Translational perspective
Exosomes secreted by cardiosphere-derived cells (CDCs) were studied as a therapy for myocardial infarction (MI). In two different porcine models, exosomes from CDCs limited injury when given acutely and halted adverse remodelling when given in convalescent MI. Both models showed decreased scarring and improved function with intramyocardial delivery of exosomes. Cardiosphere-derived cell-secreted exosomes are a promising cell-free therapy for MI.
Keywords: Exosomes, Cell therapy, Myocardial infarction, Animal models
Abstract
Aims
Naturally secreted nanovesicles known as exosomes are required for the regenerative effects of cardiosphere-derived cells (CDCs), and exosomes mimic the benefits of CDCs in rodents. Nevertheless, exosomes have not been studied in a translationally realistic large-animal model. We sought to optimize delivery and assess the efficacy of CDC-secreted exosomes in pig models of acute (AMI) and convalescent myocardial infarction (CMI).
Methods and results
In AMI, pigs received human CDC exosomes (or vehicle) by intracoronary (IC) or open-chest intramyocardial (IM) delivery 30 min after reperfusion. No-reflow area and infarct size (IS) were assessed histologically at 48 h. Intracoronary exosomes were ineffective, but IM exosomes decreased IS from 80 ± 5% to 61 ± 12% (P= 0.001) and preserved left ventricular ejection fraction (LVEF). In a randomized placebo-controlled study of CMI, pigs 4 weeks post-myocardial infarction (MI) underwent percutaneous IM delivery of vehicle (n = 6) or CDC exosomes (n = 6). Magnetic resonance imaging (MRI) performed before and 1 month after treatment revealed that exosomes (but not vehicle) preserved LV volumes and LVEF (−0.1 ± 2.2% vs. −5.4 ± 3.6%, P= 0.01) while decreasing scar size. Histologically, exosomes decreased LV collagen content and cardiomyocyte hypertrophy while increasing vessel density.
Conclusion
Cardiosphere-derived cell exosomes delivered IM decrease scarring, halt adverse remodelling and improve LVEF in porcine AMI and CMI. While conceptually attractive as cell-free therapeutic agents for myocardial infarction, exosomes have the disadvantage that IM delivery is necessary.
See page 212 for the editorial comment on this article (doi:10.1093/eurheartj/ehw324)
Translational perspective.
Exosomes secreted by cardiosphere-derived cells (CDCs) were studied as a therapy for myocardial infarction (MI). In two different porcine models, exosomes from CDCs limited injury when given acutely and halted adverse remodelling when given in convalescent MI. Both models showed decreased scarring and improved function with intramyocardial delivery of exosomes. Cardiosphere-derived cell-secreted exosomes are a promising cell-free therapy for MI.
Introduction
Despite the fact that outcomes following myocardial infarction (MI) have improved in the era of prompt reperfusion, ischaemic heart disease remains the world's leading cause of death.1,2 To reverse injury post-MI, cardiosphere-derived cells (CDCs) are currently in phase 2 clinical trials with scar reduction as the major endpoint.3 Cardiosphere-derived cells have been shown to decrease scar mass, increase viable mass, and halt adverse remodelling in multiple animal models and in a phase 1 human study.4–8 Accumulating evidence indicates that the benefits of CDCs are mediated by the secretion of exosomes.9,10 These natural nano-scale lipid bilayer vesicles mediate cell–cell communication,11,12 at least partially by transferring distinctive payloads of microRNAs (miRNAs) and other non-coding RNAs13 specific to the parent cell type.14 Cardiosphere-derived cell-secreted exosomes are required for, and themselves recapitulate, the therapeutic benefits of CDCs,9 as part of a paradigm that seems to be generalizable.11,15,16 One potential advantage of exosomes over CDCs is that they are acellular and non-replicating, facilitating the development of a stable and reliable ‘off-the-shelf’ product.
Although exosomes have obvious appeal, they have yet to be tested in clinically realistic large-animal disease models. Even the fundamentals of delivery remain unexplored. As nano-sized particles, exosomes may not be well suited for intracoronary (IC) delivery, as they may not be taken up easily in a first pass. Therefore, we first compared IC and intramyocardial (IM) delivery of CDC-derived exosomes in an acute MI model (in a protocol designed to recruit cellular post-conditioning).17,18 Next, we performed a randomized placebo-controlled study in which we evaluated IM delivery of CDC-derived exosomes using the NOGA® electro-anatomic mapping system in a pig model of convalescent MI (in a protocol designed to assess therapeutic regeneration).
Methods
For detailed methods, see Supplementary material online. All experiments were performed on unconscious anaesthetized pigs. Pig models of myocardial infarction have been widely validated as reliable models for translational studies.19
Cardiosphere-derived cell exosome isolation and characterization
Human CDCs at fifth passage (from a single non-diseased human donor) were grown until confluence in regular CDC culture media,1 which was then changed to serum-free media. After 15 days, the conditioned serum-free media (containing the exosomes) was collected and filtered through a 450 nm filter. Exosomes were then isolated by ultrafiltration by centrifugation followed by overnight precipitation in 25% poly-ethylene glycol (PEG); the media containing PEG was centrifuged for 30 min at 2000 × g and the pellet containing the exosomes was resuspended in IMDM for injection (in 10 mL for intracoronary infusion and in 2 mL for intramyocardial injection). Protein concentration was measured using the Bradford protein assay, and the final suspension was analysed by nanoparticle tracking analysis (NTA, NanoSight Ltd., Amesbury, Wiltshire, UK) to determine particle number and size.
Acute study: delivery
The protocol for the acute study is depicted in Supplementary material online, Figure S1A. Closed-chest MIs were created in 22 female adult Yucatan mini-pigs. Thirty minutes after reperfusion, animals were allocated to receive IC exosomes (15 mg [≈33 × 1011 particles] infused over 30 min, n = 6), IM exosomes (15 mg divided in 10 injections, n = 4, then changed to 7.5 mg [≈16.5 × 1011 particles], n = 5, due to equivalent efficacy, Supplementary material online, Figure S2B, so total n = 9), or IC vehicle as control (n = 7) and followed for 48 h. Exosome dosing was extrapolated from previous work in small animals.10,20 Infarct size (IS), micro-vascular obstruction (MVO), and area at risk (AAR) were determined at 48 h as previously described.18 Left ventricular function was measured using left ventriculograms before treatment and at 48 h.
Acute extravasation and retention of exosomes following the different delivery methods was studied as shown in Supplementary material online, Figure S1B.
Randomized pre-clinical study in a chronic model
Study design and exosome delivery
Study design is shown in Supplementary material online, Figure S1C. A closed-chest MI was created in 13 female adult Yucatan mini-pigs as described.21 Four weeks later 12 animals were randomized to receive vehicle (IMDM, n = 6) or IM injection of CDC-derived exosomes (7.5 mg protein concentration [≈16.5 × 1011 particles], n = 6) using the NOGA® cardiac navigation system (Biosense Webster, Inc., Diamond Bar, CA, USA). Animals were then followed for one additional month and euthanized. Magnetic resonance imaging was performed before treatment (baseline) and at 1 month.
To confirm the inefficacy of IC exosomes, 4 additional pigs were infarcted and treated with IC exosomes 4 weeks post-MI (results in see Supplementary material online, Figure S5).
Statistical analysis
Continuous variables are presented as mean ± standard deviation in the text and mean ± standard error in the figures. Categorical variables are expressed as an absolute number and a percentage. Independent groups were compared using Mann–Whitney U test for two groups and using Kruskal–Wallis test for n > 2 groups. Wilcoxon test was used to compare paired groups (changes from baseline). All P values are two sided, and a P value of <0.05 was considered statistically significant.
Results
Exosome characterization
Supplementary material online, Figure S3A summarizes the CDC exosome isolation process. The number and size of the particles were measured using nanoparticle tracking analysis (NTA, NanoSight Ltd., Amesbury, Wiltshire, UK) (representative raw data on see Supplementary material online, Figure S3B). One milligram of proteins contained ∼2.2 × 1011 particles and the average diameter of the particles was 192 ± 17 nm (see Supplementary material online, Figure S3C and D). This diameter is larger than what is usually described for exosomes because of the use of nanoparticle tracking analysis on normally hydrated exosomes for measurement.22,23
Acute delivery study
Intramyocardial exosomes but not intracoronary exosomes decrease infarct size and preserve left ventricular function
Figure 1A shows representative images of AAR, MVO, and IS (assessed by Gentian violet, Thioflavin T, and triphenyl tetrazolium chloride (TTC) staining, respectively) each in the control, IC exosome, and IM exosome groups. The extent of the ischaemic injury was similar in the three groups as demonstrated by the identical /LV ratio (21 ± 4% of the LV, P= 0.49, Figure 1B). Forty-eight hours after treatment, the extent of MVO/AAR was 58 ± 6% in the control group when compared with 43 ± 6% (P= 0.03 vs. placebo) in the IC exosome group and 33 ± 14% (P< 0.001 vs. control) in the IM exosome group (Figure 1C). While MVO decreased in both exosome groups, IS at endpoint was only decreased after IM delivery of exosomes (IS/AAR = 80 ± 5% in control vs. 77 ± 5% and 61 ± 12% in IC exosomes and IM exosomes, respectively, P= 0.001, Figure 1D). Consistently, despite similar LV end-diastolic, end-systolic volumes, and LVEF before treatment, these parameters were preserved only in the IM exosome group at endpoint; chamber volumes increased, while LVEF decreased, in both the placebo and IC exosome groups (Figure 1E–G).
Figure 1.
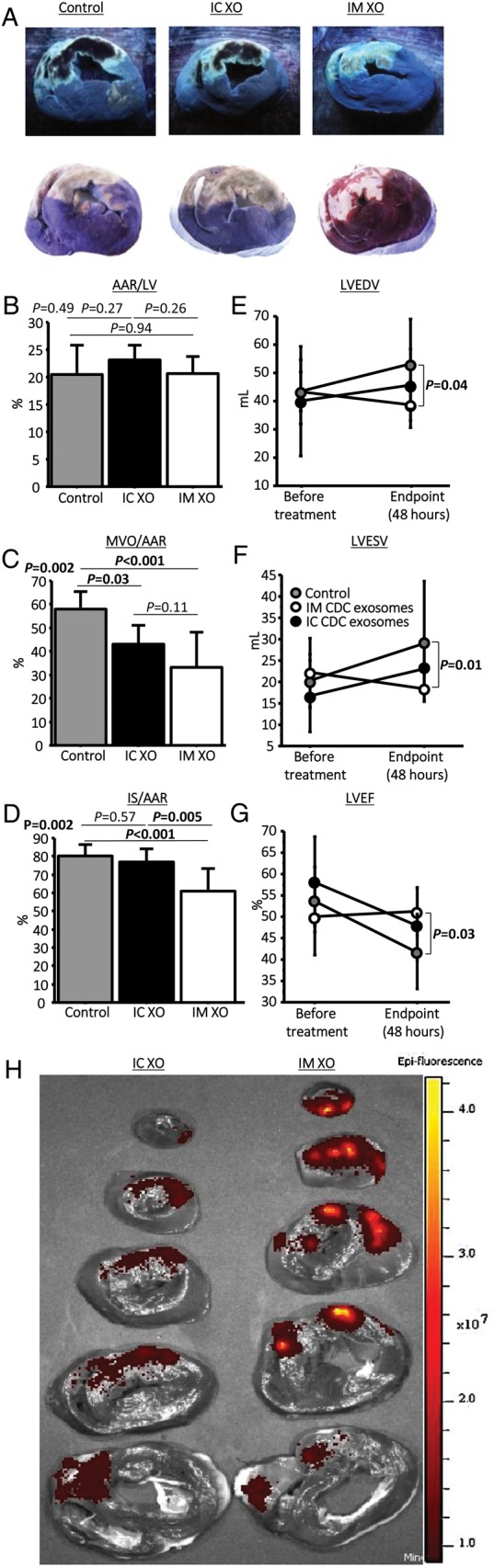
Acute myocardial infarction study: infarct size, micro-vascular occlusion, and retention. (A) Representative images of heart sections under UV-light (top panels) and after TTC staining (bottom panels) in control, intracoronary exosomes, and intramyocardial-treated pigs. Under UV light, micro-vascular obstruction appears dark and area-at-risk fluorescent; after TTC staining, scar appears white and area-at-risk red; non-ischaemic myocardium appears purple. Pooled data show that area-at-risk/left ventricular is similar in the three groups (B), both intracoronary and intramyocardial exosomes decrease micro-vascular obstruction compared with control (C), and intramyocardial exosomes but not intracoronary exosomes decrease scar size compared with control (D). Intramyocardial exosomes (but not intracoronary exosomes) preserve left ventricular end-diastolic (E) and end-systolic volume (F) and left ventricular ejection fraction. (H) Bioluminescence tracking in a heart infused via the intracoronary route with far-red labelled exosomes (left) and another heart that had been injected intramyocardially with exosomes (right); signal intensity is much higher after intramyocardial injection.
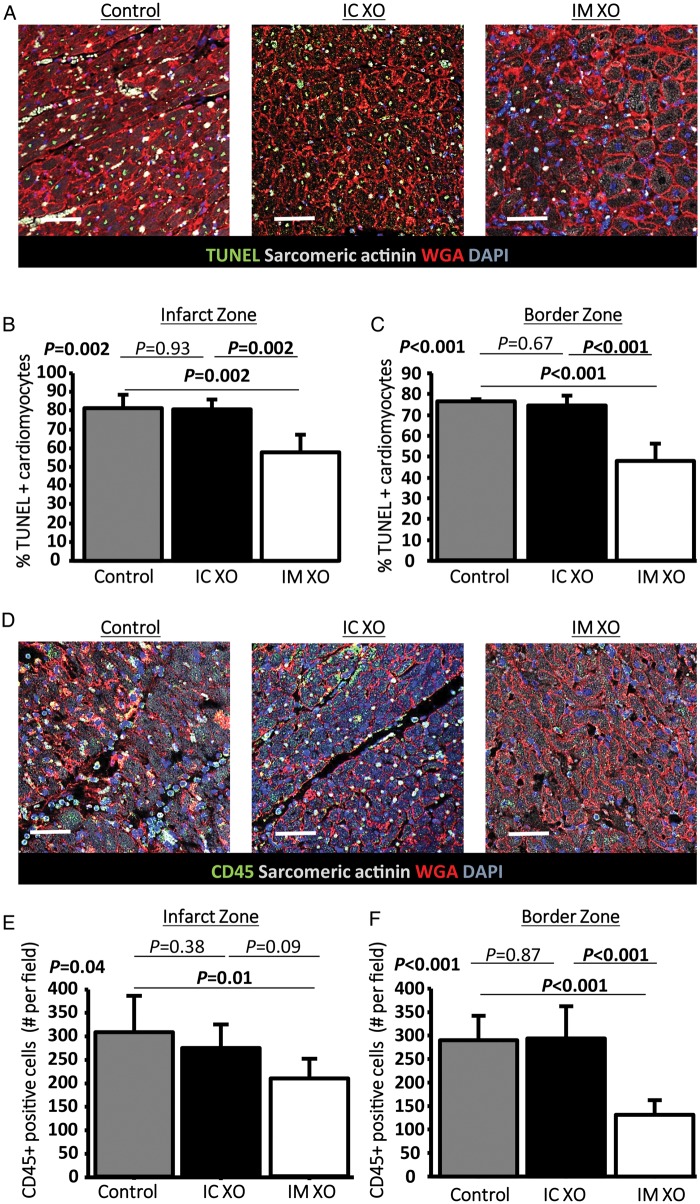
To determine whether the decreased IS and preserved function were related to decreased apoptosis or to decreased inflammatory cell infiltration, these processes were investigated using terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and CD45 staining respectively. TUNEL (representative images in Figure 2A) revealed that the proportion of TUNEL-positive cardiomyocytes was reduced in the IM exosome treated compared with control and IC exosome pigs, a finding that was observed both in the infarct (Figure 2B) and border zones (Figure 2C). In parallel, using CD45 staining (representative images, Figure 2D), we observed that leukocyte infiltration in the infarct zone (Figure 2E) and border (Figure 2F) was decreased by IM exosomes but not by IC exosomes compared with placebo.
Figure 2.
Acute myocardial infarction study: apoptosis and inflammation. (A) Representative images of TUNEL staining for apoptosis quantification in a control, an intracoronary exosome, and an intramyocardial exosome-treated pig (border zone). Pooled data show that cardiomyocyte apoptosis in the infarcted (B) and border (C) area is reduced by intramyocardial exosomes but not by intracoronary exosomes compared with control. (D) Representative images of CD45 staining for quantification of leukocyte infiltration in a control, an intracoronary exosome and an intramyocardial exosome-treated pig (border zone). Pooled data show that leukocyte infiltration in the infarcted (E) and border (F) area is reduced by intramyocardial exosomes but not by intracoronary exosomes compared with control. Scale bars = 50 μm.
Exosome retention
Having established that IM delivery of exosomes was superior to IC delivery, we hypothesized that this superior efficacy was related to higher myocardial retention of the exosomes after treatment. For this purpose, two pigs were injected with identical doses of labelled exosomes, one using IC infusion and one by IM injection. Three hours later, the hearts were harvested and imaged simultaneously for bioluminescence. Figure 1H shows that signal intensity was much higher after IM injection when compared with IC infusion of exosomes. This finding is consistent with the idea that the increased efficacy of IM exosomes is related to better retention within the heart.
Randomized pre-clinical study
With the acute study demonstrating that IM delivery of exosomes is superior to IC delivery, we proceeded to a randomized pre-clinical study using NOGA®-guided IM delivery of exosomes or placebo.
Safety and feasibility of NOGA®-guided exosome injection
A comprehensive LV map was obtained in all animals without complications. During injection, two pigs injected with vehicle and one pig injected with exosomes experienced sustained ventricular tachycardia and were cardioverted to a normal sinus rhythm. All animals were successfully injected with the assigned treatment.
Preservation of ventricular volumes and function
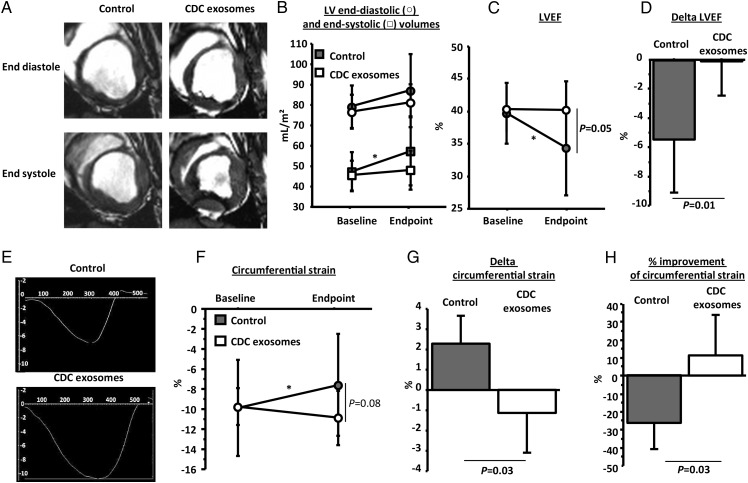
Figure 3A shows representative magnetic resonance images of the LV at end diastole and end systole in a control and an exosome-treated pig. Systolic function looks better in the exosome-treated animal. Pooled data confirm that LV end-systolic volume significantly increased in controls, but not in exosome-treated animals indicating attenuation of adverse ventricular remodelling (Figure 3B); meanwhile, LVEF was higher at endpoint in exosome-treated pigs, despite comparable volumes and LVEF in the two groups at the pre-treatment baseline (Figure 3B–D). In addition to LVEF, we further assessed systolic function by global circumferential strain measurements (representative data for the two groups shown in Figure 3E). As with LVEF, we observed that circumferential strain decreased in control but not in exosome-treated animals (Figure 3F–H).
Figure 3.
Randomized pre-clinical study in convalescent myocardial infarction: structure and function. (A) Representative short-axis end-diastolic (top) and end-systolic (bottom) magnetic resonance images at endpoint in control (left) and cardiosphere-derived cell exosome-treated (right) pigs. (B) Pooled data show that left ventricular end-diastolic and end-systolic volumes are similar in the two groups at baseline but end-systolic volume increases only in the vehicle group at endpoint (not in the cardiosphere-derived cell exosome-treated animals). Left ventricular ejection fraction is similar in the two groups at baseline but is higher in cardiosphere-derived cell exosome-treated pigs at endpoint (C), and the decrease in left ventricular ejection fraction is greater in the control-treated animals (D). (E) Representative images of global circumferential strain at endpoint in a cardiosphere-derived cell exosome and a control-treated pig. Pooled data show that circumferential strain is similar in the two groups at baseline (F) but improves (decrease of value shown in [G], absolute improvement in [H]) in the cardiosphere-derived cell exosome-treated pigs while it decreases in the control pigs at endpoint. * P< 0.05 vs. baseline (intra-group paired analysis).
Scar reduction
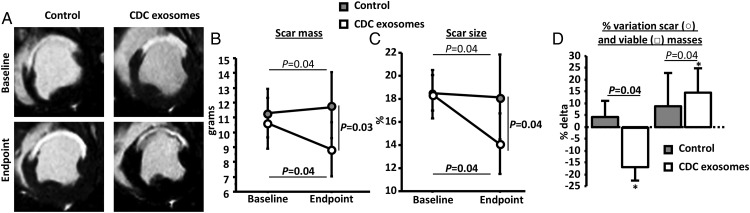
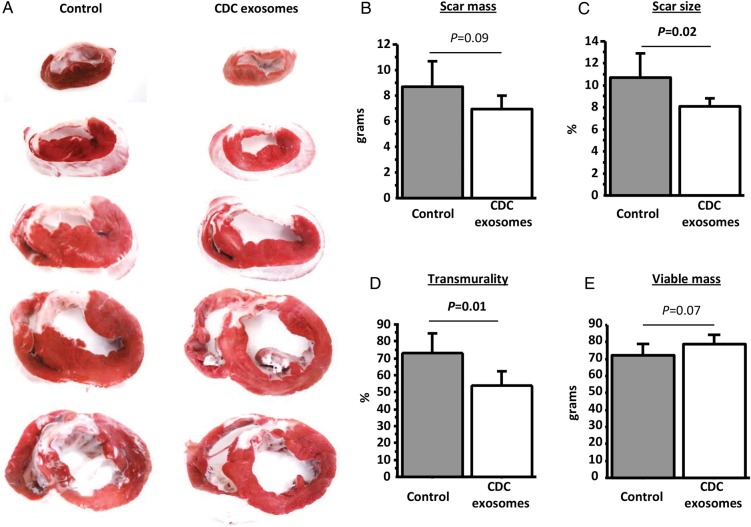
Scar mass was first measured by MRI using late-gadolinium enhancement. Figure 4A shows representative images from the two groups. At baseline, scar mass and scar size (scar mass/LV mass) were similar in the two groups (Figure 4B and C). After treatment, however, both scar mass and scar size decreased in the exosome group but not in the CDC group, leading to a smaller scar at endpoint (8.8 ± 1.7 g in the exosomes-treated group vs. 11.8 ± 2.1 g in the control group, P= 0.03, Figure 4B and C). Additionally, viable mass increased in exosome-treated animals but not in controls (Figure 4D), consistent with regrowth of myocardium. To check the MRI findings, scar was quantified by TTC staining post-mortem. Figure 5A shows heart sections for a control and an exosome-treated pig. The visible reductions in scar were evident in pooled data showing lower scar mass, scar size, and scar transmurality after exosome treatment (Figure 5B–D). In addition, we observed a non-significant trend for increased viable mass (measured by histology) in the exosome-treated pigs (Figure 5E). Prior work with CDCs in this model has shown that, while scar reductions are typically evident by 1 month, increases in viable mass develop more slowly and are usually significant only after 2 months.8,24,25
Figure 4.
Randomized pre-clinical study in convalescent myocardial infarction: scar mass and viable mass. (A) Representative MR images of short-axis late-gadolinium enhancement at baseline and endpoint in a control and a cardiosphere-derived cell exosome-treated pigs. Pooled data show that scar mass (B) and scar size (C) are similar at baseline in the two groups but decrease at endpoint in cardiosphere-derived cell exosome-treated pigs compared with control. Consequently, viable mass increases only in cardiosphere-derived cell exosome-treated pigs (D). * P< 0.05 vs. baseline (intra-group paired analysis).
Figure 5.
Randomized pre-clinical study in convalescent myocardial infarction: histological quantification of infarction. (A) Representative images of TTC stained heart from a control (left) and a cardiosphere-derived cell exosome-treated heart (right). Pooled data show that scar mass (B), scar size (C), and transmurality (D) at endpoint are lower in the cardiosphere-derived cell exosome-treated animals compared with control while viable mass tends to be higher (E).
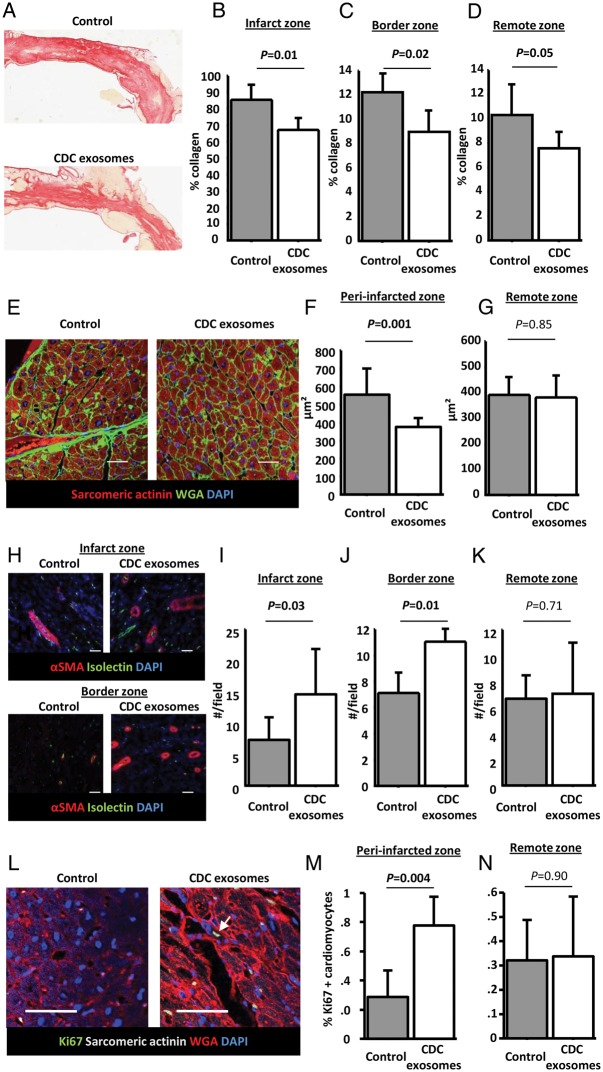
Histology: fibrosis, architecture, and angiogenesis
In addition to the MI scar, we looked for the evidence of exosome-related changes in remote fibrosis related to global remodelling. Collagen content of the infarcted, border, and remote zones was quantified using picrosirius red staining. Infarcted area from a control and an exosome-treated animal are shown in Figure 6A. Quantification revealed that collagen content was decreased not only in the infarct zone but also in the border and remote zones (Figure 6B–D). This finding suggests that exosome treatment not only decreased fibrosis at the site of injection but also had a more global anti-fibrotic effect. We also quantified cardiomyocyte cross-sectional area to look for changes in hypertrophy. Cardiomyocytes from the peri-infarct area (border zone and viable area of the infarct zone), but not those in the remote zone, were smaller in exosome-treated pigs (representative images: Figure 6E; pooled data: Figure 6F and G). Thus, in addition to decreased fibrosis, CDC exosomes prevented cardiomyocyte hypertrophy associated with adverse remodelling (although we cannot rule out a contribution of newly generated, smaller myocytes to the overall reductions in cell size).26
Figure 6.
Randomized pre-clinical study in convalescent myocardial infarction: fibrosis, vascular density, and cardiomyocyte proliferation. (A) Representative images of infarcted area stained with picrosirius red in a control and a cardiosphere-derived cell exosome-treated pig. Pooled data show that collagen content of the infarcted area (B), the border zone (C), and the remote area (D) at endpoint is lower in cardiosphere-derived cell exosomes-treated pigs compared with control. (E) Representative images of cross-sectional area in a control and a cardiosphere-derived cell exosome-treated pig. Pooled data show that cardiomyocytes of cardiosphere-derived cell exosome-treated pigs are smaller in the peri-infarcted area (F) but not in the remote area (G). (H) Representative images of arteriole density in a control and a cardiosphere-derived cell exosome-treated pig (infarct and border zone). Pooled data show that, compared with control, cardiosphere-derived cell exosomes increase vascular density at endpoint in the infarct (I) and border area (J) but not in the remote area (K). (L) Representative images of Ki67 staining in the peri-infarct area in a control and a cardiosphere-derived cell exosome-treated pig (arrow shows a Ki67 positive cardiomyocyte). Pooled data show that proliferation of cardiomyocytes in the peri-infarct area (M) but not in the remote area (N) is higher in cardiosphere-derived cell exosome-treated pigs than in control. Scale bars = 50 μm.
Since CDCs have angiogenic properties,6,21 we hypothesized that exosomes were likely to favour this process. To test this idea, we measured arteriolar density in the infarcted, remote, and border zones (representative pictures for infarct and border area shown in Figure 6H). As expected, we observed a higher number of arterioles in both the infarct and border zones of the exosome-treated pigs when compared with control (Figure 6I–J). No difference was observed in the remote zone (Figure 6K).
To confirm the regrowth of myocardium suggested by the increased viable mass and decreased cardiomyocyte size, we investigated cardiomyocyte proliferation using Ki67 staining (representative pictures shown in Figure 6L). We observed an increased proliferation of cardiomyocytes in the peri-infarct zone of exosome-treated pigs compared with control (Figure 6M), providing further evidence for regrowth of myocardium. No difference was observed in the remote zone (Figure 6N).
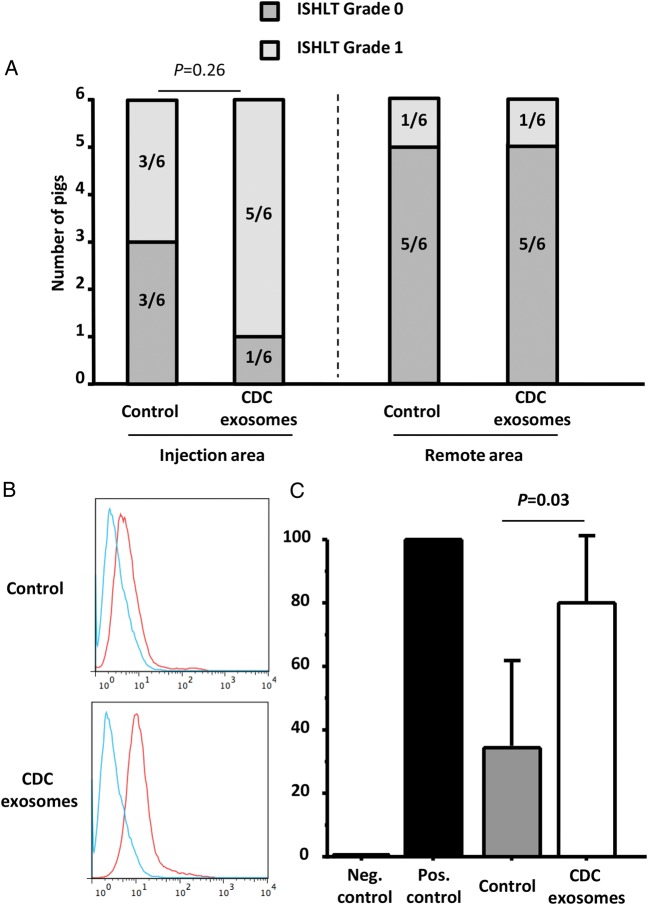
Immunogenicity
Histology revealed no significant treatment-related differences in inflammatory infiltrates in the tissue (Figure 7A). Importantly, no cardiomyocyte necrosis was observed in any animal. Higher levels of allo-antibodies were observed in CDC exosome-treated animals when compared with placebo (Figure 7B). However, all pigs (placebo and CDC exosome treated) had detectable circulating antibodies at lower levels than in the positive control. Therefore, the significance of this finding is uncertain.
Figure 7.
Randomized pre-clinical study in convalescent myocardial infarction: immunology. (A) Cellular reactions in the injection area and remote area are not different between control and exosome-treated animals. (B) Representative data of allo-antibodies quantification by flow cytometry in a control and an exosome-treated animal (blue is negative control, red is the animal serum). Pooled data show that allo-antibodies were detected in both control and exosome-treated animals, although at higher levels in the latter (C).
Mode of delivery in convalescent myocardial infarction
To verify that IC delivery was inefficacious in CMI, as it had been in AMI, we performed additional experiments (see Supplementary material online, Figure S5). Cardiosphere-derived cell exosomes delivered IC did not improve LVEF, scar mass or scar size. These data confirm the generalization that IM delivery of CDC exosomes works well and consistently, but IC delivery does not.
Discussion
Two phase 1 clinical trials of cardiosphere-derived cells have been completed,5,7,27 and other trials are ongoing for indications ranging from convalescent/chronic MI (ALLSTAR),3 to heart failure with reduced EF (DYNAMIC28), and Duchenne cardiomyopathy (HOPE-Duchenne).29 Exosomes secreted by CDCs appear to replicate the cardioprotective20 and regenerative effects of CDCs,9,10 but so far the evidence is limited to in vitro work and to rodent models. Here, we demonstrate the effects of exosomes in large-animal models of acute and convalescent MI. In both of these models, IM delivery of CDC exosomes mimicked the structural and functional benefits of CDCs, but IC delivery did not. The salutary effects included attenuation of adverse remodelling, preserved LVEF, and decreased scar size relative to placebo controls.
Delivery of exosomes for clinical translation
Given that exosomes are nano-sized particles (<200 nm [Figure 1], when compared with ∼20 µm for CDCs),4 their homing to heart tissue following IC delivery is uncertain when compared with the mechanism of CDC extravasation (vascular occlusion by CDCs followed by endothelial pocketing) into tissue.30 Here we showed that the therapeutic effects of CDC-derived exosomes are almost non-existent after IC infusion but are highly significant when IM delivery is performed. Such findings were observed both in acute (Figure 1) and chronic MI (Figure 4 and see Supplementary material online, Figure S5). Exosome tracking by bioluminescence revealed greater myocardial retention after IM injection than after IC infusion. Altogether, these results are consistent with the idea that IC exosomes are not very efficacious because first-pass retention of these tiny, non-occlusive vesicles is low. The finding of a decrease in MVO may reflect trapping of some exosomes in sluggishly perfused areas of the myocardium, where they dwell sufficiently long to be taken up and exert their bioactivity. However, this hypothesis remains speculative.
Mechanism of action of cardiosphere-derived cell exosomes
Cardiosphere-derived cells have shown efficacy in different indications including acute and convalescent MI.4–8,17,18 Here, we demonstrated that exosomes recapitulate the effect of CDCs in those two indications. Moreover, the magnitude of acute IS decrease (in the AMI model) and attenuation of adverse remodelling (in the CMI model) are comparable, if not somewhat greater, to what had been observed with CDCs in similar models (see Supplementary material online, Figure S6). This finding suggests that exosomes suffice to mediate the entire effect of CDCs; they are not just part of the story. The ability of exosomes to halt both pro-inflammatory and pro-fibrotic pathways is believed to reside in their unique miRNA (and other non-coding RNAs13) payload and their internalization by macrophages, fibroblasts, and cardiomyocytes.10,31 Indeed, exosomes can ‘reprogram’ fibroblasts, fundamentally altering the phenotype of these cells.10,32,33 Moreover, CDC-derived exosomes modify the content of exosomes secreted by recipient fibroblasts, thus leading to an amplification of the therapeutic effect. This modification of the recipient fibroblasts will lead to increased collagen degradation by MMPs and decreased collagen production (through TGF-β pathway inhibition), leading to decreased scar content.25 These concepts help to rationalize why relatively low doses of CDCs are effective, and why benefits persist for months after single doses. It also explains how exosomes are able to decrease MVO and IS even when infused 30 min after reperfusion, while other cardioprotective techniques fail to decrease IS past the first minutes of reperfusion.34
Immunogenicity
We have previously shown that allogeneic CDCs do not instigate a major immune response.6,8,35 Cardiosphere-derived cells express MHC1 but do not express MHC2, CD80, and CD86. The absence of MHC2 gives CDCs the potential to escape recognition by CD4 lymphocytes. Moreover, CD80 and CD86, which are required for the induction of effector lymphocytes, are lacking. Another reason why allogeneic CDCs do not instigate an immune response is that they secrete many paracrine factors that tend to decrease the local inflammatory reaction and the infiltration of inflammatory cells in the recipient. However, although CDCs are immunomodulatory cells, some questions remain unanswered particularly if repeat dosing is to be considered. One of the potential advantages of exosomes over CDCs is that they are acellular and therefore likely to be less immunogenic than CDCs. Here, we injected human exosomes into non-immunosuppressed pigs. Xenogeneic CDC therapy has been shown to be ineffective and highly immunogenic.6,35 Here, treatment with xenogeneic exosomes recapitulates the entire benefit profile of auto- or allogeneic CDCs without apparent adverse effects. Histological grade 1 rejection was observed in both exosome-treated and control pigs, suggesting that this inflammatory reaction is more likely to be related to the MI, the needle puncture, or the IMDM (the vehicle used to resuspend the exosomes) rather than the exosomes themselves. Given our favourable results with the worst-case scenario of xenogeneic exosome therapy in a large-animal model, the use of allogeneic CDC-derived exosomes in humans is likely to be safe. The presence of allo-antibodies is difficult to interpret since some are also observed in vehicle-treated animals. Whether such antibodies will undermine the efficacy of repeated treatment with CDC-derived exosomes is unclear and will require additional studies. Such studies are needed, as repeat dosing may be necessary in diseases with ongoing myocardial degeneration (e.g. genetic cardiomyopathies).
Limitations
One limitation of this study is that the MIs were performed on young healthy animals. Although the two models recapitulate the key features of MI with adverse ventricular remodelling, the effect of exosomes on older human patients with cardiovascular risk factors remains uncertain. The effects of exosomes may differ in senescent cells with altered genetic or epigenetic content. Another limitation is that, in the AMI study, LVEF and LV volumes were measured using contrast ventriculography. Magnetic resonance imaging (which has been used in the CMI model) is the gold standard, but practical timing constraints make it impossible to interpolate an MRI between the time of reflow and the time of therapy. Nevertheless, histological assessments of MVO and scar size (and not LV remodelling) were the key endpoints in the AMI model.
Conclusion
Human CDC-derived exosomes can decrease acute ischaemia-reperfusion injury, and halt chronic post-MI adverse remodelling in pigs when delivered by IM injection. These findings suggest that CDC-derived exosomes may be an attractive cell-free product for the treatment of ischaemic heart disease in humans.
Supplementary material
Supplementary material is available at European Heart Journal online.
Authors’ contributions
R.G. performed statistical analysis; E.M., L.M., B.G. handled funding and supervision; R.G., J.D., J.V., E.S., G.d.C., R.M., E.T., D.L., M.K., R.R.S. acquired the data; R.G., J.D., E.M. conceived and designed the research; R.G., J.D., E.M. drafted the manuscript; J.V., E.S., G.d.C., R.M., E.T., D.L., M.K., R.R.S., L.M., B.G., E.M. made critical revision of the manuscript for key intellectual content.
Funding
This study was funded by NIH R01HL124074 to E.M. and the Cedars-Sinai Board of Governors Heart Stem Cell Center. R.G. received a grant from the French Society of Cardiology. J.D. is supported by NIH T32HL116273.
Conflict of interest: E.M. and L.M. own equity in Capricor Inc. L.M., R.R.S. and M.K. are employed by Capricor Inc.
Supplementary Material
References
- 1. Schmidt M, Jacobsen JB, Lash TL, Botker HE, Sorensen HT. 25 year trends in first time hospitalisation for acute myocardial infarction, subsequent short and long term mortality, and the prognostic impact of sex and comorbidity: a Danish nationwide cohort study. Br Med J 2012;344:e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organisation. Global atlas on cardiovascular disease prevention and control. 2011. [Google Scholar]
- 3. Allogeneic Heart Stem Cells to Achieve Myocardial Regeneration (ALLSTAR) (NCT01458405) http://clinicaltrials.gov/ct2/show/NCT01458405?term=allstar&rank=1 (28 May 2016, date last accessed). [DOI] [PMC free article] [PubMed]
- 4. Johnston PV, Sasano T, Mills K, Evers R, Lee ST, Smith RR, Lardo AC, Lai S, Steenbergen C, Gerstenblith G, Lange R, Marban E. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation 2009;120:1075–1083, 7 p following 1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LEJ, Berman D, Czer LSC, Marbán L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marbán E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. The Lancet 2012;379:895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Malliaras K, Li TS, Luthringer D, Terrovitis J, Cheng K, Chakravarty T, Galang G, Zhang Y, Schoenhoff F, Van Eyk J, Marban L, Marban E. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation 2012;125:100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Malliaras K, Makkar RR, Smith RR, Cheng K, Wu E, Bonow RO, Marban L, Mendizabal A, Cingolani E, Johnston PV, Gerstenblith G, Schuleri KH, Lardo AC, Marban E. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J Am College Cardiol 2014;63:110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Malliaras K, Smith RR, Kanazawa H, Yee K, Seinfeld J, Tseliou E, Dawkins JF, Kreke M, Cheng K, Luthringer D, Ho CS, Blusztajn A, Valle I, Chowdhury S, Makkar RR, Dharmakumar R, Li D, Marban L, Marban E. Validation of contrast-enhanced magnetic resonance imaging to monitor regenerative efficacy after cell therapy in a porcine model of convalescent myocardial infarction. Circulation 2013;128:2764–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ibrahim AG, Cheng K, Marban E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports 2014;2:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tseliou E, Fouad J, Reich H, Slipczuk L, de Couto G, Aminzadeh M, Middleton R, Valle J, Weixin L, Marban E. Fibroblasts rendered antifibrotic, antiapoptotic, and angiogenic by priming with cardiosphere-derived extracellular membrane vesicles. J Am Coll Cardiol 2015;66:599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, Ito A, Liu T, Kamide C, Agrawal H, Perlman H, Qin G, Kishore R, Losordo DW. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res 2011;109:724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sahoo S, Losordo DW. Exosomes and cardiac repair after myocardial infarction. Circ Res 2014;114:333–344. [DOI] [PubMed] [Google Scholar]
- 13. Cambier L, De Couto G, Ibrahim A, Marban E. Y RNA Fragments Enriched in Exosomes From Cardiosphere-derived Cells Mediate Cardioprotection and Macrophage Polarization. AHA Scientific Sessions 2015 Abstract 16009 2015. [Google Scholar]
- 14. Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol 2002;2:569–579. [DOI] [PubMed] [Google Scholar]
- 15. Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, Torre T, Siclari F, Moccetti T, Vassalli G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res 2014;103:530–541. [DOI] [PubMed] [Google Scholar]
- 16. Vicencio JM, Yellon DM, Sivaraman V, Das D, Boi-Doku C, Arjun S, Zheng Y, Riquelme JA, Kearney J, Sharma V, Multhoff G, Hall AR, Davidson SM. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J Am Coll Cardiol 2015;65:1525–1536. [DOI] [PubMed] [Google Scholar]
- 17. de Couto G, Liu W, Tseliou E, Sun B, Makkar N, Kanazawa H, Arditi M, Marban E. Macrophages mediate cardioprotective cellular postconditioning in acute myocardial infarction. J Clin Invest 2015;125:3147–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kanazawa H, Tseliou E, Malliaras K, Yee K, Dawkins JF, De Couto G, Smith RR, Kreke M, Seinfeld J, Middleton RC, Gallet R, Cheng K, Luthringer D, Valle I, Chowdhury S, Fukuda K, Makkar RR, Marban L, Marban E. Cellular postconditioning: allogeneic cardiosphere-derived cells reduce infarct size and attenuate microvascular obstruction when administered after reperfusion in pigs with acute myocardial infarction. Circ Heart Fail 2015;8:322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heusch G, Skyschally A, Schulz R. The in-situ pig heart with regional ischemia/reperfusion - ready for translation. J Mol Cell Cardiol 2011;50:951–963. [DOI] [PubMed] [Google Scholar]
- 20. de Couto G, Durvasula P, Ibrahim A, Marban E. Intracoronary delivery of exosomes secreted by cardiosphere-derived cells confers cardioprotection with delayed administration after ischemia-reperfusion injury in rats. AHA Scientific Sessions 2014 Abstract 15845 2014. [Google Scholar]
- 21. Gallet R, Tseliou E, Dawkins J, Middleton R, Valle J, Angert D, Reich H, Luthringer D, Kreke M, Smith R, Marban L, Marban E. Intracoronary delivery of self-assembling heart-derived microtissues (cardiospheres) for prevention of adverse remodeling in a pig model of convalescent myocardial infarction. Circ Cardiovasc Interv 2015;8:pii: e002391. doi: 10.1161/CIRCINTERVENTIONS.115.002391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chernyshev VS, Rachamadugu R, Tseng YH, Belnap DM, Jia Y, Branch KJ, Butterfield AE, Pease LF III, Bernard PS, Skliar M. Size and shape characterization of hydrated and desiccated exosomes. Anal Bioanal Chem 2015;407:3285–3301. [DOI] [PubMed] [Google Scholar]
- 23. Phuyal S, Skotland T, Hessvik NP, Simolin H, Overbye A, Brech A, Parton RG, Ekroos K, Sandvig K, Llorente A. The ether lipid precursor hexadecylglycerol stimulates the release and changes the composition of exosomes derived from PC-3 cells. J Biol Chem 2015;290:4225–4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tseliou E, de Couto G, Terrovitis J, Sun B, Weixin L, Marban L, Marban E. Angiogenesis, cardiomyocyte proliferation and anti-fibrotic effects underlie structural preservation post-infarction by intramyocardially-injected cardiospheres. PLoS ONE 2014;9:e88590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tseliou E, Reich H, de Couto G, Terrovitis J, Sun B, Liu W, Marban E. Cardiospheres reverse adverse remodeling in chronic rat myocardial infarction: roles of soluble endoglin and TGF-beta signaling. Basic Res Cardiol 2014;109:443. [DOI] [PubMed] [Google Scholar]
- 26. Malliaras K, Zhang Y, Seinfeld J, Galang G, Tseliou E, Cheng K, Sun B, Aminzadeh M, Marban E. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Mol Med 2013;5:191–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Makkar R, Schatz R, Traverse J, Hamer A, Beattie K, Smith RR, Kivel F, Marban L, Marban E. ALLogeneic Heart STem Cells to Achieve Myocardial Regeneration (ALLSTAR): the One Year Phase I Results. AHA Scientific Session 2014 Abstract 20536 2014. [Google Scholar]
- 28. Dilated cardiomYopathy iNtervention With Allogeneic MyocardIally-regenerative Cells (DYNAMIC) (NCT02293603) https://clinicaltrials.gov/ct2/show/nct02293603 (28 May 2016, date last accessed). [DOI] [PubMed]
- 29. Halt cardiomyOPathy progrEssion in Duchenne (HOPE-Duchenne) (NCT02485938) https://clinicaltrials.gov/ct2/show/NCT02485938 (28 May 2016, date last accessed).
- 30. Cheng K, Shen D, Xie Y, Cingolani E, Malliaras K, Marban E. Brief report: Mechanism of extravasation of infused stem cells. Stem Cells 2012;30:2835–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Couto G, Makkar N, Marbán E. Cardiosphere-derived cell exosomes confer acute cardioprotection following ischemia-reperfusion injury through macrophage polarization. AHA Scientific Sessions 2015 2015. [Google Scholar]
- 32. Quesenberry PJ, Aliotta J, Deregibus MC, Camussi G. Role of extracellular RNA-carrying vesicles in cell differentiation and reprogramming. Stem Cell Res Ther 2015;6:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gray WD, French KM, Ghosh-Choudhary S, Maxwell JT, Brown ME, Platt MO, Searles CD, Davis ME. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ Res 2015;116:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heusch G. Treatment of myocardial ischemia/reperfusion injury by ischemic and pharmacological postconditioning. Compr Physiol 2015;5:1123–1145. [DOI] [PubMed] [Google Scholar]
- 35. Tseliou E, Pollan S, Malliaras K, Terrovitis J, Sun B, Galang G, Marban L, Luthringer D, Marban E. Allogeneic cardiospheres safely boost cardiac function and attenuate adverse remodeling after myocardial infarction in immunologically mismatched rat strains. J Am Coll Cardiol 2013;61:1108–1119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.