Neutrophils are the first immune cells arriving at the site of infection or injury. These early responders are effective in fighting pathogens via several mechanisms: they phagocytose antibody- or complement-coated pathogens and kill them by NADPH oxidase-dependent mechanisms or antibacterial proteins that are released from granules into the phagosome. The content of these granules, i.e. various inflammatory mediators including neutrophil-derived lipocalin (NGAL), is also released to fight extracellular pathogens. Neutrophil extracellular traps (NETs), composed of chromatin, histones, and proteases, are yet another mechanism to capture and kill microorganisms.1 In sterile injury after ischaemia, danger-associated molecules released by necrotic tissues trigger the same Toll-like receptor-induced activation of neutrophils. After myocardial infarction (MI), neutrophils are the first to enter the damaged tissue in large numbers, and peak at day 1 after onset of ischaemia.2 While neutrophils initially help to clear cellular debris, their inflammatory mediators result in tissue damage and further leucocyte recruitment.3 Neutrophils may support recruitment and activation of Ly-6Chi monocytes,4 which dominate ischaemic tissue on days 1–4 after injury. Ly-6Chi monocytes are initially sourced from reservoirs in the spleen, which they depart in an angiotensin II-dependent manner.5 These monocytes, and derived macrophages, perpetuate inflammation by releasing inflammatory cytokines, proteases, and reactive oxygen species. Monocytes/macrophages phagocytose dead cardiomyocytes and neutrophils, thereby clearing cellular debris in the infarct. The ingestion of apoptotic neutrophils may also influence the phenotype of the macrophage.6 Several days after injury, reparative macrophages dominate the cellular infiltrate. These cells promote tissue repair, for instance by releasing angiogenic factors. The biphasic monocyte/macrophage response is critical for myocardial healing after ischaemia. Lack of inflammatory phase 1 results in impaired removal of cellular debris, while lack of reparative phase 2 results in impaired angiogenesis and inefficient fibrosis.7 Increased or prolonged Ly-6Chi monocyte recruitment, as observed in ApoE–/– mice after coronary ligation, promotes heart failure.8 Based on the promotion of inflammation and tissue damage by neutrophils, they are currently seen as ‘bad guys’ in the setting of ischaemic heart disease. In mice with atherosclerosis, neutrophil numbers correlate with plaque inflammation and their depletion reduces disease burden.3 In patients, high circulating neutrophil numbers, neutrophil/lymphocyte ratios, and granule proteins (e.g. myeloperoxidase and NGAL) correlate with coronary artery disease severity and are discussed as prognostic markers for acute coronary syndromes.2 Earlier studies demonstrated that neutrophil depletion reduces ischaemia/reperfusion injury, but this effect decreases with prolonged ischaemia. Inhibiting neutrophil mediators post-MI is likewise beneficial.9
In this issue of the journal, Horckmans et al.10 posit that neutrophils orchestrate post-MI monocyte/macrophage phenotype and number, and that neutrophils can have beneficial effects on myocardial healing. Depleting neutrophils with a monoclonal Ly-6G antibody in wild-type mice with coronary ligation results in a lack of Ly-6Chi monocyte release from the splenic reservoir, lower Ly-6Chi monocyte numbers in the infarct, increased infarct macrophage proliferation, and altered macrophage phenotype. Based on M1/M2 marker profiling, the authors conclude that in the absence of neutrophils, macrophages assume more M2-like phenotypes, which ultimately worsened outcome. To our knowledge, this is the first study reporting a detrimental effect of M2 macrophages in acute MI. Of note, the in vitro M1/M2 macrophage classification is an oversimplification that we should probably abandon, as in vivo macrophages are highly heterogeneous and comprise a spectrum of phenotypes.11 Nevertheless, the observed up-regulation of arginase, Ym1, and interleukin-4 (IL-4) indicates that cardiac macrophages are less inflammatory after neutrophil depletion. These macrophages are probably responsible for the detected increase in myofibroblasts and collagen deposition. According to Horckmans et al., this excessive fibrosis increased stiffness and reduced cardiac function, ultimately resulting in heart failure and kidney injury. The authors identify NGAL as a key neutrophil mediator which induces MerTK (myeloid–epithelial–reproductive tyrosine kinase) expression in cardiac macrophages, enabling efficient efferocytosis of cardiomyocytes and tissue digestion.12 In other words, neutrophils and their NGAL production induce recruitment of splenic Ly-6Chi monocytes, promote clearance of debris (inflammatory phase), and thereby enable resolution of inflammation and proper wound healing (reparative phase; Figure 1). This work leads to a number of interesting questions. How do neutrophils promote splenic monocyte release? Do neutrophils influence macrophage proliferation directly and, if so, how? Do neutrophils directly compromise or enhance myocyte health at early or late time points after MI? Are their functions different in ischaemic vs. remote myocardium?
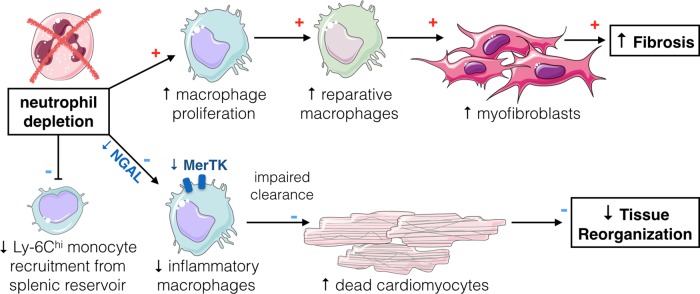
Figure 1.
Neutrophil–macrophage cross-talk in acute myocardial infarction. Neutrophil depletion reduces Ly-6Chi monocyte recruitment from the splenic reservoir. Lack of neutrophil neutrophil-derived lipocalin (NGAL) reduces myeloid–epithelial–reproductive tyrosine kinase (MerTK) expression in cardiac macrophages, thereby impairing their clearance of cellular debris. Cardiac macrophages proliferate in the absence of neutrophils and adopt a reparative phenotype, inducing prominent fibrosis.
That neutrophils also play a protective role after MI might come as a surprise, but could make sense from an evolutionary perspective: initial inflammatory responses to kill pathogens need to be followed by inflammation resolution to restore tissue homeostasis. That the same cell induces both inflammation and its resolution could function as a failsafe mechanism that had previously been discussed for monocytes/macrophages.6 Overall, neutrophils may therefore have a dual role post-MI and pursue both damaging and protective functions. Indeed, neutrophils play a fundamental role in degrading necrotic tissue and accelerating proinflammatory cytokine production, which then leads to clearance of debris by macrophages. To put the current findings into a more clinically relevant context, it is interesting to consider the neutral effects of neutrophil depletion in ApoE–/– mice with MI.8 In ApoE–/– mice the inflammatory phase increases to a detrimental level, which probably models the clinical situation with higher fidelity.
Horckmans et al. add another dimension of neutrophil responses. Either modification of neutrophil function or substitution of protective factors expressed by neutrophils (e.g. NGAL) could prove beneficial for infarct healing. First, however, it remains to be established whether the reported data translate into patients with atherosclerosis and ischaemia. The study underscores that therapies targeting inflammatory diseases should focus on restoring balance. Similar to what was observed for monocytes/macrophages,7 neutrophil accumulation produces a variety of inflammatory signals that promote tissue destruction and amplify leucocyte recruitment. On the other hand, these actions facilitate debridement, by neutrophils themselves and by regulating macrophage activity. Ultimately, neutrophils may therefore also enable tissue reorganization and infarct healing.
Acknowledgments
This work was supported by a fellowship from the Netherlands Organisation for Scientific Research (NWO, Rubicon Grant: 835.15.014) and from the National Institutes of Health (R01HL117829). The figure was made using Servier Medical Art.
Conflict of interest: none declared.
References
- 1. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013;13:159–175. [DOI] [PubMed] [Google Scholar]
- 2. Carbone F, Nencioni A, Mach F, Vuilleumier N, Montecucco F. Pathophysiological role of neutrophils in acute myocardial infarction. Thromb Haemost 2013;110:501–514. [DOI] [PubMed] [Google Scholar]
- 3. Döring Y, Drechsler M, Soehnlein O, Weber C. Neutrophils in atherosclerosis: from mice to man. Arterioscler Thromb Vasc Biol 2015;35:288–295. [DOI] [PubMed] [Google Scholar]
- 4. Soehnlein O, Zernecke A, Eriksson EE, Rothfuchs AG, Pham CT, Herwald H, Bidzhekov K, Rottenberg ME, Weber C, Lindbom L. Neutrophil secretion products pave the way for inflammatory monocytes. Blood 2008;112:1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009;325:612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol 2005;6:1191–1197. [DOI] [PubMed] [Google Scholar]
- 7. Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 2013;339:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, Libby P, Pittet M, Weissleder R, Nahrendorf M. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol 2010;55:1629–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shinagawa H, Frantz S. Cellular immunity and cardiac remodeling after myocardial infarction: role of neutrophils, monocytes, and macrophages. Curr Heart Fail Rep 2015;12:247–254. [DOI] [PubMed] [Google Scholar]
- 10. Horckmans M, Ring L, Duchene J, Santovito D, Schloss M, Drechsler M, Weber C, Soehnlein O, Steffens S. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J 2017;38:187–197. [DOI] [PubMed] [Google Scholar]
- 11. Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014;41:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wan E, Yeap XY, Dehn S, Terry R, Novak M, Zhang S, Iwata S, Han X, Homma S, Drosatos K, Lomasney J, Engman DM, Miller SD, Vaughan DE, Morrow JP, Kishore R, Thorp EB. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid–epithelial–reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ Res 2013;113:1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]