Abstract
Aims
Despite patient reports of neurocognitive disorders with lipid-lowering treatments (LLTs), large clinical trials have found no significant association between neurocognitive disorders and LLTs. We assessed incidence of neurocognitive treatment-emergent adverse events (TEAEs) from 14 Phase 2 and 3 trials of the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor alirocumab.
Methods and results
Patients (most on background maximally tolerated statin) received alirocumab 75/150 mg every 2 weeks (n = 3340; 4029 patient-years of exposure), placebo (n = 1276), or ezetimibe (n = 618). Data were pooled by the control used. Neurocognitive TEAEs were reported by 22 (0.9%) alirocumab-treated patients vs. 9 (0.7%) with placebo in placebo-controlled trials [hazard ratio (HR) 1.24, 95% confidence interval (CI) 0.57–2.68] and 10 (1.2%) with alirocumab vs. 8 (1.3%) with ezetimibe in ezetimibe-controlled trials (HR 0.81, 95% CI 0.32–2.08). Rates of neurocognitive TEAEs were similar in patients receiving alirocumab with LDL cholesterol (LDL-C) levels <25 mg/dL (<0.65 mmol/L; n = 5/839; 0.6%; 0.5/100 patient-years) vs. ≥25 mg/dL (n = 26/2501; 1.0%; 0.8/100 patient-years). One patient (0.1%; ezetimibe-controlled pool) receiving alirocumab had a neurocognitive TEAE leading to discontinuation vs. two (0.2%) patients receiving placebo and three (0.4%) patients receiving ezetimibe. Neurocognitive TEAE incidence was also similar between alirocumab and controls when stratified by age.
Conclusions
Neurocognitive TEAE incidences were low (≤1.2%), with no significant differences between alirocumab vs. controls up to 104 weeks. No association was found between neurocognitive TEAEs and LDL-C <25 mg/dL based on the completed Phase 2 and 3 trials examined, although long-term effects of very low LDL-C levels induced by PCSK9 inhibitors are currently unknown.
Keywords: Cholesterol-lowering drugs, Patient safety, Cognitive function , LDL, PCSK9
Introduction
Due to rare post-marketing reports of memory loss or confusion with statin treatment, the US Food and Drug Administration (FDA) changed the labelling of statins in 2012 to indicate that neurocognitive disorders had been associated with statin use.1 However, the case reports of neurocognitive effects with statins have not been confirmed in large randomized controlled trials or meta-analyses, and no significant association between neurocognitive disorders and low levels of low-density lipoprotein cholesterol (LDL-C) have been shown.2–7 Despite this, a potential association between lipid-lowering treatments (LLTs) and neurocognitive disorders remains an area of debate.
Alirocumab is a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor, a new class of LLT. In Phase 2 and 3 clinical trials, alirocumab demonstrated large reductions in LDL-C.8–20 In 2014, the FDA directed companies developing PCSK9 inhibitors to monitor neurocognitive events and to consider neurocognitive testing during late-stage trials.21 To assess the potential association between alirocumab and neurocognitive disorders, we examined safety data collected from 14 completed clinical trials of alirocumab.
Methods
Trial participants and study designs
This analysis included data from 14 double-blind trials (4 Phase 2 and 10 Phase 3). Methods and results of the trials have been reported previously.8–20 Patients had either heterozygous familial hypercholesterolaemia (HeFH) or non-FH and were randomized to alirocumab 75 or 150 mg every 2 weeks vs. placebo or ezetimibe. Double-blind treatment periods were 8–12 weeks for Phase 2 and 24–104 weeks for Phase 3 trials. Most patients received study treatment as add-on to background maximally tolerated statin. Further details are provided in the Supplementary material online, Tables S1 and S2. All study protocols were approved by the appropriate institutional review board, and all patients provided informed, written consent.
Recording of safety data
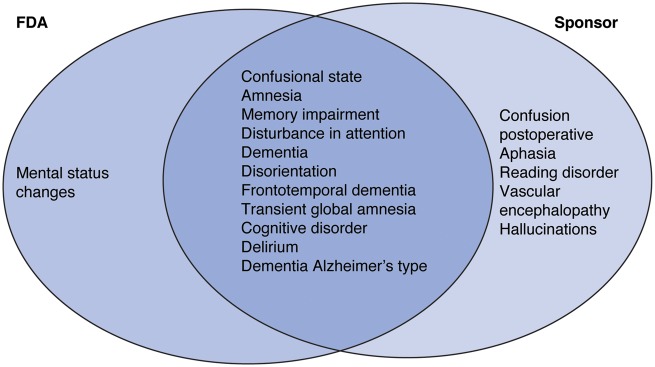
This analysis focuses on treatment-emergent adverse events (TEAEs), defined as those events occurring from the first dose of study treatment up to 70 days after the last dose. All safety data were collected while patients and investigators were blinded to treatment allocations. TEAEs were categorized using standard Medical Dictionary of Regulatory Activities (MedDRA) preferred terms (lowest level term reviewed). Two different groupings or custom MedDRA queries (CMQs) were used to define neurocognitive TEAEs in this analysis (Figure 1), a broad set of terms defined by the study sponsors (sponsor CMQ) and a more focused set of terms which the FDA proposed (FDA CMQ).5
Figure 1.
Individual preferred terms included in custom Medical Dictionary of Regulatory Activities (MedDRA) query groupings of neurocognitive events by the US Food and Drug Administration (FDA)5 and alirocumab study sponsors. Adverse events were reported by patients in the trials and recorded by the trial investigators, then encoded according to standard MedDRA adverse event (preferred) terms. For the purposes of this analysis, two groupings of preferred terms were categorized as ‘neurocognitive events’, one defined by the FDA and the other defined by the alirocumab study sponsors.
Neurocognitive events were self-reported by the patients and recorded by the investigators. No formal neurocognitive testing was included in trials. The trials included in the analysis did not employ a screening tool for evaluating cognitive impairment, and there were no selection criteria related to concomitant medications or cognitive status that may affect cognition. Patients who prematurely discontinued study treatment were followed up where possible for at least 10 weeks or until recovery or stabilization of any TEAEs. All TEAEs were recorded, regardless of their severity or potential relationship to alirocumab. Severity of neurocognitive events was determined by investigators.
Statistical analysis
Data were analysed in two pools according to the control used in the trials (placebo or ezetimibe) and also in an overall pool of all studies. Event rates were calculated by dividing the number of patients by the duration of exposure (in 100 patient-years). To compare treatment groups in the placebo and in the ezetimibe pools, the hazard ratio (HR) and 95% confidence interval (CI) levels were calculated using a Cox model stratified in the study. Event rates were also calculated in patients treated with alirocumab who had two consecutive LDL-C levels <25 mg/dL (<0.65 mmol/L) separated by at least 21 days, with HR and 95% CI calculated using a Cox model stratified on the propensity score to achieve LDL-C <25 mg/dL (quintile) and including the covariate for two consecutive LDL-C <25 mg/dL (yes/no) as fixed effect and study as random effect. Further details on the propensity score analysis are given in the Supplementary material online. The Kaplan–Meier curves are presented for each pool to describe the cumulative incidence of neurocognitive events. An analysis was also performed by age group (<65, ≥65 to <75, and ≥75 years). Average percentage and absolute LDL-C reductions during treatment were determined for patients with and without neurocognitive TEAEs from the area under the curve and taking into account all LDL-C levels to the end of treatment period or occurrence of a neurocognitive TEAE, whichever came first. Previous reports have suggested an association between low HDL-C and memory decline;22,23 hence, neurocognitive TEAEs were also analysed by baseline HDL-C levels (post hoc analysis). To examine the effects of alirocumab on neurocognitive TEAEs in studies of longer duration, a sensitivity analysis was also performed including only those patients treated for at least 52 weeks.
Results
Patient population
Safety data were available for a total of 5234 patients (alirocumab, n = 3340; placebo, n = 1276; and ezetimibe, n = 618). Patients received alirocumab for a total of 4029 patient-years. Baseline characteristics were similar across alirocumab and control groups (see Supplementary material online, Table S1). The mean age of study participants was 58.5–62.1 years (range across the groups in the placebo-controlled and ezetimibe-controlled pools), with 59.8–67.2% males; the majority (86.2–90.1%) were White. Across the groups, 66.2–75.3% of patients had a history of clinical atherosclerotic cardiovascular disease and 5.8–8.6% had a history of ischaemic stroke. In the pool of placebo-controlled trials, which included four trials specifically recruiting patients with HeFH, 36.5% of patients receiving alirocumab and 36.4% receiving placebo had HeFH; corresponding figures were 4.6% for alirocumab and 7.0% for ezetimibe in the ezetimibe-controlled pool (see Supplementary material online, Table S1). Alirocumab produced significant and substantial reductions in LDL-C vs. control in all trials (see Supplementary material online, Table S2).
Neurocognitive treatment-emergent adverse events
Results analysed using the sponsor CMQ definition of neurocognitive events are presented in the main article with analyses using the FDA CMQ definition in the Supplementary material online (except where stated). The majority of neurocognitive TEAEs identified in this analysis under the FDA definition were also present in the sponsor definition, except for ‘mental status changes’, which was not included in the sponsor definition; several terms included in the sponsor definition were not included in the FDA definition (Figure 1). Overall rates of neurocognitive TEAEs, including those classified as serious and those leading to treatment discontinuation, were low and similar between groups (Figure 2A). The most common individual events were confusional state, amnesia, and memory impairment, which were observed in six patients or fewer (≤0.5%) in each group (see Supplementary material online, Tables S3 and S4).
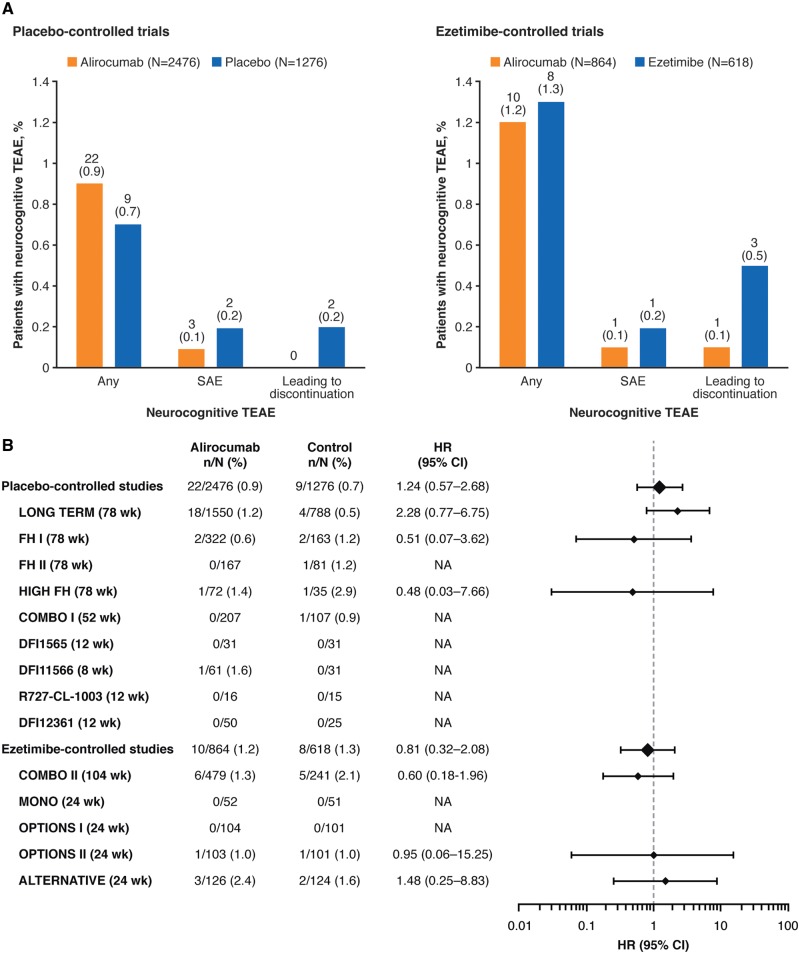
Figure 2.
Overall percentage rates of neurocognitive treatment-emergent adverse events (TEAEs) (A) and hazard ratios for incidence of neurocognitive TEAEs (B) in alirocumab-treated patients compared with the controls. Neurocognitive TEAEs categorized using the sponsor custom MedDRA query as illustrated in Figure 1. In (A), values on chart are n (%) = number and percentage of patients with at least one neurocognitive disorder TEAE. Hazard ratios (HR) were calculated using a Cox model in each study. CI, confidence interval.
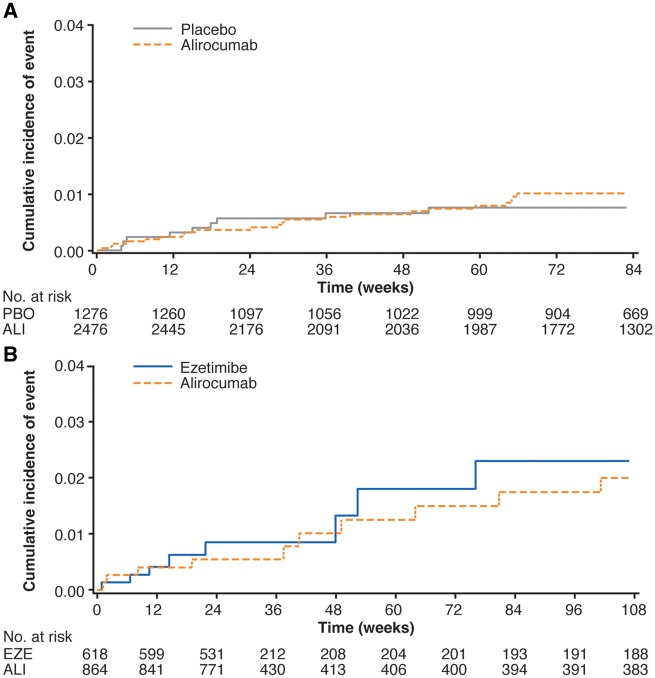
Neurocognitive TEAEs were reported by 22 (0.9%; 95% CI 0.6–1.3%) patients in the alirocumab group vs. 9 (0.7%; 95% CI 0.3–1.3%) patients in the placebo group in the placebo-controlled pool (HR 1.24; 95% CI 0.57–2.68; Figure 2B). In the ezetimibe-controlled pool, neurocognitive TEAEs were reported by 10 (1.2%; 95% CI 0.6–2.1%) patients in the alirocumab group vs. 8 (1.3%; 95% CI 0.6–2.4%) patients in the ezetimibe group (HR 0.81; 95% CI 0.32–2.08; Figure 2B). Although for the largest trial (LONG TERM, 1550 patients allocated to alirocumab in the safety population) neurocognitive TEAEs were observed for 18 (1.2%) alirocumab vs. 4 (0.5%) placebo-treated patients (HR 2.28; 95% CI 0.77–6.75), and no difference between alirocumab and control groups was observed in the pools, which include 1632 patients from the other Phase 3 trials in addition to those from LONG TERM (Figure 2B). Neurocognitive TEAEs as defined using the FDA CMQ were reported in 16 (1.0%) alirocumab-treated patients in LONG TERM, compared with 6 (0.8%) placebo-treated patients (HR 1.35; 95% CI 0.53–3.46; Supplementary material online, Table S5). Cumulative incidence curves for the time to first neurocognitive event were similar for alirocumab and control groups (Figure 3; Supplementary material online, Figure S1).
Figure 3.
The Study-adjusted Kaplan–Meier cumulative incidence curve for the time to first neurocognitive event during the treatment in pools of (A) placebo-controlled studies and (B) ezetimibe-controlled studies. Neurocognitive treatment-emergent adverse events (TEAEs) categorized using the Sponsor custom MedDRA query. Patients are censored at the end of TEAE period (last injection of study treatment + 70 days).
The majority of neurocognitive TEAEs were considered to be mild or moderate in severity (Table 1 and Supplementary material online, Table S6). Neurocognitive events led to treatment discontinuation for no patients who received alirocumab and for two (0.2%) patients who received placebo in the placebo-controlled pool. One of the placebo-treated patients who discontinued had dementia of unknown origin (classified as moderate in severity), whereas the other had delirium (classified as moderate) and dementia (classified as severe and leading to death). In the ezetimibe-controlled pool, neurocognitive events led to discontinuation in one patient in the alirocumab group (0.1%; memory impairment, classified as mild) and three (0.5%) patients in the ezetimibe group (disturbance in attention, mild; transient global amnesia, moderate; and confusional state, moderate).
Table 1.
Severity of neurocognitive TEAEs
| Placebo-controlled pool |
Ezetimibe-controlled pool |
Pool of all studies |
||||
|---|---|---|---|---|---|---|
| Alirocumab (n = 2476) | Placebo (n = 1276) | Alirocumab (n = 864) | Ezetimibe (n = 618) | Alirocumab (n = 3340) | Control (n = 1894) | |
| Any neurocognitive TEAE, n (%) | 22 (0.9) | 9 (0.7) | 10 (1.2) | 8 (0.9) | 32 (1.0) | 17 (0.9) |
| Mild | 14 (63.6) | 4 (44.4) | 9 (90.0) | 4 (50.0) | 23 (0.7) | 8 (0.4) |
| Moderate | 8 (36.4) | 4 (44.4) | 1 (10.0) | 4 (50.0) | 9 (0.3) | 8 (0.4) |
| Severe | 0 | 1 (11.1) | 0 | 0 | 0 | 1 (0.1) |
Neurocognitive TEAEs categorized using the Sponsor CMQ.
CMQs, custom Medical Dictionary of Regulatory Activities queries; TEAEs, treatment-emergent adverse events.
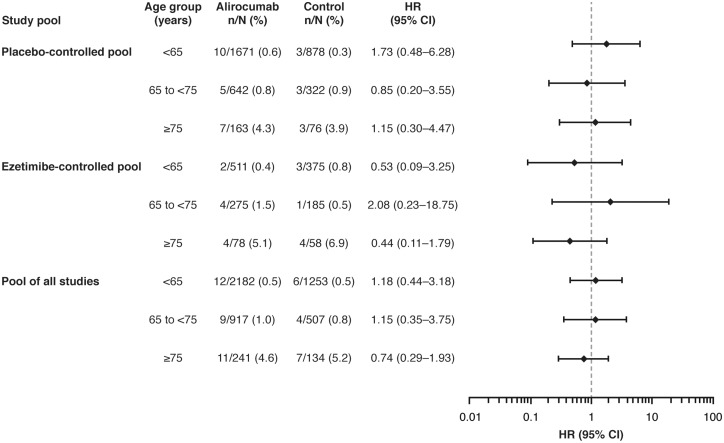
Subanalysis by age group and concomitant medication
The percentages of patients with neurocognitive TEAEs were similar between alirocumab and control groups in each age category, although the percentage of neurocognitive TEAEs was higher in both alirocumab and control groups in patients ≥75 years of age vs. those <65 years of age and ≥65 to <75 years of age (Figure 4 and Supplementary material online, Table S7). Results were similar when comparing patients <75 vs. ≥75 years of age (see Supplementary material online, Table S8). The percentage of patients with neurocognitive TEAEs was low, regardless of the receipt of medications with potential neurocognitive effects, and there were no statistically significant differences between the alirocumab and control groups (see Supplementary material online, Table S9).
Figure 4.
Neurocognitive treatment-emergent adverse events (TEAEs) based on age categories. Neurocognitive TEAEs categorized using the Sponsor custom MedDRA query. Interaction P-values between age groups: placebo-controlled pool, P = 0.7345; ezetimibe-controlled pool, P = 0.4964; pool of all studies, P = 0.9009.
Lipid levels and neurocognitive treatment-emergent adverse events
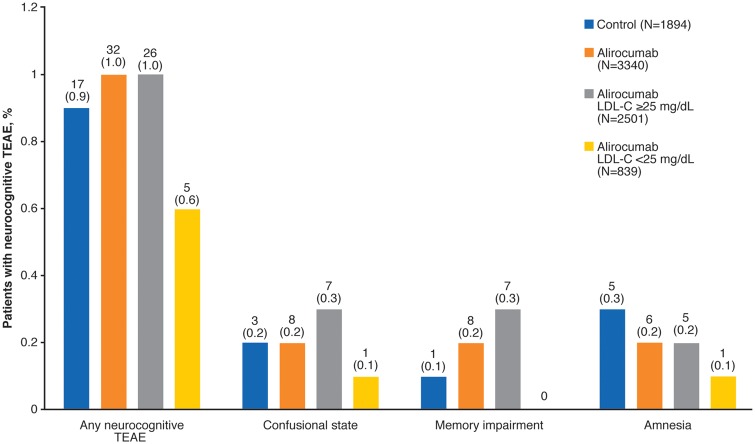
In total, 839 (25.1%) patients who received alirocumab achieved two consecutive LDL-C values <25 mg/dL (<0.65 mmol/L). One patient in the control group achieved such LDL-C levels and was not considered for this analysis. Incidence of neurocognitive TEAEs was similar in alirocumab-treated patients with two consecutive LDL-C values <25 mg/dL vs. those with LDL-C levels ≥25 mg/dL (HR 0.39; 95% CI 0.14–1.10; Figure 5). Similarly, low rates of individual events were also observed across these subgroups (Figure 5; further details in Supplementary material online, Table S10). In those with two consecutive LDL-C levels <25 mg/dL, four events (confusional state, amnesia, dementia, and frontotemporal dementia) were considered to be of moderate severity and one event (aphasia) was considered to be mild. It is conceivable that the magnitude of LDL-C reduction rather than the achieved level may have an association with adverse effects; however, mean LDL-C reductions during alirocumab treatment were somewhat smaller in patients with neurocognitive TEAEs than for patients without a neurocognitive TEAE (see Supplementary material online, Table S11). The incidence of neurocognitive TEAEs in those with HDL-C above and below the median level was similar in both men and women and across treatment groups (see Supplementary material online, Table S12).
Figure 5.
Neurocognitive treatment-emergent adverse events (TEAEs) in patients with two consecutive LDL cholesterol levels <25 mg/dL (<0.65 mmol/L). Neurocognitive TEAEs categorized using the Sponsor custom MedDRA query. Values on chart are n (%).
Sensitivity analysis in patients treated ≥52 weeks
This analysis included six trials with double-blind treatment periods of 52 weeks or more (five placebo-controlled trials and one ezetimibe-controlled; see Supplementary material online, Table S2). A large proportion of the pooled patient population were treated for 52 weeks or more: 1991 (80.4%) and 1000 (78.4%) patients who received alirocumab or placebo, respectively, in the placebo-controlled trials and 410 (47.5%) and 209 (33.8%) patients who received alirocumab or ezetimibe, respectively, in the ezetimibe-controlled trials. The occurrence of neurocognitive TEAEs in patients treated ≥52 weeks was similar to the overall analysis, with no apparent differences between the alirocumab and control groups (see Supplementary material online, Tables S13 and S14). Among patients treated ≥52 weeks, neurocognitive TEAEs were reported in 14 (0.7%) and 6 (0.6%) of patients treated with alirocumab and placebo, respectively (HR 1.17; 95% CI 0.45–3.06), and in 6 (1.5%) and 4 (1.9%) of patients treated with alirocumab and ezetimibe, respectively (HR 0.75; 95% CI 0.21–2.67; results using Sponsor CMQ; Supplementary material online, Table S13). Furthermore, among patients treated ≥52 weeks, incidence of neurocognitive TEAEs was similar in alirocumab-treated patients with two consecutive LDL-C values <25 mg/dL vs. those with LDL-C levels ≥25 mg/dL [4 (0.6%) vs. 15 (0.9%); HR 0.51; 95% CI 0.15–1.71].
Discussion
In this large pooled analysis of completed Phase 2 and 3 trials, across 4029 patient-years of exposure to alirocumab, the overall rate of neurocognitive TEAEs was low, and there was no significant difference in the incidence of neurocognitive TEAEs between the alirocumab and control (placebo and ezetimibe) groups, including when stratified by age, nor between alirocumab-treated patients with LDL-C <25 mg/dL vs. ≥25 mg/dL. One patient receiving alirocumab discontinued due to a neurocognitive TEAE (mild memory impairment), compared with two placebo- and three ezetimibe-treated patients. Furthermore, in a sensitivity analysis including only patients treated for 52 weeks or more, we saw no evidence of an increase in neurocognitive TEAEs in the alirocumab-treated patients compared with the controls.
A recent meta-analysis by Khan et al.24 of PCSK9 inhibitor trials found a higher rate of neurocognitive events with PCSK9 inhibitors vs. placebo in a pool of the two largest trials: the alirocumab ODYSSEY LONG TERM trial16 and an open-label study of evolocumab.25 There was no difference between PCSK9 inhibitor and control in a pool of 10 smaller Phase 3 studies.24 The meta-analysis by Khan et al.24 included a larger set of alirocumab trials (10 in total) compared with a previous similar analysis by Lipinski et al.26 (which included 6 alirocumab trials); however, both analyses were limited by data the author groups did not have access to, such as severity of the events and rate of discontinuations. As we report here, the majority of neurocognitive events reported in the alirocumab Phase 2 and 3 trials were considered to be mild and the rate of discontinuation due to neurocognitive events was low. Furthermore, this analysis includes data up to the end of study for longer studies such as COMBO II (104 weeks), which were previously unavailable.
In this analysis of relatively short Phase 2 and 3 trials (compared with the forthcoming alirocumab outcomes trial), there was no difference in rates of neurocognitive TEAEs between those with two consecutive LDL-C levels <25 mg/dL and those without. Similarly, there appeared to be no relationship between LDL-C levels and incidence of neurocognitive TEAEs over ∼1–2 years of treatment with evolocumab.25,27 This is consistent with the results from statin studies, including the JUPITER trial that found no increase in the rate of memory impairment or nervous system disorders in patients who achieved LDL-C levels <30 mg/dL (0.78 mmol/L) with rosuvastatin treatment.3 Furthermore, no increase in neurocognitive events was found over a perod of 6 years in patients who achieved LDL-C <30 mg/dL (0.78 mmol/L) in the IMPROVE-IT trial (including patients treated with ezetimibe/simvastatin combination or simvastatin alone).28 Additionally, a study examining a single nucleotide polymorphism in the PCSK9 gene associated with lower LDL-C levels found no association between this and neurocognitive function.29 These observations remain to be analysed in larger and longer term studies.
An association between low HDL-C and memory decline in middle-aged adults has been reported previously22,23 and may be a confounding factor when assessing neurocognitive function in patients with dyslipidaemia. However, in this analysis, there were no significant differences in neurocognitive TEAE rates in those above or below median HDL-C levels across all treatment groups and both genders.
Factors such as coronary heart disease and age are independent risk factors for Alzheimer’s disease and many other age-related conditions associated with cognitive decline.30 This makes analysis of the relationship between PCSK9 inhibitors, LDL-C levels, and cognitive decline a complex one since the population treated with these drugs is typically older, often with comorbidities and concomitant medication use. In this analysis, there was no significant effect of medications with potential neurocognitive effects on the rate of neurocognitive TEAEs. Furthermore, there was no difference in neurocognitive TEAEs between alirocumab and control groups when stratified by age (<65, ≥65 to <75, and ≥75 years), although the incidence of neurocognitive TEAEs was overall higher in patients (both alirocumab- and control-treated) who were aged ≥75 years.
Limitations
Although an association between alirocumab treatment and neurocognitive TEAEs was not found in these relatively short-term Phase 2 and 3 trials (longest follow-up was 2 years), the long-term effects of very low levels of LDL-C induced by PCSK9 inhibitors are unknown. Also, it is acknowledged that the overall number of neurocognitive events observed in alirocumab trials to date is too small to draw definitive conclusions. Due to the small number of neurocognitive events and the relatively small number of patients in this study, the CIs for the HRs are wide and do not exclude HRs >2. A study of a greater dimension in terms of number of events and patients would permit more precise estimations of the HRs of neurocognitive events in alirocumab vs. control. Moreover, the statistical analysis did not explicitly take into consideration the competing risk of death. However, the incidence of death was low and similar between alirocumab and control arms (alirocumab vs. placebo: 15 (0.6%) vs. 9 (0.7%), alirocumab vs. ezetimibe: 6 (0.7%) vs. 6 (1.0%); number and percentage of patients who died prior to any neurocognitive event as per sponsor CMQ; results were similar with the FDA CMQ). The effect of alirocumab on neurocognitive events will require further investigation in the ongoing large cardiovascular outcomes trial of alirocumab (ODYSSEY OUTCOMES; NCT01663402). Results from a recent outcomes trial of a different PCSK9 inhibitor (>27 000 patients) showed no significant difference between evolocumab and placebo in terms of physician-reported neurocognitive events, although mean follow-up was limited (2.2 years).31
No dedicated tool was used to assess neurocognitive TEAEs; all were patient reported. However, although formal neuropsychological testing with well-validated tests have been shown to detect small differenes in performance on a group-mean basis, in some instances, only fairly large changes in cognitive performance on the part of individual changes can be reliably detected. For example, Woods et al.32 found that a minimum of 50% change in recall memory performance was required to meet the criteria for being a reliable change in an individual patient. Such a large change on the part of an individual seems unlikely to go undetected by clinical observation and would likely be noticed and self-reported by patients as in the current analysis.
No specific survey was used for assessment and severity was not assessed using a validated scale. The study designs did not capture or screen for neurocognitive deficits at entry; neurocognitive decline was not assessed during the studies and the use of concomitant medications that could influence neurocognitive state was not a prespecified analysis in any of the studies.
In ODYSSEY OUTCOMES, a Neurocognitive Expert Panel Charter has been established to review in a blinded manner all neurocognitive TEAEs and to explore possible aetiology. Furthermore, a dedicated study of neurocognitive events using formal testing and comparing alirocumab with placebo over 2 years is now under way (clinicaltrials.gov identifier: NCT02957682). In a substudy of the evolocumab outcomes trial (NCT02207634), which utilized tests of neurocognitive function, as well as a patient questionnaire, no difference was found between evolocumab or placebo in neurocognitive tests.27
Self-report in the controlled context of a clinical trial can also lead to a bias towards over-detection, with events reported which might not be noted as a problem or reported during real-world clinical use. However, randomization should reduce problems with this type of bias.
Ultimately, any potential risk of neurocognitive TEAEs associated with PCSK9 inhibition must be weighed against the potential benefits of therapy, including decreased risk of major adverse cardiovascular events.21
Conclusion
Overall, this analysis of 4 Phase 2 and 10 Phase 3 trials of alirocumab found low rates of neurocognitive TEAEs distributed evenly across the alirocumab, placebo, and ezetimibe groups. There was no association found between neurocognitive TEAEs and LDL-C levels <25 mg/dL, reductions in LDL-C, or HDL-C levels. However, further monitoring in long-term outcomes studies is needed, with specific assessment of any effect on cognition over a longer period and consideration given to potential contributing factors such as concomitant medication and comorbid conditions. A further study is now under way to specifically evaluate neurocognitive function and neurocognitive TEAEs occurring with longer term exposure to alirocumab, using formal testing.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
The authors would like the following people from the sponsors for providing critical review: Sanofi: Corinne Hanotin, MD, Michael Howard, MBA, and L. Veronica Lee, MD; Regeneron: Robert Pordy, MD, Carol Hudson, BPharm, and Eva-Lynne Greene, MA. Medical writing support was provided by Eloise Aston, MSc, and Rob Campbell, PhD, of Prime, Knutsford, Cheshire, UK, funded by Sanofi and Regeneron. Responsibility for all opinions, conclusions, and interpretation of data lies with the authors. No author was paid for services involved in writing this manuscript.
Funding
This analysis was funded by Sanofi and Regeneron Pharmaceuticals, Inc.
Conflict of interest: P.D.H. has received personal fees for consultancy from Sanofi and Regeneron Pharmaceuticals, Inc., for review of adverse event data monitoring as presented in this paper. M.N.S. has received research grant support from AstraZeneca, Avid Pharmaceuticals, Axovant, Genentech, Lilly Pharmaceuticals, Merck & Co, Pfizer, Piramal Imaging, Roche Diagnostics Corporation, and vTv Therapeutics. He has been a consultant/on advisory boards for Axovant, Biogen, FORUM Pharmaceuticals, Fujirebio Diagnostics, Humana, Lilly Pharmaceuticals, and Sanofi. He is a shareholder in Brain Health, Muses Labs and Versanum. J.E.H. has received consultancy fees from Abbvie, Amgen, Anavex, AstraZeneca, Avonex, Avraham, Axon Neuroscience, Axovant, Biogen Idec, Boehringer Ingelheim, Bracket, Catenion, CRF Health, DeNDRoN, Eisai, Eli Lilly, EnVivo Pharma, Enzymotec, ePharmaSolutions, Forum Pharma, GfHEu, Heptares, Janssen AI, Johnson & Johnson, Kaasa Health, Kyowa Hakko Kirin, MedAvante, Merck, Mind Agilis, MyCognition, Neurim, Neurocog, Neurotrack, Novartis, Nutricia, Orion Pharma, Pharmanet/i3, Pfizer, Prana Biotech, PriceSpective, Probiodrug, Prophase, Prostrakan, Regeneron, Reviva, Roche, Sanofi, Servier, Takeda, TransTech Pharma & Velacor. He has options in Neurotrack Inc., holds patents with MyCognition, and has received honoraria for CME activities from Lundbeck. H.N.G. has received research support and consultancy fees from Sanofi, Regeneron Pharmaceuticals, Inc., Merck, and Amgen. M.J.C. has received research grant support from CSL, Kowa and Pfizer. He is a member of the Speakers’ Bureau of Amgen, Kowa, MSD, Regeneron and Sanofi and has received honoraria from Amgen, MSD, Regeneron, Pfizer, and Sanofi. G.M. is an employee and a stockholder of Regeneron Pharmaceuticals Inc. A.M. is an employee and stockholder of Sanofi. J.M. is a consultant for Sanofi mandated by IviData Stats. M.F. has received research support from and/or participated in a speakers’ bureau for Amgen, Merck, Mylan, Pfizer, Regeneron, and Sanofi and acted as a consultant/advisory board member for Akcea/Ionis, Amgen, AstraZeneca, Eli Lilly, Kowa, Merck, Pfizer, Regeneron, and Sanofi.
References
- 1. US Food and Drug Administration. FDA Drug Safety Communication: important safety label changes to cholesterol-lowering statin drugs. http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm (6 November 2017).
- 2. Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, Blumenthal R, Danesh J, Smith GD, DeMets D, Evans S, Law M, MacMahon S, Martin S, Neal B, Poulter N, Preiss D, Ridker P, Roberts I, Rodgers A, Sandercock P, Schulz K, Sever P, Simes J, Smeeth L, Wald N, Yusuf S, Peto R.. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016;388:2532–2561. [DOI] [PubMed] [Google Scholar]
- 3. Everett BM, Mora S, Glynn RJ, MacFadyen J, Ridker PM.. Safety profile of subjects treated to very low low-density lipoprotein cholesterol levels (<30 mg/dl) with rosuvastatin 20 mg daily (from JUPITER). Am J Cardiol 2014;114:1682–1689. [DOI] [PubMed] [Google Scholar]
- 4. Ott BR, Daiello LA, Dahabreh IJ, Springate BA, Bixby K, Murali M, Trikalinos TA.. Do statins impair cognition? A systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med 2015;30:348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Richardson K, Schoen M, French B, Umscheid CA, Mitchell MD, Arnold SE, Heidenreich PA, Rader DJ, deGoma EM.. Statins and cognitive function: a systematic review. Ann Intern Med 2013;159:688–697. [DOI] [PubMed] [Google Scholar]
- 6. Shepherd J, Blauw GJ, Murphy MB, Bollen ELEM, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Meinders AE, Norrie J, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey G, Westendorp RGJ.. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002;360:1623–1630. [DOI] [PubMed] [Google Scholar]
- 7. Swiger KJ, Manalac RJ, Blumenthal RS, Blaha MJ, Martin SS.. Statins and cognition: a systematic review and meta-analysis of short- and long-term cognitive effects. Mayo Clin Proc 2013;88:1213–1221. [DOI] [PubMed] [Google Scholar]
- 8. Bays H, Gaudet D, Weiss R, Ruiz JL, Watts GF, Gouni-Berthold I, Robinson J, Zhao J, Hanotin C, Donahue S.. Alirocumab as add-on to atorvastatin versus other lipid treatment strategies: ODYSSEY OPTIONS I randomized trial. J Clin Endocrinol Metab 2015;100:3140–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cannon CP, Cariou B, Blom D, McKenney JM, Lorenzato C, Pordy R, Chaudhari U, Colhoun HM.. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur Heart J 2015;36:1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Farnier M, Jones P, Severance R, Averna M, Steinhagen-Thiessen E, Colhoun HM, Du Y, Hanotin C, Donahue S.. Efficacy and safety of adding alirocumab to rosuvastatin versus adding ezetimibe or doubling the rosuvastatin dose in high cardiovascular-risk patients: the ODYSSEY OPTIONS II randomized trial. Atherosclerosis 2016;244:138–146. [DOI] [PubMed] [Google Scholar]
- 11. Ginsberg HN, Rader DJ, Raal FJ, Guyton JR, Baccara-Dinet MT, Lorenzato C, Pordy R, Stroes E.. Efficacy and safety of alirocumab in patients with heterozygous familial hypercholesterolemia and LDL-C of 160 mg/dl or higher. Cardiovasc Drugs Ther 2016;30:473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kastelein JJ, Ginsberg HN, Langslet G, Hovingh GK, Ceska R, Dufour R, Blom D, Civeira F, Krempf M, Lorenzato C, Zhao J, Pordy R, Baccara-Dinet MT, Gipe DA, Geiger MJ, Farnier M.. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J 2015;36:2996–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kereiakes DJ, Robinson JG, Cannon CP, Lorenzato C, Pordy R, Chaudhari U, Colhoun HM.. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: the ODYSSEY COMBO I study. Am Heart J 2015;169:906–915.e913. [DOI] [PubMed] [Google Scholar]
- 14. McKenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand AC, Stein EA.. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol 2012;59:2344–2353. [DOI] [PubMed] [Google Scholar]
- 15. Moriarty PM, Thompson PD, Cannon CP, Guyton JR, Bergeron J, Zieve FJ, Bruckert E, Jacobson TA, Kopecky SL, Baccara-Dinet MT, Du Y, Pordy R, Gipe DA.. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol 2015;9:758–769. [DOI] [PubMed] [Google Scholar]
- 16. Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ.. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1489–1499. [DOI] [PubMed] [Google Scholar]
- 17. Roth EM, McKenney JM, Hanotin C, Asset G, Stein EA.. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med 2012;367:1891–1900. [DOI] [PubMed] [Google Scholar]
- 18. Roth EM, Taskinen MR, Ginsberg HN, Kastelein JJ, Colhoun HM, Robinson JG, Merlet L, Pordy R, Baccara-Dinet MT.. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: results of a 24 week, double-blind, randomized Phase 3 trial. Int J Cardiol 2014;176:55–61. [DOI] [PubMed] [Google Scholar]
- 19. Stein EA, Gipe D, Bergeron J, Gaudet D, Weiss R, Dufour R, Wu R, Pordy R.. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet 2012;380:29–36. [DOI] [PubMed] [Google Scholar]
- 20. Teramoto T, Kobayashi M, Uno K, Takagi Y, Matsuoka O, Sugimoto M, Inoue S, Minami F, Baccara-Dinet MT.. Efficacy and safety of alirocumab in Japanese subjects (Phase 1 and 2 Studies). Am J Cardiol 2016;118:56–63. [DOI] [PubMed] [Google Scholar]
- 21. Swiger KJ, Martin SS.. PCSK9 inhibitors and neurocognitive adverse events: exploring the FDA directive and a proposal for N-of-1 trials. Drug Saf 2015;38:519–526. [DOI] [PubMed] [Google Scholar]
- 22. Singh-Manoux A, Gimeno D, Kivimaki M, Brunner E, Marmot MG.. Low HDL cholesterol is a risk factor for deficit and decline in memory in midlife: the Whitehall II study. Arterioscler Thromb Vasc Biol 2008;28:1556–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kontush A, Chapman MJ.. HDL: close to our memories? Arterioscler Thromb Vasc Biol 2008;28:1418–1420. [DOI] [PubMed] [Google Scholar]
- 24. Khan AR, Bavishi C, Riaz H, Farid TA, Khan S, Atlas M, Hirsch G, Ikram S, Bolli R.. Increased risk of adverse neurocognitive outcomes with proprotein convertase subtilisin-kexin type 9 inhibitors. Circ Cardiovasc Qual Outcomes 2017;10:e003153. [DOI] [PubMed] [Google Scholar]
- 25. Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, Ballantyne CM, Somaratne R, Legg J, Wasserman SM, Scott R, Koren MJ, Stein EA.. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1500–1509. [DOI] [PubMed] [Google Scholar]
- 26. Lipinski MJ, Benedetto U, Escarcega RO, Biondi-Zoccai G, Lhermusier T, Baker NC, Torguson R, Brewer HB Jr, Waksman R.. The impact of proprotein convertase subtilisin-kexin type 9 serine protease inhibitors on lipid levels and outcomes in patients with primary hypercholesterolaemia: a network meta-analysis. Eur Heart J 2016;37:536–545. [DOI] [PubMed] [Google Scholar]
- 27. Giugliano RP, Mach F, Zavitz K, Kurtz C, Im K, Kanevsky E, Schneider J, Wang H, Keech AC, Pedersen TR, Sabatine MS, Sever P, Robinson JG, Honarpour N, Wasserman SM, Ott BR.. Cognitive function in a randomized trial of evolocumab. N Engl J Med 2017;377:633–643. [DOI] [PubMed] [Google Scholar]
- 28. Giugliano RP, Wiviott SD, Blazing MA, De Ferrari GM, Park JG, Murphy SA, White JA, Tershakovec AM, Cannon CP, Braunwald E.. Long-term safety and efficacy of achieving very low levels of low-density lipoprotein cholesterol: a prespecified analysis of the IMPROVE-IT trial. JAMA Cardiol 2017;2:547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Postmus I, Trompet S, de Craen AJ, Buckley BM, Ford I, Stott DJ, Sattar N, Slagboom PE, Westendorp RG, Jukema JW.. PCSK9 SNP rs11591147 is associated with low cholesterol levels but not with cognitive performance or noncardiovascular clinical events in an elderly population. J Lipid Res 2013;54:561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A.. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ 2001;322:1447–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR.. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 32. Woods SP, Delis DC, Scott JC, Kramer JH, Holdnack JA.. The California Verbal Learning Test-second edition: test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Arch Clin Neuropsychol 2006;21:413–420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.