Abstract
Background
Studies of antiretroviral therapy (ART) use during pregnancy in HIV-infected women have suggested that ART exposure may be associated with adverse birth outcomes. However, there are few data from sub-Saharan Africa where HIV is most common, and few studies involving the World Health Organization’s (WHO’s) recommended first-line regimens.
Methods
We enrolled consecutive HIV-infected pregnant women and a comparator cohort of uninfected women at a primary-level antenatal care facility in Cape Town, South Africa. Gestational assessment combined clinical history, examination and ultrasonography; outcomes included preterm (PTD), low birthweight (LBW) and small for gestational age (SGA) deliveries. In analysis we compared birth outcomes between HIV-infected and -uninfected women, and HIV-infected women who initiated ART before vs during pregnancy.
Results
In 1554 women (mean age 29 years) with live singleton births at time of analysis, 82% were HIV-infected, 92% of whom received a first-line regimen of tenofovir, emtricitabine and efavirenz. Overall, higher levels of PTD [22% vs 13%; odds ratio (OR) 1.94, 95% confidence interval (CI): 1.34, 2.82] and LBW (14% vs 9%; OR 1.62, 95% CI: 1.05, 2.29) were observed in HIV-infected vs uninfected women, although SGA deliveries were similar (9% vs 11%; OR 1.06, 95% CI: 0.71, 1.61). Adjusting for demographic characteristics and HIV disease measures, HIV-infected (vs HIV-uninfected) women had persistently increased odds of PTD [adjusted odds ratio (AOR) 2.03; CI 1.33, 3.10]; associations with LBW were attenuated (AOR 1.47; CI 0.90, 2.40). Among all HIV-infected women, there appeared to be no association between the timing of ART initiation (before or during pregnancy) and adverse birth outcomes.
Conclusions
These findings suggest that current WHO-recommended ART regimens appear relatively safe in pregnancy, although more data are required to understand the aetiology of preterm delivery in HIV-infected women using ART.
Keywords: HIV, antiretroviral therapy, perinatal outcomes, prematurity, low birthweight, small for gestational age
Background
Use of triple-drug antiretroviral therapy (ART) during pregnancy is the central intervention promoting the health of HIV-infected women and their children. Widespread ART access has significantly reduced the number of new paediatric HIV infections and improved the long-term health of HIV-infected mothers, representing one of the greatest successes of the public health response to the HIV epidemic.1
However, there are persistent questions regarding the potential adverse effects of in utero ART exposure. Whereas the association between untreated, advanced HIV disease and adverse birth outcomes is well documented,2,3 a number of studies have suggested increased levels of preterm (PTD),4–7 low birthweight (LBW)8–10 and/or small for gestational age (SGA)4,11 deliveries among women receiving ART. Findings vary by the class of antiretroviral (ARVs) agents used, with protease inhibitors (PIs) more commonly implicated than nucleoside and non-nucleoside reverse transcriptase inhibitors (NRTIs and NNRTIs, respectively).12–15 However, overall findings for the putative association between antenatal ART use and adverse birth outcomes are highly mixed, with many studies finding no evidence of associations with PTD, LBW and/or SGA.11,16–21
With approximately 1.4 million HIV-infected women becoming pregnant annually,22 the possibility of an increased risk of adverse birth outcomes has generated considerable concern. The current evidence base is subject to several notable limitations. Few studies have focused on African populations where most pregnant women using ART live, and where rates of PTD are often high.23,24 In addition, most studies investigate ARVs not widely used in low- and middle-income countries (LMIC), and there are few data examining the World Health Organization (WHO) recommended regimen of two NRTIs [tenofovir (TDF) and emtricitabine (FTC)], with the NNRTI efavirenz (EFV)]. Accurate pregnancy dating is critical for defining adverse outcomes in perinatal epidemiology, but the quality of gestational dating in the existing literature is variable, and subsequent potential for bias poorly understood. Finally, the choice of comparison groups varies between studies, and in studies without HIV-negative or HIV-infected, ART-unexposed comparator groups, it can be difficult to attribute adverse effects to ART exposure rather than HIV disease.8
Given the large numbers of ART-exposed pregnancies around the world and the conflicting evidence to date, better understandings of the potential associations between commonly used ART regimens and adverse birth outcomes are critical.25 In particular, with national treatment programmes in high-burden countries implementing a first-line regimen of TDF + FTC + EFV for all HIV-infected women regardless of disease status or CD4 cell count, data on how this regimen may affect major birth outcomes are urgently required. Therefore, we examined the associations between ART use and birth outcomes in a well-characterized cohort of women seeking routine public sector antenatal care in Cape Town, South Africa.
Methods
Study setting
This prospective cohort study was conducted among consecutive HIV-infected and HIV-uninfected women seeking antenatal care (ANC) at a large, community-based public sector primary care facility in Cape Town, South Africa, enrolled between April 2013 and August 2015. The facility serves a catchment population of approximately 350 000 where ANC uptake is high (95%); in 2014, the antenatal HIV seroprevalence was estimated at 30%.26
All women in this setting have gestational age estimated based on last menstrual period (LMP) and symphysis-fundal height (SFH) at the first ANC visit, as part of routine clinical care at their first ANC visit.
All women without a previous HIV diagnosis underwent HIV testing, with ART eligibility based on CD4 cell count <350 cells/µl or WHO stage III/IV disease (from April to June 2013) or universal ART eligibility, regardless of CD4 cell count or disease stage (July 2013 onwards). HIV-infected women conceiving while on ART continued their current regimen throughout pregnancy; regimens included PIs (used in this setting predominantly after failure of first-line therapy) or NNRTIs such as EFV or nevirapine (NVP, used in previous first-line regimens). For women initiating ART in pregnancy, a fixed-dose combination of TDF + FTC + EFV was used throughout. Following ART initiation, clinical follow-up was through an integrated primary care service providing antenatal and HIV care.
Study procedures
This analysis draws on data from a larger multicomponent study of antiretroviral services for HIV-infected women during pregnancy and postpartum [https://clinicaltrials.gov/ct2/show/NCT01933477].27 HIV-uninfected women were enrolled consecutively into a separate comparator cohort with identical study procedures. The parent study was reviewed and approved by the University of Cape Town Faculty of Health Sciences Human Research Ethics Committee and Columbia University Medical Center Institutional Review Board. Written informed consent was obtained from all participants at their first ANC visit, and this consent included access to their clinical records for this birth outcomes analysis.
Inclusion and exclusion criteria
Consecutive women (aged ≥ 18 years) attending their first antenatal care visit, who were identified as HIV-infected through routine rapid antibody tests, were eligible for enrolment into the HIV-infected cohort. Women not eligible for ART at their first ANC visit (receiving zidovudine prophylaxis) were excluded from this analysis. For the comparator HIV-uninfected cohort, women were eligible for enrolment based on the same criteria and a negative test on the same routine rapid antibody test.
Data collection
All women (HIV-infected and HIV-uninfected) completed questionnaires including demographics and obstetric and medical history. HIV-infected women provided 5 ml of blood for viral load (VL) testing using Abbot Realtime HIV-1 assay (Abbot Laboratories, Waltham, MA). At their first visit, an obstetric ultrasound (US) was performed on all women by an experienced research sonographer using a standardized assessment protocol and blinded to other clinical details. Follow-up study interviews, separate from routine clinical care, were scheduled around the second ANC visit, late third trimester and within 7 days postpartum. Obstetric outcomes, including date and mode of delivery and birthweight, were abstracted from obstetric records at delivery facilities.
Variables and outcomes
In analysis, gestation was based on completed weeks using the best available measure (US or combination of LMP/SFH at later gestations). HIV/ART status (the exposure of interest) was categorized as: (i) HIV-uninfected; (ii) ART initiated before pregnancy; and (iii) ART initiated during pregnancy in the (a) first trimester (<14 weeks), (b) first half of the second trimester (14–20 weeks), (c) second half of the second trimester (21–27 weeks) or (d) third trimester (≥28 weeks). Regimens were categorized as either PI or NNRTI; NNRTI regimens were either EFV-based [TDF + 3TC (lamivudine) + EFV], NVP-based (TDF + 3TC + NVP) or involving other NNRTIs.
All deliveries before September 2015 were included in analysis. PTD was defined as delivery at <37 weeks’ gestation, categorized as late preterm (34–37 weeks), moderately preterm (32–34 weeks) or very preterm (<32weeks). LBW was defined as birthweight < 2500 g and very low birthweight (VLBW) as <1500 g. Using the INTERGROWTH-21st Project Standards, infants with birthweights <10th percentile for gestational age were classified SGA; those between 10th and 90th percentiles were classified appropriate for gestational age (AGA); and those >90th percentile were classified large for gestational age (LGA).28,29 Composite pregnancy loss was defined as any loss before delivery, and included: ectopic pregnancies as determined by the research sonographer; miscarriages defined as pregnancy loss <28 weeks;30 and stillbirths defined as fetal death occurring before/during labour and delivery (based on a 1-min APGAR score of 0).
Data analysis
Statistical analyses (STATA 14.0, Stata Corporation, College Station, TX, USA) focused on three exposure comparisons: HIV-infected vs HIV-uninfected women (Comparison A); among HIV-infected women, those initiating ART before pregnancy vs those initiating during pregnancy (Comparison B); and among women initiating ART during pregnancy, comparisons across gestational ages at ART initiation (Comparison C). Pregnancy outcome analyses were restricted to live singleton births. In bivariable analyses, proportions were compared using chi-square and rank sum tests. Birth outcomes (PTD, LBW and SGA) were compared using unadjusted and adjusted logistic regression; results are presented as odds ratios (OR) with 95% confidence intervals (CI). Confounders identified a priori included age, maternal height, parity and previous PTD; and among HIV-infected women, pre-ART CD4 count and pre-ART viral load (VL). Subgroup analyses involved restrictions by EFV or PI use, and by gestation at first ANC visit. Model fit was assessed using likelihood ratio tests and Akaike’s Information Criterion; throughout, statistical tests were two-sided (alpha = 0.05).
Results
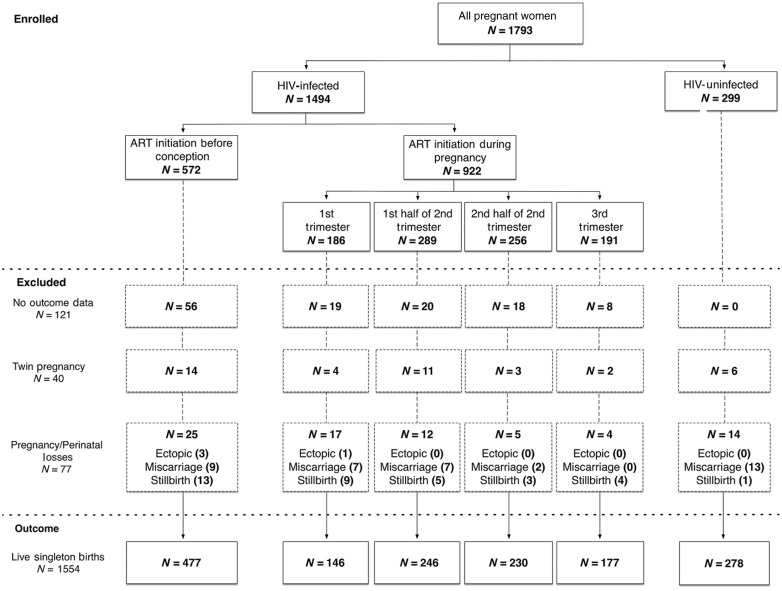
A total of 1793 women who had delivered at the time of analysis were included: 1494 (83%) HIV-infected and 299 (17%) HIV-uninfected. Among HIV-infected women, 572 (38%) initiated ART before the current pregnancy and 922 (62%) initiated during pregnancy: 186 during the first trimester, 289 during the first half and 256 during the second half of the second trimester and 191 during the third trimester (Figure 1). TDF + FTC + EFV was the most commonly used regimen and 6% reported PI use.
Figure 1.
Birth outcomes by HIV/ART exposure status among women in the cohort.
Table 1 compares demographic and clinical characteristics of women at their first ANC visit. Compared with HIV-uninfected women, women who were HIV-infected were older, less educated, more likely to be unemployed and more likely to have previous adverse birth outcomes, but gestation at first ANC visit did not vary systematically between these groups. Among HIV-infected women, those initiating ART before pregnancy were older and less educated than women initiating during pregnancy. Neither pre-ART CD4 cell count nor pre-ART HIV VL appeared associated with timing of ART initiation in pregnancy among women newly initiating ART.
Table 1.
Characteristics of pregnant women at first antenatal visit stratified by HIV/ART status
| HIV- uninfected N = 299 | HIV- infected N = 1494 | HIV-infected |
P-value* | |||||
|---|---|---|---|---|---|---|---|---|
| Initiation before pregnancy N = 572 | Initiation during pregnancy N = 922 |
|||||||
| First trimester N = 186 | First half of second trimester N = 289 | Second half of second trimester N = 256 | Third trimester N = 191 | |||||
| Maternal characteristics | ||||||||
| Age, years | <0.001 | |||||||
| ≤24 | 103 (34) | 285 (19) | 55 (10) | 51 (27) | 69 (24) | 62 (24) | 48 (25) | |
| 25–29 | 84 (28) | 472 (32) | 152 (27) | 72 (39) | 107 (37) | 72 (28) | 69 (36) | |
| ≥30 | 110 (37) | 720 (48) | 358 (63) | 63 (34) | 110 (38) | 116 (45) | 73 (38) | |
| Median (IQR) | 27 (23–32) | 29 (26–34) | 31 (28–35) | 27 (24–31) | 28 (25–32) | 29 (25–32) | 29 (25–33) | |
| Education (finished high school) | 119 (40) | 402 (27) | 127 (22) | 60 (32) | 90 (31) | 78 (30) | 47 (25) | <0.001 |
| Employment status | 0.002 | |||||||
| Employed | 139 (46) | 557 (37) | 214 (37) | 88 (47) | 118 (41) | 89 (35) | 48 (25) | |
| SES | 0.66 | |||||||
| Lowest | 91 (30) | 451 (30) | 180 (31) | 50 (27) | 73 (25) | 80 (31) | 68 (36) | |
| Medium | 94 (31) | 539 (36) | 216 (38) | 65 (35) | 99 (34) | 89 (35) | 70 (37) | |
| Highest | 95 (32) | 504 (34) | 176 (31) | 71 (38) | 117 (40) | 87 (34) | 53 (28) | |
| Obstetric characteristics, gestation, weeks | ||||||||
| Median (IQR) | 21 (16 – 27) | 21 (15–27) | 21 (15–28) | 10 (8–12) | 18 (16–19) | 24 (22–25) | 32 (26–35) | – |
| Height, cm | 0.9 | |||||||
| ≤155 | 85 (28) | 444 (30) | 163 (29) | 60 (32) | 92 (32) | 79 (31) | 50 (26) | |
| 156–161 | 90 (30) | 464 (31) | 170 (30) | 72 (39) | 89 (31) | 69 (27) | 64 (34) | |
| ≥162 | 73 (24) | 353 (24) | 142 (25) | 33 (18) | 68 (24) | 59 (23) | 51 (27) | |
| Mean (SD) | 158 (8) | 158 (7) | 158 (7) | 158 (7) | 157 (7) | 158 (7) | 158 (6) | |
| Gravidity | 0.005 | |||||||
| 1 | 72 (24) | 244 (16) | 63 (11) | 38 (20) | 64 (22) | 48 (19) | 31 (16) | |
| 2 | 101 (34) | 544 (36) | 196 (34) | 81 (44) | 109 (38) | 90 (35) | 68 (36) | |
| ≥3 | 14 (47) | 706 (47) | 313 (55) | 67 (36) | 116 (40) | 118 (46) | 92 (48) | |
| Median (IQR) | 2 (2–3) | 2 (2–3) | 3 (2–3) | 2 (2–3) | 2 (2–3) | 2 (2–3) | 2 (2–3) | |
| Parity | 0.006 | |||||||
| 0 | 75 (25) | 259 (17) | 68 (12) | 43 (23) | 65 (22) | 50 (20) | 33 (17) | |
| 1 | 100 (33) | 558 (37) | 202 (35) | 79 (42) | 117 (40) | 94 (37) | 66 (35) | |
| ≥2 | 122 (41) | 677 (45) | 302 (53) | 64 (34) | 107 (37) | 112(44) | 92 (48) | |
| Median (IQR) | 1 (0–2) | 1 (1–2) | 2 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–2) | |
| Previous miscarriagea | 8 (3) | 204 (14) | 104 (18) | 36 (19) | 34 (12) | 20 (8) | 10 (5) | <0.001 |
| Previous preterma | 6 (2) | 107 (7) | 47 (7) | 11 (6) | 21 (7) | 8 (3) | 20 (10) | 0.001 |
| HIV | ||||||||
| Current ART regimen, self-report | <0.001 | |||||||
| TDF + 3TC + EFV | 1116 (87) | 197 (34) | 186 (100) | 288 (99) | 256 (100) | 189 (99) | ||
| TDF + 3TC + NVP | 57 (4) | 56 (10) | 0 | 0 | 0 | 1 (0.5) | ||
| Other NNRTI-based regimen | 72 (6) | 71 (12) | 0 | 1 (0.4) | 0 | 0 | ||
| PI-based regimen | 33 (3) | 32 (6) | 0 | 0 | 0 | 1 (0.5) | ||
| CD4 cell count, (cells/µl) | 0.38 | |||||||
| ≤200 | 213 (14) | 65 (12) | 29 (16) | 44 (16) | 47 (19) | 28 (15) | ||
| 201–350 | 426 (29) | 167 (30) | 52 (29) | 90 (32) | 70 (28) | 47 (26) | ||
| 351–500 | 384 (26) | 154 (28) | 42 (23) | 75 (27) | 65 (26) | 48 (26) | ||
| >500 | 423 (28) | 167 (30) | 58 (32) | 72 (25) | 67 (26) | 59 (31) | ||
| Median (IQR) | 396 | 379 | 361 | 357 | 397 | |||
| (271–524) | (256–552) | (246–504) | (235–506) | (258–576) | ||||
| Median HIV RNA viral load (log10 copies/ml) | 3.35 | 1.59 | 3.97 | 4.12 | 3.99 | 3.79 | <0.001 | |
| (1.59–4.25) | (1.59–1.6) | (3.41–4.49) | (3.44–4.12) | (3.41–4.64) | (3.18–4.42) | |||
All variables, with the exception of height and ART regimen, had <3% missing data. For height, 16% (n = 284) of data was missing with similar proportions of missing data across all comparison groups. For ART regimen, 14% (n = 216) of data was missing and this was among the women who initiated ART before pregnancy
*IQR, interquartile range; SES, socioeconomic status; SD, standard deviation.
aAmong women with a previous pregnancy.
P-values refer to the comparisons across exposure categories: HIV-uninfected, initiation before pregnancy, initiation during pregnancy (all four time periods).
Figure 1 shows the cohort disposition through delivery. Overall 121 pregnancies (7%) were missing outcome data, principally among those receiving ART before pregnancy. Following exclusion of 40 twin deliveries and 77 pregnancy losses (4%), 1554 live singleton births were available for analysis. No difference was observed in the composite pregnancy loss outcome by HIV status or timing of ART initiation. HIV-uninfected women experienced a higher proportion of miscarriages (n = 13; 4%) compared with their HIV-infected counterparts (n = 25; 2%); the opposite was observed with stillbirths, with HIV-infected women experiencing a higher proportion (n = 34; 2%) compared with HIV-uninfected women (n = 1; 0.3%) (Figure 1).
Birth outcomes by HIV/ART status
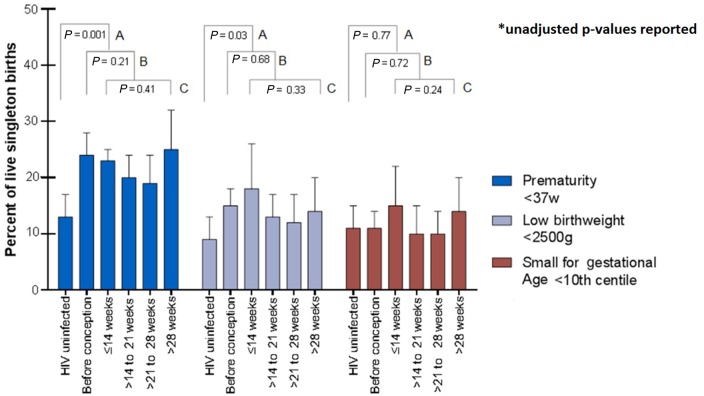
Comparing outcomes overall between HIV-infected (n = 1276) and uninfected (n = 278) women (Comparison A), a higher incidence of any PTD (OR 1.94, 95% CI: 1.34, 2.82; 22% vs 13%) and any LBW (OR 1.62, 95% CI: 1.05, 2.29; 14% vs 9%) was observed among the HIV-infected women. SGA deliveries were similar (OR 1.06, 95% CI: 0.71, 1.61; 9% vs 11%) (Figure 2). In both groups, most preterm deliveries were either late (59% and 58%) or moderately preterm (32% and 36%); similarly, most newborns were LBW (87% and 88%) rather than VLBW (Table 2). Following adjustment for age, parity, height and previous PTD, HIV infection was associated with an increased odds of PTD [adjusted odds ratio (AOR) 2.03, 95% CI: 1.33, 3.10] but not LBW (AOR 1.47, 95% CI: 0.90, 2.40) (Table 3).
Figure 2.
Incidence of preterm, low birthweight and small for gestational age deliveries by HIV status and timing of ART initiation before and during pregnancy among 1554 women who had live singleton deliveries; unadjusted P-values reported.
Table 2.
Birth outcomes by HIV/ART status among women with live singleton births (N = 1554)
| HIV-uninfected N = 278 | HIV-infected N = 1276 | HIV-infected vs uninfected P-value | HIV-infected |
ART initiation before vs duringaP-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Initiation before pregnancy N = 477 | ART initiation during pregnancy N = 799 |
||||||||
| First trimester N = 146 | First half of second trimester N = 246 | Second half of second trimester N = 230 | Third trimester N = 177 | ||||||
| Gestational age (weeks) | <0.0001 | 0.001 | |||||||
| Term (≥37) | 242 (87) | 986 (78) | 358 (75) | 111 (76) | 197 (80) | 187 (81) | 133 (75) | ||
| Any preterm (<37) | 36 (13) | 285 (22) | 115 (24) | 34 (23) | 49 (20) | 43 (19) | 44 (25) | ||
| Late preterm (34–37) | 21 (8) | 167 (13) | 69 (14) | 19 (13) | 29 (12) | 24 (10) | 26 (15) | ||
| Moderately preterm (32–34) | 13 (5) | 91 (7) | 37 (8) | 9 (6) | 16 (7) | 11 (5) | 18 (10) | ||
| Very preterm (28–32) | 2 (0.7) | 27 (2) | 9 (2) | 6 (4) | 4 (2) | 8 (3) | 0 | ||
| Birthweight (grams) | 0.03 | 0.68 | |||||||
| Normal (≥) | 252 (91) | 1085 (85) | 402 (84) | 118 (81) | 214 (87) | 199 (87) | 152 (86) | ||
| Any LBW (<2500) | 26 (9) | 181 (14) | 70 (15) | 27 (18) | 31 (13) | 28 (12) | 25 (14) | ||
| LBW (2500–1500) | 23 (8) | 157 (12) | 61 (13) | 23 (16) | 27 (11) | 21 (9) | 25 (14) | ||
| Very LBW (<1500) | 3 (1) | 24 (2) | 9 (2) | 4 (3) | 4 (2) | 7 (3) | 0 | ||
| Mean (SD) | 3199 (548) | 3052 (580) | 0.003 | 3090 (602) | 3080 (641) | 3120 (533) | 3130 (574) | 3150 (531) | 0.63 |
| Size for gestational age (centile) | 0.013 | 0.011 | |||||||
| LGA (>90th) | 35 (13) | 112 (9) | 56 (12) | 13 (9) | 13 (5) | 11 (5) | 19 (11) | ||
| AGA (10th–90th) | 211 (76) | 984 (77) | 356 (75) | 105 (72) | 202 (82) | 190 (83) | 131 (74) | ||
| SGA (<10th) | 31 (11) | 112 (9) | 53 (11) | 22 (15) | 25 (10) | 22 (10) | 25 (14) | ||
AGA, appropriate for gestational age.
aP-values refer to comparisons between women who initiated ART before pregnancy vs during pregnancy (not expanded into the four time periods). All the variables had <4% missing data, with similar proportions of missing data across the comparison groups.
Table 3.
Adjusted associations between HIV/ART status and adverse birth outcomes
| Outcome measure | Comparison Aa (Ref. category: HIV-uninfected) |
Comparison Bb (Ref. category: Before pregnancy) |
Comparison Cb (Ref. category: P1: <14weeks) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| AOR (95% CI) | P-value | AOR (95% CI) | P-value | AOR (95% CI) | P-value | ||||
| Preterm delivery (<37 weeks) | HIV-infected | 2.03 (1.33–3.10) | 0.001 | During pregnancy | 0.70 (0.45–1.07) | 0.102 | P2 | 0.91 (0.52–1.60) | 0.75 |
| P3 | 0.79 (0.44–1.42) | 0.442 | |||||||
| P4 | 1.41 (0.79–2.51) | 0.244 | |||||||
| Low birthweight (<2500 g) | HIV-infected | 1.47 (0.90–2.40) | 0.124 | During pregnancy | 0.72 (0.43–1.21) | 0.217 | P2 | 0.70 (0.36–1.35) | 0.283 |
| P3 | 0.68 (0.34–1.34) | 0.264 | |||||||
| P4 | 1.15 (0.59–2.28) | 0.669 | |||||||
| Small for gestational age (<10th centile) | HIV-infected | 0.91 (0.58–1.43) | 0.695 | During pregnancy | 1.05 (0.58–1.91) | 0.861 | P2 | 0.77 (0.38–1.56) | 0.461 |
| P3 | 0.74 (0.35–1.54) | 0.414 | |||||||
| P4 | 1.62 (0.79–3.30) | 0.184 | |||||||
Comparison A (HIV-infected vs HIV-uninfected), Comparison B (ART initiated before pregnancy vs ART initiated during pregnancy), Comparison C (ART initiated during pregnancy at four time points: P1 (<14 weeks), P2 (14–20 weeks), P3 (21–27 weeks), P4 (>28weeks).
aAdjusted for age, maternal height, parity and previous PTD.
bAdjusted for age, maternal height, parity and previous PTD, CD4 count and VL.
Among HIV-infected women (comparisons B and C), there was a similar distribution of gestational age and birthweight subgroups, with most being late or moderately preterm and/or LBW (Table 2). Birth outcomes did not vary appreciably in comparison B (initiating ART before pregnancy, n = 477 vs initiating in pregnancy, n = 799): PTD (AOR 0.70, 95% CI: 0.45, 1.07); LBW (AOR 0.72, 95% CI: 0.43, 1.21); SGA (AOR 1.05, 95% CI: 0.58, 1.91) (Figure 2, Table 3). Results were similar for comparison C (Figure 2, Table 3). In addition, the findings did not change appreciably when those comparisons were restricted to women who initiated ART after the July 2013 ART eligibility guideline changes.
Among term infants, similar proportions were AGA (87% vs 88%) and SGA (13% vs 12%), comparing those born to HIV-infected and HIV-uninfected women. Likewise among preterm infants. the proportions who were AGA (87% vs 84%) and SGA (13% vs 16%) were similar in the HIV-infected and HIV-uninfected women.
Subgroups of antiretroviral agents
Because the associations between ART and birth outcomes may depend on choice of ARV, we carried out the same comparisons restricted to subgroups by antiretroviral agents. The incidences of PTD, LBW and SGA were not appreciably different among women on EFV-based regimens compared with the total HIV-infected sample (Supplementary Table 1, available as Supplementary data at IJE online). Following adjustment for age, parity, height and previous PTD, HIV infection was associated with an increased odds of PTD (AOR 1.97, 95% CI: 1.27, 3.04) but not LBW (AOR 1.51, 95% CI: 0.91, 2.48). Among HIV-infected women (Comparison B), a higher incidence of PTD was observed among women conceiving on EFV-containing regimens (29%) compared with those initiating EFV-containing regimens during pregnancy (21%). This difference in PTD persisted following adjustment for confounders (AOR 0.60, 95% CI: 0.39, 0.94) (Supplementary Table 2, available as Supplementary data at IJE online). When comparisons were restricted to women initiating ART in pregnancy (Comparison C), no differences were observed.
When analysis was restricted to women on PI-based regimens, a higher incidence of PTD (34% vs 13%) was observed in the HIV-infected (n = 29) compared with HIV-uninfected women (n = 278) (Supplementary Table 3, available as Supplementary data at IJE online). This incidence in women on PI-based regimens (34%) was higher than that observed in HIV-infected women in the unrestricted (22%) and EFV-based analyses (23%). In multivariable analysis, HIV infection was associated with an increased odds of PTD (AOR 4.46, 95% CI: 1.55, 12.83) but not LBW or SGA (Supplementary Table 4, available as Supplementary data at IJE online). When women on PI-based regimens were compared with women on any NNRTI regimens, a higher incidence of PTD in women on PI-based regimens was noted (36% vs 24%) (Supplementary Table 5, available as Supplementary data at IJE online).
Subgroups by gestation at first ANC visit
When analysis was restricted to women who entered ANC before 20 weeks of gestation, in whom gestational estimation is likely to be most accurate, results for each comparison mirrored those of the main analysis. A higher incidence of PTD (22% vs 9%) and LBW (15% vs 7%) was observed in HIV-infected (n = 582) compared with HIV-uninfected women (n = 128), whereas the frequency of SGA deliveries (12% vs 11%) was similar (Supplementary Table 6, available as Supplementary data at IJE online). In multivariable analysis, HIV infection was associated with an increased odds of PTD (AOR 2.75, 95% CI: 1.38, 5.48) (Supplementary Table 7, available as Supplementary data at IJE online); however, the association with LBW (AOR 2.19, 95% CI: 0.97, 4.94) did not persist. Among HIV-infected women there were no differences observed between women initiating ART before pregnancy compared with those initiating during pregnancy (Comparison B). Similarly when comparisons were restricted to women initiating ART in pregnancy (Comparison C), no differences were observed between groups.
Discussion
In this cohort of HIV-infected and -uninfected pregnant women seeking ANC at a large South African public sector primary care facility, PTD appeared consistently associated with HIV infection and ART use, with HIV-infected women receiving ART being approximately twice as likely to deliver preterm compared with HIV-uninfected women. We found few appreciable differences in adverse birth outcomes between women initiating ART during pregnancy vs those initiating ART before pregnancy, though in subgroup analyses restricted to EFV-based regimens, PTD appeared to be more likely in women conceiving on ART compared with those initiating during pregnancy.
Our finding of a higher incidence of PTD in HIV-infected women regardless of timing of ART use is consistent with several previous studies from African populations as well as from high-income countries.4,17,20 However, the PTD incidence among both HIV-infected (22%) and HIV-uninfected (13%) women observed here is higher than previous estimates for South Africa (approximately 10%).31 These results raise concern, as PTD is the most common cause of neonatal morbidity and mortality globally,32 particularly in LMICs.4,33 Nonetheless, a larger proportion of our PTDs occurred later in gestation (>32weeks), which is somewhat reassuring.24,34
We did not observe any differences in the proportions of term or preterm SGA or any significant associations with SGA across any of the three major analytical comparisons. The lack of association between HIV status and SGA is different from a study in Botswana during the NVP-based ART era;4 however, our findings were similar to those from another recent study in Botswana which evaluated TDF + FTC + EFV.11 In LMIC, SGA is usually a result of intrauterine growth restriction (IUGR) which leads to LBW, as opposed to being normally grown but small because of PTD (constitutionally small).35 IUGR in these settings tends to be caused by extrinsic factors and is late onset (>32 weeks).36 Given our higher frequency of late PTD (34–37 weeks), our SGA findings could be a result of a reduction in the time for the effects of late onset growth restriction to take place because of earlier delivery. Consequently any reductions in birthweight would be insufficient to achieve the definition of SGA.
Overall among all HIV-infected women, we found that timing of initiation of widely used NNRTI-based regimens, before or during pregnancy, was not associated with adverse birth outcomes (PTD, LBW and SGA). A study in Cameroon came to similar conclusions in terms of PTD.19 However, our overall results differ from a number of previous studies that have demonstrated an increased risk of PTD and/or LBW in women initiating ART before pregnancy9,37 and in women initiating during pregnancy.5 There are concerns that previous studies comparing timing of ART initiation and adverse birth outcomes did not take into account gestational age at ART initiation, as women initiating ART later in pregnancy do not have equal opportunity to experience different outcome compared with those who initiated earlier or before pregnancy. As part of our subgroup analyses, we restricted the analysis to women who initiated before 26 weeks and those who experienced an outcome after 26 weeks; these results were similar to the results of the overall analysis.
One explanation for the lack of associations observed in our study could be the relatively high overall incidence of PTD (22%), possibly obscuring a weak signal for increased adverse birth outcomes among the women who initiated ART earlier during pregnancy. It should be noted that in subgroup analyses, when restricted to EFV-based regimens, women who initiated ART before pregnancy were at increased risk of PTD compared with those initiating during pregnancy, which is consistent with previous studies that demonstrated an increased risk of PTD and/or LBW in women on ART initiated before pregnancy.9,37 Given missing regimen data, particularly among younger women, this subgroup analysis requires cautious interpretation, given that previous studies have shown that differences in birth outcomes between initiating ART before pregnancy compared with those initiating during pregnancy may be largely attributable to differences in other risk factors for adverse outcomes, such as gravidity4 and maternal age.
Despite the global use of TDF + FTC + EFV, few studies to date have investigated the effect of this regimen in pregnancy on birth outcomes. Our results are consistent with a recent study of a national programme using this regimen in Botswana,11 suggesting this regimen is unlikely to worsen rates of adverse birth outcomes. Despite observing no overall differences in adverse birth outcomes by timing of ART initiation or with NNRTI use, there is some suggestion in these results of increased risk linked to the use of PI-based regimens, consistent with previous studies.14,38 PIs may cause increased adverse birth outcomes via mechanisms related to interference with the adrenal system, implicated in the spontaneous onset of labour,15 and/or reductions in progesterone levels during pregnancy which could affect fetal growth.39 To investigate this potential effect here, we compared women using PI-based to those using NNTRI-based regimens, and found a similarly increased risk of PTD among women using PI-based regimens, albeit with limited precision.
We found notable differences in miscarriages and stillbirths between HIV-infected and HIV-uninfected women. Stillbirths appeared more likely among HIV-infected women, consistent with a meta-analysis demonstrating a nearly 4-fold increase in stillbirths among HIV-exposed pregnancies.3 Conversely, miscarriage appeared more likely in the HIV-uninfected women than HIV-infected women, a finding that is unexpected given that HIV status is often associated with early pregnancy loss.3 In considering the latter finding, it is critical to note that these data–with enrolment of women as they present for routine care at a range of gestations–are not ideal for examining early pregnancy loss, and in turn, this finding should be approached with caution.
Interpretation of these data requires consideration of several strengths and limitations. This study of a public sector primary care population allowed examination of the impact of ART initiation across a range of gestations, compared with clinical trials where gestation at ART initiation is often fixed. Furthermore, the observational nature of our study provides good external validity of experiences in pregnancy. These results are also substantially strengthened by the use of high quality measures of gestation,40 which contrasts with the reliance on SFH and/or LMP throughout previous analyses. Ultrasonography for gestational age determination has been shown to be highly reproducible up to the early second trimester.40 Since we enrolled women entering ANC throughout pregnancy, we conducted subanalyses restricted to women entering ANC < 20 weeks, which did not affect our findings. A major limitation of our study is that our sample size is limited for certain subgroup analyses (including by ART regimen). We were also unable to directly measure birthweight and relied on data abstraction from routine records; although this approach is widely used in research, it may contribute to random measurement error, potentially attenuating findings for LBW and SGA outcomes. In addition, we had missing regimen data for women initiating ART before pregnancy; this is a result of the design of the parent study that collected less information on these women compared with those initiating during pregnancy.
This research focuses on widely used NNRTI-based regimens which include TDF + FTC + EFV, the first-line regimen currently recommended by the WHO and the most commonly used combination of antiretroviral drugs globally. However as new antiretroviral agents become more widely available, it will be critical to continue to evaluate birth and long-term outcomes associated with in utero ART exposure. This includes both ongoing epidemiological research and investigations of the pathophysiological mechanisms that may lead HIV infection and/or antiretroviral use to cause prematurity and/or growth restriction.
In summary, with the large and rapidly increasing numbers of HIV-infected women receivinging ART during pregnancy around the world, our study suggests that current NNRTI-based regimens are unlikely to further increase adverse birth outcomes. However, given the limited data on TDF + FTC + EFV, and the results of the EFV-based subgroup analyses, more studies investigating this regimen according to timing of ART initiation are required in representative cohorts. These data highlight the high incidence of PTD among HIV-infected women on ART, pointing to a significant public health problem and an important consideration for the long-term health of HIV-exposed infants and children globally.
Supplementary Data
Supplementary data are available at IJE online.
Funding
This research was supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the National Institute of Child Health and Human Development (NICHD), grant number 1R01HD074558. Additional funding came from the South African Medical Research Council (Clinician Researcher Development Fund), the National Institute of Health (NIH) Fogarty International Center Grant #5R25TW009340 and the NIH Office of AIDS Research.
Conflict of interest: None declared.
Key Messages
Several studies have suggested that antiretroviral therapy (ART) use in pregnancy may contribute to adverse birth outcomes, but there are few data from sub-Saharan Africa, where HIV is most prevalent.
In this cohort of 1554 women enrolled in routine public sector care in Cape Town, HIV-infected women had higher incidence of adverse birth outcomes (preterm and low birthweight delivery) compared with HIV-uninfected women. There appeared to be no associations between the timing of antiretroviral initiation before or during pregnancy and birth outcomes, although some of the comparisons may have been limited by lack of power.
Whereas these data suggest that first-line ART regimens (TDF + FTC + EFV) appear to be safe during pregnancy, the high incidence of preterm delivery among HIV-infected women on ART remains a significant public health problem.
Supplementary Material
References
- 1. Abrams EJ, Myer L. Can we achieve an AIDS-free generation? Perspectives on the global campaign to eliminate new pediatric HIV infections. J Acquir Immune Defic Syndr 2013;63(Suppl 2):S208–12. [DOI] [PubMed] [Google Scholar]
- 2. Rollins NC, Coovadia HM, Bland RM. et al. Pregnancy outcomes in HIV-infected and uninfected women in rural and urban South Africa. J Acquir Immune Defic Syndr 2007;44:321–28. [DOI] [PubMed] [Google Scholar]
- 3. Brocklehurst P, French R. The association between maternal HIV infection and perinatal outcome: a systematic review of the literature and meta‐analysis. BJOG 1998;105:836–48. [DOI] [PubMed] [Google Scholar]
- 4. Chen JY, Ribaudo HJ, Souda S. et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis 2012;206:1695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Short CE, Douglas M, Smith JH, Taylor GP. Preterm delivery risk in women initiating antiretroviral therapy to prevent HIV mother‐to‐child transmission. HIV Med 2014;15:233–38. [DOI] [PubMed] [Google Scholar]
- 6. Thorne C, Patel D, Newell ML. Increased risk of adverse pregnancy outcomes in HIV-infected women treated with highly active antiretroviral therapy in Europe. AIDS 2004;18:2337–39. [DOI] [PubMed] [Google Scholar]
- 7. Townsend CL, Cortina-Borja M, Peckham CS, Tookey PA. Antiretroviral therapy and premature delivery in diagnosed HIV-infected women in the United Kingdom and Ireland. AIDS 2007;21:1019–26. [DOI] [PubMed] [Google Scholar]
- 8. Ekouevi DK, Coffie P, Becquet R. et al. Antiretroviral therapy in pregnant women with advanced HIV disease and pregnancy outcomes in Abidjan, Cote d'Ivoire. AIDS 2008;22:1815–20. [DOI] [PubMed] [Google Scholar]
- 9. Li N, Sando MM, Spiegelman D. et al. Antiretroviral therapy in relation to birth outcomes among HIV-infected women: a cohort study. J Infect Dis 2016;213:1057–64. [DOI] [PubMed] [Google Scholar]
- 10. Tuomala RE, Shapiro DE, Mofenson LM. et al. Antiretroviral therapy during pregnancy and the risk of an adverse outcome. N Engl J Med 2002;364:1863–70. [DOI] [PubMed] [Google Scholar]
- 11. Zash R, Souda S, Chen JY. et al. Reassuring birth outcomes with tenofovir/emtricitabine/efavirenz used for prevention of mother to child transmission of HIV in Botswana. J Acquir Immune Defic Syndr 2016;71:428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grosch-Woerner I, Puch K, Maier RF. et al. Increased rate of prematurity associated with antenatal antiretroviral therapy in a German/Austrian cohort of HIV‐1‐infected women. HIV Med 2008;9:6–13. [DOI] [PubMed] [Google Scholar]
- 13. Martin F, Taylor GP. Increased rates of pre-term delivery are associated with the initiation of highly active antiretroviral therapy during pregnancy: a single centre cohort study. J Infect Dis 2007;196:558–61. [DOI] [PubMed] [Google Scholar]
- 14. Powis KM, Kitch D, Ogwu A. et al. Increased risk of preterm delivery among HIV-infected women randomized to protease versus nucleoside reverse transcriptase inhibitor-based ART during pregnancy. J Infect Dis 2011;204:506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sibiude J, Warszawski J, Tubiana R. et al. Premature delivery in HIV-infected women starting protease inhibitor therapy during pregnancy: role of the ritonavir boost? Clin Infect Dis 2012;54:1348–60. [DOI] [PubMed] [Google Scholar]
- 16. Bengtson AM, Chibwesha CJ, Westreich D. et al. Duration of cART before delivery and low infant birthweight among HIV-infected women in Lusaka, Zambia. J Acquir Immune Defic Syndr 2016;71:563–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boer K, Nellen JF, Patel D. et al. The AmRo study: pregnancy outcome in HIV‐1‐infected women under effective highly active antiretroviral therapy and a policy of vaginal delivery. BJOG 2007;114:148–55. [DOI] [PubMed] [Google Scholar]
- 18. Kesho Bora Study Group. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis 2011;11:171–80. [DOI] [PubMed] [Google Scholar]
- 19. Njom Nlend A, Nga Motazé A, Moyo Tetang S, Zeudja C, Ngantcha M, Tejiokem M. Preterm birth and low birthweight after in utero exposure to antiretrovirals initiated during pregnancy in Yaoundé, Cameroon. PLoS One 2016;11:e0150565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Olagbuji BN, Ezeanochie MC, Ande AB, Oboro VO. Obstetric and perinatal outcome in HIV positive women receiving HAART in urban Nigeria. Arch Gynecol Obstet 2010;281:991–94. [DOI] [PubMed] [Google Scholar]
- 21. Tuomala RE, Watts DH, Li D. et al. Improved obstetric outcomes and few maternal toxicities are associated with antiretroviral therapy, including highly active antiretroviral therapy during pregnancy. J Acquir Immune Defic Syndr 2005;38:449–73. [DOI] [PubMed] [Google Scholar]
- 22. WHO. Prevent HIV, Test and Treat All – WHO Support for Country Impact. Progress Report 2016. http://apps.who.int/iris/bitstream/10665/251713/1/WHO-HIV-2016.24-eng.pdf?ua=1 (30 November 2016, date last accessed).
- 23. Beck S, Wojdyla D, Say L. et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ 2010;88:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blencowe H, Cousens C, Chou D. et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health 2013;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Watts DH, Mofenson LM. Antiretrovirals in pregnancy: a note of caution. J Infect Dis 2012;206:1639–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Myer L, Phillips TK, Manuelli V. et al. Evolution of antiretroviral therapy services for HIV-infected pregnant women in Cape Town, South Africa. J Acquir Immune Defic Syndr 2015;69:e57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Myer L, Phillips TK, Zerbe A. et al. Optimizing antiretroviral therapy (ART) for maternal and child health (MCH): Rationale and design of the MCH-ART Study. J Acquir Immune Defic Syndr 2016;72(Suppl 2):S189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Villar J, Ismail LC, Victora CG. et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014;384:857–68. [DOI] [PubMed] [Google Scholar]
- 29. Villar J, Giuliani F, Bhutta ZA. et al. Postnatal growth standards for preterm infants: the Preterm Postnatal Follow-up Study of the INTERGROWTH-21st Project. Lancet Glob Health 2015;3:e681–91. [DOI] [PubMed] [Google Scholar]
- 30. WHO. Neonatal and Perinatal Mortality: Country, Regional and Global Estimates. http://apps.who.int/iris/handle/10665/43444 (10 February 2017, date last accessed).
- 31. Blencowe H, Cousens S, Oestergaard MZ. et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012;379:2162–72. [DOI] [PubMed] [Google Scholar]
- 32. WHO. WHO Recommendations on Interventions to Improve Preterm Birth Outcomes. http://apps.who.int/iris/bitstream/10665/183037/1/9789241508988_eng.pdf (14 October 2016, date last accessed). [PubMed]
- 33. Katz J, Lee AC, Kozuki N. et al. Mortality risk in preterm and small-for-gestational age infants in low-income and middle-income countries: a pooled country analysis. Lancet 2013;382:417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Howson C, Kinney MV, McDougall L, Lawn JE. Born too soon: preterm birth matters. Reprod Health 2013;10(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Villar J, Belizán J. The relative contribution of prematurity and fetal growth retardation to low birthweight in developing and developed societies. Am J Obstet Gynecol 1982;143:793–98. [DOI] [PubMed] [Google Scholar]
- 36. Faraci M, Renda E, Monte S. et al. Fetal growth restriction: current perspectives. J Prenat Med 2011;5:31–33. [PMC free article] [PubMed] [Google Scholar]
- 37. Machado E, Hofer C, Nogueira S, Costa T, Lambert J. Pregnancy outcome in HIV-1-infected women receiving combination therapy prior to conception. Sex Transm Infect 2009;85:82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fowler MG, Qin M, Fiscus SA. et al. Benefits and risks of antiretroviral therapy for perinatal HIV prevention. N Engl J Med 2016;375:1726–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Papp E, Mohammadi H, Loutfy MR. et al. HIV protease inhibitor use during pregnancy is associated with decreased progesterone levels; a potential mechanism contributing to fetal growth restriction. J Infect Dis 2015;211:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Butt K, Lim K, Bly S. et al. Determination of gestational age by ultrasound. J Obstet Gynaecol 2014;36:171–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.