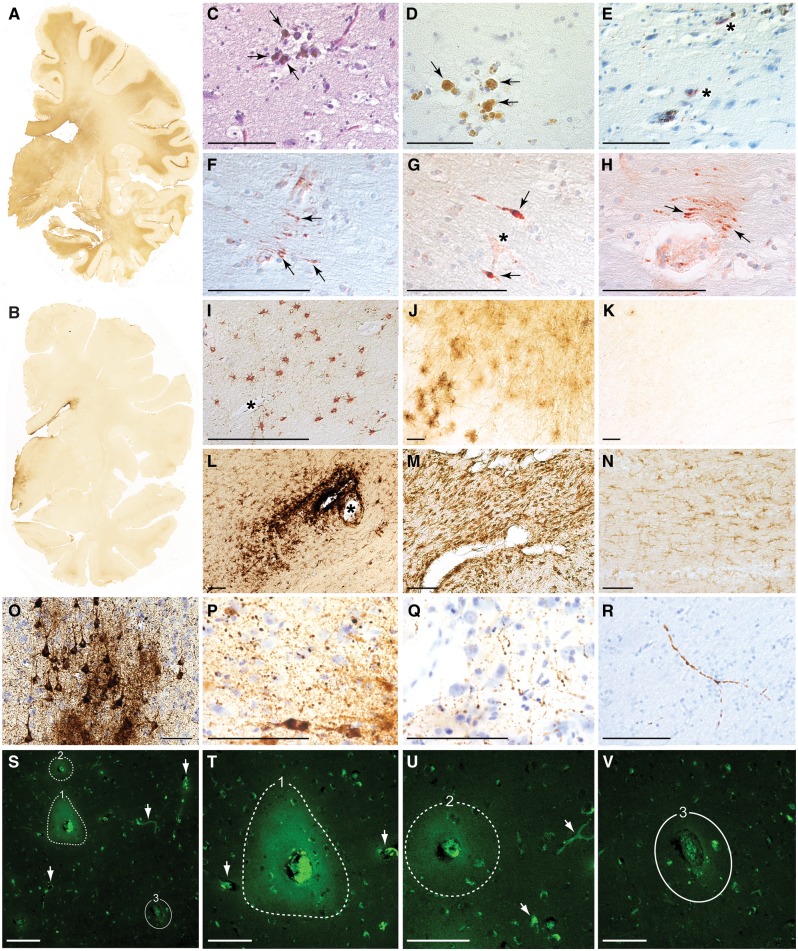
Figure 1.
Post-mortem pathologies in brains from teenage athletes in the acute-subacute period after mild closed-head impact injury. (A) Coronal brain section immunostained for the astrocytic marker glial fibrillary acid protein (GFAP) in Case 3, a 17-year-old male high school American football player who died by suicide 2 days after a closed-head impact injury. Widespread GFAP immunoreactivity (brown staining) indicative of reactive astrocytosis was diffusely present in white matter fibre tracts throughout the brain. Representative whole-mount brain section, 50 µm thickness. (B) Coronal brain section immunostained for GFAP in control Case 8, a 22-year-old male former high school American football player without history of recent head injury who died by suicide. GFAP immunoreactivity is restricted to the periventricular area and diencephalon. Representative whole-mount brain section, 50 µm thickness. (C and D) Haemosiderin-laden macrophages (arrows) surrounding a small blood vessel consistent with prior microhaemorrhage in Case 1, an 18-year-old male high school American football player who died by suicide 4 months after a closed-head impact injury. Representative Luxol fast blue haematoxylin and eosin (C) and haematoxylin (D) staining, 10 µm paraffin sections. Scale bars = 100 µm. (E) Case 1, microhaemorrhage surrounded by neurites immunoreactive for phosphorylated tau protein (asterisks) detected by monoclonal antibody AT8 directed against hyperphosphorylated tau protein (pSer202, pThr205) with haematoxylin counterstain, 10 µm paraffin section. Scale bar = 100 µm. (F–H) Perivascular anti-amyloid precursor protein (APP)-immunoreactive axonal swellings (arrows) in the corpus callosum from Case 3. Representative APP immunostaining with haematoxylin counterstain, 10 µm paraffin sections. Asterisk in G marks a small blood vessel. Scale bars = 100 µm. (I and J) Perivascular astrocytosis in white matter from Case 3. Representative GFAP immunostaining with haematoxylin counterstain, 10 µm paraffin section (I) and 50 µm free-floating section (J). Asterisk in I marks a small blood vessel. Scale bars = 100 µm. (K) Minimal GFAP-immunoreactive astrocytosis in the white matter from control Case 8. Representative GFAP immunostaining, 50 µm free-floating section. Scale bar = 100 µm. (L) Perivascular clusters of activated microglia around a small blood vessel in the subcortical white matter from Case 4, a 17-year-old male high school American football player who sustained three closed-head impact injuries 26 days, 6 days, and 1 day before death. Representative LN3 immunostaining directed against human leukocyte antigen DR-II (HLA-DR II), 50 µm free-floating section. Asterisk indicates small blood vessel. Scale bar = 100 µm. (M) Microgliosis in brainstem white matter in Case 1. Representative LN3 immunostaining, 50 µm free-floating section. Scale bar = 100 µm. (N) Few activated microglia in brainstem white matter in control Case 8. Representative LN3 immunostaining, 50 µm free-floating section. Scale bar = 100 µm. (O) Phosphorylated tau protein-containing neurofibrillary tangles, pretangles, and neurites in the sulcal depths of the cerebral cortex consistent with neuropathological diagnosis of early-stage CTE in Case 4. Representative CP13 immunostaining directed against hyperphosphorylated tau protein (pSer202), 50 µm free-floating section. Scale bar = 100 µm. (P and Q) Perivascular dot-like neurites immunoreactive for CP13-immunoreactive phosphorylated tau protein in frontal cortex sulcal depths consistent with early-stage CTE in Case 4. Representative CP13 immunostaining, 50 µm free-floating section. Scale bar = 100 µm. (R) Dystrophic axons immunoreactive for CP13-immunoreactive phosphorylated tau protein in frontal cortex white matter in Case 4. Representative CP13 immunostaining, 50 µm free-floating section. Scale bar = 100 µm. (S) Persistent vascular leakage in Case 1 demonstrated by anti-IgG immohistofluorescence in the brain parenchyma surrounding two blood vessels (dashed lines, 1 and 2) in the dorsolateral frontal cortex. These findings are consistent with focal blood–brain barrier disruption. Another blood vessel of similar calibre in the same field (denoted by solid line, number 3) does not exhibit evidence of blood–brain barrier disruption, consistent with the high degree of focality. Other smaller blood vessels (white arrows) are also devoid of perivascular IgG immunoreactivity, thereby confirming specificity of the microvascular pathology. Scale bar = 200 µm. (T) High magnification photomicrograph of the same field in Case 1 showing perivascular IgG immunoreactivity in the brain parenchyma surrounding blood vessel 1 (dashed line, 1). The intensely immunoreactive material in the centre of the lesion is residual blood in the vessel lumen. Two nearby blood vessels (arrows) do not show evidence of blood–brain barrier disruption. Scale bar = 100 µm. (U) High magnification photomicrograph of the same field in Case 1 showing perivascular IgG immunoreactivity in the brain parenchyma surrounding blood vessel 2 (dashed line, 2). Scale bar = 100 µm. (V) High magnification photomicrograph of the same field in Case 1 showing the absence of perivascular IgG immunoreactivity in the brain parenchyma surrounding blood vessel 3 (solid line, 3). Scale bar = 100 µm.