||||
| Women | 58.0 | 62.0 | 0.394 | ||
| Black | 7.0 | 8.0 | 0.493 | ||
| DM | 12.0 | 25.0 | <0.001 | ||
| ApoE-e4 | 27.0 | 28.0 | 0.666 | ||
| Folate deficiencya | 0.0 | 1.0 | 0.018 | ||
| B12 deficiencyb | 5.0 | 5.0 | 0.831 | ||
| Depressed moodc | 10.0 | 11.0 | 0.760 | ||
| Stroke | 2.0 | 4.0 | 0.196 | ||
| Cardiovascular diseased | 12.0 | 25.0 | <0.001 | ||
aDefined as deficient if folate <3 ng/mL.
bDefined as deficient if B12 <200 pg/mL.
cDefined as having depressed mood if CES-D >16.
dDefined as present if there was self-reported history of coronary artery disease, congestive heart failure, myocardial infarction, transient ischemic attack or angina pectoris.
CKD and cognitive performance
Mean (SD) cognitive scores for each measure are located in Supplementary data, Appendix 2, Table 1a. This appendix also contains proportions of persons with modest (Supplementary data, Table 2a) and severe (Supplementary data, Table 3a) cognitive impairment on each measure. Participants in the CKD group had significantly higher percentages of impairment than the reference group on at least one neurocognitive test in each of the eight domains of functioning examined (all P < 0.05).
Tables 3 and 4 present the odds ratios (ORs) associated with CKD for modest and severe cognitive impairment, respectively. In the unadjusted model, participants with CKD were significantly more likely to have modest impairment on all tests, with the exception of Logical Memory Delayed Recall, Digit Span Tests Forward and Backward and CLOX 1. The number of significant associations was attenuated with use of the demographic- and comorbidity-adjusted models. With use of the latter, the CKD group remained at significantly higher risk of modest impairment on the Block Design and Object Assembly tests, measures of VSOM, and the Trail Making Test B, a measure of scanning and tracking (all P < 0.05). In sensitivity analyses utilizing the comorbidity-adjusted model without CRP, modest impairment on the COWA and Hooper VOT were also observed for the CKD group (both P < 0.05). The interaction between the CRP and CKD groups did not significantly predict modest impairment on any neurocognitive test when added to the demographic-adjusted model.
Table 3.
ORs and 95% confidence intervals (CIs) associated with modest cognitive impairment (1.0 SD below the mean) for persons in the CKD group (eGFR <60 mL/min/1.73 m2)
| Cognitive domain | Neurocognitive test | Zero order |
Demographic-adj.a |
Comorbidity-adj.b |
|||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Verbal episodic memory | Logic Mem IR | 1.73* | [1.10–2.72] | 1.14 | [0.70–1.85] | 1.14 | [0.69–1.88] |
| Logic Mem DR | 1.56 | [1.00–2.45] | 1.01 | [0.63–1.63] | 1.01 | [0.61–1.66] | |
| Hopkins VLT | 2.74*** | [1.84–4.10] | 1.66* | [1.07–2.59] | 1.48 | [0.92–2.36] | |
| Visual-spatial organization/memory | Vis Repro IR | 2.87*** | [1.91–4.32] | 1.67* | [1.06–2.62] | 1.41 | [0.87–2.29] |
| Vis Repro DR | 2.80*** | [1.87–4.19] | 1.43 | [0.92–2.24] | 1.23 | [0.77–1.98] | |
| Matrix Reasoning | 2.40*** | [1.63–3.55] | 1.40 | [0.90–2.20] | 1.13 | [0.70–1.82] | |
| Block Design | 3.51*** | [2.37–5.21] | 2.42*** | [1.55–3.78] | 2.16** | [1.35–3.48] | |
| Object Assembly | 2.86*** | [1.92–4.24] | 2.03*** | [1.32–3.11] | 1.82* | [1.15–2.87] | |
| Hooper VOT | 3.20*** | [2.07–4.94] | 1.72* | [1.06–2.79] | 1.59 | [0.96–2.65] | |
| Scanning and tracking | Trail Making Ac | 2.43*** | [1.61–3.69] | 1.27 | [0.80–2.02] | 1.06 | [0.65–1.74] |
| Trail Making Bc | 3.23*** | [2.12–4.92] | 1.88** | [1.17–3.02] | 1.68* | [1.02–2.78] | |
| Digit Symbol | 2.66*** | [1.76–4.04] | 1.45 | [0.90–2.34] | 1.15 | [0.69–1.93] | |
| Symbol Search | 3.05*** | [2.02–4.59] | 1.65* | [1.04–2.62] | 1.36 | [0.83–2.23] | |
| Working memory | Digit Span F | 1.61 | [0.98–2.63] | 1.61 | [0.95–2.73] | 1.46 | [0.84–2.56] |
| Digit Span B | 1.40 | [0.88–2.23] | 1.35 | [0.81–2.25] | 1.24 | [0.72–2.13] | |
| Letter Number | 2.10*** | [1.34–3.27] | 1.23 | [0.75–2.00] | 0.98 | [0.58–1.65] | |
| Abstract reasoning | Similarities | 2.23*** | [1.48–3.37] | 1.74* | [1.07–2.82] | 1.66 | [1.00–2.75] |
| Executive functioning | COWA | 2.49*** | [1.63–3.79] | 1.85** | [1.18–2.91] | 1.58 | [0.98–2.54] |
| CLOX 1 | 1.52 | [0.90–2.59] | 0.95 | [0.54–1.69] | 0.88 | [0.48–1.63] | |
| CLOX 2 | 1.57* | [1.03–2.39] | 1.07 | [0.68–1.67] | 1.02 | [0.64–1.64] | |
| General mental state | MMSE | 1.64* | [1.01–2.67] | 1.12 | [0.64–1.93] | 1.05 | [0.59–1.87] |
| Language | Boston Naming | 1.97** | [1.23–3.15] | 1.63 | [0.95–2.79] | 1.49 | [0.85–2.63] |
aDemographic-adjusted model: age, sex, education and race.
bComorbidity-adjusted model: age, sex, education, race, diabetes, SBP, BMI, cigarettes/week, HDL, CVD, stroke and CRP.
cLog (base 10).
*P < 0.05.
**P < 0.01.
***P < 0.001.
Table 4.
ORs and 95% CIs associated with severe cognitive impairment (1.5 SD below the mean) for persons in the CKD group (eGFR <60 mL/min/1.73 m2)
| Cognitive domain | Neurocognitive test | Zero order |
Demographic-adj.a |
Comorbidity-adj.b |
|||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Verbal episodic memory | Logic Mem IR | 1.47 | [0.77–2.79] | 0.83 | [0.42–1.64] | 0.78 | [0.38–1.57] |
| Logic Mem DR | 1.40 | [0.77–2.54] | 0.78 | [0.41–1.48] | 0.80 | [0.41–1.54] | |
| Hopkins VLT | 1.97* | [1.12–3.49] | 0.97 | [0.52–1.81] | 0.73 | [0.38–1.41] | |
| Visual-spatial organization/memory | Vis Repro IR | 3.26*** | [1.98–5.38] | 1.64 | [0.94–2.85] | 1.25 | [0.69–2.25] |
| Vis Repro DR | 2.48*** | [1.52–4.06] | 1.20 | [0.70–2.06] | 0.95 | [0.53–1.71] | |
| Matrix Reasoning | 2.05** | [1.25–3.36] | 1.08 | [0.61–1.91] | 0.75 | [0.40–1.42] | |
| Block Design | 3.03*** | [1.80–5.10] | 2.04* | [1.14–3.65] | 1.54 | [0.80–2.93] | |
| Object Assembly | 2.77*** | [1.68–4.55] | 1.73* | [1.01–2.97] | 1.62 | [0.92–2.86] | |
| Hooper VOT | 3.41*** | [1.99–5.85] | 1.71 | [0.95–3.10] | 1.58 | [0.84–2.96] | |
| Scanning and tracking | Trail Making Ac | 2.48** | [1.36–4.52] | 1.22 | [0.63–2.36] | 1.02 | [0.50–2.07] |
| Trail Making Bc | 2.90*** | [1.73–4.87] | 1.61 | [0.91–2.84] | 1.35 | [0.74–2.47] | |
| Digit Symbol | 2.70*** | [1.55–4.70] | 1.44 | [0.78–2.67] | 1.04 | [0.53–2.04] | |
| Symbol Search | 2.70*** | [1.47–4.96] | 1.38 | [0.71–2.67] | 1.09 | [0.54–2.20] | |
| Working memory | Digit Span F | 1.61 | [0.98–2.63] | 1.61 | [0.95–2.73] | 1.46 | [0.84–2.56] |
| Digit Span B | 0.64 | [0.15–2.81] | 0.55 | [0.12–2.57] | 0.43 | [0.08–2.18] | |
| Letter Number | 2.29** | [1.35–3.90] | 1.40 | [0.78–2.52] | 1.27 | [0.69–2.35] | |
| Abstract reasoning | Similarities | 1.93* | [1.11–3.37] | 1.45 | [0.77–2.73] | 1.28 | [0.66–2.48] |
| Executive functioning | COWA | 3.07*** | [1.70–5.53] | 2.18* | [1.15–4.11] | 1.77 | [0.91–3.47] |
| CLOX 1 | 1.19 | [0.54–2.62] | 0.65 | [0.28–1.49] | 0.60 | [0.25–1.44] | |
| CLOX 2 | 2.06* | [1.06–4.02] | 1.12 | [0.55–2.28] | 1.01 | [0.47–2.15] | |
| General mental state | MMSE | 2.26** | [1.27–4.04] | 1.57 | [0.82–3.01] | 1.44 | [0.73–2.85] |
| Language | Boston Naming | 2.91*** | [1.67–5.10] | 2.40** | [1.27–4.54] | 2.65** | [1.35–5.19] |
aDemographic-adjusted model: age, sex, education and race.
bComorbidity-adjusted model: age, sex, education, race, diabetes, SBP, BMI, cigarettes per week, HDL, CVD, stroke and CRP.
cLog (base 10).
*P < 0.05.
**P < 0.01.
***P < 0.001.
In unadjusted models of severe cognitive impairment (at least 1.5 SD below mean performance), participants with CKD were more likely to have impaired performance than the non-CKD reference group on nearly all measures, with the exception of: Logical Memory Immediate and Delayed Recall, Digit Span Forward and Backward and CLOX 1. With use of the comorbidity-adjusted model, only the Boston Naming a measure of language abilities, retained a significant OR (P < 0.01).
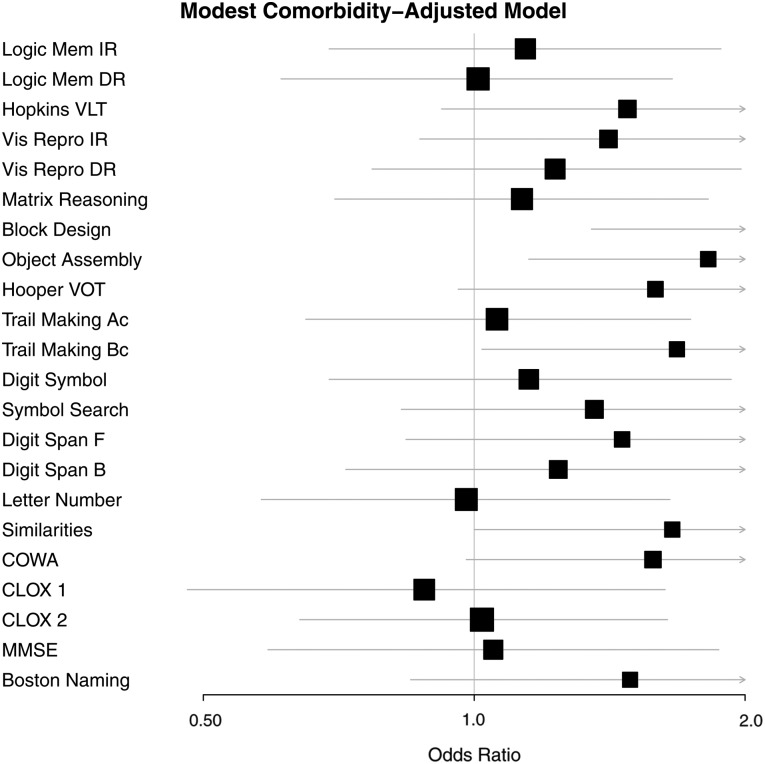
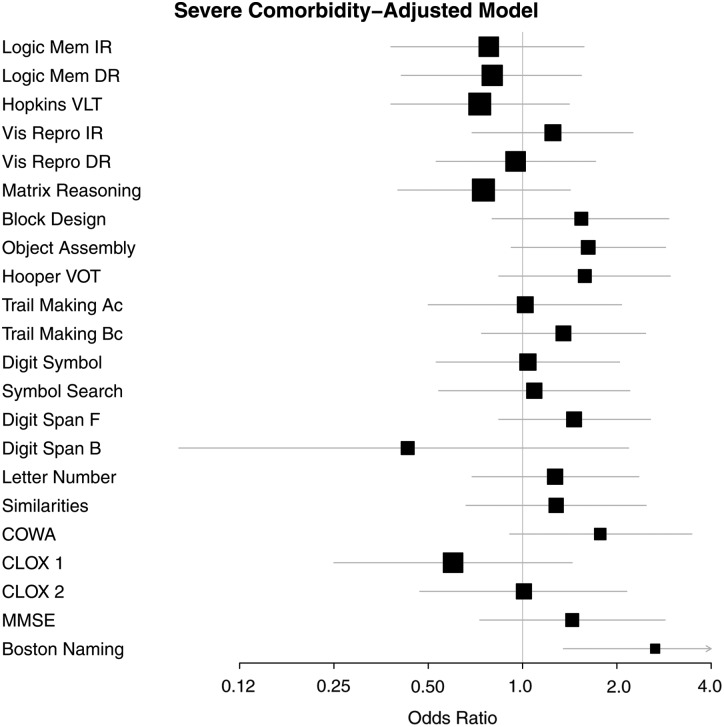
Figures 1 and 2 provide a graphic summary of the results for the comorbidity-adjusted model and both impairment criteria (1.0 SD and 1.5 SD, respectively) using forest plots.
FIGURE 1.
Comorbidity-adjusted model odds ratios (OR) and 95 percent confidence intervals (CI) associated with modest impairment (1.0 SD below the mean) on each test for participants in the CKD group.
FIGURE 2.
Comorbidity-adjusted model odds ratios (OR) and 95 percent confidence intervals (CI) associated with severe impairment (1.5 SD below the mean) on each test for participants in the CKD group.
Additional analyses
Supplementary data, Appendix 2, Table 4a shows results of simple linear regression analyses (i.e. correlations presented in regression metric) where continuously distributed eGFR (truncated with scores ≥60 = 60) was related to the continuous distribution of cognitive scores. The pattern of results for the various models was similar to those reported for the logistic regression analyses in Tables 3 and 4.
DISCUSSION
Previous studies provided strong support for an association between early-stage CKD and lower cognitive function for one or a few measures of cognitive ability or composite scores reflecting broad domains of cognitive performance [1, 2, 5, 9]. Here, we are concerned with levels of lower cognitive performance recognized by psychologists as diagnostically meaningful in studies of disease and aging [2, 4, 24]. We report relationships between CKD and cognitive ability for what may be the largest battery of tests employed in studies of cardiovascular risk factors and cognitive performance [30].
We calculated the risk of impaired performance using two criteria for impairment, 1.0 SD (modest) and 1.5 SD (severe) below the mean for each measure. We used cross-sectional data because one of our goals was to provide data that would allow early-stage CKD to be used as a reference group in cross-sectional studies of patients with ESRD, including studies of longitudinal changes in cognitive performance. The participants in our study represent a community-based, non-clinic sample with early-stage CKD.
Using the modest and severe impairment criteria and the unadjusted model, the risk for cognitive impairment was significantly higher for persons with CKD for 18 out of 22 and 17 out of 22 cognitive tests, respectively. With use of the comorbidity-adjusted model, significant differences in risk between participants with and without CKD were attenuated for all but three neurocognitive tests using the modest impairment criteria and for all but one using the severe impairment criteria.
While direct effects of kidney disease on cognition have been suggested [1, 2, 5, 9], some of the variables we include in the comorbidity-adjusted model could mediate the relationship between CKD and cognitive performance [29]. Persons with CKD have a higher burden of traditional and non-traditional CVD-RFs than those without, which may expose them to systemic and cerebrovascular changes that increase the risk for cognitive impairment [29, 31, 32].
Cerebrovascular disease is common at all stages of CKD and has been associated with greater burden of white matter hyperintensities, atrophy and cerebral infarcts in the brain [29, 32]. Neurocognitive manifestations of cerebrovascular disease affect executive functioning [32]. In the present study, CKD was associated with greater odds of modest and severe cognitive impairment on two tests indexing executive functioning in the zero-order model. These results were attenuated with adjustment for CVD-RFs. While this is of theoretical interest, the main purpose of our study was to present data useful to nephrologists wishing to evaluate cognitive deficit in their own patients. Kidney disease patients go to their physicians with many of the risk factors we include in the comorbidity-adjusted model. Thus, we argue that the most clinically relevant data in terms of test selection and evaluation from among the 22 neurocognitive tests employed in this study should be taken from the analyses utilizing the demographic-adjusted model.
The level of risk for modest impairment associated with CKD ranged from 1.65 (for performance on the Symbol Search test) to 2.42 (Block Design test) when the demographic-adjusted model was employed. With control of the comorbidity-adjusted model, risk for impairment on the Block Design test, Object Assembly and Trail Making B were the only remaining statistically significant tests. These tests index VSOM and scanning and tracking, functions essential for problem solving and the ability to shift set, and should be tests of significant interest to clinicians in terms of the ability of patients to be flexible and to adjust to changing demands of treatment.
CKD-associated risk for severe cognitive impairment was observed for Block Design (OR = 2.04), Object Assembly (OR = 1.73) and COWA (OR = 2.18), measures of VSOM and executive functioning, when the demographically adjusted model was employed. Risk for impairment was also observed in the Boston Naming Test, a measure of language ability (OR = 2.40). Poor naming performance on this test is a good predictor of subsequent dementia and is included in the Framingham Heart Study Dementia Battery for this reason [33]. Risk remained significant for the Boston Naming Test (OR = 2.65) with use of the comorbidity-adjusted model.
Limitations
The absence of hemoglobin determinations due to our inability to anticipate an examination of kidney function and cognition at the time of data collection was a limitation of our study. We used eGFR data obtained on only one occasion to define CKD. However, our eGFR criterion was consistent with previous community-based studies and clinical trials that defined CKD in this manner [6, 34, 35]. We also lacked data on albuminuria, which has been suggested as a risk factor of cognitive impairment and decline independent of renal filtration function [36]. Finally, although our goal in this study was to examine cross-sectional data for early-stage CKD in order to evaluate this group as a potentially useful control group for studies of ESRD patients, it must be acknowledged that these cross-sectional data do not permit us to determine causality in the relationship between CKD and cognitive function.
Strengths
Our community-based study permitted us to examine relationships between CKD and cognition in a non-clinical sample, unselected for kidney disease status, with blinded testing procedures. Data on multiple risk factors for CKD and cognitive impairment were present for use in statistical models, allowing us to take into account variables deemed important by the literature. Finally, an extensive neurocognitive test battery was employed. This has allowed us to provide nephrologists and researchers with specific data on the abilities that are at the highest risk for impairment with respect to early-stage CKD.
Perspectives
While we continue to argue that early-stage CKD patients are ideal candidates for a reference group in studies of ESRD [8], it is clear that even non-dialysis-dependent CKD is associated with deficit in some of the most important cognitive abilities related to patient–physician communication. The extensive data presented in this study will also allow investigators who must use only one or a few tests due to time restrictions to select those tests revealing the greatest vulnerability to CKD for use with their own patients.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by grants R01HL67358 and R01HL081290 from the National Heart, Lung and Blood Institute, National Institutes of Health (USA) and research grant R01AG03055 from the National Institute on Aging, National Institutes of Health (USA) to the University of Maine.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this paper have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Etgen T, Chonchol M, Förstl H et al. . Chronic kidney disease and cognitive impairment: a systematic review and meta-analysis. Am J Nephrol 2012; 35: 474–482 [DOI] [PubMed] [Google Scholar]
- 2. Elias MF, Dore GA, Davey A. Kidney disease and cognitive function. In: Toyota K. (ed). Brain, Stroke and Kidney. Vol. 175 of Contributions to Nephrology. Basel, Switzerland: Karger Publishers, 2013, 42–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bugnicourt JM, Godefroy O, Chillon JM et al. . Cognitive disorders and dementia in CKD: the neglected kidney-brain axis. J Am Soc Nephrol 2013; 24: 353–363 [DOI] [PubMed] [Google Scholar]
- 4. Schneider SM, Malecki AK, Muller K et al. . Effect of a single dialysis session on cognitive function in CKD5D patients: a prospective clinical study. Nephrol Dial Transplant 2015; 30: 1551–1559 [DOI] [PubMed] [Google Scholar]
- 5. Davey A, Elias MF, Robbins MA et al. . Decline in renal functioning is associated with longitudinal decline in global cognitive functioning, abstract reasoning and verbal memory. Nephrol Dial Transplant 2013; 28: 1810–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kurella M, Chertow GM, Luan J et al. . Cognitive impairment in chronic kidney disease. J Am Geriatr Soc 2004; 52: 1863–1869 [DOI] [PubMed] [Google Scholar]
- 7. Jassal SV, Roscoe J, LeBlanc D et al. . Differential impairment of psychomotor efficiency and processing speed in patients with chronic kidney disease. Int Urol Nephrol 2008; 40: 849–854 [DOI] [PubMed] [Google Scholar]
- 8. Elias MF, Seliger S, Torres RV. Improved cognitive performance after a single dialysis session: where do we go from here? Nephrol Dial Transplant 2015; 30: 1414–1417 [DOI] [PubMed] [Google Scholar]
- 9. Elias MF, Elias PK, Seliger SL et al. . Chronic kidney disease, creatinine and cognitive functioning. Nephrol Dial Transplant 2009; 24: 2446–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Petersen RC. Conceptual overview. In: Petersen RC. (ed). Mild Cognitive Impairment: Aging to Alzheimer's Disease. New York, NY: Oxford University press, 2003, 1–14 [Google Scholar]
- 11. Elias MF, Robbins MA, Budge MM et al. . Homocysteine, folate, and vitamins B6 and B12 blood levels in relation to cognitive performance: The Maine-Syracuse Study. Psychosom Med 2006; 68: 547–554 [DOI] [PubMed] [Google Scholar]
- 12. Elias MF, Robbins MA, Budge MM et al. . Homocysteine and cognitive performance: modification by the ApoE genotype. Neurosci Lett 2008; 430: 64–69 [DOI] [PubMed] [Google Scholar]
- 13. McKhann G, Drachman D, Folstein M et al. . Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology 1984; 34: 939–944 [DOI] [PubMed] [Google Scholar]
- 14. World Health Organization. The ICD 10 Classification of Mental and Behavioral Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization, 1992 [Google Scholar]
- 15. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977; 1: 385–401 [Google Scholar]
- 16. Levey AS, Stevens LA, Schmid CH et al. . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stevens LA, Schmid CH, Greene T et al. . Comparative performance of the CKD epidemiology collaboration (CKD-EPI) and the modification of diet in renal disease (MDRD) study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis 2010; 56: 486–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hixson JE, Vernier DR. Restricting isotyping of human Apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res 1990; 31: 545–548 [PubMed] [Google Scholar]
- 19. Elias MF, Sullivan LM, D'Agostino RB et al. . Framingham stroke risk profile and lowered cognitive performance. Stroke 2004; 35: 404–409 [DOI] [PubMed] [Google Scholar]
- 20. Elias MF, Sullivan LM, Elias PK et al. . Left ventricular mass, blood pressure and lowered cognitive performance in the Framingham offspring. Hypertension 2007; 49: 439–445 [DOI] [PubMed] [Google Scholar]
- 21. Wechsler D. Manual for the Wechsler Adult Intelligence Scale. New York, NY: Psychological Corporation, 1955 [Google Scholar]
- 22. Wechsler D. Wechsler Memory Scale-Revised Manual. New York, NY: Psychological Corporation, 1987 [Google Scholar]
- 23. Wechsler D. Wechsler Adult Intelligence Scale-III. San Antonio, TX: Psychological Corporation, 1997 [Google Scholar]
- 24. Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment, 4th edn New York, NY: Oxford University Press, 2004 [Google Scholar]
- 25. Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation, 2nd edn Tucson, AZ: Neuropsychology Press, 1993 [Google Scholar]
- 26. Chan RC, Shum D, Toulopoulou T et al. . Assessment of executive functions: Review of instruments and identification of critical issues. Arch Clin Neuropsychol 2008; 23: 201–216 [DOI] [PubMed] [Google Scholar]
- 27. Strauss E, Sherman EM, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms and Commentary. Oxford: Oxford University Press, 2006 [Google Scholar]
- 28. Sperandei S. Understanding logistic regression analysis. Biochem Med 2014; 24: 12–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seliger SL, Wendell CR, Waldstein SR et al. . Renal function and long-term decline in cognitive function: the Baltimore Longitudinal Study of Aging. Am J Nephrol 2015; 41: 305–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Waldstein SR, Elias MF (eds). Neuropsychology of Cardiovascular Disease, 2nd edn New York, NY: Taylor and Francis, 2015 [Google Scholar]
- 31. Madero M, Gul A, Sarnak MJ. Cognitive function in chronic kidney disease. Semin Dial 2008; 21: 29–37 [DOI] [PubMed] [Google Scholar]
- 32. Drew DA, Weiner DE. Cognitive impairment in chronic kidney disease: keep vascular disease in mind. Kidney Int 2014; 85: 505–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Downer B, Fardo DW, Schmitt FA. A summary score for the Framingham Heart Study neuropsychological battery. J Aging Health 2015; 27: 1199–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hailpern SM, Melamed ML, Cohen HW et al. . Moderate chronic kidney disease and cognitive function in adults 20 to 59 years of age: Third National Health and Nutrition Examination Survey (NHANES III). J Am Soc Nephrol 2007; 18: 2205–2213 [DOI] [PubMed] [Google Scholar]
- 35. Mann JF, Sheridan P, McQueen MJ et al. . Homocysteine lowering with folic acid and B vitamins in people with chronic kidney disease—results of the renal Hope-1 study. Nephrol Dial Transplant 2008; 23: 645–653 [DOI] [PubMed] [Google Scholar]
- 36. Fried L. Albuminuria and cognitive impairment. Clin J Am Soc Nephrol 2012; 7: 376–378 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.