Abstract
Background
Simultaneously adhering to multiple healthy lifestyle factors has been related to up to 90% reduction in type 2 diabetes (T2DM) incidence in White populations; however, little is known about whether such protective effects persist in other non-White populations.
Methods
We examined the associations of six lifestyle factors with T2DM in the China Kadoorie Biobank of 461 211 participants aged 30–79 years without diabetes, cardiovascular diseases or cancer at baseline. We defined low-risk lifestyle factors as non-smoking or having stopped for reasons other than illness; alcohol consumption of <30 g/day; upper quarter of the physical activity level; diet rich in vegetables and fruits, low in red meat and with some degree of replacement of rice with wheat; body mass index (BMI) of 18.5–23.9 kg/m2; and waist-to-hip ratio (WHR) <0.90 (men)/<0.85 (women).
Results
During a median of 7.2 years of follow-up, we identified 8784 incident T2DM. In multivariable-adjusted analyses, two important risk factors for developing T2DM were higher BMI and WHR. Compared with participants without any low-risk factors, the hazard ratio [95% confidence interval (CI)] for those with at least three low-risk factors was 0.20 (0.19, 0.22). Approximately 72.6% (64.2%, 79.3%) of the incident diabetes were attributable to the combination of BMI, WHR, diet and physical activity. The population attributable risk percentage (PAR%) of diabetes appeared to be similar for men and women, and higher among urban, older and obese participants.
Conclusions
Our findings indicate that adherence to a healthy lifestyle may substantially lower the burden of T2DM in the Chinese population.
Keywords: diabetes mellitus, type 2, cohort studies, health behaviour, lifestyle
Key Messages
In mostly White populations from the USA, simultaneously adhering to multiple healthy lifestyle factors has been related to up to 90% reduction in type 2 diabetes (T2DM) incidence. However, whether such protective effects persist in other non-White populations remains unknown.
In this prospective cohort study of Chinese adults, approximately three-quarters of incident T2DM over a period of fewer than 10 years could have been avoided by adherence to a healthy lifestyle. The protective effects of adherence to a healthy lifestyle were persistent in populations of different characteristics.
Adherence to a healthy lifestyle could prevent the majority of cases of T2DM, including in less developed regions.
Background
Since 1980, the diabetes burden has shown a faster increase in low- and middle-income countries than in high-income countries.1 China is heading towards a diabetes epidemic, with the prevalence rising from 2.5% in 19942 to 11.6% in 2010.3 Such soaring of diabetes prevalence has paralleled a dramatic shift in dietary behaviour and lifestyle from traditional, healthy patterns towards unhealthy patterns.
A series of landmark randomized clinical trials in high-risk individuals with impaired glucose tolerance have demonstrated that type 2 diabetes (T2DM) could be largely prevented through dietary and lifestyle modifications,4 which have shown at least similar effectiveness as drug treatment5 and exhibited sustaining effects for many years after the intervention period.4 Because long-term pharmacologic intervention is costly, only applicable to a specific high-risk population and may lead to side effects, adherence to a healthy lifestyle remains a mainstream approach for the prevention of diabetes.6,7
Several modifiable, healthy lifestyle factors consistently linked to a lower risk of T2DM include lower weight,8 healthy dietary patterns,9 being physically active,10 non-smoking11 and moderate alcohol consumption.12 In mostly White populations from the USA, simultaneously adhering to these healthy lifestyle factors has been related to up to roughly 90% reduction in T2DM incidence.13–15 Only a few prospective studies in the Chinese population have associated healthy lifestyle factors with a lower risk of incident diabetes.16 However, how much of the burden of T2DM that could be prevented through adherence to a combination of healthy lifestyle factors remains unknown in the Chinese population.
In the current study, we prospectively examined the joint association of several modifiable dietary and lifestyle factors with the risk of T2DM and estimated the proportion of the disease that could potentially be prevented by the adoption of a healthy lifestyle in a large cohort of 0.5 million adult Chinese—the China Kadoorie Biobank (CKB).17,18
Methods
Study population
The CKB cohort was established during 2004–08, when 512 891 adults aged 30–79 years were enrolled from 10 study areas geographically spread across China, with valid baseline data including completed questionnaire, physical measurements and a written informed consent form. The study areas included five urban areas (Harbin, Qingdao, Suzhou, Liuzhou and Haikou) and five rural areas (Gansu, Henan, Sichuan, Zhejiang and Hunan). The Ethical Review Committee of the Chinese Center for Disease Control and Prevention (Beijing, China) and the Oxford Tropical Research Ethics Committee, University of Oxford (UK) approved the study. Further details of the CKB cohort have been described in previous publication.17,18
For the present analysis, we excluded participants (n = 30 300) who had a self-reported history of diabetes or screen-detected diabetes, defined as measured fasting blood glucose ≥7.0 mmol/L or random blood glucose ≥11.1 mmol/L at baseline. We also excluded participants with previously diagnosed heart disease (n = 15 472), stroke (n = 8884) or cancer (n = 2577), and those who had missing data for body mass index (BMI, n = 2) or were lost to follow-up shortly after baseline (n = 3). After these exclusions, the analysis included 461 211 participants.
Assessment of lifestyle factors and other covariates
We assessed a range of lifestyle factors in the baseline questionnaire. We asked the frequency, type and amount of tobacco smoked per day for ever smokers, and years since quitting and the reason for quitting for former smokers. Questions about alcohol consumption included typical drinking frequency, type of alcoholic beverage drunk habitually and volume of alcohol drunk on a typical drinking day in the past 12 months. Physical activity was assessed by asking the usual type and duration of activities in occupational, commuting, domestic and leisure-time-related domains in the past 12 months. We multiplied the metabolic equivalent tasks (METs) value for a particular type of activity by hours spent on that activity per day and summed the MET-hours for all activities to get the daily level of physical activity. We assessed habitual intakes of 12 conventional food groups in the past 12 months using a short qualitative food frequency questionnaire. The reproducibility of the assessment has been validated in previous studies.19–21
Trained staff measured weight, height and circumferences of waist and hip using calibrated instruments. We calculated BMI as weight in kilograms divided by height in metres squared. Waist-to-hip ratio (WHR) was the ratio of waist circumference to hip circumference.
The baseline questionnaire also inquired about the socio-demographic characteristics, personal and family medical history, and women’s reproductive information. A participant was considered as having a family history of diabetes if he reported at least one first-degree relative with that disease.
Definition of low-risk lifestyle
We considered six dietary and lifestyle factors to define a low-risk lifestyle, namely smoking, alcohol consumption, physical activity, diet, BMI and WHR, consistently with previous studies.13–15 These factors have also been addressed in recent guidelines22,23 for the prevention of T2DM and associated with the increase of diabetes in China.24 We defined participants who reported not smoking or having stopped for reasons other than illness as being at low risk for smoking status. In the CKB cohort, about half of former smokers quit because of illness.25 We included former smokers who stopped smoking for illness in the current smoker category to avoid misleadingly elevated risk. The low-risk group for alcohol consumption was defined as those who drank greater than zero but less than 30 g of alcohol per day. Participants who engaged in a sex-specific upper quarter of the physical activity level were defined as being at low risk for physical activity.
For dietary factors, we considered four food items including vegetables, fruits, red meat and wheat. High consumption of white rice has been associated with an increased risk of T2DM.26 Because the daily frequency and amount of rice consumed were not collected, we used the weekly frequency of wheat consumption as a surrogate measure. For example, if participants reported eating wheat every day, it indicates that they had partial or complete replacement for white rice with wheat. Specifically, in the present study, we defined participants who reported eating vegetables, fruits and wheat every day and red meat less than daily as being at low risk for the dietary pattern.
For general adiposity measured by BMI, the low-risk group was defined as those who had a BMI of 18.5–23.9 kg/m2, according to the standard classification specific for the Chinese population.27 For central adiposity measured by WHR, the low-risk group was defined as those who had a WHR < 0.90 in men and <0.85 in women.
Ascertainment of T2DM
We identified incident diabetes since the participants’ enrollment into the study at baseline using linkage with local disease and death registries, with the recently established national health insurance system and by active follow-up.17 Trained staff, blinded to the baseline information, coded all cases with the 10th revision of the International Classification of Diseases (ICD-10). For the present analysis, we included diabetes cases coded as E11 and E14. Other cases clearly defined as non-T2DM were excluded. Because the vast majority of our participants were aged over 40 years among whom the number of any non-T2DM was small, misclassification of other types of diabetes was minimal. The validity of reported diabetes diagnosis has been adjudicated in a random sample of 831 cases by clinical research fellows in the Oxford International Coordinating Center of the CKB reviewing their medical records during 2012–13, of which 98.6% were reconfirmed.
Statistical analysis
We calculated person-years at risk from the baseline date to the diagnosis of diabetes, death, loss to follow-up or 31 December 2013, whichever came first. Loss to follow-up in the CKB study referred to a participant whose permanent registered residence had moved out of the jurisdiction of the Regional Coordinating Center. By 31 December 2013, 2411 (0.5%) participants were lost to follow-up. Cox proportional hazards model was used to estimate the hazard ratio (HR) and 95% confidence interval (CI), with age as the underlying time scale, and stratified jointly by study area and age at baseline in a 5-year interval.
Multivariable models were adjusted for age, sex, education, marital status, family history of diabetes, menopausal status (for women only) and, in analyses of the individual lifestyle factors, each of the other lifestyle factors. The linear trend test for individual factors was performed by assigning the sex-specific median to each category and then modelling this as a continuous variable in a separate model, and, for combined lifestyle factors, by treating the number of low-risk factors as a continuous variable. The test for interaction was performed by using likelihood ratio test comparing models with and without cross-product term.
We calculated the population attributable risk percentage (PAR%)28,29—an estimate of the percentage of incident diabetes in this population during follow-up that hypothetically would not have occurred if all participants had been in the low-risk group, assuming a causal relation. In these analyses, we used a single binary variable and compared participants in the low-risk group for each factor with all the others, following a method previously suggested by Wacholder et al.30 We conducted analyses stratified according to the sex, residence, age, family history of diabetes and adiposity. We also repeated the analysis among never-regular smokers and never-regular drinkers.
We calculated PAR% using SAS (version 9.4, SAS Institute Inc.). All other statistical analyses were performed using Stata (version 13.1, StataCorp).
Results
Of 461 211 participants, 0.3%, 7.2% and 31.4% had at least five, four and three low-risk lifestyle factors, respectively. Participants of women, younger age, being more educated and urban residents were more likely to adhere to a healthy lifestyle (Table 1).
Table 1.
Age- and study area-adjusted baseline characteristics of 461 211 participants according to number of low-risk lifestyle factors
| Baseline characteristics | Number of low-risk lifestyle factorsa |
|||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ≥5 | |
| Men (n = 189 153) | ||||||
| No. of participants, n (%)b | 27 888 (14.7) | 57 293 (30.3) | 62 758 (33.2) | 34 310 (18.1) | 6570 (3.5) | 334 (0.2) |
| Age, year | 50.8 | 52.3 | 52.0 | 50.7 | 48.9 | 49.6 |
| Urban area, % | 50.2 | 44.2 | 37.7 | 37.7 | 37.5 | 55.3 |
| Middle school and above, % | 60.1 | 58.4 | 56.6 | 56.1 | 56.5 | 57.0 |
| Married, % | 94.2 | 93.0 | 92.6 | 92.8 | 93.1 | 92.2 |
| Family history of diabetes, % | 7.3 | 6.4 | 5.4 | 4.8 | 4.8 | 5.8 |
| Eating both vegetables and fruits daily, % | 12.1 | 13.1 | 13.3 | 14.8 | 21.5 | 41.6 |
| Eating red meat less than daily, % | 61.8 | 66.5 | 69.0 | 70.9 | 75.9 | 84.1 |
| Eating wheat daily, % | 41.5 | 40.7 | 41.0 | 41.1 | 43.6 | 53.0 |
| Having low-risk lifestyle factorsa, % | ||||||
| Smoking | – | 28.2 | 30.9 | 54.4 | 88.3 | 98.6 |
| Alcohol consumption | – | 2.3 | 4.6 | 8.8 | 23.0 | 68.7 |
| Physical activity | – | 16.4 | 23.5 | 48.8 | 77.4 | 89.0 |
| Dietary pattern | – | 1.3 | 2.5 | 4.4 | 12.6 | 34.3 |
| BMI | – | 33.8 | 74.2 | 92.5 | 98.1 | 99.6 |
| WHR | – | 17.8 | 64.3 | 90.0 | 97.6 | 99.0 |
| Women (n = 272 058) | ||||||
| No. of participants, n (%)b | 2022 (0.7) | 72 664 (26.7) | 93 719 (34.5) | 77 349 (28.4) | 25 395 (9.3) | 909 (0.3) |
| Age, year | 59.0 | 53.1 | 51.3 | 47.6 | 44.5 | 42.7 |
| Urban area, % | 30.0 | 41.0 | 40.9 | 46.2 | 44.4 | 73.4 |
| Middle school and above, % | 30.6 | 41.4 | 43.4 | 45.8 | 46.0 | 55.6 |
| Married, % | 88.0 | 90.5 | 89.9 | 89.0 | 88.7 | 84.0 |
| Family history of diabetes, % | 6.1 | 7.2 | 6.8 | 6.0 | 5.5 | 5.2 |
| Post-menopausal, % | 55.0 | 54.1 | 54.5 | 54.1 | 53.8 | 53.5 |
| Eating both vegetables and fruits daily, % | 14.7 | 18.8 | 20.7 | 21.6 | 24.8 | 90.1 |
| Eating red meat less than daily, % | 68.7 | 70.3 | 73.8 | 74.4 | 79.7 | 99.0 |
| Eating wheat daily, % | 34.4 | 36.5 | 37.2 | 37.8 | 41.8 | 76.1 |
| Having low-risk lifestyle factorsa, % | ||||||
| Smoking | – | 96.6 | 98.2 | 99.4 | 99.9 | 99.8 |
| Alcohol consumption | – | 0.1 | 0.5 | 1.0 | 2.8 | 18.3 |
| Physical activity | – | 0.6 | 22.1 | 27.8 | 81.6 | 98.1 |
| Dietary pattern | – | 0.1 | 4.0 | 5.0 | 15.0 | 78.1 |
| BMI | – | 2.1 | 48.0 | 87.9 | 98.8 | 99.9 |
| WHR | – | 1.1 | 26.7 | 78.4 | 98.8 | 99.6 |
BMI, body mass index; WHR, waist-to-hip ratio. The results are presented as adjusted means or percentages, with adjustment for age and study area, as appropriate. All baseline characteristics were associated with the number of low-risk lifestyle factors, with P < 0.001 for trend across categories, except for eating wheat daily in men (P = 0.001) and post-menopausal status in women (P = 0.057).
aLow-risk lifestyle factors were defined as: non-smoking or having stopped for reasons other than illness; drinking greater than zero but less than 30 g of alcohol per day; engaging in a sex-specific upper quarter of the physical activity level; eating vegetables, fruits and wheat every day and red meat less than daily; having a BMI between 18.5 and 23.9 kg/m2; and having a WHR < 0.90 in men and <0.85 in women.
bThe numbers in parentheses indicate the proportion of participants who had a different number of low-risk lifestyle factors.
During a median of 7.2 years (3 291 895 person-years) of follow-up, we identified 8784 incident T2DM. After mutual adjustment for all lifestyle factors and other covariates, two important risk factors for developing T2DM were higher BMI and WHR (Table 2; and Supplementary Table 1, available as Supplementary Data at IJE online). Heavy smoking was associated with an increased risk of diabetes, whereas light-to-moderate alcohol consumption, high physical activity and a diet rich in vegetables and fruits, low in red meat and with part or complete replacement of rice with wheat every day were associated with a reduced risk of diabetes, even after adjustment for the BMI and WHR. The inverse association between physical activity and diabetes was slightly attenuated after including BMI and WHR in the model.
Table 2.
HRs (95% CIs) for incident T2DM by lifestyle factors among 461 211 participants
| (ncase = 8784) | PY (%) | Cases | Multivariable-adjusteda |
Further adjustment for BMI and WHR |
||
|---|---|---|---|---|---|---|
| HR (95% CI) | Ptrendb | HR (95% CI) | Ptrendb | |||
| Smoking | ||||||
| Never | 68.0 | 6076 | 1.00 | 1.00 | ||
| Former | 2.8 | 287 | 1.07 (0.94, 1.21) | 0.99 (0.87, 1.12) | ||
| Current (cigarettes/day) | ||||||
| <15 | 10.7 | 848 | 0.98 (0.90, 1.07) | 0.001 | 1.06 (0.97, 1.16) | 0.018 |
| 15– | 13.4 | 1043 | 0.94 (0.86, 1.02) | 1.02 (0.93, 1.11) | ||
| 25– | 5.2 | 530 | 1.16 (1.04, 1.29) | 1.20 (1.08, 1.34) | ||
| Alcohol consumption | ||||||
| Never | 84.8 | 7619 | 1.00 | 1.00 | ||
| Current weekly | 6.0 | 397 | 0.94 (0.85, 1.05) | 0.84 (0.75, 0.93) | ||
| Current daily (g/day) | ||||||
| <15 | 0.5 | 38 | 0.68 (0.49, 0.94) | 0.400 | 0.71 (0.52, 0.98) | 0.923 |
| 15– | 1.8 | 141 | 0.74 (0.63, 0.88) | 0.75 (0.63, 0.89) | ||
| 30– | 3.0 | 261 | 0.83 (0.73, 0.94) | 0.83 (0.73, 0.94) | ||
| 60– | 4.0 | 328 | 0.79 (0.70, 0.89) | 0.79 (0.70, 0.88) | ||
| Physical activity (MET-hours/day) | ||||||
| <11.0 | 24.2 | 2363 | 1.00 | <0.001 | 1.00 | <0.001 |
| 11.0– | 25.9 | 2208 | 0.88 (0.83, 0.94) | 0.92 (0.86, 0.97) | ||
| Men 20.0–, Women 18.0– | 25.0 | 2176 | 0.89 (0.83, 0.95) | 0.98 (0.92, 1.04) | ||
| Men 33.5–, Women 29.5– | 24.9 | 2037 | 0.76 (0.71, 0.81) | 0.86 (0.81, 0.93) | ||
| Vegetables and fruits | ||||||
| Less than daily (either or both) | 82.0 | 7367 | 1.00 | 1.00 | ||
| Daily (both) | 18.0 | 1417 | 0.93 (0.87, 0.99) | 0.91 (0.85, 0.97) | ||
| Red meat | ||||||
| Daily | 29.0 | 2522 | 1.00 | 1.00 | ||
| Less than daily | 71.0 | 6262 | 0.87 (0.83, 0.92) | 0.92 (0.88, 0.97) | ||
| Wheat | ||||||
| Less than daily | 60.8 | 6726 | 1.00 | 1.00 | ||
| Daily | 39.2 | 2058 | 0.93 (0.86, 1.01) | 0.90 (0.84, 0.98) | ||
| BMI (kg/m2) | ||||||
| <18.5 | 4.4 | 230 | – | 0.96 (0.84, 1.10) | <0.001 | |
| 18.5– | 53.6 | 2872 | – | 1.00 | ||
| 24.0– | 32.4 | 3748 | – | 1.79 (1.70, 1.89) | ||
| 28.0– | 9.7 | 1934 | – | 3.04 (2.84, 3.25) | ||
| WHR | ||||||
| Men < 0.90, women < 0.85 | 43.9 | 2053 | – | 1.00 | <0.001 | |
| Men 0.90–, women 0.85– | 28.2 | 2276 | – | 1.43 (1.35, 1.53) | ||
| Men 0.95–, women 0.90– | 27.9 | 4455 | – | 2.16 (2.03, 2.30) | ||
HR, hazard ratio; CI, confidence interval; PY, person-years; BMI, body mass index; WHR, waist-to-hip ratio; MET, metabolic equivalent task. aMultivariable model was adjusted for age, sex, education, marital status and family history of diabetes. Lifestyle factors including smoking, alcohol consumption, physical activity and intakes of vegetables and fruits, red meat and wheat were included simultaneously in the same model.
bLinear trend test for smoking was only performed in current smokers and alcohol consumption in current daily drinkers.
When we dichotomized the six lifestyle factors, all low-risk factors except non-smoking were independently associated with a lower risk of diabetes in the whole cohort (Table 3). In this population, 34.4% (95% CI: 31.9%, 36.8%) of the incident diabetes could be attributed to higher or lower than normal BMI and 39.8% (37.4%, 42.3%) could be attributed to higher WHR. We repeated the analysis using BMI < 24.0 kg/m2 as the low-risk definition; the PAR% (95% CI) was 36.0% (33.5%, 38.4%) for overweight/obesity and 34.7% (31.8%, 37.5%) for higher WHR. After adjustment for BMI and WHR, the PAR% (95% CI) for an unhealthy diet and lack of physical activity was 26.5 (15.3, 36.9) and 9.1 (5.2, 13.1), respectively. Subgroup analyses according to baseline factors showed that the PAR% for low physical activity was slightly higher in the urban population, and the PAR% for the unhealthy diet was higher in the urban and older populations (Supplementary Table 2, available as Supplementary Data at IJE online).
Table 3.
HRs (95% CIs) and PARs% (95% CIs) for incident T2DM by low-risk lifestyle factorsa among 461 211 participants
| PY in low-risk group (%) | Cases in low-risk group | Multivariable-adjustedb |
Further adjustment for BMI and WHR |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | PAR% (95% CI) | HR (95% CI)c | PAR% (95% CI) | |||
| Whole cohort (ncase = 8784) | ||||||
| Smoking | 70.7 | 6363 | 1.04 (0.97, 1.11) | NA | 0.97 (0.91, 1.03) | 1.3 (–0.5, 3.0) |
| Alcohol consumption | 2.2 | 179 | 0.76 (0.66, 0.88) | 21.5 (9.7, 32.7) | 0.77 (0.66, 0.89) | 21.5 (9.7, 32.7) |
| Physical activity | 24.9 | 2037 | 0.83 (0.79, 0.88) | 12.2 (8.3, 16.0) | 0.88 (0.83, 0.93) | 9.1 (5.2, 13.1) |
| Dietary pattern | 3.5 | 189 | 0.72 (0.62, 0.83) | 27.3 (16.3, 37.7) | 0.74 (0.63, 0.86) | 26.5 (15.3, 36.9) |
| BMI | 53.6 | 2872 | – | – | 0.49 (0.47, 0.52) | 34.4 (31.9, 36.8) |
| WHR | 43.9 | 2053 | – | – | 0.49 (0.46, 0.51) | 39.8 (37.4, 42.3) |
| Men (ncase = 3259) | ||||||
| Smoking | 31.7 | 1 072 | 1.06 (0.99, 1.15) | NA | 0.99 (0.92, 1.07) | 0.2 (–4.9, 5.2) |
| Alcohol consumption | 4.6 | 154 | 0.80 (0.68, 0.94) | 17.4 (4.4, 29.9) | 0.81 (0.69, 0.96) | 16.5 (3.2, 29.1) |
| Physical activity | 25.3 | 733 | 0.78 (0.72, 0.86) | 15.5 (9.2, 21.7) | 0.85 (0.77, 0.93) | 11.4 (4.9, 17.8) |
| Dietary pattern | 2.5 | 57 | 0.78 (0.59, 1.02) | 22.7 (1.5, 41.9) | 0.78 (0.59, 1.02) | 23.2 (2.2, 42.3) |
| BMI | 55.5 | 1090 | – | – | 0.47 (0.44, 0.51) | 35.2 (31.0, 39.4) |
| WHR | 47.0 | 877 | – | – | 0.48 (0.44, 0.53) | 38.1 (33.7, 42.3) |
| Women (ncase = 5525) | ||||||
| Smoking | 97.4 | 5291 | 0.90 (0.78, 1.04) | 0.7 (0.1, 1.3) | 0.87 (0.75, 1.00) | 0.8 (0.2, 1.4) |
| Alcohol consumption | 0.6 | 25 | 0.55 (0.37, 0.82) | 42.4 (17.5, 62.3) | 0.56 (0.38, 0.83) | 41.5 (16.2, 61.7) |
| Physical activity | 24.7 | 1304 | 0.88 (0.82, 0.94) | 8.8 (3.8, 13.8) | 0.91 (0.85, 0.97) | 6.8 (1.7, 11.8) |
| Dietary pattern | 4.2 | 132 | 0.72 (0.60, 0.86) | 27.3 (14.0, 39.7) | 0.72 (0.60, 0.86) | 28.2 (15.0, 40.4) |
| BMI | 52.3 | 1782 | – | – | 0.51 (0.48, 0.54) | 33.6 (30.6, 36.5) |
| WHR | 41.8 | 1 176 | – | – | 0.49 (0.46, 0.53) | 40.3 (37.5, 43.0) |
HR, hazard ratio; CI, confidence interval; PAR%, population attributable risk percentage; PY, person-years; BMI, body mass index; WHR, waist-to-hip ratio; NA, no meaningful PAR% estimate was obtained because the estimated relative risk for this factor in the model had negative coefficient.
aLow-risk lifestyle factors were defined as: non-smoking or having stopped for reasons other than illness; drinking greater than zero but less than 30 g of alcohol per day; engaging in a sex-specific upper quarter of the physical activity level; eating vegetables, fruits and wheat every day and red meat less than daily; having a BMI between 18.5 and 23.9 kg/m2; and having a WHR < 0.90 in men and <0.85 in women.
bMultivariable model was adjusted for age, sex (for whole cohort only), education, marital status, family history of diabetes, menopausal status (for women only), and follow-up period and study area (only in pooled logistic regression model). Lifestyle factors including smoking, alcohol consumption, physical activity and dietary pattern were included simultaneously in the same model.
cAll associations of low-risk lifestyle factors with the risk of incident diabetes were consistently observed in both men and women, with P > 0.05 for interaction with sex, except for smoking (P = 0.023 for interaction).
When these lifestyle factors were considered jointly, the risk of developing diabetes decreased significantly with an increasing number of the low-risk factors in both men and women (all P for linear trend < 0.001) (Figure 1 and Supplementary Table 3, available as Supplementary Data at IJE online). Compared with participants who were not in the low-risk group for any factors, a combination of three or more healthy lifestyle factors was associated with an 80% reduction in the risk of diabetes. The respective adjusted HR (95% CI) was 0.20 (0.19, 0.22), 0.23 (0.20, 0.26) and 0.22 (0.18, 0.27) for the whole cohort, men and women.
Figure 1.
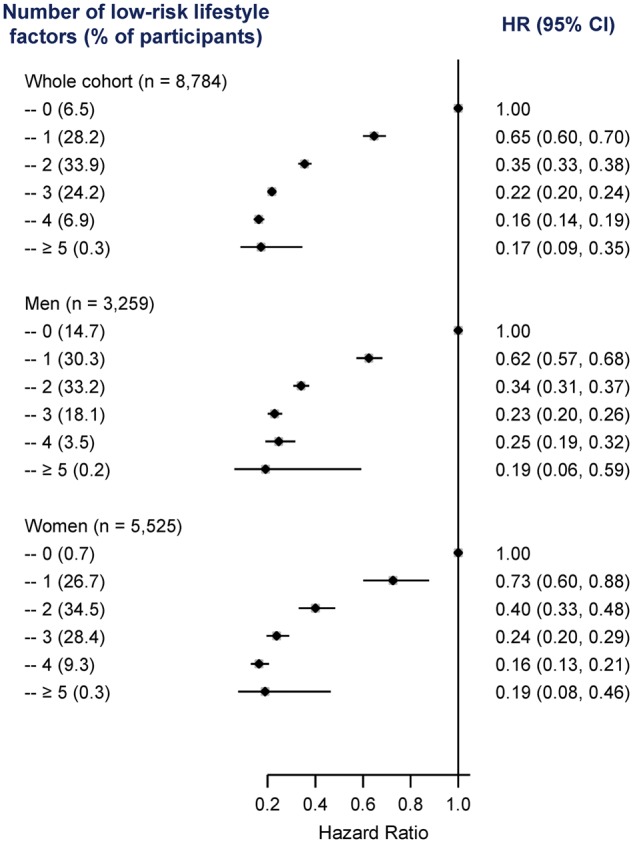
HRs (95% CIs) for incident T2DM by number of low-risk lifestyle factors among 461 211 participants. HR, hazard ratio; CI, confidence interval. Horizontal lines represent 95% CI. ‘n’ in parentheses indicates the number of new cases. Low-risk lifestyle factors include maintaining healthy adiposity, consuming a healthy diet, high physical activity, not smoking and light-to-moderate alcohol consumption.
To assess the robustness of the findings, we examined potential confounding of socio-economic status by adding occupation and household income to the model. To minimize potential bias due to subclinical conditions, we performed sensitivity analyses by further excluding diabetes cases that occurred in the first 2 years of follow-up and the results did not materially change (data not shown).
The combined PAR% (95% CI) of diabetes in relation to the lack of physical activity and unhealthy diet was 36.2% (19.9%, 50.5%) without adjustment for BMI and WHR in the model, and reduced slightly to 33.2% (16.3%, 48.2%) after adjustment for BMI and WHR (Table 4). When we included BMI and WHR in the definition of low-risk lifestyle, the PAR% (95% CI) increased to 72.6% (64.2%, 79.3%), suggesting that approximately three-quarters of the incident diabetes might have been prevented if all participants had been in the low-risk group for these four factors. The addition of non-smoking and light-to-moderate alcohol consumption to the low-risk definition added little to the PAR% (78.8%; 95% CI: 67.9%, 86.3%). We also calculated PAR% of different orders in which six low-risk lifestyle factors were added to the cumulative model in Table 4. Non-smoking had no contribution to the PAR% regardless of when it was added to the model (data not shown). Light-to-moderate alcohol consumption added more to the PAR% if it was put at the beginning of the order. For example, when we included non-smoking and light-to-moderate alcohol consumption first, the PAR% (95% CI) was 22.5% (7.8%, 36.2%).
Table 4.
PARs% (95% CIs) for incident T2DM by specific combination of low-risk lifestyle factorsa among 461 211 participants
| Physical activity, dietary pattern | +BMI | + WHR | + Smoking | + Alcohol consumption | |
|---|---|---|---|---|---|
| Whole cohort | 33.2 (16.3, 48.2) | 56.2 (44.0, 66.3) | 72.6 (64.2, 79.3) | 73.0 (63.7, 80.2) | 78.8 (67.9, 86.3) |
| Subgroup analyses | |||||
| Sex | |||||
| Men | 32.0 (0.5, 57.7) | 55.9 (32.8, 72.8) | 71.3 (54.5, 82.6) | 71.4 (49.7, 84.7) | 76.1 (51.1, 89.2) |
| Women | 33.0 (12.4, 51.0) | 55.6 (40.3, 67.8) | 72.7 (62.2, 80.6) | 72.9 (62.3, 80.9) | 84.1 (71.5, 91.5) |
| Residence | |||||
| Rural | 15.7 (–29.8, 55.4) | 42.1 (6.5, 68.2) | 64.0 (39.7, 80.0) | 64.7 (37.1, 81.8) | 70.8 (37.2, 88.0) |
| Urban | 38.0 (19.3, 54.0) | 62.6 (49.1, 73.2) | 76.2 (66.2, 83.6) | 76.5 (65.6, 84.3) | 82.6 (70.1, 90.2) |
| Age (years) | |||||
| <50 | 18.3 (–14.7, 47.7) | 49.5 (25.2, 67.9) | 66.9 (49.0, 79.4) | 67.2 (47.2, 80.6) | 75.7 (50.2, 89.0) |
| 50–59 | 39.2 (11.0, 61.5) | 59.4 (38.3, 74.6) | 73.8 (58.5, 84.0) | 74.7 (58.0, 85.3) | 77.2 (54.7, 89.3) |
| ≥60 | 38.3 (8.6, 61.7) | 58.3 (35.9, 74.4) | 75.5 (60.3, 85.5) | NA | 82.4 (65.8, 91.4) |
| Family history of diabetes | |||||
| No | 33.8 (14.9, 50.4) | 56.4 (42.7, 67.5) | 72.6 (63.2, 79.9) | 73.0 (62.5, 80.8) | 78.4 (66.0, 86.6) |
| Yes | 26.8 (–14.1, 59.9) | 53.8 (20.6, 75.8) | 72.5 (47.5, 86.7) | 73.3 (46.1, 87.9) | 82.5 (49.8, 94.7) |
| Adiposity | |||||
| No | 40.4 (–3.8, 71.4) | – | – | NA | NA |
| Yesb | 32.7 (14.3, 48.9) | 62.5 (50.9, 71.9) | 79.9 (71.8, 85.9) | 80.5 (71.8, 86.7) | 85.7 (76.5, 91.5) |
| Never-regular (daily) smokers | 29.4 (9.2, 47.2) | 53.1 (38.1, 65.3) | 70.1 (59.5, 78.4) | – | 77.1 (63.1, 86.2) |
| Never-regular (weekly) drinkers | 31.2 (12.6, 47.7) | 54.2 (40.4, 65.5) | 71.0 (61.4, 78.6) | 71.4 (61.1, 79.3) | – |
PAR%, population attributable risk percentage; CI, confidence interval; BMI, body mass index; WHR, waist-to-hip ratio. Multivariable model was adjusted for age, sex, education, marital status, family history of diabetes, menopausal status (for women only), follow-up period and study area. Besides the lifestyle factors of interest, other lifestyle factors were also included simultaneously in the same model. NA, no meaningful PAR% estimate was obtained for additionally added factor because the estimated relative risk for this factor in the model had negative coefficient.
aLow-risk lifestyle factors were defined as: non-smoking or having stopped for reasons other than illness; drinking greater than zero but less than 30 g of alcohol per day; engaging in a sex-specific upper quarter of the physical activity level; eating vegetables, fruits and wheat every day and red meat less than daily; having a BMI between 18.5 and 23.9 kg/m2; and having a WHR < 0.90 in men and <0.85 in women.
bBMI ≥ 24.0 kg/m2 or WHR ≥ 0.90 (men)/0.85 (women).
The PAR% estimates in relation to physical activity, diet, BMI and WHR in combination seems similar for men and women, and for participants with or without a family history of diabetes. The potential reduction in the incident diabetes among never-regular smokers and never-regular drinkers were consistent with those observed in the whole cohort. The PAR% appeared higher among urban residents, older populations and obese participants.
Discussion
In this large prospective cohort of 0.5 million middle-to-older-aged Chinese people, adhering to a healthy lifestyle, i.e. maintaining a normal BMI and a lower WHR, eating a diet rich in vegetables and fruits and low in red meat and white rice, being physically active, abstaining from smoking and consuming alcohol in moderation were associated with a significantly reduced risk of T2DM. Participants at low risk for three or more lifestyle factors had an 80% lower risk of diabetes than those without any of the low-risk factors. If observed associations were causal, approximately three-quarters of incident T2DM in this population during a median 7.2 years of follow-up could have been avoided by adherence to a healthy lifestyle.
Previous diabetes-prevention trials have shown that lifestyle interventions could reduce the risk of T2DM in high-risk populations.5 Our findings, consistent with previous prospective cohort studies conducted in mostly White populations from the USA13–15 and Europe,31 provided robust evidence for the beneficial effects of adherence to a healthy lifestyle on the reduction of diabetes risk in the Chinese population. Findings from the Nurses’ Health Study (NHS) of 16 years of follow-up showed that 91% (95% CI: 83%, 95%) of diabetes cases in these middle-aged women of high socio-economic status could be attributed to overweight, a poor diet, lack of exercise, smoking and abstinence from alcohol.13 A similar PAR% of 89% (23%, 99%) was reported in an older US population aged 65 years and older.14 In another study conducted in a pooled sample of two Finnish cohorts aged 40–79 years, five modifiable lifestyle factors defined by BMI, exercise, smoking, alcohol consumption and serum vitamin D explained 82% (70%, 90%) of the diabetes cases during a 10-year period.31 The Multiethnic Cohort study with 12-year follow-up showed that the PAR% (95% CI) for overweight, physical inactivity, high meat intake, no alcohol consumption and smoking was greatest among Caucasians [86% (64%, 95%) for men and 90% (66%, 97%) for women], followed by Native Hawaiians, and lowest among Japanese Americans [70% (44%, 85%) for men and 73% (42%, 88%) for women].15 Even after lowering the BMI cut-off from 25 kg/m2 to 23 kg/m2, the gap between Japanese Americans [77% (55%, 89%) for men and 78% (51%, 91%) for women] and Caucasians persisted.
The PAR% for the combination of BMI, WHR, diet, physical activity, smoking and alcohol consumption in our Chinese population was close to that of the Japanese Americans in the Multiethnic Cohort15 and appeared to be lower than that of the White populations.13–15 A possible explanation for this difference is that major lifestyle factors included in the present study were mirrored from the White populations. Other Asian-specific lifestyle risk factors might exist.24
PAR% is a population-specific calculation that depends on both the prevalence of risk factor and its association with disease risk. In the present study, the difference in the prevalence of several lifestyle factors across subgroups might lead to the observed subgroup variations in the PAR% for individual low-risk factors. For example, the PAR% for unhealthy diet differed between urban and rural residence. However, the overall proportion of disease that was attributable to the combination of these lifestyle factors differed less than might have been expected. Our findings suggested that adopting a healthy lifestyle could significantly reduce the risk of diabetes in people with family history of diabetes, similarly to the general population. In addition, our data showed that the protective effects of adherence to healthy lifestyle were persistent regardless of sex, age, urban or rural residence and adiposity status.
We found that obesity was among the most important risk factors for T2DM in Chinese people, in agreement with previous studies in White populations.13–15,31 It is well recognized that the maintenance of healthy weight confers the greatest benefits in T2DM prevention. Physical activity and diet are critical for weight loss and maintenance. However, the present study found that, even after adjustment for adiposity measures, high physical activity and a healthy diet were still associated with a reduced risk for diabetes, similarly to findings reported in previous cohort studies in the general population13–15 and prevention trials in high-risk individuals.4 This observation suggested that the beneficial effects of increased physical activity and dietary modifications were at least through mechanisms beyond weight loss.
It is worth mentioning that the PAR% results of different orders in which six low-risk lifestyle factors were added to the cumulative model were slightly different, as health-related behaviours usually influence each other in a clustered way. The choice of the order in the present study was a public health consideration. Non-smoking was not associated with a lower risk of T2DM in the present population. Light-to-moderate alcohol consumption was shown to have an important protective effect on diabetes. However, even light-to-moderate drinking might increase the risk of other outcomes such as cancer.32,33 We would be cautious on the recommendation of consuming alcohol regarding overall human health.34,35 Therefore, we included these two factors in the model last.
To the best of our knowledge, this is by far the largest prospective study quantifying the burden of T2DM that could be prevented through adherence to a combination of healthy dietary and lifestyle factors. Our study for the first time provided evidence for the joint beneficial effects of multiple lifestyle factors on prevention of diabetes in the nationally representative general Chinese population. The inclusion of a geographically spread population living in urban and rural areas, with different socio-demographic characteristics, makes our results broadly applicable. The large sample size permitted subgroup analyses by several characteristics. We have carefully controlled for potential confounding factors and sought to minimize the reverse causation bias by excluding participants with major chronic diseases at baseline that might lead to lifestyle changes. The anthropometric information was measured rather than self-reported in our cohort, providing more accurate estimates of BMI and WHR.
Several limitations merit consideration. Measurement errors in self-reported lifestyle factors are inevitable; however, misclassification in this prospective study should be non-differential on subsequent disease status and tend to underestimate the true relative risks. The lifestyle factors were measured once at baseline and might not necessarily reflect the long-term exposure. Accordingly, the present study possibly underestimates the importance of individual and combined effects of lifestyle factors on diabetes risk. Lack of comprehensive assessment of food consumption limited our ability to capture the complexity of the dietary patterns. Nevertheless, the limited food items instead of the complex dietary score may simplify the public health interpretation of our findings. Since the identification of incident diabetes in this study depended mainly on the health insurance system, some cases of asymptomatic diabetes might be undiagnosed. Although the present study might underestimate the incidence of diabetes, such non-differential misclassification might lead to attenuation of effect estimates.
Conclusions
In summary, the present study is thus far the largest prospective cohort of Chinese adults to provide critical quantitative estimates of the potential effects of modifying diet and lifestyle on the fast-growing burden of T2DM in China. Our findings suggest that the majority of cases of T2DM could be prevented by adherence to a healthy lifestyle, i.e. maintaining lean body weight without central adiposity, following healthy diet habits and being physically active. Our study lends robust support to lifestyle intervention on the reduction of diabetes in the Chinese population.
Supplementary Data
Supplementary data are available at IJE online.
Funding
This work was supported by grants (81390540, 81390544, 81390541) from the National Natural Science Foundation of China. The CKB baseline survey and the first re-survey were supported by a grant from the Kadoorie Charitable Foundation in Hong Kong. The long-term follow-up is supported by grants from the UK Wellcome Trust (088158/Z/09/Z, 104085/Z/14/Z) and by a grant from the Chinese Ministry of Science and Technology (2011BAI09B01). Dr Lv is supported by the State Scholarship Fund of China Scholarship Council (201506015053). Dr Qi is supported by NIH grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, HL126024, HL132254), the National Institute of Diabetes and Digestive and Kidney Diseases (DK091718, DK100383, DK078616), the Boston Obesity Nutrition Research Center (DK46200) and USA—Israel Binational Science Foundation Grant 2011036. Dr Qi was a recipient of the American Heart Association Scientist Development Award (0730094N). The funders had no role in the study design, data collection, data analysis and interpretation, writing of the report or the decision to submit the article for publication.
Supplementary Material
Acknowledgements
The most important acknowledgement is to the participants in the study and the members of the survey teams in each of the 10 regional centres, as well as to the project development and management teams based at Beijing, Oxford and the 10 regional centres. L.Q., L.L. and J.L. conceived and designed the paper. L.L., Z.C. and J.C., as the members of CKB steering committee, designed and supervised the conduct of the whole study, obtained funding and, together with Y.G., Z.B., L.Y., Y.C., X.H. and W.H., coordinated the data acquisition (for baseline, resurveys and long-term follow-up) and standardization. J.L. and C.Y. analysed the data. J.L. drafted the manuscript. L.Q. and L.L. contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content and approved the final version of the manuscript. All authors reviewed and approved the final manuscript. J.L. will act as guarantor for the paper.
Conflict of interest: The authors have no conflicts of interest to declare.
References
- 1. Zhou B, Lu Y, Hajifathalian K. et al. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016;387:1513–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pan XR, Yang WY, Li GW, Liu J. Prevalence of diabetes and its risk factors in China, 1994. National Diabetes Prevention and Control Cooperative Group. Diabetes Care 1997;20:1664–9. [DOI] [PubMed] [Google Scholar]
- 3. Xu Y, Wang L, He J. et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013;310:948–59. [DOI] [PubMed] [Google Scholar]
- 4. Tuomilehto J, Schwarz P, Lindstrom J. Long-term benefits from lifestyle interventions for type 2 diabetes prevention: time to expand the efforts. Diabetes Care 2011;34(Suppl 2):S210–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gillies CL, Abrams KR, Lambert PC. et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ 2007;334:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schulze MB, Hu FB. Primary prevention of diabetes: what can be done and how much can be prevented?. Annu Rev Public Health 2005;26:445–67. [DOI] [PubMed] [Google Scholar]
- 7. Alberti KG, Zimmet P, Shaw J. International Diabetes Federation: a consensus on Type 2 diabetes prevention. Diabet Med 2007;24:451–63. [DOI] [PubMed] [Google Scholar]
- 8. Vazquez G, Duval S, Jacobs DR Jr, Silventoinen K. Comparison of body mass index, waist circumference, and waist/hip ratio in predicting incident diabetes: a meta-analysis. Epidemiol Rev 2007;29:115–28. [DOI] [PubMed] [Google Scholar]
- 9. de Koning L, Chiuve SE, Fung TT. et al. Diet-quality scores and the risk of type 2 diabetes in men. Diabetes Care 2011;34:1150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aune D, Norat T, Leitzmann M. et al. Physical activity and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis. Eur J Epidemiol 2015;30:529–42. [DOI] [PubMed] [Google Scholar]
- 11. Pan A, Wang Y, Talaei M, Hu FB, Wu T. Relation of active, passive, and quitting smoking with incident type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2015;3:958–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baliunas DO, Taylor BJ, Irving H. et al. Alcohol as a risk factor for type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 2009;32:2123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hu FB, Manson JE, Stampfer MJ. et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001;345:790–7. [DOI] [PubMed] [Google Scholar]
- 14. Mozaffarian D, Kamineni A, Carnethon M. et al. Lifestyle risk factors and new-onset diabetes mellitus in older adults: the cardiovascular health study. Arch Intern Med 2009;169:798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steinbrecher A, Morimoto Y, Heak S. et al. The preventable proportion of type 2 diabetes by ethnicity: the multiethnic cohort. Ann Epidemiol 2011;21:526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu X, Cui L, Wang A. et al. Cumulative Exposure to Ideal Cardiovascular Health and Incident Diabetes in a Chinese Population: The Kailuan Study. J Am Heart Assoc 2016;5:pii: e004132. doi: 10.1161/JAHA.116.004132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen Z, Chen J, Collins R. et al. China Kadoorie Biobank of 0.5 million people: survey methods, baseline characteristics and long-term follow-up. Int J Epidemiol 2011;40:1652–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li LM, Lv J, Guo Y. et al. [The China Kadoorie Biobank: related methodology and baseline characteristics of the participants]. Zhonghua Liu Xing Bing Xue Za Zhi 2012;33:249–55. [PubMed] [Google Scholar]
- 19. Lv J, Qi L, Yu C. et al. Consumption of spicy foods and total and cause specific mortality: population based cohort study. BMJ 2015;351:h3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu C, Shi Z, Lv J. et al. Major dietary patterns in relation to general and central obesity among Chinese adults. Nutrients 2015;7:5834–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Du H, Li L, Bennett D. et al. Fresh fruit consumption and major cardiovascular disease in China. N Engl J Med 2016;374:1332–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paulweber B, Valensi P, Lindstrom J. et al. A European evidence-based guideline for the prevention of type 2 diabetes. Horm Metab Res 2010;42(Suppl 1):S3–36. [DOI] [PubMed] [Google Scholar]
- 23. American Diabetes Association. 4. Prevention or delay of type 2 diabetes. Diabetes Care 2016;39(Suppl 1):S36–8. [DOI] [PubMed] [Google Scholar]
- 24. Ma RC, Lin X, Jia W. Causes of type 2 diabetes in China. Lancet Diabetes Endocrinol 2014;2:980–91. [DOI] [PubMed] [Google Scholar]
- 25. Chen Z, Peto R, Zhou M. et al. Contrasting male and female trends in tobacco-attributed mortality in China: evidence from successive nationwide prospective cohort studies. Lancet 2015;386:1447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hu EA, Pan A, Malik V, Sun Q. White rice consumption and risk of type 2 diabetes: meta-analysis and systematic review. BMJ 2012;344:e1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen C, Lu FC, Department of Disease Control Ministry of Health PRC. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci 2004;17(Suppl):1–36. [PubMed] [Google Scholar]
- 28. Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer Causes Control 2007;18:571–9. [DOI] [PubMed] [Google Scholar]
- 29. Hertzmark E, Wand H, Spiegelman D. The SAS PAR Macro. 2012. https://www.hsph.harvard.edu/donna-spiegelman/software/par/ (20 April 2017, date last accessed).
- 30. Wacholder S, Benichou J, Heineman EF. et al. Attributable risk: advantages of a broad definition of exposure. Am J Epidemiol 1994;140:303–9. [DOI] [PubMed] [Google Scholar]
- 31. Laaksonen MA, Knekt P, Rissanen H. et al. The relative importance of modifiable potential risk factors of type 2 diabetes: a meta-analysis of two cohorts. Eur J Epidemiol 2010;25:115–24. [DOI] [PubMed] [Google Scholar]
- 32. Corrao G, Bagnardi V, Zambon A. et al. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev Med 2004;38:613–19. [DOI] [PubMed] [Google Scholar]
- 33. Bagnardi V, Rota M, Botteri E. et al. Light alcohol drinking and cancer: a meta-analysis. Ann Oncol 2013;24:301–8. [DOI] [PubMed] [Google Scholar]
- 34. Colhoun H, Ben-Shlomo Y, Dong W. et al. Ecological analysis of collectivity of alcohol consumption in England: importance of average drinker. BMJ 1997;314:1164–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smyth A, Teo KK, Rangarajan S. et al. Alcohol consumption and cardiovascular disease, cancer, injury, admission to hospital, and mortality: a prospective cohort study. Lancet 2015;386:1945–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


