Abstract
Background
Partial nephrectomy is considered the preferred care for localized kidney tumors and may yield better patient and kidney survival and similar oncological outcomes compared with radical nephrectomy. We sought to reexamine these hypotheses in a large nationally representative cohort of US veterans who underwent radical or partial nephrectomy.
Methods
We identified 7073 US veterans who had a partial or radical nephrectomy between 2004 and 2013. We collected data on estimated glomerular filtration rate (eGFR) prior to admission for nephrectomy surgery, immediately after surgery and 180 days postsurgery. We evaluated the association of nephrectomy type and eGFR at different time points with long-term mortality risk in adjusted survival models.
Results
Patients who underwent radical (compared to partial) nephrectomy had a 2-fold greater decline in eGFR (−21.8 ± 17.7 versus −10.3 ± 17.4 mL/min/1.73 m2) immediately after surgery. This larger drop in eGFR resulted in a larger proportion of radical nephrectomy patients having an eGFR <60 mL/min/1.73 m2 at ≥180 days postsurgery. Radical (compared to partial) nephrectomy patients also exhibited a 2.2-fold higher mortality [adjusted death hazard ratio 2.21 (95% confidence interval 1.91–2.55)]. Low eGFRs prior to surgery and 180 days postsurgery were associated with higher risk of postnephrectomy death.
Conclusions
Worse postnephrectomy kidney function and higher mortality were observed with radical nephrectomy, and a low presurgical eGFR and a greater decrease in eGFR postsurgery were associated with worse mortality irrespective of the type of nephrectomy. Additional studies are needed to examine predictors of postnephrectomy outcomes.
Keywords: chronic kidney disease, eGFR, partial and radical nephrectomy, renal cell carcinoma, survival analysis
INTRODUCTION
Renal cancer is one of the 10 most frequent cancers reported in the USA. The American Cancer Society estimates that there will be 64 000 new cases this year [1]. The treatment of choice for nonmetastatic renal cancer is nephrectomy. Radical nephrectomy (RN) was popularized by Robson et al. in 1969 [2]. Between 1990 and 2000, a different procedure referred to as partial nephrectomy (PN) [3, 4] was perfected, also referred to as ‘nephron-sparing surgery’ [5]. In 2009, the American Urological Association Guideline for Management of the Clinical T1 Renal Mass [6] recommended nephron-sparing approaches to be considered in all patients with a clinical T1 renal mass based on data demonstrating an increased risk of chronic kidney disease (CKD) associated with RN and a direct correlation between CKD and morbid cardiovascular events and mortality. In the past 5 years there has been an increased trend in performing PN and authors have criticized the slow implementation of the guidelines [7].
The paradigm proposed by the guidelines implies that the difference in mortality between RN and PN will likely be accounted for by the increased cardiovascular risk conferred by a lower estimated glomerular filtration rate (eGFR) after surgery in RN patients. This is based on relatively short-term observational studies and supported by long-term data showing an increased risk of cardiovascular disease and death in kidney transplant donors [8]. In this study we explored this hypothesis in a large, nationally representative contemporary cohort of US veterans. We hypothesized that patients who received PN had better patient and kidney survival than those who received RN, likely due to the preservation of nephron mass.
MATERIALS AND METHODS
Cohort definition
The institutional review committees at the Memphis and Long Beach Veterans Affairs Medical Centers approved the study. Our study utilized data from a cohort study examining risk factors in patients with incident CKD [Racial and Cardiovascular Risk Anomalies in CKD (RCAV) study] [9, 10]. From a cohort of 3 582 478 US veterans with a stable eGFR ≥60 mL/min/1.73 m2 at baseline (1 October 2004 and 30 September 2006), we identified 7835 patients who had either a PN or RN between 1 October 2004 and 30 September 2012. We then removed patients who had more than one nephrectomy surgery (n = 166), patients who had end-stage renal disease (ESRD) before the date of the nephrectomy (n = 44) and patients with no serum creatinine measurement and therefore no eGFR before hospital admission for nephrectomy (n = 286) or after the nephrectomy procedure (n = 129). Our final cohort consisted of 7073 patients (Supplementary data, Figure S1).
Sociodemographic characteristics, comorbid conditions, blood pressure and laboratory characteristics were obtained as previously described [11, 12] but recorded at the time of surgery (or baseline). Information about age, sex and race were obtained from the VA Corporate Data Warehouse (CDW) and from Medicare through the VA–Medicare data merge project [13].
Nephrectomy: intervention assessment
Patients were identified as having a nephrectomy if they had a surgical procedure listed in the VA Inpatient Medical SAS Datasets [International Classification of Diseases, Ninth Revision (ICD-9) codes 5551 or 5552 (RN) or 554 (PN)]. Only patients who had a single procedure throughout follow-up were included (e.g. patients who had a PN followed by an RN were excluded).
The eGFR was calculated according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [14] using extracted serum creatinine measurements from the national Veterans Affairs (VA) Corporate Data Warehouse LabChem data files.
Outcomes
Data on all-cause mortality were obtained from the VA Vital Status Files (VSF), which contained dates of death or last medical or administrative encounter from all sources in the VA system, with 98.3% sensitivity and 99.8% specificity when compared with the National Death Index as the gold standard [15].
Statistical analysis
Descriptive analyses were performed and skewed variables (mean income) were log transformed. The start of the follow-up period was the date of nephrectomy surgery or the eGFR measurement date after surgery. Patients were followed until death or were censored at the date of the last health care or administrative visit, or on 26 July 2013.
Individual eGFR measurements and the differences between measurements prior to admission for surgery (presurgery), immediately after surgery, 180 days postnephrectomy (postsurgery) and last measurement after nephrectomy were collected and summarized. With the exception of measurements collected immediately after surgery, all measurements were taken from outpatient data only. The postsurgery date of 180 days was used because this is the time needed for renal function to stabilize according to prior literature [16]. Slopes of eGFR change per year were measured in patients who had at least three eGFR measurements 180 days postsurgery until the end of follow-up using mixed models. Multivariable linear regression models examining the effect of RN versus PN on postsurgery eGFR were performed.
In survival analyses, we examined the long-term mortality risk associations of RN (versus PN) and presurgery and postsurgery eGFR as continuous predictors and in groups (≥75 (reference), 60–<75, 45–<60 and <45 mL/min/1.732). Short-term mortality risk analyses (or death risks within the first 180 days postsurgery) were evaluated comparing RN versus PN. Associations were examined using Kaplan–Meier methods and unadjusted and adjusted Cox proportional hazard regression models. Associations of continuous eGFR with mortality were also modeled using restricted cubic splines.
Adjusted models included the following covariates based on a priori considerations: age, gender, race/ethnicity, marital status, service connectedness (a measure indicating whether one or more of a patient's comorbidities were caused by their military service, resulting in certain privileges such as preferential access to care and lower copayments), per capita income, ever use of an angiotensin-converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB) over the cohort follow-up time, body mass index (BMI), systolic and diastolic blood pressure, comorbidities (hypertension, diabetes, myocardial infarction, angina, cerebrovascular disease, congestive heart failure, peripheral artery disease, liver disease, peptic ulcer disease, rheumatic disease, malignancy, hemiplegia, HIV/AIDS and depression) and year of nephrectomy procedure.
Mediation analysis was conducted to assess possible mediation of the effect of RN versus PN on mortality by the change in eGFR postnephrectomy (eGFR 180 days postsurgery—presurgery eGFR) using recently published causal analytical methods [17]. In the sensitivity analysis, we additionally evaluated eGFR 180 days postnephrectomy as the mediator. For these analyses, associations of RN versus PN with mortality for each 1 mL/min/1.73 m2 decrease in eGFR (or for each 10 mL/min/1.73 m2 lower postsurgery eGFR in sensitivity analyses) were evaluated using logistic regression models. This mediation analyses additionally involved estimating and combining the regressions of eGFR change (mediator) on RN versus PN exposure and of mortality (outcome) on RN versus PN exposure and eGFR change to obtain the natural direct and indirect effects as well as the controlled direct effect of RN versus PN. The total effect of RN on mortality is commonly decomposed into the pure natural direct and total natural indirect effect. The pure natural direct effect of RN (versus PN) was defined as the effect of RN on mortality if (individual patients') eGFR change attained the value it would have had under non-exposure to RN (i.e. under PN instead). The total natural indirect effect of RN on mortality captured how much the mortality outcomes would change on average under RN (versus PN) intervention through the continuous mediator of eGFR change. The controlled direct effect can be interpreted as the effect of RN (versus PN) on mortality had patients' eGFR change 180 days postnephrectomy been fixed at a uniform level. The proportion of the total effect of RN on mortality mediated by eGFR change 180 days postnephrectomy was also estimated and reported.
We also performed sensitivity analyses by excluding patients whose indication for nephrectomy was other than confirmed renal cancer. Statistical analyses were performed using Stata MP Version 12 (StataCorp, College Station, TX, USA).
RESULTS
Baseline characteristics
We selected 7073 patients, 4795 of whom underwent RN and 2278 of whom underwent PN between 1 October 2004 and 30 September 2012. The median observation time from the date of surgery to the end of follow-up was 45 [interquartile range (24–69)] months. Characteristics of RN versus PN patients at the time of nephrectomy are shown in Table 1. The two groups were similar in terms of comorbidities. RN patients were older and had lower prenephrectomy eGFR (P < 0.001 each).
Table 1.
Differences in baseline characteristics between RN and PN
| Variables | PN | RN |
|---|---|---|
| N | 2278 | 4795 |
| Age (years), mean ± SD | 62 ± 9 | 64 ± 10 |
| Gender (% female) | 3.5 | 3.7 |
| Marital status (%) | ||
| Married | 51 | 51 |
| Single | 11 | 9 |
| Divorced | 33 | 33 |
| Widow | 5 | 7 |
| Race (%) | ||
| White | 73 | 79 |
| African American | 22 | 16 |
| Hispanic | 3 | 3 |
| Other | 2 | 2 |
| Presurgery eGFR (mL/min/1.73 m2), mean ± SD | 81 ± 19 | 77 ± 20 |
| Mean annual income ($), median (IQR) | 20 722 (11 540– 31 685) | 20 807 (12 083– 31 876) |
| Service connectedness (% yes) | 48 | 44 |
| Ever ACEI/ARB use (%) | 72 | 68 |
| Systolic BP (mmHg), mean ± SD | 135 ± 18 | 135 ± 19 |
| Diastolic BP (mmHg), mean ± SD | 77 ± 11 | 76 ± 12 |
| BMI (kg/m2), mean ± SD | 30.0 ± 6.1 | 29.2 ± 6.3 |
| Comorbidities (%) | ||
| Hypertension | 82 | 80 |
| Diabetes | 37 | 35 |
| Cardiovascular disease | 31 | 31 |
| Myocardial infarction | 29 | 30 |
| Angina | 7 | 6 |
| Percutaneous coronary intervention | <1 | <1 |
| Coronary artery bypass graft | 1 | 1 |
| Congestive heart failure | 8 | 10 |
| Cerebrovascular disease | 9 | 9 |
| Peripheral arterial disease | 5 | 5 |
| Chronic lung disease | 33 | 34 |
| Depression | 21 | 14 |
| Kidney cancer, n (%) | 2145 (94) | 4158 (87) |
| Charlson Comorbidity Index, median (IQR) | 3 (2–4) | 3 (2–4) |
Deterioration of kidney function after radical versus partial nephrectomy
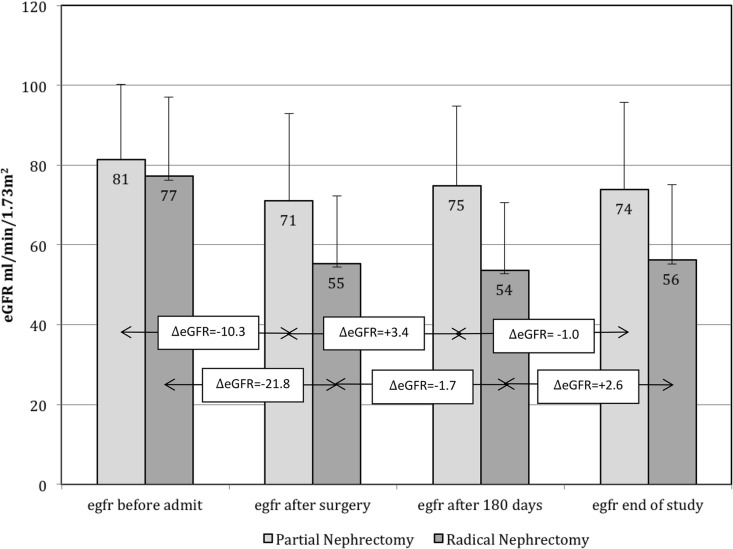
Presurgery eGFRs were obtained at a median of 14 (IQR 7–25) days prior to the nephrectomy surgery date. The postsurgery eGFRs were obtained within 1 week of surgery (99% of patients had an eGFR recorded the day after surgery). Postsurgery eGFRs were obtained at a median of 226 (IQR 198–282) days or 7 (IQR 6–9) months after surgery, and the last measurement obtained after surgery was at a median 39 (IQR 21–60) months after surgery. Table 2 presents the mean eGFR for the total cohort and by nephrectomy type at each time point, and the course of eGFR changes by nephrectomy type are shown in Figure 1. As expected, eGFR decreased after nephrectomy, and the decline was 2-fold greater for RN versus PN, i.e. −21.8 ± 17.7 versus −10.3 ± 17.4 mL/min/1.73 m2, respectively. After the initial eGFR decline postsurgery, the average eGFR remained relatively stable in both groups for the remainder of the observation period. As a consequence of the greater decrease in eGFR, patients who had an RN were more likely than PN patients to exhibit a postsurgery eGFR <60 mL/min/1.73 m2 (Supplementary data, Figure S2). In multivariable adjusted linear regression analyses, RN compared with PN was associated with a 19 mL/min/1.73 m2 lower (worse) postsurgery eGFR {β = −19.51 [95% confidence interval (CI) −20.44 to −18.59], P < 0.001} and a 18 mL/min/1.732 lower eGFR when further adjusted for presurgery eGFR [β = −18.35 (95% CI −19.01 to −17.59), P < 0.001].
Table 2.
eGFRs (mL/min/1.73 m2) recorded at time points throughout the observation period in PN and RN patients
| Time point | Total | PN | RN |
|---|---|---|---|
| Presurgery | 78.5 ± 19.4 | 81.3 ± 18.9 | 77.2 ± 19.6 |
| Immediately after surgery | 60.4 ± 20.2 | 71.0 ± 22.2 | 55.3 ± 17.0 |
| At least 180 days postsurgery | 60.5 ± 20.5 | 74.8 ± 20.3 | 53.6 ± 16.7 |
| Last eGFR obtained postsurgery | 61.9 ± 21.8 | 73.8 ± 21.9 | 56.2 ± 19.3 |
Values are given as mean ± SD.
FIGURE 1.
Changes in GFR throughout the observation period in PN and RN patients.
In 3666 RN and 1714 PN patients with at least three available eGFR values [median 12 (IQR 7–19) measurements] 180 days postsurgery, the average slope was positive for both groups, showing a continuous albeit slow process of recovery or compensation 180 days postsurgery throughout the observation time, with a relatively larger increase in the RN group (RN: +0.65 ± 2.29 versus PN: +0.04 ± 2.47 mL/min/1.73 m2/year; P < 0.0001).
RN versus PN survival
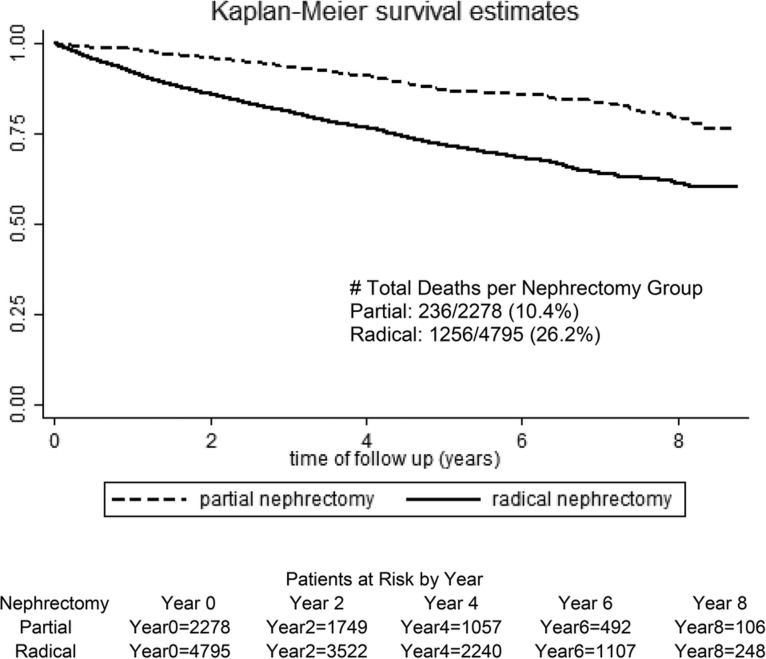
In a Kaplan–Meier plot of survival from surgery to the end of follow-up, there was a survival advantage for PN, as shown in Figure 2. In adjusted Cox proportional hazard models, patients who underwent RN had a >2.2-fold increased risk of death as compared with those who had a PN [HR 2.21 (95% CI 1.91–2.55)]. In examining short-term mortality risk, or risk of death within 180 days postsurgery, RN patients had a 2.8-fold higher death risk compared with those who had a PN in fully adjusted models [hazard ratio (HR) 2.82 (95% CI 1.89–4.19)]. A sensitivity analysis after restricting the data to only patients with confirmed renal cancer (eliminating nephrectomy for other indications, n = 770) showed similar results [HR 2.27 (95% CI 1.95–2.65)].
FIGURE 2.
Unadjusted Kaplan–Meier estimates of survival in RN versus PN patients.
Impact of eGFR on mortality
Lower presurgery eGFR was associated with a higher mortality risk (Supplementary data, Figure S3) in both unadjusted and adjusted models where we evaluated the association of eGFR groups (<45, 45–<60, 60–<75, ≥75 mL/min/1.73 m2) with mortality and used the highest (best) eGFR group (≥75 mL/min/1.73 m2) as a reference. For presurgery, each 10 mL/min/1.73 m2 lower eGFR prior to surgery was associated with a significantly higher mortality risk [HR 1.11 (95% CI 1.08–1.15)], with similar associations in PN [HR 1.09 (95% CI 1.01–1.18)] and RN [HR 1.11 (95% CI 1.07–1.14)] strata (P for interaction = 0.41).
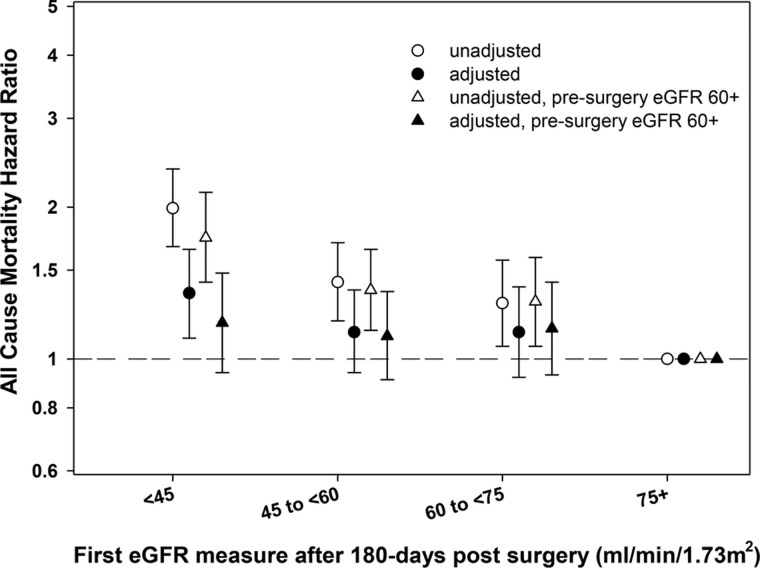
Compared with patients with a postsurgery (obtained 180 days postnephrectomy) eGFR ≥75 mL/min/1.73 m2, patients with a lower (worse) postsurgery eGFR had a significant trend toward higher mortality risk (P for trend <0.001; Figure 3 and Supplementary data, Figure S4), where patients with a postsurgery eGFR <45 mL/min/1.73 m2 had a 35% higher risk of mortality in adjusted models [HR 1.35 (95% CI 1.10–1.65)]. However, in patients with presurgery eGFR ≥60 mL/min/1.73 m2 (normal kidney function), this association was attenuated and there was no clear association between lower (worst) eGFR and mortality in adjusted models. Similarly, each 10 mL/min/1.73 m2 decrease in postsurgery eGFR was also associated with a significantly higher risk of mortality [HR 1.08 (95% CI 1.04–1.12) and HR 1.05 (95% CI 1.01–1.09)] after additional adjustment for presurgery eGFR. There was a significant interaction between continuous pre- and postsurgery eGFR for associations with mortality risk (P = 0.009).
FIGURE 3.
Hazard ratio for mortality in groups for eGFR 180 days postnephrectomy (eGFR ≥75 mL/min/1.73 m2 as reference) in unadjusted and adjusted models in the total cohort and in a subcohort of patients with presurgery normal kidney function (eGFR ≥60 mL/min/1.73 m2).
Change in eGFR from pre- to postsurgery
Each 10% decrease in eGFR from pre- to postsurgery with adjustment for presurgery eGFR was associated with a 4% higher risk of mortality [HR 1.04 (95% CI 1.01–1.07), P = 0.007]. The interaction between percent change and presurgery eGFR was not significant for the mortality outcome (P = 0.20).
Mediation analyses
We analyzed if eGFR changes postsurgery (presurgery to 180 days postsurgery) could account for the increased mortality in RN versus PN via mediation analyses, including adjustment for presurgery eGFR. The natural direct effect odds ratio (OR) of RN versus PN and mortality was 2.83 (95% CI 2.29–3.50), while the natural indirect effect OR per 1 mL/min/1.73 m2 decrease in eGFR on mortality was 0.88 (95% CI 0.79–0.99). The controlled direct effect OR was 2.94 (95% CI 2.31–3.75) and the mediated total effect OR was 2.49 (95% CI 2.09–2.98). These results demonstrate that the difference in the magnitude of the eGFR decrease postnephrectomy accounts for ∼23% of the increased risk of mortality for RN versus PN. In the sensitivity analysis, where eGFR 180 days postsurgery was evaluated as the mediator, results were similar (see Supplementary data).
DISCUSSION
This study confirms that mortality is higher after RN than after PN and that eGFR is lower after RN. The data also showed that after a decrease in the nephron pool by PN or RN surgery, the eGFR stabilized or slightly improved in the majority of patients for the remainder of the observation period. The typical progressively declining trajectory of eGFR observed in patients with CKD from other (medical) causes was absent in the majority of nephrectomy patients and was no more frequent after RN, in spite of this procedure producing a more profound decrease in postsurgery eGFR.
Our data do not support the hypothesis that the increased mortality in RN patients as compared with PN patients is attributable to the eGFR changes induced by surgery (nephron sparing). The results of our mediation analysis indicate that eGFR 180 days postsurgery accounts for only 32% of the increased risk in mortality seen in RN versus PN patients.
The only randomized clinical trial for head-to-head comparison of RN and PN [18] concluded that both methods provide excellent oncologic results. Survival was better for PN patients in the intent-to-treat population, but this was not significant in the targeted population (those with clear cell carcinoma). The trial was stopped early because of poor enrollment and was criticized for having randomization problems [19, 20].
The current recommendations [6] are based on the ‘nephron-sparing’ concept. They are supported by retrospective studies showing lower survival for RN. The increased relative risk of mortality associated with RN versus PN ranges from 15–25% [21, 22] to 250% [23]. Two meta-analyses [24, 25] published simultaneously confirmed the improved survival for PN patients. Studies showing increased long-term risk in kidney transplant donors also support this concept [8].
There is little evidence that the better survival of PN patients is attributable to nephron sparing. In patients who have undergone a nephrectomy, reduced GFR was shown to be associated with decreased overall survival [26]. A small study showed an increased mortality in patients with low eGFR after PN [27], but the data were not adjusted for presurgery eGFR. An increased risk of cardiovascular events after RN was also reported [28, 29].
Studies comparing the course of GFR decline in patients with low GFR attributable to CKD or to nephrectomy showed a much more benign course for postnephrectomy patients [30, 31]. In a large study, the eGFR after RN showed a slow improvement over the first 5 years [32].
There are a few possible interpretations for the lack of association between eGFR and mortality in RN and PN. First, one could speculate that RN had a higher rate of cancer-related mortality. Multiple studies have shown, however, that the difference in mortality is attributable to noncancer overall survival [21, 22, 33, 34].
Second, one could speculate that the mortality is higher in patients undergoing RN, unrelated to GFR level, and perhaps the result of a bias by indication (higher-risk patients being triaged towards the RN procedure). A recent analysis by Schuch et al. [35] using sophisticated methodology showed that patients undergoing PN had improved overall survival compared with noncancer patients, suggesting that PN patients have a survival advantage over RN patients and a possible selection bias is responsible for improved overall survival in PN versus RN patients. However, in a follow-up study the authors showed that PN had a higher risk of morbidity when compared with noncancer patients [36].
There are only a few studies that examine the risk of nonfatal and fatal cardiovascular events after nephrectomy. In two studies, cardiovascular death in RN was more than twice that in PN [34, 37]. In another study, there were an increased number of cardiovascular events but no increase in the time to the first event or the risk of cardiovascular death [38]. In our opinion, it is difficult to determine with certainty the mechanisms for better survival in PN versus RN without an in depth analysis of the cause of death and preexisting comorbidities related to it.
The strengths of this study include use of the large VA database with detailed preprocedure comorbidity data and vital status at the end of the study available in all patients. The study is limited, however, by its retrospective observational aspect. The study was also limited in the duration of follow-up and it is conceivable that after a longer observation time, the reduced eGFR of RN patients could account for decreased survival. Another limitation is the small number of women included in the cohort. Information on important confounders such as tumor size, cancer stage and pathology type and on important outcome characteristics such as cause of death and date of oncological recurrence were not available. It is possible that more advanced renal cancer may have contributed to survival differences in RN versus PN. Lastly, the possibility of additional unidentified confounders or residual confounding could also limit the interpretation of our results.
CONCLUSIONS
We have shown that RN (versus PN) is associated with a higher risk of long-term mortality and a lower postnephrectomy eGFR. In this regard, this study supports the current guidelines of PN for management of nonmetastatic renal cancer. We have shown, however, that after a relatively short-term observation time, increased mortality cannot be accounted for by the difference in eGFR induced by the procedure (RN or PN). The cause of the difference in survival between RN and PN reported by most authors remains unknown. Further studies including large randomized clinical trials with additional oncological data are needed to examine pathophysiological mechanisms postnephrectomy and to determine the most optimal management strategy in renal cancer.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
Supplementary Material
ACKNOWLEDGEMENTS
E.S. and C.P.K. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. This study is supported by grant 1R01DK096920 from the National Institutes of Health to C.P.K. and K.K.-Z. and is the result of work supported with resources and the use of facilities at the Memphis VA Medical Center and the Long Beach VA Medical Center. Support for VA/Centers for Medicare and Medicaid Services data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004). The VA and NIH did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. C.P.K. and K.K.-Z. are employees of the Department of Veterans affairs. Opinions expressed in this article are those of the authors' and do not necessarily represent the opinion of the Department of Veterans Affairs.
CONFLICT OF INTEREST STATEMENT
K.K.-Z. has received honoraria and/or support from Abbott, AbbVie, Alexion, Amgen, American Society of Nephrology, AstraZeneca, AVEO, Chugai, DaVita, Fresenius, Genetech, Haymarket Media, Hospira, Kabi, Keryx, National Institutes of Health, National Kidney Foundation, Relypsa, Resverlogix, Sanofi, Shire, Vifor and ZS-Pharma. C.P.K. has received honoraria from Sanofi-Aventis, Relypsa and ZS Pharma. All other authors have no conflicts of interest or financial disclosures. The results of this article have not been published previously in whole or part, except for abstract submission to the 46th American Society of Nephrology Kidney Week, November 11–14, 2014, Philadelphia, PA, USA.
REFERENCES
- 1. American Cancer Society. What are the key statistics about kidney cancer? http://www.cancer.org/cancer/kidneycancer/detailedguide/kidney-cancer-adult-key-statistics 2014
- 2. Robson CJ, Churchill BM, Anderson W. The results of radical nephrectomy for renal cell carcinoma. J Urol 1969; 101: 297–301 [DOI] [PubMed] [Google Scholar]
- 3. Lee CT, Katz J, Shi W, et al. Surgical management of renal tumors 4 cm. or less in a contemporary cohort. J Urol 2000; 163: 730–736 [PubMed] [Google Scholar]
- 4. Licht MR, Novick AC, Goormastic M. Nephron sparing surgery in incidental versus suspected renal cell carcinoma. J Urol 1994; 152: 39–42 [DOI] [PubMed] [Google Scholar]
- 5. Novick AC. The role of nephron-sparing surgery for renal cell carcinoma. Eur Urol 1990; 18(Suppl 2): 24–25 [DOI] [PubMed] [Google Scholar]
- 6. Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol 2009; 182: 1271–1279 [DOI] [PubMed] [Google Scholar]
- 7. Schiffmann J, Bianchi M, Sun M, et al. Trends in surgical management of T1 renal cell carcinoma. Curr Urol Rep 2014; 15: 383. [DOI] [PubMed] [Google Scholar]
- 8. Mjoen G, Hallan S, Hartmann A, et al. Long-term risks for kidney donors. Kidney Int 2014; 86: 162–167 [DOI] [PubMed] [Google Scholar]
- 9. Kovesdy CP, Norris KC, Boulware LE, et al. Association of race with mortality and cardiovascular events in a large cohort of US veterans. Circulation 2015; 132: 1538–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu JL, Molnar MZ, Naseer A, et al. Association of age and BMI with kidney function and mortality: a cohort study. Lancet Diabetes Endocrinol 2015; 3: 704–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Molnar MZ, Mucsi I, Novak M, et al. Association of incident obstructive sleep apnoea with outcomes in a large cohort of US veterans. Thorax 2015; 70: 888–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Molnar MZ, Alhourani HM, Wall BM, et al. Association of hepatitis C viral infection with incidence and progression of chronic kidney disease in a large cohort of US veterans. Hepatology 2015; 61: 1495–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stroupe KT, Tarlov E, Zhang Q, et al. Use of Medicare and DOD data for improving VA race data quality. J Rehabil Res Dev 2010; 47: 781–795 [DOI] [PubMed] [Google Scholar]
- 14. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sohn MW, Arnold N, Maynard C, et al. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr 2006; 4: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clark WF, Na Y, Rosansky SJ, et al. Association between estimated glomerular filtration rate at initiation of dialysis and mortality. CMAJ 2011; 183: 47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods 2013; 18: 137–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol 2011; 59: 543–552 [DOI] [PubMed] [Google Scholar]
- 19. Ganesamoni R, Mavuduru R, Agarwal MM. Re: Hendrik van Poppel, Luigi da Pozzo, Walter Albrecht, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol 2011; 59: 543–52. Eur Urol 2011; 60: e9; author reply e10 [DOI] [PubMed] [Google Scholar]
- 20. Kluth LA, Xylinas E, Shariat SF. Words of wisdom: re: a prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol 2013; 63: 399–400 [DOI] [PubMed] [Google Scholar]
- 21. Zini L, Perrotte P, Capitanio U, et al. Radical versus partial nephrectomy: effect on overall and noncancer mortality. Cancer 2009; 115: 1465–1471 [DOI] [PubMed] [Google Scholar]
- 22. Tan HJ, Norton EC, Ye Z, et al. Long-term survival following partial vs radical nephrectomy among older patients with early-stage kidney cancer. JAMA 2012; 307: 1629–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weight CJ, Lieser G, Larson BT, et al. Partial nephrectomy is associated with improved overall survival compared to radical nephrectomy in patients with unanticipated benign renal tumours. Eur Urol 2010; 58: 293–298 [DOI] [PubMed] [Google Scholar]
- 24. MacLennan S, Lam T, Imamura M, et al. Re: comparative effectiveness for survival and renal function of partial and radical nephrectomy for localized renal tumors: a systematic review and meta-analysis: S. P. Kim, R. H. Thompson, S. A. Boorjian, C. J. Weight, L. C. Han, M. H. Murad, N. D. Shippee, P. J. Erwin, B. A. Costello, G. K. Chow and B. C. Leibovich J Urol 2012; 188: 51–57. J Urol 2013; 189: 1166–1167 [DOI] [PubMed] [Google Scholar]
- 25. Kim SP, Murad MH, Thompson RH, et al. Comparative effectiveness for survival and renal function of partial and radical nephrectomy for localized renal tumors: a systematic review and meta-analysis. J Urol 2012; 188: 51–57 [DOI] [PubMed] [Google Scholar]
- 26. Pettus JA, Jang TL, Thompson RH, et al. Effect of baseline glomerular filtration rate on survival in patients undergoing partial or radical nephrectomy for renal cortical tumors. Mayo Clin Proc 2008; 83: 1101–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sejima T, Iwamoto H, Masago T, et al. Oncological and functional outcomes after radical nephrectomy for renal cell carcinoma: a comprehensive analysis of prognostic factors. Int J Urol 2013; 20: 382–389 [DOI] [PubMed] [Google Scholar]
- 28. Takeshita H, Yokoyama M, Fujii Y, et al. Impact of renal function on cardiovascular events in patients undergoing radical nephrectomy for renal cancer. Int J Urol 2012; 19: 722–728 [DOI] [PubMed] [Google Scholar]
- 29. Capitanio U, Terrone C, Antonelli A, et al. Nephron-sparing techniques independently decrease the risk of cardiovascular events relative to radical nephrectomy in patients with a T1a-T1b renal mass and normal preoperative renal function. Eur Urol 2014; 67: 683–689 [DOI] [PubMed] [Google Scholar]
- 30. Lane BR, Campbell SC, Demirjian S, et al. Surgically induced chronic kidney disease may be associated with a lower risk of progression and mortality than medical chronic kidney disease. J Urol 2013; 189: 1649–1655 [DOI] [PubMed] [Google Scholar]
- 31. Demirjian S, Lane BR, Derweesh IH, et al. Chronic kidney disease due to surgical removal of nephrons: relative rates of progression and survival. J Urol 2014; 192: 1057–1062 [DOI] [PubMed] [Google Scholar]
- 32. Chung JS, Son NH, Byun SS, et al. Trends in renal function after radical nephrectomy: a multicentre analysis. BJU Int 2014; 113: 408–415 [DOI] [PubMed] [Google Scholar]
- 33. Crepel M, Jeldres C, Sun M, et al. A population-based comparison of cancer-control rates between radical and partial nephrectomy for T1A renal cell carcinoma. Urology 2010; 76: 883–888 [DOI] [PubMed] [Google Scholar]
- 34. Patel HD, Kates M, Pierorazio PM, et al. Balancing cardiovascular (CV) and cancer death among patients with small renal masses: modification by CV risk. BJU Int 2014; 115: 58–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shuch B, Hanley J, Lai J, et al. Overall survival advantage with partial nephrectomy: a bias of observational data? Cancer 2013; 119: 2981–2989 [DOI] [PubMed] [Google Scholar]
- 36. Shuch B, Hanley JM, Lai JC, et al. Adverse health outcomes associated with surgical management of the small renal mass. J Urol 2014; 191: 301–308 [DOI] [PubMed] [Google Scholar]
- 37. Kates M, Badalato GM, Pitman M, et al. Increased risk of overall and cardiovascular mortality after radical nephrectomy for renal cell carcinoma 2 cm or less. J Urol 2011; 186: 1247–1253 [DOI] [PubMed] [Google Scholar]
- 38. Huang WC, Elkin EB, Levey AS, et al. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors—is there a difference in mortality and cardiovascular outcomes? J Urol 2009; 181: 55–61; discussion 61–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.