Abstract
Background
Prior studies have suggested that arteriovenous fistula (AVF) or graft (AVG) creation may be associated with slowing of estimated glomerular filtration rate (eGFR) decline. It is unclear if this is attributable to the physiological benefits of a mature access on systemic circulation versus confounding factors.
Methods
We examined a nationwide cohort of 3026 US veterans with advanced chronic kidney disease (CKD) transitioning to dialysis between 2007 and 2011 who had a pre-dialysis AVF/AVG and had at least three outpatient eGFR measurements both before and after AVF/AVG creation. Slopes of eGFR were estimated using mixed-effects models adjusted for fixed and time-dependent confounders, and compared separately for the pre- and post-AVF/AVG period overall and in patients stratified by AVF/AVG maturation. In all, 3514 patients without AVF/AVG who started dialysis with a catheter served as comparators, using an arbitrary 6-month index date before dialysis initiation to assess change in eGFR slopes.
Results
Of the 3026 patients with AVF/AVG (mean age 67 years, 98% male, 75% diabetic), 71% had a mature AVF/AVG at dialysis initiation. eGFR decline accelerated in the last 6 months prior to dialysis in patients with a catheter (median, from −6.0 to −16.3 mL/min/1.73 m2/year, P < 0.001), while a significant deceleration of eGFR decline was seen after vascular access creation in those with AVF/AVG (median, from −5.6 to −4.1 mL/min/1.73 m2/year, P < 0.001). Findings were independent of AVF/AVG maturation status and were robust in adjusted models.
Conclusions
The creation of pre-dialysis AVF/AVG appears to be associated with eGFR slope deceleration and, consequently, may delay the onset of dialysis initiation in advanced CKD patients.
Keywords: arteriovenous access, chronic kidney disease, eGFR decline, hemodialysis
INTRODUCTION
Each year, as many as 115 000 patients transition from advanced non-dialysis-dependent chronic kidney disease (NDD-CKD) to end-stage renal disease (ESRD) in the USA [1], the majority of whom are treated with in-center hemodialysis and require a vascular access [2], such as an arteriovenous fistula (AVF) or graft (AVG), or a tunneled central venous catheter. Existing guidelines have encouraged the timely creation of an arteriovenous access as the preferred vascular access type rather than a central venous catheter [2, 3], based on the evidence that using an arteriovenous access can provide greater blood flow rates [4] and is associated with lower infection risk, fewer hospitalizations, prolonged survival and improved quality of life compared with using a central venous catheter [5–11]. The creation of an arteriovenous access may also have systemic physiological benefits, such as decreased total peripheral resistance and both systolic and diastolic blood pressure (BP), and increased stroke volume, left ventricular ejection fraction and cardiac output [12, 13]. Furthermore, a recent study has demonstrated that successful AVF creation prior to dialysis initiation may be associated with a slowing of estimated glomerular filtration rate (eGFR) decline [14], possibly due to functional and structural changes of endothelium induced by the local shear wall stress downstream from the created access [15, 16]. However, it remains unclear if the observed association was specific to arteriovenous access creation and its associated physiological benefits versus confounding factors observed in late-stage NDD-CKD that influence eGFR independent of vascular access maturation.
In this study, we hypothesized that patients with an AVF/AVG are more likely to experience deceleration of eGFR decline after creation of AVF/AVG versus those without AVF/AVG, and that patients with a mature AVF/AVG would benefit more from its physiological effects on kidney function than those with a non-mature AVF/AVG. To test these hypotheses, we investigated the association of AVF/AVG creation with change in eGFR slopes using a large nationally representative cohort of US veterans with advanced CKD transitioning to dialysis.
MATERIALS AND METHODS
Cohort definition
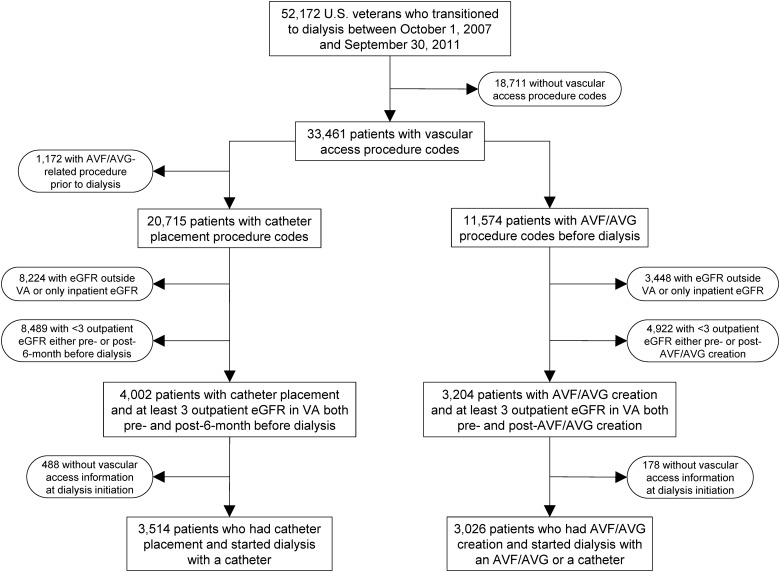
We analyzed data from the Transition of Care in CKD (TC-CKD) study, a retrospective cohort study examining US veterans transitioning to dialysis from 1 October 2007 through 30 September 2011 [17]. A total of 52 172 US veterans were identified from the US Renal Data System (USRDS) [1] as an initial cohort. The algorithm for the cohort definition is shown in Figure 1. At first, patients without any vascular access procedure codes, as identified by the International Classification of Diseases, Ninth Revision Clinical Modification (ICD-9-CM) procedure codes and Current Procedural Terminology (CPT) codes (Supplementary data, Table S1), were excluded (n = 18 711). Of the remaining 33 461 patients with vascular access procedure codes, 11 574 patients with AVF/AVG creation procedure codes prior to dialysis initiation were identified. In order to quantify the trajectory (slope) of eGFR over time, we used outpatient serum creatinine measurements available from Veterans Affairs (VA) medical centers because of the potential fluctuation of serum creatinine levels among hospitalized patients. Therefore, patients without serum creatinine measurement(s) in the VA medical system or those with only inpatient serum creatinine measurement(s) were excluded (n = 3448). Patients were also excluded if they had fewer than three outpatient serum creatinine measurements either before the AVF/AVG creation or during the interval between the AVF/AVG creation and dialysis initiation (n = 4922). The final cohort consisted of 3026 patients with an AVF/AVG (Figure 1). We also identified 3514 patients without a pre-dialysis AVF/AVG creation as comparators, who started dialysis with a tunneled catheter and had at least three outpatient serum creatinine measurements both before and after the 6-month index date prior to dialysis initiation (Figure 1).
FIGURE 1.
Flow chart of the study population. AVF, arteriovenous fistula; AVG, arteriovenous graft; eGFR, estimated glomerular filtration rate; VA, Veterans Affairs.
Data collection
Data from the USRDS Patient and Medical Evidence files were used to determine baseline demographic characteristics and type of vascular access at the time of dialysis initiation. Laboratory variables including serum creatinine were collected as previously described [18, 19]. Baseline values for laboratory variables (except for serum creatinine) were defined as the last quarterly average of each variable before dialysis initiation or the second quarterly average from the last if the last one was not available. Data regarding medication exposure were obtained from both Centers for Medicare & Medicaid Services (CMS) Data (Medicare Part D) and VA pharmacy dispensation records [20]. Patients who received at least one dispensation of outpatient medications within 6 months prior to dialysis initiation were recorded as having been treated with these medications. Information on angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs) use and systolic BP were collected for the entire evaluation period as time-dependent variables. Information about vascular access procedures and comorbidities was extracted from the VA Inpatient and Outpatient Medical SAS Datasets [21], using the ICD-9-CM diagnostic and procedure codes and CPT codes, as well as from VA/CMS data. Cardiovascular disease was defined as the presence of diagnostic codes for coronary artery disease, angina, myocardial infarction or cerebrovascular disease. We calculated the Charlson comorbidity index score using the Deyo modification for administrative data sets, without including kidney disease [22]. eGFR was calculated based on the Chronic Kidney Disease Epidemiology Collaboration equation [23], from all available outpatient serum creatinine measurements starting not more than 7 years before dialysis initiation.
Statistical analysis
Data are presented as number (percent) for categorical variables and mean ± standard deviation or median [interquartile range (IQR)] as appropriate. Continuous variables were compared using t tests or Mann–Whitney U tests as appropriate. Categorical variables were analyzed with χ2 test. Progression of CKD was assessed by estimating slopes of eGFR (annual change in eGFR) from mixed-effects models with random intercepts and slopes using the XTMIXED command in STATA [24]. This model estimates the slopes of eGFR over time, taking into account the varying number and spacing of eGFR measurements, as well as the variable follow-up for each subject [24]. The effect of potential confounders on eGFR slopes was analyzed in an adjusted multilevel mixed-effects model, which included fixed (age, sex, race, diabetes mellitus and Charlson comorbidity index) and time-dependent variables (systolic BP and ACEIs/ARBs use).
In order to assess change in eGFR slopes, we defined a cutoff time point as the date of the pre-dialysis AVF/AVG procedure for patients who underwent such a procedure. Among patients without an AVF/AVG, we used an index date of 6 months prior to dialysis initiation, which corresponded to the median time interval from AVF/AVG creation to dialysis initiation. eGFR slopes were calculated separately for these two time periods in each patient and compared using paired t tests. To account for potential differences in the effect of AVF/AVG on eGFR slopes by AVF/AVG maturation status, we stratified patients with an AVF/AVG into two groups according to whether or not an AVF/AVG was used as the primary vascular access at the time of dialysis initiation (per USRDS records). Analyses were repeated in a propensity score-matched cohort to account for dissimilarities in clinical characteristics between the groups with and without an AVF/AVG, including pre-AVF/AVG or pre-index date eGFR slopes and eGFR levels at the time of AVF/AVG creation or index date.
The changes in eGFR slope were also examined separately for AVF and AVG and in subgroups of patients categorized by age, race, body mass index, and the presence of diabetes mellitus or cardiovascular disease. Of the variables included in the multivariable-adjusted mixed-effects model, none was missing in the final cohort. Analyses were conducted using STATA MP Version 14 (STATA Corporation, College Station, TX, USA). The study was approved by the Institutional Review Boards of the Memphis and Long Beach VA Medical Centers, with exemption from informed consent.
RESULTS
Patients' baseline characteristics at the time of dialysis initiation are presented in Table 1. Among 3026 patients with an AVF/AVG, the mean ± standard deviation age was 67.0 ± 10.8 years, 97.9% were male, 35.2% were African-American and 74.8% were diabetic. Of the patients with an AVF/AVG, 2147 (71%) had a mature AVF/AVG at the time of dialysis initiation, among whom there was a lower prevalence of diabetes mellitus and congestive heart failure versus those with a non-mature AVF/AVG (Supplementary data, Table S2). Compared with patients without an AVF/AVG (n = 3514), those with an AVF/AVG were more likely to be married and had a lower prevalence of cardiovascular disease, congestive heart failure, liver disease and malignancy. They were also more likely to use vitamin D analogs, phosphate binders and erythropoietin stimulating agents (ESAs); were less likely to use ACEIs/ARBs, aspirin, anti-platelet agents other than aspirin, and warfarin; had higher serum albumin, urea nitrogen and creatinine levels; and had lower serum cholesterol and pre-dialysis eGFR levels.
Table 1.
Patient characteristics at the initiation of dialysis according to the absence or presence of AVF/AVG
| Variable | Without AVF/AVG (n = 3514) |
With AVF/AVG (n = 3026) |
P-value |
|---|---|---|---|
| Age (years) | 67.0 ± 10.8 | 67.1 ± 10.7 | 0.38 |
| Sex (male) | 3439 (97.9) | 2964 (98.0) | 0.81 |
| Race | 0.84 | ||
| White | 2201 (62.6) | 1895 (62.6) | |
| African-American | 1236 (35.2) | 1067 (35.3) | |
| Asian | 49 (1.4) | 36 (1.2) | |
| Other | 28 (0.8) | 28 (0.9) | |
| Marital status | <0.001 | ||
| Married | 1599 (45.5) | 1614 (53.3) | |
| Divorced | 1049 (29.9) | 789 (26.1) | |
| Single | 396 (11.3) | 264 (8.7) | |
| Widow | 367 (10.4) | 285 (9.4) | |
| Body mass index (kg/m2) | 30.1 ± 7.1 | 30.5 ± 6.6 | 0.027 |
| Systolic BP (mmHg) | 144.2 ± 21.4 | 144.4 ± 21.9 | 0.39 |
| Diastolic BP (mmHg) | 73.7 ± 12.2 | 73.8 ± 12.3 | 0.46 |
| Diabetes mellitus | 2629 (74.8) | 2265 (74.9) | 0.99 |
| Hypertension | 3463 (98.6) | 3017 (99.7) | <0.001 |
| Cardiovascular disease | 1671 (47.6) | 1276 (42.2) | <0.001 |
| Congestive heart failure | 2076 (59.1) | 1513 (50.0) | <0.001 |
| Liver disease | 560 (15.9) | 400 (13.2) | 0.002 |
| Malignancies | 956 (27.2) | 710 (23.5) | 0.001 |
| Charlson comorbidity index | 5 (3, 7) | 5 (3, 6) | <0.001 |
| Medications | |||
| ACEI/ARB use | 1993 (56.7) | 1472 (48.7) | <0.001 |
| Diuretic use | 2995 (85.2) | 2589 (85.6) | 0.71 |
| Statin use | 2393 (68.1) | 2066 (68.3) | 0.88 |
| Vitamin D analog use | 1091 (31.1) | 1553 (51.3) | <0.001 |
| Phosphate binder usea | 1432 (40.8) | 1543 (51.0) | <0.001 |
| Bicarbonate use | 1023 (29.1) | 909 (30.0) | 0.41 |
| ESA use | 1545 (44.0) | 1627 (53.8) | <0.001 |
| Aspirin use | 1666 (47.4) | 1129 (37.3) | <0.001 |
| Other anti-platelet use | 512 (14.6) | 363 (12.0) | 0.002 |
| Warfarin use | 357 (10.2) | 208 (6.9) | <0.001 |
| Laboratory parameters | |||
| Serum albumin (g/dL) | 3.2 ± 0.6 | 3.5 ± 0.5 | <0.001 |
| Serum cholesterol (mg/dL) | 155.7 ± 54.4 | 147.6 ± 46.1 | <0.001 |
| Serum bicarbonate (mEq/L) | 22.4 ± 3.9 | 22.2 ± 3.7 | 0.062 |
| Serum potassium (mEq/L) | 4.5 ± 0.5 | 4.5 ± 0.6 | 0.089 |
| Serum calcium (mg/dL) | 8.5 ± 0.7 | 8.7 ± 0.8 | <0.001 |
| Serum phosphorus (mg/dL) | 5.2 ± 1.3 | 5.3 ± 1.3 | 0.008 |
| Serum ALP (U/L) | 87 (68, 115) | 81 (64, 107) | <0.001 |
| Serum intact PTH (pg/mL) | 214 (122, 361) | 237 (138, 392) | <0.001 |
| Hemoglobin (g/dL) | 10.2 ± 1.4 | 10.5 ± 1.3 | <0.001 |
| Blood WBC (1000/mm3) | 8.0 ± 3.6 | 7.6 ± 2.4 | <0.001 |
| Serum urea nitrogen (mg/dL) | 66.7 ± 23.0 | 74.5 ± 21.4 | <0.001 |
| Serum creatinine (mg/dL) | 5.3 ± 2.4 | 6.1 ± 2.1 | <0.001 |
| eGFR (mL/min/1.73 m2) | 13.0 (9.6, 17.7) | 10.3 (8.0, 12.9) | <0.001 |
| Last outpatient eGFR (mL/min/1.73 m2) | 12.3 (8.6, 18.2) | 9.8 (7.5, 1.72) | <0.001 |
| Last eGFR (mL/min/1.73 m2) | 11.0 (7.7, 15.4) | 9.6 (7.2, 12.6) | <0.001 |
| Number of serum creatinine measurements | |||
| Pre-AVF/AVGb | 18 (11, 28) | 20 (12, 29) | 0.015 |
| Post-AVF/AVGb | 5 (4, 7) | 7 (4, 12) | <0.001 |
| Time period (years) | |||
| Pre-AVF/AVGb | 1.7 (0.7, 2.9) | 1.4 (0.5, 2.6) | <0.001 |
| Post-AVF/AVGb | 0.5 (0.5, 0.5) | 0.5 (0.2, 1.2) | <0.001 |
Data are presented as number (percentage), mean ± standard deviation or median (interquartile range).
AVF, arteriovenous fistula; AVG, arteriovenous graft; eGFR, estimated glomerular filtration rate; BP, blood pressure; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ESA, erythropoietin stimulating agent; ALP, alkaline phosphatase; WBC, white blood cell count; PTH, parathyroid hormone.
aPhosphate binders include calcium acetate, sevelamer or lanthanum.
bA 6-month time point prior to dialysis was used as the index date in patients without AVF/AVG.
Before the AVF/AVG creation [median (IQR) 1.4 (0.5, 2.6) years] and during the interval between the AVF/AVG creation and dialysis initiation [median (IQR) 0.5 (0.2, 1.2) years], there were a median (IQR) of 20 (12, 29) and 7 (4, 12) serum creatinine measurements per patient, respectively. In patients without an AVF/AVG, there were a median (IQR) of 18 (11, 28) and 5 (4, 7) serum creatinine measurements before [median (IQR) 1.7 (0.7, 2.9) years] and after [median (IQR) 0.5 (0.5, 0.5) years] the 6-month index date prior to dialysis, respectively.
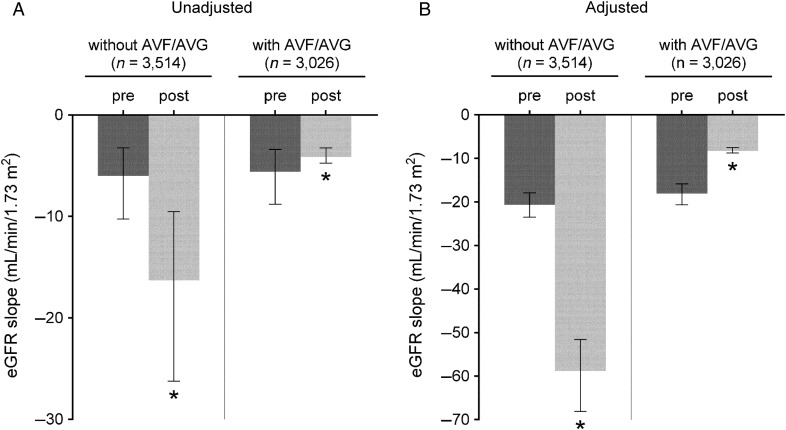
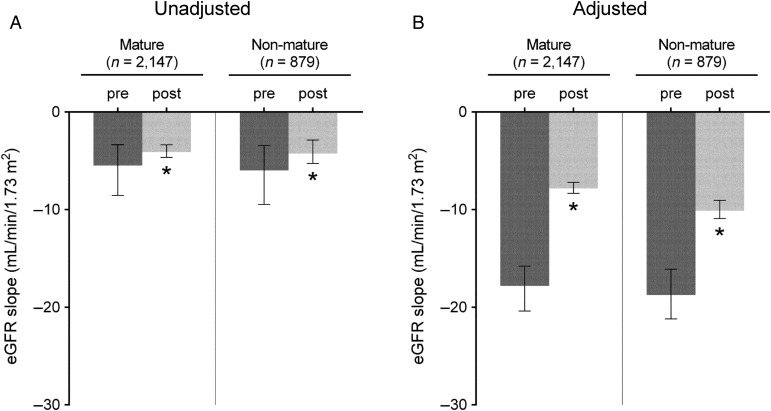
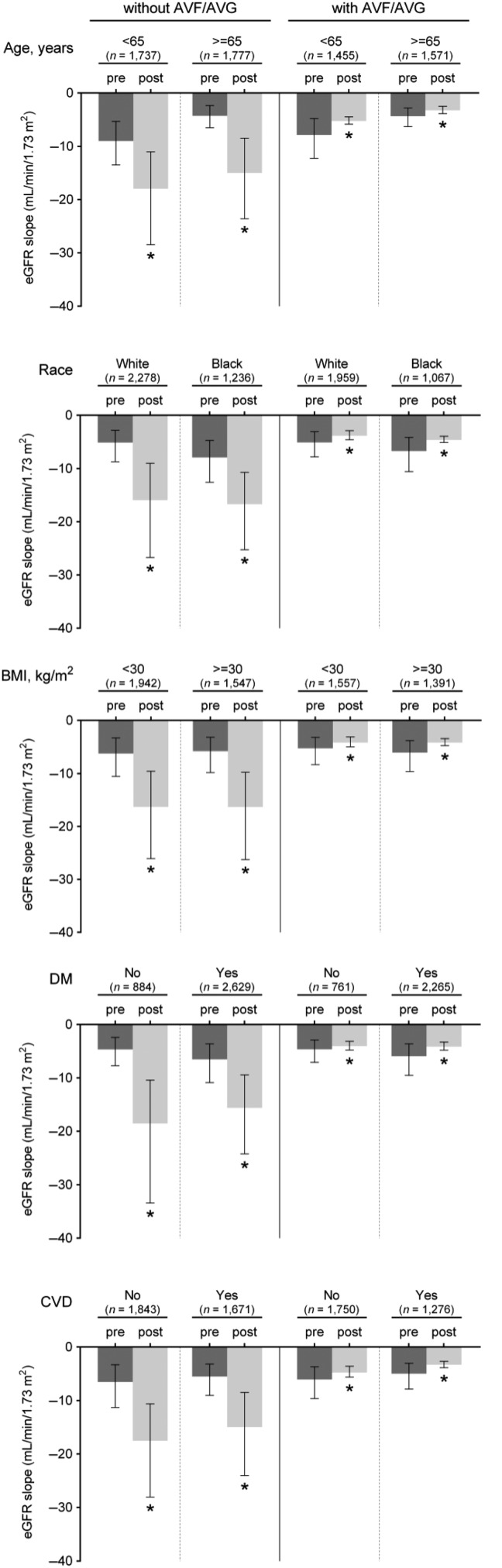
Figure 2 shows the predicted slopes of eGFR in multilevel mixed-effects models pre- and post-AVF/AVG creation in patients with an AVF/AVG, paralleled with those without an AVF/AVG. In unadjusted models, the eGFR decline accelerated in patients without an AVF/AVG [median (IQR), −6.0 (−10.2, −3.3) versus −16.3 (−26.2, −9.5) mL/min/1.73 m2/year (P < 0.001) preceding and following the 6-month index date prior to dialysis initiation, respectively], while a significant deceleration of eGFR decline was seen after the AVF/AVG creation in those with an AVF/AVG [median (IQR), −5.6 (−8.8, −3.4) versus −4.1 (−4.8, −3.2) mL/min/1.73 m2/year (P < 0.001) before and after AVF/AVG creation, respectively] (Figure 2A). After adjustment for confounders, the estimated median (IQR) eGFR slopes before and after the 6-month index date in patients without an AVF/AVG were −20.6 (−23.5, −17.9) and −58.8 (−68.1, −51.6) mL/min/1.73 m2/year (P < 0.001), respectively, whereas the estimated median (IQR) eGFR slopes before and after AVF/AVG creation in those with an AVF/AVG were −18.1 (−20.6, −15.9) and −8.3 (−8.8, −7.5) mL/min/1.73 m2/year (P < 0.001), respectively (Figure 2B). These associations were similarly observed independent of AVF/AVG maturation status (Figure 3) and access type (Supplementary data, Figure S1) and in all examined subgroups (Figure 4), as well as in the propensity-matched cohort (Supplementary data, Table S3 and Figure S2).
FIGURE 2.
eGFR slopes (median, IQR) before and after AVF/AVG creation in patients with AVF/AVG, in contrast to non-AVF/AVG patients. Slopes were estimated from unadjusted (A) and multivariable-adjusted (B) mixed-effects models. Models were adjusted for fixed (age, sex, race, diabetes mellitus and Charlson comorbidity index) and time-dependent confounders (systolic BP and ACEIs/ARBs use). *P < 0.001. eGFR, estimated glomerular filtration rate; IQR, interquartile range; AVF, arteriovenous fistula; AVG, arteriovenous graft; BP, blood pressure; ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers.
FIGURE 3.
eGFR slopes (median, IQR) before and after AVF/AVG creation in patients categorized by AVF/AVG maturation status. Slopes were estimated from unadjusted (A) and multivariable-adjusted (B) mixed-effects models. Models were adjusted for fixed (age, sex, race, diabetes mellitus and Charlson comorbidity index) and time-dependent confounders (systolic BP and ACEIs/ARBs use). *P < 0.001. eGFR, estimated glomerular filtration rate; IQR, interquartile range; AVF, arteriovenous fistula; AVG, arteriovenous graft; BP, blood pressure; ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers.
FIGURE 4.
eGFR slopes (median, IQR) before and after AVF/AVG creation in selected subgroups in patients with AVF/AVG, in contrast to non-AVF/AVG patients. Slopes were estimated from unadjusted mixed-effects models. *P < 0.001. eGFR, estimated glomerular filtration rate; IQR, interquartile range; AVF, arteriovenous fistula; AVG, arteriovenous graft; BMI, body mass index; DM, diabetes mellitus; CVD, cardiovascular disease.
DISCUSSION
In this retrospective cohort study of late-stage NDD-CKD patients transitioning to dialysis, we compared changes in eGFR slopes between the pre- and post-AVF/AVG period overall and in patients stratified by AVF/AVG maturation. We found a significant deceleration of eGFR decline after AVF/AVG creation independent of its maturation status, whereas eGFR decline accelerated in the last 6 months prior to dialysis in patients without an AVF/AVG. These findings were similarly observed in selected subgroups and were robust even after adjustment for known confounders and in the propensity-matched cohort.
Timely creation of a primary arteriovenous access before the anticipated need for hemodialysis therapy allows adequate time for the access to mature as well as sufficient time for a potential vascular access revision procedure if the first attempt fails [2]. A functional AVF/AVG provides convenient dialysis access to circulation with adequate blood flow, and also has certain systemic physiological benefits [12, 13, 25–29]. In pre-dialysis CKD patients, a successful AVF creation has been shown to be associated with reduction in arterial stiffness and BP, and an increase in stroke volume, left ventricular ejection fraction and cardiac output, in contradistinction to those with an unsuccessful AVF creation [12, 13]. These beneficial physiological effects have been suggested to be partly attributable to the functional and structural alterations of vascular endothelium such as increased production of nitric oxide and cell proliferation in response to the shear wall stress downstream from the fistula, leading to the mitigation of arterial stiffening both locally and systemically [13, 15]. These studies, however, have largely focused on the cardiovascular effects of a functional AVF; to our knowledge, there is only one study which investigated its potential physiological effects on kidney function. Golper et al. [14] evaluated the rate of eGFR decline before and after successful AVF creation among 123 patients with advanced NDD-CKD, and demonstrated that there was a significant slowing of eGFR decline from −5.9 to −0.5 mL/min/1.73 m2/year after AVF creation. They concluded that there may be an association between successful AVF creation and the slowing of eGFR decline, based on the assumption of potential ‘downstream’ vasodilatory effects of a functional AVF on renal vascular beds through vascular endothelium, which may lead to perfusion of previously under-perfused renal tissues, resulting in a recruitment of untapped renal functional reserve [30]. There seems to be biological plausibility for the observed deceleration of eGFR decline after the creation of a functional AVF; however, the previous study by Golper et al. [14] examined the change in eGFR slopes only in patients with successful AVF creation, and hence, no conclusion could be drawn as to whether the observed association was specific to AVF creation and attributable to the physiological effects of a mature access versus confounding factors observed in the late-stage NDD-CKD that may influence eGFR independent of vascular access maturation.
Our study is the first to examine the change in eGFR slopes before and after AVF/AVG creation separately by its maturation status, and in contrast to non-AVF/AVG patients, and the first to provide evidence regarding the association between AVF/AVG creation and a deceleration of eGFR decline independent of AVF/AVG maturation status. Because we detected improved outcomes after AVF/AVG creation even in patients with a non-mature AVF/AVG, we must consider the possibility that the deceleration of eGFR decline associated with AVF/AVG creation may be attributable to factors unrelated to the physiological effects of a mature vascular access, such as more attentive nephrologist care and improved patient health status or behavioral compliance with therapy. Although most of the patients agreeing to a timely AVF/AVG creation are compliant by nature, we cannot rule out the possibility of their compliance and/or nephrologist care improving even further after AVF/AVG creation and hence positively affecting their disease course. Nonetheless, there still seem to be other plausible physiological mechanisms that could explain the potential reno-protective effects of AVF/AVG creation even in those patients whose vascular access is not mature enough for successful hemodialysis cannulation.
Recently, growing evidence from experimental and clinical studies has indicated that remote ischemic preconditioning (RIPC), represented by limb ischemic preconditioning by alternating cycles of inflating and deflating BP cuffs on either the arm or leg, is a promising and feasible approach for renoprotection and prophylaxis against acute kidney injury (AKI) [31–34]. It has been postulated that even a brief ischemic stimulus of a remote site releases RIPC-induced humoral factors such as adenosine, erythropoietin or nitric oxide into the systemic circulation, which subsequently protects other target organs, including the kidney [31, 34]. Other underlying mechanisms of RIPC may include systemic immune modulation and anti-inflammatory effects on immune-competent cells following RIPC [34]. In addition, autonomic reflexive and other neurogenic pathways that are stimulated by the release of opioid and bradykinin may be involved in RIPC-induced reno-protective effects [34]. Moreover, the reno-protective effects of RIPC have been shown to be more beneficial in patients with preexisting CKD and comorbid conditions who are at higher risk for developing superimposed AKI [35]. Regarding AVF/AVG creation, temporal and repetitive clamping of the feeding artery and permanent ligation of small arterial branches are inevitable or necessary during the surgical procedure [36]. Furthermore, the AVF/AVG creation itself may cause local limb ischemia in the distal part of access [36], and hence the post-operative state of AVF/AVG creation could be considered as a similar state to RIPC. Therefore, the sequential local ischemic conditions caused by the creation of AVF/AVG could also serve as a potential explanation for the observed deceleration of eGFR slopes after AVF/AVG creation in advanced CKD patients, even in patients with a non-mature AVF/AVG.
Although many studies have demonstrated that hemodialysis through an arteriovenous access is associated with better clinical outcomes than a central venous catheter [5–10], a number of barriers must be overcome to achieve successful arteriovenous access construction; chief among these is late referral of patients for permanent access creation [37]. In addition, there remains uncertainty regarding the benefits of early arteriovenous access creation as the preferred management strategy in pre-dialysis CKD patients progressing to ESRD, given the risk of premature access-related complications and undefined cost-effectiveness of the procedure [38]. Given these circumstances, our results could be a novel incentive for advanced CKD patients to pursue timely creation of an AVF/AVG.
Our study is notable for its large sample size of late-stage NDD-CKD patients transitioning to dialysis, and for being representative of veterans in the entire geographic United States; however, several limitations need to be acknowledged. This study was observational, and hence, the results do not allow us to infer causality but merely associations between AVF/AVG creation and eGFR slopes. Most of our patients consisted of male veterans; therefore, the results may not be generalizable to women or patients from other geographical areas. Data related to AVF/AVG blood flow measured in a quantitative manner were not available; hence, the AVF/AVG maturation status was defined based on procedure codes and the type of vascular access at the time of dialysis initiation. Information about vascular access procedures was obtained from diagnostic codes recorded during care in a VA facility. Thus, there might be misclassification of vascular access status, such that patients who had undergone AVF/AVG creation at a non-VA facility which did not mature would have not been captured and might have been misclassified as comparators. However, this misclassification would tend to underestimate the true change in eGFR slopes of patients without an AVF/AVG. We adjusted predicted eGFR slopes for a variety of important covariates as potential confounders, but we cannot eliminate the possibility of unmeasured confounders, such as proteinuria, muscle mass, changes in volume status and quality of care, which might affect eGFR slopes over time.
In conclusion, the creation of an AVF/AVG is associated with an improvement in the rate of eGFR decline over time in advanced CKD patients irrespective of AVF/AVG maturation status. These findings highlight the potential beneficial effects of AVF/AVG creation on kidney function and suggest that timely creation of AVF/AVG prior to dialysis may contribute to delayed onset of dialysis initiation in advanced CKD patients. Further studies are needed to clarify the underlying mechanisms.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
Supplementary Material
ACKNOWLEDGEMENTS
This study is supported by grant 5U01DK102163 from the National Institutes of Health (NIH) to K.K.-Z and C.P.K., and by resources from the US Department of Veterans Affairs. The data reported here have been supplied by the United States Renal Data System (USRDS). Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004). C.P.K. and K.K.-Z. are employees of the Department of Veterans Affairs. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the Department of Veterans Affairs or the US government.
CONFLICT OF INTEREST STATEMENT
None declared. The results of this paper have not been published previously in whole or part.
REFERENCES
- 1. United States Renal Data System, 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2014 [Google Scholar]
- 2. Vascular Access Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis 2006; 48(Suppl 1): S248–S273 [DOI] [PubMed] [Google Scholar]
- 3. Fluck R, Kumwenda M. Renal Association Clinical Practice Guideline on vascular access for haemodialysis. Nephron Clin Pract 2011; 118(Suppl 1): c225–c240 [DOI] [PubMed] [Google Scholar]
- 4. Fan PY, Schwab SJ. Vascular access: concepts for the 1990s. J Am Soc Nephrol 1992; 3: 1–11 [DOI] [PubMed] [Google Scholar]
- 5. Dixon BS, Novak L, Fangman J. Hemodialysis vascular access survival: upper-arm native arteriovenous fistula. Am J Kidney Dis 2002; 39: 92–101 [DOI] [PubMed] [Google Scholar]
- 6. Astor BC, Eustace JA, Powe NR. et al. Type of vascular access and survival among incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for ESRD (CHOICE) Study. J Am Soc Nephrol 2005; 16: 1449–1455 [DOI] [PubMed] [Google Scholar]
- 7. Dhingra RK, Young EW, Hulbert-Shearon TE. et al. Type of vascular access and mortality in U.S. hemodialysis patients. Kidney Int 2001; 60: 1443–1451 [DOI] [PubMed] [Google Scholar]
- 8. Pastan S, Soucie JM, McClellan WM. Vascular access and increased risk of death among hemodialysis patients. Kidney Int 2002; 62: 620–626 [DOI] [PubMed] [Google Scholar]
- 9. Pisoni RL, Arrington CJ, Albert JM. et al. Facility hemodialysis vascular access use and mortality in countries participating in DOPPS: an instrumental variable analysis. Am J Kidney Dis 2009; 53: 475–491 [DOI] [PubMed] [Google Scholar]
- 10. Polkinghorne KR. Vascular access and all-cause mortality: a propensity score analysis. J Am Soc Nephrol 2004; 15: 477–486 [DOI] [PubMed] [Google Scholar]
- 11. Wasse H, Kutner N, Zhang R. et al. Association of initial hemodialysis vascular access with patient-reported health status and quality of life. Clin J Am Soc Nephrol 2007; 2: 708–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Korsheed S, Eldehni MT, John SG. et al. Effects of arteriovenous fistula formation on arterial stiffness and cardiovascular performance and function. Nephrol Dial Transplant 2011; 26: 3296–3302 [DOI] [PubMed] [Google Scholar]
- 13. Utescu MS, LeBoeuf A, Chbinou N. et al. The impact of arteriovenous fistulas on aortic stiffness in patients with chronic kidney disease. Nephrol Dial Transplant 2009; 24: 3441–3446 [DOI] [PubMed] [Google Scholar]
- 14. Golper TA, Hartle PM, Bian A. Arteriovenous fistula creation may slow estimated glomerular filtration rate trajectory. Nephrol Dial Transplant 2015; 30: 2014–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Korsheed S, Crowley LE, Fluck RJ. et al. Creation of an arteriovenous fistula is associated with significant acute local and systemic changes in microvascular function. Nephron Clin Pract 2013; 123: 173–179 [DOI] [PubMed] [Google Scholar]
- 16. Locatelli F, Zoccali C. Arteriovenous fistula as a nephroprotective intervention in advanced CKD: scientific discovery and explanation, and the evaluation of interventions. Nephrol Dial Transplant 2015; 30: 1939–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sumida K, Molnar MZ, Potukuchi PK. et al. Association of slopes of estimated glomerular filtration rate with post-end-stage renal disease mortality in patients with advanced chronic kidney disease transitioning to dialysis. Mayo Clin Proc 2016; 91: 196–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kovesdy CP, Norris KC, Boulware LE. et al. Association of race with mortality and cardiovascular events in a large cohort of US veterans. Circulation 2015; 132: 1538–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kovesdy CP, Alrifai AZ, Gosmanova EO. et al. Age and outcomes associated with blood pressure in patients with incident CKD. Clin J Am Soc Nephrol 2016; 6: 821–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. VA Information Resource Center (VIReC): VIReC Research User Guide; VHA Pharmacy Prescription Data, 2nd edn Hines, IL: VA Information Resource Center, 2008 [Google Scholar]
- 21. US Department of Veterans Affairs: VIReC Research User Guide; VHA Medical SAS Inpatient Datasets FY2006-2007. Hines, IL: VA Information Resource Center, 2007 [Google Scholar]
- 22. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45: 613–619 [DOI] [PubMed] [Google Scholar]
- 23. Levey AS, Stevens LA, Schmid CH. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Singer JD, Willett JB. Applied Longitudinal Data Analysis. Modeling Change and Event Occurrence. Oxford: Oxford University Press, 2003 [Google Scholar]
- 25. Mahgoub MA, Guo JH, Gao SP. et al. Hyperdynamic circulation of arteriovenous fistula preconditions the heart and limits infarct size. Ann Thorac Surg 1999; 68: 22–28 [DOI] [PubMed] [Google Scholar]
- 26. Sandhu JS, Wander GS, Gupta ML. et al. Hemodynamic effects of arteriovenous fistula in end-stage renal failure. Ren Fail 2004; 26: 695–701 [DOI] [PubMed] [Google Scholar]
- 27. Iwashima Y, Horio T, Takami Y. et al. Effects of the creation of arteriovenous fistula for hemodialysis on cardiac function and natriuretic peptide levels in CRF. Am J Kidney Dis 2002; 40: 974–982 [DOI] [PubMed] [Google Scholar]
- 28. Ori Y, Korzets A, Katz M. et al. Haemodialysis arteriovenous access—a prospective haemodynamic evaluation. Nephrol Dial Transplant 1996; 11: 94–97 [PubMed] [Google Scholar]
- 29. Ori Y, Korzets A, Katz M. et al. The contribution of an arteriovenous access for hemodialysis to left ventricular hypertrophy. Am J Kidney Dis 2002; 40: 745–752 [DOI] [PubMed] [Google Scholar]
- 30. Bosch JP, Saccaggi A, Lauer A. et al. Renal functional reserve in humans. Effect of protein intake on glomerular filtration rate. Am J Med 1983; 75: 943–950 [DOI] [PubMed] [Google Scholar]
- 31. Liu Z, Gong R. Remote ischemic preconditioning for kidney protection: GSK3beta-centric insights into the mechanism of action. Am J Kidney Dis 2015; 66: 846–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Endre ZH. Renal ischemic preconditioning: finally some good news for prevention of acute kidney injury. Kidney Int 2011; 80: 796–798 [DOI] [PubMed] [Google Scholar]
- 33. Yang Y, Lang XB, Zhang P. et al. Remote ischemic preconditioning for prevention of acute kidney injury: a meta-analysis of randomized controlled trials. Am J Kidney Dis 2014; 64: 574–583 [DOI] [PubMed] [Google Scholar]
- 34. Gassanov N, Nia AM, Caglayan E. et al. Remote ischemic preconditioning and renoprotection: from myth to a novel therapeutic option? J Am Soc Nephrol 2014; 25: 216–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Er F, Nia AM, Dopp H. et al. Ischemic preconditioning for prevention of contrast medium-induced nephropathy: randomized pilot RenPro Trial (Renal Protection Trial). Circulation 2012; 126: 296–303 [DOI] [PubMed] [Google Scholar]
- 36. Konner K, Nonnast-Daniel B, Ritz E. The arteriovenous fistula. J Am Soc Nephrol 2003; 14: 1669–1680 [DOI] [PubMed] [Google Scholar]
- 37. Astor BC, Eustace JA, Powe NR. et al. Timing of nephrologist referral and arteriovenous access use: The CHOICE Study. Am J Kidney Dis 2001; 38: 494–501 [DOI] [PubMed] [Google Scholar]
- 38. Hiremath S, Knoll G, Weinstein MC. Should the arteriovenous fistula be created before starting dialysis? A decision analytic approach. PLoS One 2011; 6: e28453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.