Why was the cohort set up?
The infant mortality rate(IMR) of 40 per 1000 live births in India is seven times higher than that in the USA1,2 and contributes one-quarter of the world’s newborn deaths.3 Despite efforts to reduce the number of infant deaths through social welfare programmes and other interventions,4 IMR remains unacceptably high in India, though infant mortality rates have stabilized in recent years.5
Predictors of infant mortality in India include socio-demographic factors including maternal age and prenatal exposures including malnutrition.6–8 Further, prematurity, low birthweight (LBW), neonatal infections and birth asphyxia have been associated with an increased risk of infant mortality in this population. Indeed, the major causes of under-five morbidity and mortality are LBW and preterm birth.9 Newborns weighing less than 1500 g have approximately a 100-fold higher mortality risk than newborns at optimum weight.10–12 In India, efforts to reduce infant mortality have largely focused on increasing BW.5,13,14 Despite a large-scale national programme providing prenatal nutritional supplements for over three decades, about 30% of Indian births remain LBW.15
In rural India, micronutrient deficiencies such as low levels of iron, folic acid and vitamin B12 and malnutrition in pregnant women have been associated with an increased risk of a LBW or a small for gestational age (SGA) infant.16,17 Further, younger maternal age and inadequate prenatal care have been associated with LBW.18,19 Other exposures in this population which may increase the risk of infant morbidity and mortality include consanguineous marriage,20–23 chronic parasitic infections that can further exacerbate malnutrition and anaemia,24–26 and contaminated drinking water.27–28
The LIFE cohort was established in 2009 with broad aims to examine how environmental, infectious, lifestyle, metabolic and genetic factors impact on birth outcomes and early childhood health and development. With continued follow-up, we will also examine long-term effects into adolescence and early adulthood. In addition, we plan to examine chronic disease in mothers and fathers as this generation undergoes the epidemiological transition. The ultimate goal is to identify modifiable risk factors and develop interventions to improve the health of children and their families in this region. Specific research objectives that are currently being investigated include the relationship of gestational weight gain (GWG) with birth outcomes, maternal anaemia with birth outcomes, risk factors for infant mortality and quantifying the burden of childhood developmental disorders and delays. We are also working to determine the contribution of maternal vaginal and intestinal infections to preterm birth, spontaneous abortion, stillbirth, LBW and infant mortality. This study was approved by the SHARE INDIA/MediCiti Institute of Medical Sciences Ethics Committee.
The LIFE cohort is based in a rural to peri-urban area of Telangana State, India, about 40 km from the city of Hyderabad. The study is being conducted by SHARE INDIA, a non-government research and health care organization which includes a medical school, a nursing school and a hospital that serves the local population residing in the two adjoining ‘mandals’, Medchal and Shamirpet. The Rural Effective Affordable Comprehensive Healthcare (REACH) Project, a major project of SHARE INDIA, has been providing maternal and child health services since 1999 for a population of 43 270 in 40 villages in Medchal Mandal.28 With these efforts, an IMR of about 40 was achieved in the project area 10 years ago, compared with a much higher rate of 64 for the rest of undivided rural Andhra Pradesh at that time.29 Since then it has plateaued at this level in the Medchal region.30
In 2008, SHARE INDIA in collaboration with the University of Pittsburgh planned and developed the LIFE Pilot Study. A series of focus groups were conducted to discuss the idea of the LIFE Study with local women and community leaders. We developed and piloted recruitment methods and materials, questionnaires and protocols in two villages of Medchal Mandal. These efforts revealed community concern about several issues including cost of medical care, exposures to water and air pollution, and culturally sensitive maternal health issues including postpartum depression and infertility. A general desire of husbands to participate in the study was noted. These revelations led to some changes in study design and protocols, which were then piloted and finalized.
Funding for the LIFE Cohort Study has come primarily from SHARE INDIA Research Foundation and their fundraising efforts. A sub-study of pre-pregnancy and prenatal vaginal infections and adverse pregnancy outcomes has been favourably reviewed by the NIH and ICMR.
Who is in the cohort?
The LIFE cohort comprises married women between 15 and 35 years of age (mean 22 years), recruited beforepregnancy or in the first trimester of pregnancy, from 2009 to 2011. Recruitment utilized the existing infrastructure of the REACH Project, described earlier. In each of the villages in the mandal, a community health volunteer (CHV) has been recruited to visit each family once a month. These CHVs focus on women in the village to ascertain pregnancy (by interview) and to educate and encourage the women to seek regular antenatal care and other health care services. REACH has enumerated all household members in these communities and mapped each dwelling by a geographical information system (GIS). During each visit, CHVs conduct interviews to collect and update information on demography and pregnancy. Since 2004, CHVs have been collecting data on infant deaths and birthweights in the population. Socio-demographic variables such as access to electricity, means of transportation and possession of audio-visual devices were collected from REACH database (Table 1).
Table 1.
Comparison of characteristics of women who enrolled and those who refused enrolment in the LIFE pilot study
| LIFE cohort N = 1227 n (%) | Refused N = 395 n (%) | p-Valueβ | ||
|---|---|---|---|---|
| Women’s age n (%) | ||||
| < 19 years | 70 (5.7) | 22 (5.6) | ||
| 19–24 years | 972 (79.2) | 247 (62.5) | ||
| ≥ 25 years | 185 (15.1) | 126 (31.9) | <0.001 | |
| Men’s age | ||||
| < 19 years | 3 (0.2) | 2 (0.5) | ||
| 19–24 years | 440 (35.9) | 88 (22.3) | ||
| ≥ 25 years | 784 (63.9) | 305 (77.2) | <0.001 | |
| Parity | ||||
| 0 | 204 (16.6) | 84 (21.2) | ||
| 1 | 232 (18.9) | 82 (20.8) | ||
| ≥ 2 | 791 (64.5) | 229 (58.0) | <0.001 | |
| Number of people living in home | ||||
| ≤ 4 | 673 (54.8) | 80(20.3) | ||
| 4–8 | 467(38.1) | 71(18.0) | ||
| ≥ 9 | 87(7.1) | 244(61.8) | <0.001 | |
| Distance of the village to MediCiti hospital in km | ||||
| ≤ 14 km | 633 (51.6) | 153 (38.7) | ||
| > 14 km | 594 (48.4) | 242(61.3) | <0.001 | |
| Family type | ||||
| Joint | 723 (59.0) | 277 (70.1) | ||
| Nuclear | 504 (41.0) | 118 (29.9) | <0.0001 | |
| Religion | ||||
| Hindu | 1099 (89.5) | 343 (87.0) | ||
| Muslim | 71 (5.8) | 35 (8.9) | ||
| Christian | 57 (4.7) | 16 (4.1) | 0.084 | |
| Caste (%) | ||||
| Scheduled caste | 282 (23.0) | 45 (11.5) | ||
| Scheduled tribe | 87 (7.1) | 62 (15.6) | ||
| Backward caste | 664 (54.1) | 202 (51.2) | ||
| None of the above | 194 (15.8) | 85 (21.7) | <0.0001 | |
| Availability of electricity (%) | ||||
| Yes | 1215 (99.0) | 391 (99.0) | ||
| No | 12 (1.0) | 4 (1.0) | 0.973 | |
| Availability of personal transport (%)α | ||||
| Yes | 612 (49.9) | 234 (59.3) | ||
| No | 615 (50.1) | 158 (40.0) | <0.0001 | |
| Audio-visual devices (%)α | ||||
| Television | 993 (80.9) | 262 (66.4) | <0.0001 | |
| Radio | 43 (3.8) | 10 (2.5) | 0.2456 | |
| NA | 191 (15.6) | 123 (31.1) | <0.0001 | |
NA, Data not available, αSource: REACH database, βUnpaired t-test for continuous and overall chi-square test of proportions for categorical variables. Availability of personal transport was not measured for 3 participants.
Recruitment of investigators for the LIFE Pilot Study began in the fall of 2009. They were given a fortnight’s intensive training by lectures and demonstration. The required number of barcodes for all study data forms and specimens was generated on the first day of survey for each study participant. Inter-laboratory exchange of biological specimens was carried out as part of quality control. Instruments were periodically checked for accuracy.
Women were included in the LIFE Study if they were currently married, between 15 and 35 years of age, and lived in an eligible REACH village. Women were excluded if she had or was planning tubectomy, oophorectomy or hysterectomy or her husband had undergone vasectomy, if the couple was using birth control method and planning for no further children or if the woman was pregnant beyond the first trimester. Women and their husbands were first approached by the local CHVs. If the woman agreed to participate, LIFE field staff members would visit her home, present the study, answer questions and obtain written informed consent. Enrolment was considered complete when the participant completed the enrolment questionnaire and provided laboratory samples (Table 8).
Table 8.
Laboratory tests completed
| Registration | 1st trimester | 3rd trimester | Delivery | 1 month postpartum | |
|---|---|---|---|---|---|
| Blood analysis | |||||
| Lipid profile | ✓ | ✓ | ✓ | ✓ | |
| Haemoglobin | ✓ | ✓ | ✓ | ✓ | ✓ |
| Cord haemoglobin | ✓ | ||||
| Haematocrit | ✓ | ✓ | ✓ | ✓ | ✓ |
| Cord haematocrit | ✓ | ||||
| Fasting blood sugar | ✓ | ✓ | ✓ | ✓ | |
| Random blood sugar | ✓ | ||||
| HBA1c | ✓ | ||||
| Serum creatinine | ✓ | ✓ | ✓ | ✓ | ✓ |
| Thyroid function (TSH, FT3, FT4) | ✓ | ✓ | ✓ | ✓ | |
| Malaria | ✓ | ✓ | ✓ | ||
| Stool analysis: | |||||
| Microscopy for ova and cysts | ✓ | ✓ | ✓ | ✓ | ✓ |
| Vaginal swab analysis: | |||||
| Microscopy for clue cells (bacterial vaginosis) | ✓ | ✓ | ✓ | ✓ | ✓ |
| Gonorrhea/chlamydia PCR | |||||
| Urine analysis: | |||||
| Albumin | ✓ | ✓ | ✓ | ✓ | ✓ |
| Sugar | ✓ | ✓ | ✓ | ✓ | ✓ |
| Nitrite | ✓ | ✓ | ✓ | ✓ | ✓ |
| Placenta analysis: | |||||
| Swabs | ✓ | ||||
| Biopsy | ✓ |
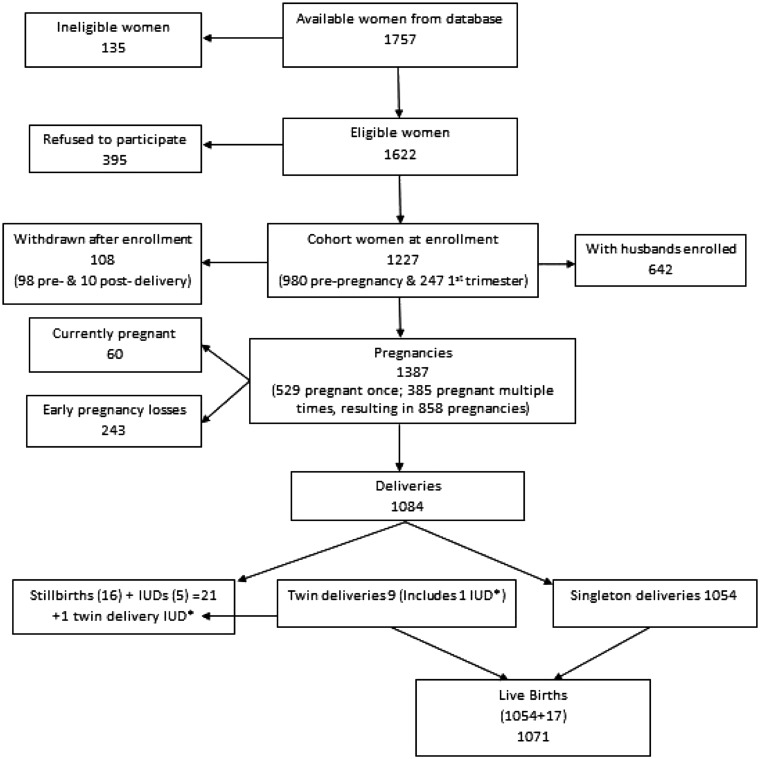
Based on the REACH database, 1757available women were deemed eligible to participate. Of these, 135 were found ineligible and 395 refused to participate. The remaining 1227 women were enrolled along with 642 husbands. Of the 585 non-participating husbands, 215 refused because they felt the study was relevant for pregnant women, 176 were unavailable due to working conditions and 194 chose not to participate for miscellaneous reasons. After enrolment, 108 women withdrew from the study. A flow chart for participant recruitment is included in Figure 1, and Table 1 compares REACH database characteristics of eligible women who agreed to participate with those who did not. Women who refused to participate were slightly older, had slightly older husbands, had more people living in their home, were less likely to have a television, and were more likely to have a joint family, be Muslim, be in a scheduled tribe and have personal transportation. Table 2 presents the LIFE study database demographic and health characteristics of the women and men in the study.
Figure 1.
Flow chart of participant recruitment. IUD, Intrauterine death.
Table 2.
Cohort Descriptive Statistics at Enrollment
| Women (N = 1227) n (%) | Men (N = 642) n (%) | |
|---|---|---|
| Age mean (SD) | 22.0 (3.0) | 27.8 (4.0) |
| <19 years | 70 (5.7) | 0 (0) |
| 19 – 24 years | 972 (79.2) | 212 (33.0) |
| ≥25 years | 185 (15.1) | 430 (67.0) |
| Illiterate | 202 (16.5) | 60 (9.4) |
| Religion | ||
| Hindu | 1098 (89.5) | 575 (89.5) |
| Muslim | 71 (5.8) | 40 (6.3) |
| Christian | 58 (4.7) | 31 (4.2) |
| Caste | ||
| Scheduled Caste | 283 (23.1) | 149 (23.2) |
| Scheduled Tribe | 87 (7.1) | 31 (4.9) |
| Backward Caste | 664 (54.1) | 352 (54.8) |
| None of the above | 193 (15.7) | 110 (17.1) |
| Occupation | ||
| Work in home(Homemaker) | 918 (74.8) | 9 (1.4) |
| Work outside home | 309 (25.2) | 633 (98.6) |
| Type of work outside home | ||
| Agricultural work | 497 (40.5) | 68 (10.6) |
| Labor | 39 (3.2) | 43 (6.7) |
| Factory worker | 67 (5.5) | 5 (0.8) |
| Domestic service | 9 (0.7) | 8 (1.3) |
| Retail | 16 (1.3) | 18 (2.8) |
| Private service | 504 (41.1) | 379 (59.0) |
| Artisan | 36 (2.9) | 15 (2.3) |
| Government service | 4 (0.3) | 5 (0.8) |
| Other | 55 (4.5) | 92 (14.3) |
| Education level, less than 7th Standard | 130 (10.6) | 10.6 |
| Age at marriage in years, mean (SD) | 18.9 (2.48) | 24.5(3.40) |
| Consanguineous marriage | 280 (22.8) | NA |
| Health status | ||
| BMI (kg/m2) (n = 980α) | ||
| Underweight (< 18.5) | 337 (34.4) | 99 (15.4) |
| Normal (≥ 18.5 and < 23) | 437 (44.6) | 269 (41.9) |
| Overweight/obese (≥ 23) | 206 (21.0) | 274 (42.7) |
| Alcohol consumption | 274 (22.3) | 443 (69) |
| Smoking, active | 0 (0.0) | 140 (21.8) |
| Smoking, passive | 255 (20.8) | 50 (7.8) |
| Medical conditions | ||
| Asthma | 4 (0.3) | 16 (2.5) |
| Goitre | 7 (0.6) | 1 (0.2) |
| Hypertension | 4.7 (N = 980α) | 18.2 (642) |
| Diabetes | 1.1 % (N = 980α) | 3.1 (642) |
Hypertension defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg and/or currently on medication. Diabetes defined as fasting blood sugar (FBS) >125 and/or currently on medication.
αExcludes pregnant women at the time of registration.
As of the end of 2014, there had been 1387 pregnancies. Sixty women were currently pregnant. There were 1084 deliveries [1071 live births and 22 stillbirths and intra-uterine death (IUD) deliveries] and 243 early pregnancy losses (Figure 1). Outcomes of 1387 pregnancies are shown in Table 3. In the study, 385 women contributed more than one pregnancy. A total of 27 infant and child deaths have occurred.
Table 3.
Pregnancy characteristics
| n (%) | |
|---|---|
| Pregnant at enrolment | 247(20.1) |
| Enrolled women who had at least 1 pregnancy | 938(76.4) |
| Total number of pregnancies | 1387 |
| Early pregnancy lossβ | 243 (17.6) |
| Total number of deliveries (n = 1048) | |
| Singleton live deliveries | 1054 (97.2) |
| Twin deliveries | 9 (0.8) |
| Stillbirths and IUD | 21 (1.9) |
| Birthweight for live births (n = 1071) | |
| Low birthweight (< 2.5 kg) | 217 (20.3) |
| Normal birthweight (≥ 2.5 kg) | 854 (79.7) |
| Gestational age at birthα(for live singleton and twin deliveries) (n = 1063) | |
| Extremely preterm (< 28 weeks) | 3 (0.3) |
| Very preterm (28–< 32 weeks) | 14 (1.3) |
| Moderate to late preterm (32–< 37 weeks) | 151 (14.2) |
| Term (≥ 37 weeks) | 895 (84.2) |
| Pregnancy complications among women who deliveredat MediCiti (n = 768) | |
| Medical disorders | |
| Hyperthyroidism | 5 (0.6) |
| Hypothyroidism | 20 (2.6) |
| Bronchial asthma | 1 (0.1) |
| Cholestasis of pregnancy | 2 (0.2) |
| Heart disease | 1 (0.1) |
| Pre-existing hypertension | 3 (0.4) |
| Anaemia (moderate and severe) | 24 (3.1) |
| Obstetric complications | |
| Gestational diabetes | 10 (1.3) |
| Gestational hypertension | 40 (5.2) |
| Pre-eclampsia | 49 (6.4) |
| Placenta praevia | 2 (0.2) |
| Abruptio placentae | 5 (0.6) |
| Intrauterine growth restriction | 16 (2.0) |
| Oligohydramnios | 49 (6.4) |
| Polyhydramnios | 10 (1.3) |
| Labour complications | |
| Malpresentations | 16 (2.0) |
| Preterm labour | 31 (4.0) |
| Premature rupture of membranes | 15 (1.9) |
| Cephalo-pelvic disproportion | 90 (11.7) |
| Cord prolapse | 2 (0.2) |
| Fetal distress | 22 (2.9) |
| Manual removal of placenta | 1 (0.1) |
| Post-partum haemorrhage | 5(0.6) |
αCategories based on World Health Organization sub-categories of preterm birth.
βEarly pregnancy loss rate is calculated by dividing total early pregnancy losses with all pregnancies > 20 weeks of gestation (n = 1377; current pregnancies < 20 weeks of gestation were excluded).
How often have they been followed up?
Women who were not pregnant at enrolment are followed with serial pregnancy tests based on the reported last menstrual period (LMP). If and when they become pregnant, they are asked to complete two study visits during the pregnancy (first and third trimester) and one study visit at the time of delivery. At each of these visits a questionnaire is administered, anthropometric measurements (height and weight) are taken and biological samples are collected from the mother. During the visit at the time of delivery, samples (cord blood and meconium) are also collected from the newborn along with anthropometric data. Amnion and chorion swabs are collected and gross and microscopic placenta analyses are carried out. After the 1-month postnatal visit, mothers and children are followed at 6-monthly intervals until the child is 24 months old and thereafter annually. A questionnaire is administered to the mother and a developmental screening of the child is completed at each of these visits. At 6, 12, 18 and 24 months, the Developmental Assessment Scales for Indian Infants (DASII), a modification of the Bayley Scales of Infant Development, was used.31 At 36, 48 and 60 months, we used the Ages and Stages Questionnaires (ASQ-3).32
Attrition
Data and specimen collection rates are high. During the first and third trimester, 91.2% of women submitted at least one biological sample and 97.4% completed the questionnaire (Table 4). At the time of delivery, collection of biological samples has been most successful among the women who delivered at MediCiti Hospital. To date, 768 women (70.8%) have delivered at MediCiti, 13 women (1.2%) have delivered at home and the rest have delivered at other area hospitals. About 90.3% and 85.0% of women provided at least one biological sample at delivery and at the 1-month postpartum visit, respectively. Follow-up rates for the children are all greater than 80% at each of the study visits. Data analysis and participant follow-up are ongoing.
Table 4.
Biological samples collected at each visit among women who delivered at MediCiti (768 deliveries)
| Visits | Blood | Urine | Stool | Vaginal swab | Cord blood | Meconium | Placenta biopsyα | Amnion & chorion swab | Breast milk |
|---|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Registration visit | 768 (100) | 756 (98.4) | 722 (94.0) | 741 (96.5) | NA | NA | NA | NA | NA |
| 1st trimester visit | 707 (92.0) | 706 (91.9) | 612 (79.7) | 696 (90.6) | NA | NA | NA | NA | NA |
| 3rd trimester visit | 743 (96.8) | 737 (96.8) | 661 (86.1) | 743 (96.8) | NA | NA | NA | NA | NA |
| Delivery (PBα, n = 503) | 760 (98.9) | 736 (95.8) | 692 (90.2) | 733 (95.4) | 719 (93.6) | 654 (85.2) | 360 (71.5) | 493 (98.0) | 657 (85.6) |
| Postnatal visit | 710 (92.4) | 705 (91.8) | 535 (69.6) | 703 (91.5) | NA | NA | NA | NA | 614 (80.0) |
NA, not applicable. PB, Placenta biopsy.
As of 31 December 2014, 108 women (9%) have withdrawn from the study. This included 98 and 10 women who withdrew before the first trimester and after delivery, respectively. Although those who withdrew after delivery are not being followed for subsequent pregnancies, their children are continuing to be followed. The protocol for following women is rigorous and if an appointment is missed, four attempts are made to contact her and reschedule appointment. If the woman expresses a desire to withdraw from the study, she is asked to provide a reason which was recorded. If a woman has moved away or cannot be contacted for any other reason, efforts are made to identify the reasons behind withdrawal, which are recorded and are shown in Table 5.
Table 5.
Follow-up of children at each visit
| Time of visit | Data collection, n (%) |
|||
|---|---|---|---|---|
| Completed | Pending | Not collected | Total (N) | |
| Postnatal (6 weeks) | 948 (89.8) | 13 (1.2) | 95 (9.0) | 100.0 (1056) |
| 6 months | 759 (77.0) | 5 (0.5) | 221 (22.4) | 100.0 (986) |
| 12 months | 700 (74.6) | 6 (0.6) | 233 (24.8) | 100.0 (938) |
| 18 months | 680 (79.9) | 14 (1.6) | 157 (18.4) | 100.0 (851) |
| 24 months | 583 (78.1) | 24 (3.2) | 139 (18.6) | 100.0 (746) |
| 36 months | 348 (78.7) | 27 (6.11) | 67 (15.1) | 100.0 (442) |
| 48 months | 79 (69.3) | 14 (12.3) | 21 (18.4) | 100.0 (114) |
What has been measured?
Table 6 summarizes data collection and follow-up visits for the mothers, fathers and children enrolled in LIFE. Three basic types of data were collected (Table 7). Some laboratory tests were performed at the time of collection (Table 8) and specimens were preserved for future testing. Any patient who undergoes testing as part of the LIFE Pilot Study is subsequently treated if there are abnormalities. Treatment and outcomes are recorded.
Table 6.
Reasons for withdrawal from the LIFE study (N = 108)
| Reason | N |
|---|---|
| Before delivery | |
| Refused laboratory visit | 38 |
| Preferred other hospital/did not like LIFE study visits | 18 |
| Deceased | 2 |
| Marital and/or family problems | 10 |
| Unable to contact | 29 |
| Induced abortion and planning no further children | 1 |
| Total before delivery | 98 |
| After delivery | |
| Unable to contact | 7 |
| Moved to mother’s residence | 2 |
| Husband died | 1 |
| Total after delivery | 10 |
| Total: all withdrawals | 108 |
Table 7.
LIFE study data collection
| Mother | Registration | 1st Tri-mester | 3rd Tri-mester | Delivery | Months postpartum |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6 | 12 | 18 | 24 | 36 | 48 | |||||
| Questionnaire data: | |||||||||||
| Demographics | ✓ | ||||||||||
| Occupation | ✓ | ✓ | ✓ | ||||||||
| Education/literacy | ✓ | ||||||||||
| General health | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Past medical history | ✓ | ✓ | |||||||||
| Recent health status | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Recent medication use | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Reproductive health history | ✓ | ||||||||||
| Depression screening | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Dental health | ✓ | ||||||||||
| Family medical history | ✓ | ||||||||||
| Birth history | ✓ | ||||||||||
| Family structure | ✓ | ||||||||||
| Physical activity | ✓ | ✓ | ✓ | ||||||||
| Gender roles | ✓ | ✓ | ✓ | ||||||||
| Water source, usage | ✓ | ✓ | ✓ | ||||||||
| Pesticide exposure | ✓ | ✓ | ✓ | ||||||||
| Tobacco exposure | ✓ | ✓ | ✓ | ✓ | |||||||
| Other chemical exposures | ✓ | ||||||||||
| Alcohol consumption | ✓ | ||||||||||
| Vehicle/pollution exposure | ✓ | ✓ | |||||||||
| Animals/livestock exposure | ✓ | ✓ | ✓ | ||||||||
| Pregnancy status | ✓ | ✓ | ✓ | ||||||||
| Health during pregnancy | ✓ | ✓ | ✓ | ||||||||
| Prenatal care utilization | ✓ | ✓ | |||||||||
| Vitamins/supplement usage | ✓ | ✓ | ✓ | ||||||||
| Previous pregnancy history | ✓ | ||||||||||
| Pregnancy practices/lifestyle changes | ✓ | ✓ | |||||||||
| Anthropometric measurements: | |||||||||||
| Weight | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Height | ✓ | ||||||||||
| Waist circumference | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Hip circumference | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Blood pressure | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Biological samples: | |||||||||||
| Fasting blood | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Urine | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Stool | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Vaginal swab | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Placenta | ✓ | ||||||||||
| Breast milk | ✓ | ✓ | |||||||||
| Cord blood | ✓ | ||||||||||
| Record abstraction | ✓ | ||||||||||
| Prenatal care medical record | ✓ | ||||||||||
The LIFE Biobank has over 6000 archived samples of maternal blood, urine, stool, vaginal swabs, cord blood, breast milk and placental swabs. We have also preserved blood, urine and stool from the fathers and meconium from the newborns.
What has it found? Key findings and publications.
Gestational weight gain(GWG) is a predictor of maternal and infant outcomes. Since rural to peri-urban South Indian women tend to be underweight and have limited GWG, international guidelines on GWG may not be appropriate. Further, as data on GWG in Indian populations are sparse, we examined GWG and pregnancy outcomes in the LIFE cohort. Women in our study were frequently classified as underweight (34% of entire cohort (Table 2) and 36% of women who became pregnant). Although GWG was high among underweight women (9.7 kg) compared with normal-weight women (8.5 kg) and overweight/obese women (6.0 kg), the percentage of LBW was high among underweight women (17.6%).35 Analyses examining the relationships between GWG and a range of maternal, pregnancy and infant outcomes in this cohort is ongoing and can be used to determine optimal pre-pregnancy weight and GWG among lean South Indians.
First-cousin marriages are common practice in many parts of South India, including Telangana State. In the LIFE Study, 23% of women reported being in a consanguineous marriage (Table 8). Although there is an increased risk of stillbirth and congenital malformations among consanguineous couples who conceive, little is known about the relationship between consanguineous marriage and early pregnancy outcomes. In a preliminary analysis published in 2013 which included 286 women in the LIFE study recruited at < 8 weeks of gestation, women in first-cousin marriages experienced an increased risk of any spontaneous abortion (pregnancy loss < 22 weeks of gestation), adjusted for maternal age at pregnancy [hazard ratio adj (HRadj) 1.9, 95% confidence interval (CI) 0.9 – 3.8].36 A stronger relationship between first-cousin marriage and early spontaneous abortion at ≤ 10 weeks of gestation was found (HRadj 2.7, 95% CI 1.1 – 7.0). We conclude that in the LIFE cohort, consanguineous marriage is associated with an increased risk for early spontaneous abortion, a time frame linked with pregnancy loss due to chromosomal abnormalities. Bacterial vaginosis (BV) may lead to pelvic inflammatory disease or adverse pregnancy outcomes. As BV is often asymptomatic and has not been previously studied in Telangana State, India, we examined the prevalence of BV among a subset of women in the LIFE cohort. Among 189 women recruited before pregnancy, 42 (22.2%) women were categorized with BV by Nugent’s criteria.33,34 An additional 40 (21.2%) women had vaginal flora classified as intermediate. As BV was prevalent, routine screening and intervention in the population may be warranted. Analyses of the relationship between BV and adverse pregnancy outcomes in this cohort is ongoing.
What are the main strengths and weaknesses?
The major strength of the LIFE Pilot Study is its prospective cohort design. We were able to recruit and screen before pregnancy (80 %) or within the first trimester, largely due to cultural practices of conception soon after marriage and close spacing of births in this population. We consider this a major strength, as reproductive epidemiology studies have traditionally been plagued by the inability to recruit a population-based sample of women before conception or very early in pregnancy. This design allows for examination of a broad range of pre-conceptual and early prenatal risk factors and biomarkers of very early pregnancy loss, an outcome often difficult to study as spontaneous abortion often occurs before the first prenatal examination. Of note, this is the first study to examine the role of pre-pregnancy infection in a non-in vitro fertilization (IVF) population, thus eliminating a number of biases. Our study is also uniquely poised to examine the role of pre-conceptual and early pregnancy factors in the full range of pregnancy and child outcomes.
Guidance provided by the University of Pittsburgh Graduate School of Public Health to SHARE INDIA at various stages of the study proved to be a great strength. The Pittsburgh investigators bring expertise in epidemiological methods and global public health, whereas the Indian investigators are greatly knowledgeable about the cohort population, clinical and cultural practices in rural India and how best to work in this population and successfully recruit and follow participants.
Additional strengths of our study include a large biobank of samples for future studies and the ability for our Indian-based cohort study to shed light on issues that are important and applicable to populations in developing and developed nations around the world. Finally, the LIFE Pilot Study is able to readily access detailed medical record data on the majority of women in the cohort, as free access to health care at the MediCiti Hospital encourages access to health care by our participants at a central location. As our study staff and investigators are centred in the MediCiti hospital, there is intensive and close follow-up of LIFE participants when they come to the MediCiti hospital for delivery.
The major challenge of our study is the cultural practice of Indian women of going to their mother’s home during the last trimester of pregnancy and the delivery, especially when they are having their first child. As a result, follow-up of these women during the third trimester and at the time of delivery can be very challenging. We suspect that much of the missing data around the time of delivery are due to this phenomenon. However, our study staff have been working hard to follow these women and even travel outside the catchment area to collect data at the third trimester and delivery.
One of the limitations of the study is the difference in the quality of the data collected at the time of delivery from women who delivered at MediCiti Hospital compared with those delivered elsewhere. Data from outside hospitals are often extracted from hospital records, limiting our access to closely monitor and obtain all required samples. However, a majority of women do deliver at MediCiti Hospital.
In order to fulfill our ethical obligation, we inform all participants of any abnormal test result. Ostensibly, many of the participants with abnormal results go on to seek and receive treatment. However, we have not developed an effective method to track treatments taken by the participants at clinics or hospitals other than MediCiti. As a result, some of the conditions we are considering as factors which may impact on fetal development, pregnancy outcome or childhood development may, in fact, be treated or partially treated.
Can I get hold of the data? Where can I find out more?
Data are maintained and stored at the study research office, SHARE INDIA. Data are not freely available, but specific proposals for future collaboration are welcome. An individual wishing to access the data must collaborate with LIFE Study investigators. A written protocol must be submitted, reviewed and approved by the LIFE Data Sharing Plan Committee before initiation of new projects. For further information, contact Dr P. S. Reddy at [reddyps@verizon.net]. Updated information may be found on the research centre website at [www.sharefoundations.org].
Profile in a nutshell
The LIFE Pilot is a prospective cohort study of Indian women followed through conception, pregnancy+ and delivery, and the physical and mental health and development of their children.
The LIFE Pilot study is designed to identify the root causes of conditions excessively prevalent in India, including adverse pregnancy outcomes and childhood diseases and developmental disorders.
Since 2009, 1227 women aged between 15 and 35 years were recruited before conception or within 14 weeks of gestation. Women were followed through 1387 pregnancies and 1084 deliveries, including 22 stillbirths and intra-uterine deaths. There were 243 early pregnancy losses. Baseline data were collected from husbands of 642 women.
Anthropometric measurements, biological samples and detailed questionnaire data were collected during registration, the first and third trimesters, delivery and at 1 month postpartum. Anthropometric measurements and health questionnaire data are obtained for each child, and a developmental assessment is done at 1, 6, 12, 18, 24, 36, 48 and 60 months. At 36 months, each child is screened for development and mental health problems. Questionnaires are completed for pregnancy loss and death of children under 5 years old. The LIFE Biobank preserves over 6000 samples.
Funding
Research reported in this publication was conducted by scholars in the Fogarty International Center of the National Institutes of Health training programme under Award Number D43 TW 009078. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgements
We thank consultants Dr Satyanarayana P. and Dr Shailendra D. for their laboratory and statistical help, respectively. We also thank and acknowledge the efforts of field coordinators, interviewers and data entry operators who recruited, interviewed and entered the data of study participants. They include: Mamatha D., Deepa K., John Christopher K., Madhav Rao G., Venkatalakshmi P., Jyothi N., Sujana P., Padma G., Swathi C.H., Divyasree M., Sunitha P., Divya B., Siva Prasad P., Anjaneylu V., Balaraju V., Nagaraju N., Kiranmai K., Jayalakshmi D., Padma Renuka C.H., Anuradha K., Ramadevi Y., Mariamma P., Narender K., and Archana N.
Conflict of interest: None declared.
References
- 1. International Institute for Population Sciences (IIPS) and Macro International. National Family Health Survey (NFHS-3), 2005–06, India: Key Findings. Mumbai, India: IIPS, 2007. [Google Scholar]
- 2. UN Inter-agency Group for Child Mortality Estimation. Level and Trends in Child Mortality. Report 2010. New York, NY: UN, 2010. [Google Scholar]
- 3. UNICEF. State of the World’s Children 2008. New York, NY: United Nations Chidren’s Fund, 2007. [Google Scholar]
- 4. Ministry of health and & family welfare, Government of India. Reproductive and Child Health Project. 2010. http://mohfw.nic.in/NRHM.htm. (15 April 2013, last date accessed).
- 5. Claeson M, Bos ER, Mawji T, Pathmanathan I. Reducing child mortality in India in the new millennium. Bull World Health Organ 2000;78:1192–99. [PMC free article] [PubMed] [Google Scholar]
- 6. Pandey A, Choe MK, Luther NY, Sahu D, Chand J. Infant and Child Mortality in India. Mumbai, India: International Institute for Population Sciences and East-West Center Program on Population, 1998. [Google Scholar]
- 7. Bassani DG, Kumar R, Awasthi S. et al. Causes of neonatal and child mortality in India: a nationally representative mortality survey. Lancet 2010;376:1853–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lahariya C, Sudfeld CR, Lahariya D, Tomar SS. Causes of child deaths in India, 1985–2008: a systematic review of literature. Indian J Pediatr 2010;77:1303–11. [DOI] [PubMed] [Google Scholar]
- 9. Gutbrod T, Wolke D, Soehne B, Ohrt B, Riegel K. Effects of gestation and birth weight on the growth and development of very low birthweight small for gestational age infants: a matched group comparison. Arch Dis Child Fetal Neonatal Ed 2000;82:F208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilcox AJ, Russell IT. Birthweight and perinatal mortality: II. On weight-specific mortality. Int J Epidemiol 1983;12:319–25.ss [DOI] [PubMed] [Google Scholar]
- 11. Wilcox AJ. On the importance – and the unimportance – of birthweight. Int J Epidemiol 2001;30:1233–41. [DOI] [PubMed] [Google Scholar]
- 12. Basso O, Wilcox AJ, Weinberg CR. Birth weight and mortality: causality or confounding? Am J Epidemiol 2006;164:303–11. [DOI] [PubMed] [Google Scholar]
- 13. Ramachandran P. Nutrition and child survival in India. Indian J Pediatr 2010;77:301–05. [DOI] [PubMed] [Google Scholar]
- 14. Shrimpton R. Preventing low birthweight and reduction of child mortality. Trans R Soc Trop Med Hyg 2003;97:39–42. [DOI] [PubMed] [Google Scholar]
- 15. Brough L, Rees GA, Crawford MA, Morton RH, Dorman EK. Effect of multiple-micronutrient supplementation on maternal nutrient status, infant birth weight and gestational age at birth in a low-income, multi-ethnic population. Br J Nutr 2010;104:437–45. [DOI] [PubMed] [Google Scholar]
- 16. Scholl TO. Maternal iron status: relation to fetal growth, length of gestation, and iron endowment of the neonate. Nutr Rev 2011;69(Suppl 1):S23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Balcazar H, Hartner J, Cole G. The effects of prenatal care utilization and maternal risk factors on pregnancy outcome between Mexican Americans and non-Hispanic whites. J Natl Med Assoc 1993;85:195–202. [PMC free article] [PubMed] [Google Scholar]
- 18. Alexander GR, Kotelchuck M. Assessing the role and effectiveness of prenatal care: history, challenges, and directions for future research. Public Health Rep 2001;116:306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Charafeddine L, Ammous F, Kobeissi L. et al. In-hospital neonatal mortality and the role of consanguinity. Paediatr Perinat Epidemiol 2012;26:398–407. [DOI] [PubMed] [Google Scholar]
- 20. Bittles AH, Black ML. The impact of consanguinity on neonatal and infant health. Early Hum Dev 2010;86:737–41. [DOI] [PubMed] [Google Scholar]
- 21. Tadmouri GO, Nair P, Obeid T, Al Ali MT, Al Khaja N, Hamamy HA. Consanguinity and reproductive health among Arabs. Reprod Health 2009;6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bennett RL, Hudgins L, Smith CO, Motulsky AG. Inconsistencies in genetic counseling and screening for consanguineous couples and their offspring: the need for practice guidelines. Genet Med 1999;1:286–92. [DOI] [PubMed] [Google Scholar]
- 23. Stratton JA, Miller RD, Schmidt P. Effect of maternal parasitic disease on the neonate. Am J Reprod Immunol Microbiol 1985;8:141–42. [DOI] [PubMed] [Google Scholar]
- 24. Dreyfuss ML, Msamanga GI, Spiegelman D. et al. Determinants of low birth weight among HIV-infected pregnant women in Tanzania. Am J Clin Nutr 2001;74:814–26. [DOI] [PubMed] [Google Scholar]
- 25. Weigel MM, Calle A, Armijos RX, Vega IP, Bayas BV, Montenegro CE. The effect of chronic intestinal parasitic infection on maternal and perinatal outcome. Int J Gynecol Obstet 1996;52:9–17. [DOI] [PubMed] [Google Scholar]
- 26. Cho SI, Li Q, Yang J. et al. Drinking water source and spontaneous abortion: A cross-sectional study in a rural Chinese population. Int J Occup Environ Health 1999;5:164–69. [DOI] [PubMed] [Google Scholar]
- 27. Weselak M, Arbuckle TE, Walker MC, Krewski D. The influence of the environment and other exogenous agents on spontaneous abortion risk. J Toxicol Environ Health B Crit Rev 2008;11:221–41. [DOI] [PubMed] [Google Scholar]
- 28. Tatineni A, Vijayaraghavan K, Reddy PS, Narendranath B, Reddy RP. Health metrics improve childhood immunization coverage in a rural population of Andhra Pradesh. Indian J Public Health 2009;53:41–43. [PubMed] [Google Scholar]
- 29. International Institute for Population Sciences (IIPS) and Macro International. National Family Health Survey (NFHS-3), 2005–06: Andhra Pradesh. Mumbai, India: IIPS, 2008. [Google Scholar]
- 30. Kusneniwar GN, Mishra AK, Balasubramanian K, Reddy PS. Determinants of infant mortality in a developing region in rural Andhra Pradesh. Natl J Integr Res Med 2013;4:20–26. [PMC free article] [PubMed] [Google Scholar]
- 31. Phatak P. Developmental Assessment Scales for Indian Infants (DASII), (Revised Baroda Norms, 2997) Manual. Pune, India: Anand Agencies, 1997. [Google Scholar]
- 32. Squires J, Twombly E, Bricker D, Potter LaWanda. ASQ-3 User’s Guide 3rd edn Baltimore, MD: Paul H. Brookes, 2009. [Google Scholar]
- 33. Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol 1991;29:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eastman JM, Basany K, Balasubramanian K. et al. Prevalence of bacterial vaginosis in peri-urban villages of South India. Society for Paediatric and Perinatal Epidemiology Research Annual Meeting Book of Abstracts . Clearfield, UT: SPER, 2012. [Google Scholar]
- 35. Kalpana B, Pant R, Proma P, Bunker CH, Reddy PS. An estimation of optimal gestational weight gain in a rural population in Southern India. International Conference on Pregnancy in High Risk Patients Book of Abstracts, Kamala, India: Federation of Obstetric Gynaecological Societies of India, 2015. [Google Scholar]
- 36. Eastman JM, Balasubramanian K, Bunker CH. et al. Increased risk of early spontaneous abortion among women in first cousin marriages in rural Andhra Pradesh, India. Society for Paediatric and Perinatal Epidemiology Research Meeting Book of Abstracts. Clearfield, UT: SPER, 2013. [Google Scholar]