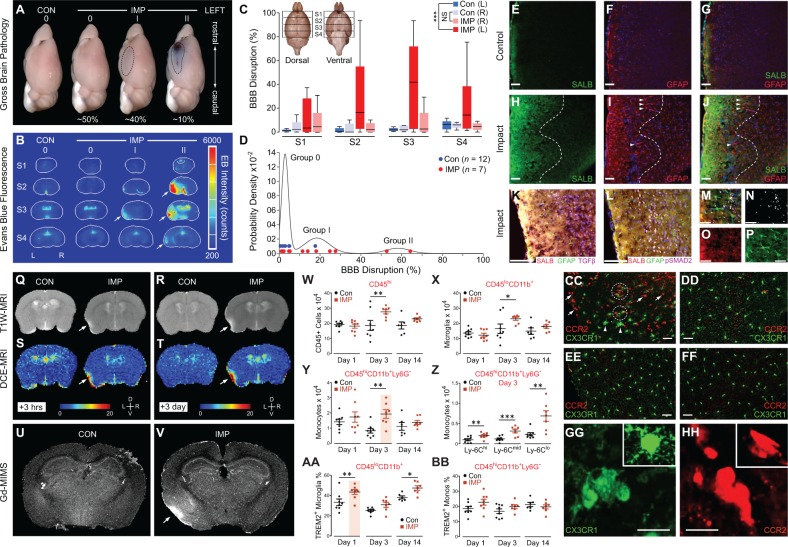
Figure 5.
Unilateral, closed-head impact injury induces focal blood–brain barrier disruption, serum albumin extravasation, astrocytosis, myeloid inflammatory cell infiltration, and TREM2+ microglial activation in cerebral cortex ipsilateral and subjacent to impact. (A) Gross pathology in representative brains from a control mouse (CON) exposed to sham (no injury) control condition compared to brains from mice subjected to experimental closed-head impact injury (IMP) with varying degrees of gross brain pathology (Grade 0, I, II, respectively) 24 h post-injury. Grade 0: absence of gross brain pathology with no evidence of macroscopic tissue damage (contusion, necrosis, hematoma, haemorrhage, or extravasated Evans blue) was observed in 100% of brains from CON mice and ∼50% of brains from IMP mice. Grade I: minimal brain pathology marked only by focal Evans blue extravasation (indicative of disruption of the blood–brain barrier, BBB) was observed in ∼40% of IMP mice but none (0%) of the CON mice. Grade II: relatively rare brains marked by complex lesions that included Evans blue extravasation and contusion observed in ˜10% of IMP mice but none (0%) in CON mice. (B) Evans blue-specific fluorescence imaging of representative mouse brain sections showing blood–brain barrier disruption 24 h post-injury. Arrows, left-lateral fluorescence signal indicating area of blood–brain barrier disruption (in Evans blue-specific fluorescence intensity counts) in cerebral cortex ipsilateral and subjacent to experimental impact injury (IMP) but not sham (no-injury) control condition (CON). Serial brain sections (anterior to caudal, S1–S4, respectively) and gross pathology injury grade (0, I, II) as indicated. (C) Quantitative analysis of blood–brain barrier disruption by coronal section Evans blue-specific fluorescence brain imaging 24 h after IMP or CON exposure. Blood–brain barrier disruption localized to the perirhinal, insular, entorhinal, and piriform cortices and basolateral amygdala of the left hemisphere ipsilateral and subjacent to the impact contact zone. Inset: rostral-to-caudal brain sections, S1–S4. ***P < 0.001; NS = not statistically different. (D) Gaussian mixed model analysis of Evans blue fluorescence brain imaging yielded three groups that corresponded to gross pathology classification (Grades 0, I, II). (E–J) Anatomical localization of extravasated serum albumin (SALB; E, G, H and J) and co-localizing reactive astrocytosis (GFAP; F, G, I and J) in left perirhinal cortex ipsilateral to impact 3 days post-injury (H–J) but not in corresponding cortex from CON mice (E–G). DAPI (blue channel: F, G, I and J), cell nuclei. Hashed lines (H–J) demarcate cortical region with maximal post-injury serum albumin extravasation (H and J) and co-localization with reactive astrocytosis (I and J). Arrowheads, GFAP-immunopositive processes of activated astrocytes. Scale bars = 100 µm. (K–P) Left perirhinal cortex at peak of reactive astrocytosis 3 days post-injury. Composite fluorescence microscopic images showing co-localization of extravasated serum albumin (SALB, red: K, L, M and O) with reactive astrocytosis (GFAP, green: K, L, M and P); TGFβ expression (TGFβ, violet: K) and phosphorylated-SMAD2, a marker downstream of TGF-β signalling (pSMAD2, violet: L, M and N). Cell nuclei (DAPI, blue: K, L and M). Yellow-white areas indicate overlapping SALB and GFAP immunoreactivity (K, L and M). High magnification (×40) composite fluorescence image (M) and fluorescence channels (N, pSMAD2; O, SALB; P, GFAP). Magnification: K and L = ×20; M–P = ×40. Scale bars in K and L = 100 µm; M–P = 50 µm. Serum albumin extravasation, GFAP-immunoreactive astrocytosis, and pSMAD2-TGFβ upregulation were not observed in the contralateral perirhinal cortex of IMP mice nor in perirhinal cortex of either hemisphere in CON mice (Supplementary Fig. 4A–F). (Q–V) Focal blood–brain barrier disruption and co-localizing serum albumin extravasation detected in the brains of living mice by dynamic contrast-enhanced MRI (DCE-MRI) neuroimaging with gadofosveset trisodium, an FDA-approved gadolinium-based contrast agent that binds serum albumin. High-field (11.7 T) T1-weighted MRI (Q and R) and DCE-MRI (S and T) with systemically administered gadofosveset trisodium. T1-weighted MRI and DCE-MRI were conducted 3 h (Q, T1-weighted MRI (T1W-MRI); S, DCE-MRI) and 3 days (R, T1-weighted MRI; T, DCE-MRI) after IMP or CON exposure. T1-weighted hyperintensity (Q and R) co-localized with blood–brain barrier permeability defect detected by DCE-MRI (S and T) in the left perirhinal cortex (arrows) 3 h and 3 days after IMP but not CON exposure. Non-specific signal was observed in the ventricles and sagittal sinus. D = dorsal, V = ventral; L = left, R = right. (U and V) Confirmation of serum albumin extravasation indicating blood–brain barrier disruption by gadolinium metallomic imaging mass spectrometry (Gd-MIMS) in perfused post-mortem brains from the same mice imaged by T1-weighted MRI (Q and R) and DCE-MRI (S and T). Enhanced gadolinium accumulation was observed in the left lateral perirhinal and piriform cortices (arrow) 2 weeks after IMP (V) but not CON (U) exposure. Gadolinium accumulation detected by Gd-MIMS co-localized with T1-weighted hyperintensity and blood–brain barrier permeability defect detected by DCE-MRI, thus confirming intracerebral blood–brain barrier disruption. (W–BB) Flow cytometry analysis showed that IMP triggers increased number of CD45+ inflammatory cells and activation of TREM2+ microglia in the brain post-injury. CD45+ inflammatory cells (W) and CD45loCD11b+ microglia (X) were significantly increased 3 days after IMP compared to CON exposure. (Y) CD45hiCD11b+Ly–6G– inflammatory cells accumulated in the brain 3 days after IMP compared to CON exposure. (Z) All three major subpopulations (Ly-6Chi, Ly-6Cmid, Ly-6Clo) were represented in CD45hiCD11b+Ly–6G– inflammatory cells detected 3 days post-injury. (AA and BB) Upregulation of TREM2 expression in microglia (AA) but not CD45+ inflammatory cells (BB) at 1 and 14 days after IMP compared to CON exposure. For flow cytometry experiments, n = 6–8 mice per group per time point. ***P < 0.001; **P < 0.01; *P < 0.05. See Supplementary Fig. 4G–I for flow cytometry population dot plots. (CC–HH) Brain accumulation of Ccr2RFP-expressing inflammatory cells (red-labelled cells) and activation of brain-resident Cx3cr1GFP-expressing microglia (green-labelled cells) were confirmed by fluorescence microscopy in perirhinal cortex ipsilateral and subjacent to experimental impact injury in Ccr2RFP/Cx3cr1GFP mice at 3 days post-injury (IMP: CC, DD, GG and HH) or control (CON: EE and FF). Representative fluorescence microscopy images show red-labelled Ccr2RFP-expressing inflammatory cells (arrows, CC) throughout the ipsilateral (left) perirhinal and adjacent cortex, basolateral amygdala, and overlying dura and leptomeninges (CC and HH) 3 days post-injury. The affected cortex was also notable for large numbers of ameboid Cx3cr1GFP-expressing microglia (arrowheads; CC and GG) that were also present, but to a lesser degree, in the contralateral (right) hemisphere (DD). Note clustering of Ccr2RFP-expressing inflammatory cells and Cx3cr1GFP-expressing microglia in the left perirhinal cortex (dashed circles, CC), the primary locus of post-traumatic brain pathology ipsilateral and subjacent to the impact. By contrast, Ccr2RFP-expressing inflammatory cells were minimally present and amoeboid Cx3cr1GFP-expressing microglia were absent in brains from Ccr2RFP/Cx3cr1GFP mice 3 days after CON exposure (EE and FF). Bars (CC–FF), 40 microns; (GG, HH), 20 microns.