Abstract
Background
Treatment response in lupus nephritis (LN) is defined clinically, without consideration of renal histology. Few studies have systematically examined histologic responses to induction therapy. In LN patients who underwent protocol kidney biopsies after induction immunosuppression, we describe the renal histology of the second biopsy and correlate histologic activity and damage with short- and long-term kidney outcomes.
Methods
Patients with suspected LN were biopsied for diagnosis (Biopsy 1), and those with proliferative LN were rebiopsied after induction (Biopsy 2). Histologic activity and damage at each biopsy were calculated as the National Institutes of Health activity and chronicity indices. Complete and partial renal responses after induction and after long-term follow-up were determined clinically.
Results
One-third of patients who achieved a complete clinical response after induction had persistently high histologic activity, and 62% of patients who had complete histologic remission on rebiopsy were still clinically active. Chronic renal damage increased after induction even in complete clinical responders. Chronicity at Biopsy 2 associated with long-term kidney function and development of chronic kidney disease.
Conclusions
Early clinical and histologic outcomes are discordant in proliferative LN, and neither correlates with long-term renal outcome. The kidney accrues chronic damage rapidly and despite clinical response in LN. Preservation of kidney function may require therapeutic targeting of both chronic damage and inflammation during LN induction treatment.
Keywords: activity index, chronicity index, kidney biopsy, lupus nephritis, outcomes
INTRODUCTION
Presently, the response of lupus nephritis (LN) to treatment is assessed according to specific clinical metrics. Although there are several definitions of complete and partial renal remission in current use, all rely mainly on an improvement in proteinuria, an improvement, stabilization or limited worsening of kidney function, and variably on the resolution of hematuria and the ability to taper corticosteroids below a target level [1]. Renal response definitions do not routinely include a kidney histology component despite a growing body of evidence suggesting discordance between clinical findings and disease activity at the tissue level (reviewed in ref. [2]).
A series of reports from Hill et al. [3–6] demonstrated that the initial kidney biopsy for the diagnosis of LN provided few clues to long-term kidney outcomes. However, a biopsy done 6 months after beginning induction therapy yielded prognostic information. In particular, doubling of serum creatinine concentration (SCr) was predicted by the persistence of glomerular and interstitial inflammation, glomerular capillary immune complexes and intratubular macrophages at the second biopsy, whereas histologic findings of chronic kidney damage, such as tubular atrophy and interstitial fibrosis, were not predictive. These reports suggested that routinely repeating a kidney biopsy after induction therapy could be helpful in deciding the next steps for treatment.
Few subsequent studies described postinduction repeat kidney biopsies for LN treatment [4, 7–13]. The bulk of repeat biopsy investigations described cohorts in which a repeat biopsy was done one or more years after the initial diagnostic biopsy, and usually for cause, such as deteriorating kidney function, worsening of proteinuria, suspicion of a renal flare or suspicion of an LN class change, as opposed to per protocol (reviewed in ref. [2]).
Since 2003, it has been our (A.M. and B.L.) standard practice to rebiopsy LN patients at the end of induction therapy, generally 6 months after the initial diagnostic biopsy and initiation of treatment. Here, we compare the histologic and clinical responses of proliferative LN to standard-of-care induction therapies.
MATERIALS AND METHODS
Patient cohort
Consecutive patients (n = 69) were seen in the nephrology clinic at a single academic center in Buenos Aires, Argentina, because they were suspected of having their first episode of LN. All had been previously diagnosed with systemic lupus erythematosus (SLE), based on having at least four American College of Rheumatology lupus criteria. Patients underwent an initial kidney biopsy (Biopsy 1) for diagnosis. After induction therapy was completed, generally by 6 months, patients who had Class III or IV LN underwent a repeat kidney biopsy per local clinical protocol (Biopsy 2).
Kidney biopsy
Kidney biopsies were processed for light, immunofluorescence and electron microscopy using standard techniques. All initial and repeat biopsies were read by a single nephropathologist (V.A.) who was blinded to the patient's clinical data. The National Institutes of Health (NIH) activity index (AI) and chronicity index (CI) were calculated for each biopsy [14].
Clinical management
All patients were induced with high-dose corticosteroids and either monthly intravenous cyclophosphamide (1 g) or mycophenolate mofetil (MMF) (2–3 g/day) for 6 months. Corticosteroids (prednisone) were initiated at 1 mg/kg/day for 15 days and then decreased every 15 days to reach a target of 10 mg/day. If patients were very active, they were given 500 mg intravenous methylprednisolone daily for 3 days. Additionally, all patients were on either an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker. Antimalarials were used in 70% of patients.
The duration of induction therapy was 6 months. Patients who improved clinically and whose repeat biopsies showed improvement continued 10 mg/day prednisone for 6 months with subsequent decreases to 5 then 2.5 mg/day as tolerated. Maintenance immunosuppression was MMF (1.5–2 g/day) or azathioprine (2 mg/kg/day). In those patients whose repeat biopsies showed persistently high levels of activity, who still had nephrotic-range proteinuria or whose renal function (further) declined, maintenance therapy was put off for 6 months and another course of induction was given. In these cases, patient who had received cyclophosphamide for induction were switched to MMF, and vice versa.
Clinical follow-up included SCr, 24-h proteinuria, complement levels, autoantibody levels, blood counts and other laboratories as indicated. For the purposes of this study, patients were stratified into complete clinical renal responders (CRRs) and partial renal responders plus non-responders (PRR/NRR) at the time of Biopsy 2 (after completion of 6 months of induction). CRR was defined as 24-h proteinuria <500 mg/day and improvement or maintenance of SCr. A PRR was defined as a decrease in proteinuria by 50% and to between 500 and 3000 mg/day, and a SCr within 15% of baseline. Patients who did not meet CRR or PRR criteria were considered clinical NRR.
Ethics
This study was approved by the ethics committee of Hospital Fernandez. Biopsy consent was taken by the internist supervising the ultrasound suite, not the investigators. The Ohio State University Office of Responsible Research Practices determined that this study did not engage Ohio State in human subjects’ research activities requiring review. All investigators adhered to the ethical principles contained in the Belmont Report.
Statistical analyses
Data are presented as mean ± SD of the mean or median and range unless otherwise indicated. Groups were compared by the paired or independent sample Student's t-test as appropriate. If the data were not normally distributed, groups were compared by the Wilcoxon matched-pairs signed rank test or the Mann–Whitney test as appropriate. Proportions were analyzed by Fisher's exact test. Segmented regression lines with a breakpoint at a CI of 4 were used to describe the linear association between high CI values at Biopsy 2 and the natural logarithm of SCr at follow-up. A P-value of <0.05 was considered significant.
RESULTS
Demographics and overall short-term responses
Our proliferative LN cohort was 87% female with a mean age of 30 ± 8 years. All patients were Caucasian and Hispanic. MMF was used for induction therapy in 53.6% of the patients and cyclophosphamide was used in 46.4%. Classes III and IV LN were diagnosed in 29 and 71% of the initial biopsies, respectively. The lesions were distributed as follows: Class III A, n = 3; Class III A/C, n = 17; Class IVG A, n = 10; Class IVG A/C, n = 35; Class IVS, n = 4. None of these patients had a Class V component, thrombotic microangiopathy or lupus podocytopathy. The mean time between Biopsy 1 and Biopsy 2 was 6.6 ± 0.7 months. The small variation in time between finishing induction and rebiopsy was due to scheduling of the second procedure. Most of the patients (84%) were biopsied between Months 6 and 7 post Biopsy 1. Clinical and histologic data at Biopsies 1 and 2 are shown in Table 1. Although this was an initial biopsy cohort, the patients already had chronic kidney damage. For comparison, we calculated the CI of an independent initial LN biopsy cohort from Ohio. The CI for Caucasian patients was 2.4 ± 1.5 (n = 23) and 2.9 ± 3.1 (n = 28) for African American patients.
Table 1.
Clinical and histologic data: overall cohort
| Biopsy 1 | Biopsy 2 | P (Biopsy 1 versus Biopsy 2) | |
|---|---|---|---|
| Proteinuria (g/day) | 2.9 ± 2.1 | 1.1 ± 1.3 | <0.0001 |
| Serum creatinine (mg/dL) | 1.0 ± 0.7 | 0.85 ± 0.30 | 0.0008 |
| AI | 8.5 ± 3.1 | 3.5 ± 2.4 | <0.0001 |
| CI | 2.6 ± 1.7 | 4.0 ± 1.5 | <0.0001 |
In general, the patients did well with induction therapy. Proteinuria and SCr improved, and the NIH AI of the biopsies declined significantly with induction treatment. There was no significant extra-renal SLE activity after induction. However, despite these improvements, the kidneys sustained chronic damage as indicated by the significant increase in the NIH CI on Biopsy 2.
Seven patients had persistent cellular crescents and subendothelial immune deposits and/or glomerular capillary necrosis at repeat biopsy. Two of these patients had achieved a complete clinical renal response (CRR), one patient had a partial renal response (PRR) and four had no renal response (NRR). These patients were given another 6 months of MMF (3 g/day), before being placed on maintenance immunosuppression.
Comparison of clinical and histologic responses
Patients were stratified by clinical response status at Biopsy 2 (Table 2). The median time to complete response for the CRR group was 4.3 months (range: 2–6 months). Kidney function, assessed by SCr, was similar at presentation in the CRR and PRR/NRR groups. Patients who achieved a CRR had significantly less proteinuria at Biopsy 1 than PRR/NRR. Complement components C3 and C4, anti-double-stranded DNA (anti-dsDNA) antibody titers and urine sediment findings were not different between CRR and PRR/NRR at Biopsy 1. Histologic activity was higher in PRR/NRR than CRR, but histologic chronicity and the type of induction immunosuppression used were not.
Table 2.
Clinical and histologic data as a function of response status after induction
| CRR (n = 28) | PRR/NRR (n = 41) | P (CRR versus PRR/NRR) | |
|---|---|---|---|
| Class 4 LN at Biopsy 1 | 71.4% | 70.7% | NS |
| Proteinuria (g/day), Biopsy 1 | 2.2 ± 1.4 | 3.5 ± 2.3 | 0.018 |
| Proteinuria (g/day), Biopsy 2 | 0.18 ± 0.16 | 1.6 ± 1.4 | <0.0001 |
| P (Biopsy 1 versus Biopsy 2) | <0.0001 | <0.0001 | |
| Serum creatinine (mg/dL), Biopsy 1 | 0.87 ± 0.17 | 1.12 ± 0.82 | NS |
| Serum creatinine (mg/dL), Biopsy 2 | 0.75 ± 0.13 | 0.92 ± 0.36 | 0.032 |
| P (Biopsy 1 versus Biopsy 2) | 0.011 | NS | |
| Complement C3 (mg/dL)a, Biopsy 1 | 60.3 ± 25.2 | 64.5 ± 30.2 | NS |
| Complement C3 (mg/dL), Biopsy 2 | 83.6 ± 31.3 | 90.2 ± 23.2 | NS |
| P (Biopsy 1 versus Biopsy 2) | 0.0039 | <0.0001 | |
| Complement C4 (mg/dL)b, Biopsy 1 | 8.4 ± 4.1 | 11.5 ± 7.1 | NS |
| Complement C4 (mg/dL), Biopsy 2 | 13.8 ± 6.0 | 16.7 ± 7.6 | NS |
| P (Biopsy 1 versus Biopsy 2) | 0.0004 | 0.003 | |
| Anti-dsDNA titer, Biopsy 1 | 462 ± 513 | 342 ± 481 | NS |
| Anti-dsDNA titer, Biopsy 2 | 246 ± 382 | 114 ± 263 | NS |
| P (Biopsy 1 versus Biopsy 2) | NS | 0.039 | |
| Active urine sedimentc, Biopsy 1 | 85.7% | 80.5% | NS |
| Active urine sediment, Biopsy 2 | 21.4% | 41.5% | NS |
| P (Biopsy 1 versus Biopsy 2) | <0.0001 | 0.0006 | |
| AI, Biopsy 1 | 7.4 ± 2.9 | 9.2 ± 3.0 | 0.031 |
| AI, Biopsy 2 | 3.3 ± 2.1 | 3.7 ± 2.6 | NS |
| P (Biopsy 1 versus Biopsy 2) | <0.0001 | <0.0001 | |
| CI, Biopsy 1 | 2.6 ± 1.7 | 2.6 ± 1.7 | NS |
| CI, Biopsy 2 | 3.7 ± 1.2 | 4.2 ± 1.6 | NS |
| P (Biopsy 1 versus Biopsy 2) | 0.011 | <0.0001 | |
| MMF (%) | 60.7 | 48.8 | NS |
| Cyclophosphamide (%) | 39.3 | 51.2 | NS |
NS, not significant.
aNormal range: 70–140 mg/dL.
bNormal range: 10–30 md/dL.
cUrine sediment was considered active if there were more than five red blood cells/high power field (hematuria must be attributable to LN and not other causes such as menses), with or without the presence of red blood cell casts or white blood cell casts (in the absence of infection). To be considered inactive, urine sediment could have no red or white blood cells casts and fewer than five red blood cells/high power field.
After induction treatment, proteinuria, urine sediment activity and complement levels improved significantly in both CRR and PRR/NRR patients. SCr and anti-dsDNA also decreased in both groups but reached significance only in CRR and PRR/NRR, respectively. The AI improved and the CI worsened at Biopsy 2 in both response groups, and the changes were similar in CRR and PRR/NRR. After induction, there were no differences in complement levels, anti-dsDNA titers or urine sediment activity between complete and incomplete clinical responders.
The AI of each patient who achieved a CRR was examined at Biopsy 2. The AI was 3 or less in 50% of the CRR patients and was 5 or higher in 29% (Figure 1). Similarly, the AI was 5 or higher in 27% of the PRR/NRR group (Table 3). There were no significant differences in serum complement levels, anti-dsDNA titers or urine sediment activity at Biopsy 1 or Biopsy 2 between CRR patients who had an AI ≤ 3 and CRR patients who had an AI ≥ 4 at Biopsy 2 (Supplementary Table 1). Numerically, more patients with an AI > 3 on Biopsy 2 still had urine red cells than patients with an AI ≤ 3 (4 and 2, respectively), but interestingly, this did not correlate with the level of activity; those patients with an AI of 6 and 8 had inactive urine sediment.
FIGURE 1.
Persistent histologic disease activity in the kidneys despite complete clinical renal remission. The AI of kidney biopsies done at completion of LN induction therapy in CRR is shown. Each patient is represented by a circle.
Table 3.
Distribution of the Components of the AI at Biopsy 2
| Clinical response at Biopsy 2 | AI2 | n | Endocapillary proliferation | Subendothelial deposits | Crescents | Necrosis | Interstitial infiltrates |
|---|---|---|---|---|---|---|---|
| CRR | ≥6 | 2 | 2a | 1 | 1 | 0 | 2 |
| CRR | 5 | 6 | 6 | 5 | 3 | 0 | 3 |
| CRR | 4 | 6 | 6 | 6 | 0 | 0 | 4 |
| CRR | ≤3 | 14 | 9 | 5 | 0 | 0 | 4 |
| CRR | ≥5 | 8 | 8 | 6 | 4 | 0 | 5 |
| PRR/NRR | ≥5 | 11 | 11 | 11 | 4 | 2 | 7 |
aThe number of patients in the AI group who have this histologic feature.
The histologic components of the AI at Biopsy 2 were examined in each patient (Table 3). The most common types of persistent histologic activity seen in biopsies of patients who achieved a CRR were endocapillary proliferation, subendothelial immune complex deposits and interstitial inflammation. Fewer patients had ongoing cellular crescents and no biopsy showed necrosis. The distribution of specific active histologic lesions was similar in the PRR/NRR patients (Table 3).
An AI of 0 at Biopsy 2 was considered to represent complete histologic remission. Patients who reached complete histologic remission were stratified into those having proteinuria <500 mg/day and those with proteinuria ≥500 mg/day (Figure 2). Of the 13 patients who had an AI of 0 at Biopsy 2, 62% had 24-h protein excretion of >500 mg/day. These patients had a median 24-h proteinuria of 1720 mg/day (range: 750–3000 mg/day). Conversely, one of the five patients with histologic remission and low-level proteinuria had 400 mg/day, while all the others had <100 mg/day proteinuria. Interestingly, complement C3 and C4 levels at Biopsies 1 and 2 were significantly higher in CRR who achieved a complete histologic remission at Biopsy 2 than CRR who still had activity on the second biopsy (Supplementary Table 1), although the increases in C3 and C4 levels over time were not different between these groups. This finding may be due to the small number of patients with CRR and complete histologic remission, but the same pattern of complement levels was observed if all 13 patients who had a complete histologic remission (regardless of clinical remission) at Biopsy 2 were compared with the rest of this cohort (data not shown). Although not statistically significant, none of the CRR patients with compete histologic remission had an active urine sediment at Biopsy 2, as opposed to six CRR patients who did not have histologic remission.
FIGURE 2.
Persistent clinical disease activity despite complete histologic renal remission. Patients whose biopsies had no disease activity (AI of 0) after completion of LN induction therapy were segregated by their levels of 24-h proteinuria. Each patient is represented by a circle.
Despite rapidly achieving clinical remission, the CI of the CRR group increased ∼1.4-fold, which was not different than the 1.6-fold increase in the PRR/NRR group. The change in CI between Biopsy 1 and Biopsy 2 for each CRR patient is shown in Figure 3. There was a wide distribution of CI scores at Biopsy 1, even though these biopsies were done at the first presentation of LN. Increases in chronicity between Biopsies 1 and 2 were not confined to patients who already had moderate to high CI, and patients who had no-low chronicity on their first biopsy appeared to have the largest increases in chronic renal damage.
FIGURE 3.
Change in renal CI between Biopsy 1 and Biopsy 2. The change in CI from Biopsy 1 (BX1, at diagnosis) to Biopsy 2 (BX2, after finishing induction treatment) for each patient who achieved a complete clinical renal remission is shown. Patients are grouped by the CI at Biopsy 1.
Long-term follow-up
Long-term follow-up data were available for 87% of this cohort. Nine patients were lost to follow-up after Biopsy 2. The median duration of observation from Biopsy 1 through December 2014 for the other 61 patients was 73 months (range: 24–144 months). One patient died at Month 24 due to complications after surgery. Three patients developed end-stage kidney disease requiring hemodialysis after severe renal flares. These flares occurred 38, 50 and 54 months after Biopsy 1, and all were in patients who had achieved a clinical CRR by Biopsy 2. Overall, six patients in the cohort had LN flares and at rebiopsy, five patients had Class IVG A/C and one patient had Class III A/C. At last follow-up, 19% of the CRR and 18.4% of the PRR/NRR patients had SCr > 1.0 mg/dL and were considered to have developed chronic kidney disease (CKD). Of the patients in the CRR group who were not lost to follow-up, did not flare and did not develop CKD, all were in complete renal remission at last follow-up. Of the patients in the PRR/NRR group who were not lost to follow-up, did not flare and did not develop CKD, 24 were considered to be in complete renal remission at last follow-up and 6 remained in partial renal remission. The median time to CRR for patients who achieved PRR/NRR after induction was 10 months (range: 7–16 months).
The seven patients who had an extended induction period based on Biopsy 2 activity were followed for 76 (29–144) months. At the end of follow-up, four patients had CRR, three had PRR, none had CKD and the residual proteinuria in the PRR patients was 700–800 mg/day.
With respect to the 13 patients who had histologic complete remission on Biopsy 2, one experienced a severe renal flare leading to end-stage kidney disease and one was lost to follow-up. The remaining 11 patients were followed for 82 (26–134) months. Seven of these patients had a SCr of 1 mg/dL or less after long-term follow-up, while four had a SCr of 1.3–2.9 mg/dL. The CI of Biopsy 2 was 6 in three of the patients who developed CKD and 4 in one patient.
Correlation between histology at repeat biopsy and long-term kidney function
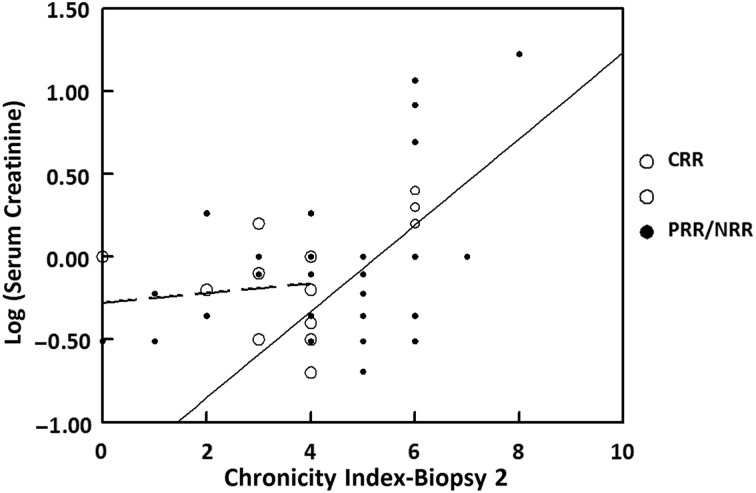
There was no relationship between AI at Biopsy 2 and long-term kidney outcomes. However, a relationship between the CI at Biopsy 2 and CKD at follow-up was observed. The median CI at Biopsy 2 of the patients who developed CKD was 6 (range: 2–8) compared with a median CI of 4 (range: 0–7) in patients who did not develop CKD (P = 0.019). Segmented regression analysis (Figure 4) showed a significant linear correlation between CI ≥ 4 at Biopsy 2 and the natural logarithm of SCr [log(SCr)] at long-term follow-up [model equation: log(last Scr) = −1.373 + 0.261 × (CI at Biopsy 2); n = 39, R2 = 0.36, P < 0.0001]. There was no relationship between CI at Biopsy 2 and SCr if the CI was <4 (R2 = 0.02, P = 0.66). The segmented regression equation for CI ≥ 4 at Biopsy 2 suggests that for every 1 unit increase in CI at Biopsy 2 in the range of 4–8, there is an increase of 30% in the last SCr.
FIGURE 4.
Serum creatinine as a function of renal CI on biopsies taken after completion of induction therapy. Each circle represents an individual patient. Patients who achieved a complete renal response after induction therapy (CRR) are represented by open circles; patients who did not achieve a complete renal response after induction therapy (PRR/NRR) are represented by closed circles. The segmented linear regression lines are for CI <4 (broken line) and CI ≥4 (solid line). The regression equation for CI <4 is log(SCr) = −0.278 + 0.029 × CI2 (r = 0.12, P = 0.66). The equation for CI ≥4 is log(SCr) = −1.37 + 0.26 × CI2 (r = 0.6, P < 0.0001).
To determine if the CI at Biopsy 2 added information about long-term kidney function beyond that available from clinical variables alone, a multivariate linear regression analysis was carried out with log (last SCr) as the response, and proteinuria, CI and log(SCr) at Biopsy 2 as predictors. Proteinuria at Biopsy 2 was not a significant predictor of the last SCr, but log(SCr) at Biopsy 2 did correlate significantly with log(last SCr) (R2 = 0.35; P < 0.0001). For patients with a CI ≥ 4 at Biopsy 2, both log(SCr) and CI at Biopsy 2 were significant independent predictors (P = 0.005 for each) of log(last SCr). The regression model equation was log(last SCr) = 0.613 log(SCr at Biopsy 2) + 0.179 (CI at Biopsy 2) − 0.875 (R2 = 0.48, P < 0.0001). For a given level of SCr at Biopsy 2, a 1-unit increase of CI in the range of 4–8 at Biopsy 2 corresponded to a 20% increase in last SCr.
DISCUSSION
This investigation compared the early histologic and clinical responses of the kidney in a cohort of SLE patients being treated for their first episode of proliferative LN with standard-of-care immunosuppression. The results show that after induction therapy, histologic and clinical responses are discordant. Furthermore, despite rapid and complete clinical responses to what are currently considered the most effective therapies for LN, many patients still developed chronic kidney damage. Finally, neither the clinical or histologic response status immediately after finishing induction therapy appeared to correlate with long-term kidney outcomes.
Nearly one-third of patients who achieved a clinical CRR after induction treatment still had high histologic activity (AI ≥ 5) at repeat biopsy. However, none of these patients developed CKD during long-term follow-up. The four CRR patients who did develop CKD had AIs of 0–4 at Biopsy 2. Importantly perhaps, three of these four patients had a CI of 6 on Biopsy 2. The inability of the AI from a postinduction kidney biopsy to predict long-term kidney outcome in LN is consistent with previous studies [7] and was the impetus for an alternative morphologic biopsy score (the Hill score) designed to increase the predictive value of the repeat biopsy [3, 4]. Although the Hill score was not formally applied to our cohort, patients whose repeat biopsy demonstrated persistence of the active inflammatory features of the Hill score that correlated with future doubling of SCr (e.g. cellular crescents and glomerular necrosis) were all given prolonged induction treatment. In the long term, these patients did well clinically. However, whether or not this good outcome was due to the prolonged induction treatment cannot be determined from these data.
On the other hand, of the patients whose second biopsy showed complete histologic remission, 62% still had clinical renal activity, characterized mainly by persistent moderate to high-grade proteinuria. Similar discordance was seen in a cohort of European, mainly white, patients reported recently by Zickert et al. [7]. At long-term follow-up, however, the majority of our patients and Zickert's patients had normal kidney function and did meet clinical criteria for a complete response. It is known that persistent proteinuria early in the treatment of LN is not a good predictor of poor long-term kidney outcomes [15]. The explanation for complete histologic resolution with persistent clinical abnormalities such as proteinuria is not clear but may simply reflect a lag between resolution of inflammation and tissue healing. Alternatively, proteinuria may persist in the absence of active inflammation if sufficient chronic kidney damage has accrued [16, 17]. This explanation is less likely here because although the CI tended to be higher in the complete histologic remitters with persistent proteinuria after induction, it was not significantly different than the CI of the complete histologic remitters who had resolution of proteinuria.
Although patients with and without histologically complete remission showed improved complement levels into the normal range by Biopsy 2, absolute complement levels were higher in patients whose second biopsies showed no activity. This raises the possibility that patients who have less complement depletion at diagnostic biopsy may have a more rapid resolution of renal inflammation than patients with highly suppressed levels.
The CI increased significantly in the whole cohort between Biopsy 1 and Biopsy 2. This was not attributable only to those patients who did not remit clinically by Biopsy 2. Patients with rapid clinical CRR after induction also developed a significant increase in CI on repeat biopsy. The tendency for CI to increase during the first 6 months of treatment has been reported previously by us and other investigators [4, 7–13]. Most of these investigations did not segregate patients by clinical response at Biopsy 2 or only did repeat biopsies in patients who still showed clinical activity. Therefore, it is not possible to assess whether chronic kidney damage accrued despite complete clinical remission from these reports. However, in contrast to our findings, one study did show that increases in CI were confined to patients who did not have a complete clinical response after induction [9].
In our cohort, the CI of Biopsy 2 was significantly associated with the patients' long-term SCr. Among all the patients who developed CKD, 63.6% had a CI of 6 or greater on Biopsy 2. Segmented regression analysis suggested that for patients with a CI of 4 or greater at Biopsy 2, 36% of the long-term variation in log(SCr) was explained by the variation in chronicity at Biopsy 2. For these patients, SCr and CI at Biopsy 2 together accounted for 48% of the long-term variation in log(SCr). While overall sample size is small, we have generated simple parsimonious regression models that are consistent with good model fitting principles and that provide enough degrees of freedom for the estimation of random error variance. In other racial/ethnic groups, the strength of the association between SCr and CI at Biopsy 2 and long-term renal function may be different than for this cohort, but we expect the general relationship to remain valid. A similar association between chronicity at repeat biopsy and poor long-term kidney function was observed in a European LN cohort [7]. However, in contrast to these results, one group did not find a significant correlation between CI at the postinduction biopsy and doubling of SCr during long-term follow-up [4]. The reason for this discrepancy is not clear.
In summary, this study provides additional evidence that clinical data and biopsy findings are discordant in LN, even very early during the treatment course. Neither the clinical nor histologic remission status immediately after induction therapy appears to correlate with long-term kidney survival. These data do not support the routine clinical use of a repeat kidney biopsy immediately after induction therapy. However, because all patients with severe activity on Biopsy 2 were given an extended induction course, we cannot exclude the possibility that a specific level or type of persistent activity on Biopsy 2 may be an indicator of poor future prognosis that requires additional induction therapy. Thus, the relevance of postinduction kidney biopsies requires further, prospective investigation. The implication of these data for clinical trial design is that 6 months is probably an inadequate time period in which to judge how a kidney will do in the long term using standard clinical and histologic evaluation. On the other hand, it seems clear that the kidney accrues chronic damage very rapidly in LN, even in patients who appear clinically to have responded well to aggressive immunosuppression. It is easy to extrapolate this finding to the observation that renal flares are correlated with future renal failure [18], if every time a patient has active LN, the kidney suffers additional chronic damage. These findings suggest that at least some patients with LN could benefit from the addition of antifibrotic agents to induction therapy when such drugs are clinically available. The need to identify these patients illustrates the desirability of being able to monitor, ideally noninvasively, renal fibrosis during the treatment of LN.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
Supplementary Material
ACKNOWLEDGEMENT
This study was partially supported by the Chronic Kidney Disease Biomarker Consortium funded by NIDDK U01-096927 (B.H.R.)
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Wofsy D, Hillson JL, Diamond B. Comparison of alternative primary outcome measures for use in lupus nephritis clinical trials. Arthritis Rheum 2013; 65: 1586–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rovin BH, Parikh SV, Alvarado A. The kidney biopsy in lupus nephritis: is it still relevant? In: Ginzler EM, Dooley MA (eds). Systemic Lupus Erythematosus. Philadelphia: Elsevier, 2014, pp. 537–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hill GS, Delahouse M, Nochy D. et al. A new morphologic index for the evaluation of renal biopsies in lupus nephritis. Kidney Int 2000; 58: 1160–1173 [DOI] [PubMed] [Google Scholar]
- 4. Hill GS, Delahousse M, Nochy D. et al. Predictive power of the second renal biopsy in lupus nephritis: significance of macrophages. Kidney Int 2001; 59: 304–316 [DOI] [PubMed] [Google Scholar]
- 5. Hill GS, Delahousse M, Nochy D. et al. Proteinuria and tubulointerstitial lesions in lupus nephritis. Kidney Int 2001; 60: 1893–1903 [DOI] [PubMed] [Google Scholar]
- 6. Hill GS, Delahousse M, Nochy D. et al. Outcome of relapse in lupus nephritis: roles of reversal of renal fibrosis and response of inflammation to therapy. Kidney Int 2002; 61: 2176–2186 [DOI] [PubMed] [Google Scholar]
- 7. Zickert A, Sundelin B, Svenungsson E. et al. Role of early repeated renal biopsies in lupus nephritis. Lupus Sci Med 2014; doi:10.1135/lupus-2014-000018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alvarado A, Malvar A, Lococo B. et al. The value of repeat kidney biopsy in quiescent Argentinian lupus nephritis patients. Lupus 2014; 23: 840–847 [DOI] [PubMed] [Google Scholar]
- 9. Singh A, Ghosh R, Kaur P. et al. Protocol renal biopsy in patients with lupus nephritis: a single center experience. Saudi J Kidney Dis Transpl 2014; 25: 801–807 [DOI] [PubMed] [Google Scholar]
- 10. Gunnarsson I, Sundelin B, Heimburger M. et al. Repeated renal biopsy in proliferative lupus nephritis—predictive role of serum C1q and albuminuria. J Rheumatol 2002; 29: 693–699 [PubMed] [Google Scholar]
- 11. Askenazi D, Myones B, Kamdar A. et al. Outcomes of children with proliferative lupus nephritis: the role of protocol renal biopsy. Pediatr Nephrol 2007; 22: 981–986 [DOI] [PubMed] [Google Scholar]
- 12. Ong LM, Hooi LS, Lim TO. et al. Randomized controlled trial of pulse intravenous cyclophosphamide versus mycophenolate mofetil in the induction therapy of proliferative lupus nephritis. Nephrology (Carlton) 2005; 10: 504–510 [DOI] [PubMed] [Google Scholar]
- 13. Traitanon O, Avihingsanon Y, Kittikovit V. et al. Efficacy of enteric-coated mycophenolate sodium in patients with resistant-type lupus nephritis: a prospective study. Lupus 2008; 17: 744–751 [DOI] [PubMed] [Google Scholar]
- 14. Austin HA III, Muenz LR, Joyce KM. et al. Prognostic factors in lupus nephritis. Contribution of renal histologic data. Am J Med 1983; 75: 382–391 [DOI] [PubMed] [Google Scholar]
- 15. Tamirou F, D'Cruz D, Sangle S. et al. Long-term follow-up of the MAINTAIN Nephritis Trial, comparing azathioprine and mycophenolate mofetil as maintenance therapy of lupus nephritis. Ann Rheum Dis 2015; doi:10.1136/annrheumdis-2014-206897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lent V, Harth J. Nephropathy in remnant kidneys: pathological proteinuria after unilateral nephrectomy. J Urol 1994; 152: 312–316 [DOI] [PubMed] [Google Scholar]
- 17. Karlen J, Linne T, Wikstad I. et al. Incidence of microalbuminuria in children with pyelonephritic scarring. Pediatr Nephrol 1996; 10: 705–708 [DOI] [PubMed] [Google Scholar]
- 18. Parikh SV, Nagaraja HN, Hebert L. et al. Renal flare as a predictor of incident and progressive CKD in patients with lupus nephritis. Clin J Am Soc Nephrol 2014; 9: 279–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.