Abstract
Aim
We sought to determine whether the optimal dual antiplatelet therapy (DAPT) duration after drug-eluting stent (DES) placement varies according to clinical presentation.
Methods and Results
We performed an individual patient data pairwise and network meta-analysis comparing short-term (≤6-months) versus long-term (1-year) DAPT as well as 3-month vs. 6-month vs 1-year DAPT. The primary study outcome was the 1-year composite risk of myocardial infarction (MI) or definite/probable stent thrombosis (ST). Six trials were included in which DAPT after DES consisted of aspirin and clopidogrel. Among 11 473 randomized patients 6714 (58.5%) had stable CAD and 4758 (41.5%) presented with acute coronary syndrome (ACS), the majority of whom (67.0%) had unstable angina. In ACS patients, ≤6-month DAPT was associated with non-significantly higher 1-year rates of MI or ST compared with 1-year DAPT (Hazard Ratio (HR) 1.48, 95% Confidence interval (CI) 0.98–2.22; P = 0.059), whereas in stable patients rates of MI and ST were similar between the two DAPT strategies (HR 0.93, 95%CI 0.65–1.35; P = 0.71; Pinteraction = 0.09). By network meta-analysis, 3-month DAPT, but not 6-month DAPT, was associated with higher rates of MI or ST in ACS, whereas no significant differences were apparent in stable patients. Short DAPT was associated with lower rates of major bleeding compared with 1-year DAPT, irrespective of clinical presentation. All-cause mortality was not significantly different with short vs. long DAPT in both patients with stable CAD and ACS.
Conclusions
Optimal DAPT duration after DES differs according to clinical presentation. In the present meta-analysis, despite the fact that most enrolled ACS patients were relatively low risk, 3-month DAPT was associated with increased ischaemic risk, whereas 3-month DAPT appeared safe in stable CAD. Prolonged DAPT increases bleeding regardless of clinical presentation. Further study is required to identify the optimal duration of DAPT after DES in individual patients based on their relative ischaemic and bleeding risks.
Keywords: Drug-eluting stent, Dual antiplatelet therapy, Stent thrombosis
Introduction
Dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 receptor inhibitor is standard therapy in patients with acute coronary syndromes (ACS) and in those undergoing drug-eluting stent (DES) implantation, although the obligate duration of DAPT continues to be debated.1 In this regard, the extent to which the net benefit of different DAPT durations may vary according to clinical presentation is still controversial. Patients with ACS have increased rates of recurrent ischaemic events compared with those with stable coronary artery disease (CAD),2 and therefore, ACS patients may be more likely to benefit from prolonged DAPT. However, data on optimal DAPT duration are relatively scant and controversial even in ACS patients, warranting further investigation.1 As many studies have challenged the notion that 1-year DAPT is necessary after DES placement, we performed an individual patient data (IPD) meta-analysis of RCTs investigating the safety and efficacy of shortening DAPT to <1 year after DES implantation in patients with and without ACS.
Methods
Study design and selection
For this meta-analysis, we included RCTs enrolling patients with stable CAD or ACS undergoing DES implantation and randomized to a short duration of DAPT (3–6 months) versus a longer duration (≥1 year), censoring data at 1 year. RCTs comparing 1-year vs. >1-year DAPT, or enrolling patients not treated with DES were excluded. These criteria were applied at the study level. Relevant RCTs were searched in August 2015 through MEDLINE, the Cochrane database, the EMBASE database, www.tctmd.com, www.clinicaltrials.gov, www.clinicaltrialresults.org, www.cardiosource.com, abstracts, and presentations from major cardiovascular meetings, using the keywords randomized clinical trial, drug-eluting stent, dual antiplatelet therapy, clopidogrel, aspirin, and thienopyridines. Two investigators (TP and DDR) independently reviewed the titles, abstracts, and studies to determine whether they met the inclusion criteria. Risk of bias was assessed using the Cochrane method. Further methodological details of the meta-analysis are provided in the Supplemental Material. The reporting of the study was in compliance with the PRISMA IPD statement.3
Endpoints and definitions
The primary objective was to investigate whether there is an interaction between DAPT duration and clinical presentation for the composite risk of 1-year myocardial infarction (MI) or definite/probable stent thrombosis (ST). Secondary pre-specified endpoints included the 1-year rates of all-cause, cardiac and non-cardiac death, MI, ST, stroke, target vessel revascularization (TVR), major bleeding, any bleeding, and various combinations of these endpoints. The endpoint definitions as applied in each trial were incorporated. Patient-level data were obtained from the principal investigators of the trials meeting the inclusion criteria and combined in a single pooled database. Interaction analyses between DAPT duration and clinical presentation were performed using frequentist IPD pairwise meta-analysis. Network meta-analysis was performed to compare outcomes of 3-month vs. 6-month vs 1-year DAPT separately in ACS and non-ACS patients.
To minimize bias by including events in the early period, landmark analyses were performed at the time of DAPT discontinuation in the short-DAPT treatment group. For this analysis, patients with ischaemic or bleeding events occurring before the landmark time-point, or those not compliant with the original randomization assignment were excluded, resulting in a ‘per-protocol’ population.
Statistical analysis
Continuous variables are displayed as means and standard deviation (SD) and were compared using two-way ANOVA stratified by trial. Categorical variables are displayed as counts and percentages and were compared with a logistic regression analysis stratified by trial. IPD meta-analysis was performed using a one-stage approach. Patient data were combined in a single dataset and fitted in a Cox regression model stratified by trial. The proportional assumptions were verified using Schoenfeld residuals. Results are reported as hazard ratio (HR) with 95% confidence interval (CI). A simple Cox regression model was used to generate cumulative hazard function curves of events for each outcome of interest. Kaplan–Meier estimates were also determined. As sensitivity analyses, we investigated the relative risk and benefit of short DAPT versus prolonged DAPT using standard parametric survival models, as well as Royston Parmar models.4 For Royston-Parmar models we evaluated 1, 2, and 3 knots of the spline function.
For the network meta-analysis treatment effect estimates for short vs. long DAPT were obtained as log HR and standard error from individual RCTs.5 These estimates were then used to obtain head-to-head comparison estimates between different DAPT regimens (3-months vs 6-months vs 1-year). A frequentist framework based on graph-theoretical method was used to calculate point estimates with 95% CI using a random-effects model (R netmeta package).6 Pair-wise inconsistency was assessed with the I2 statistic. P values <0.05 were considered significant. Statistical analyses were performed using Stata 12 SE (StataCorp, College Station, Texas) and R 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
As shown in Supplementary material online, Figure S1, of 1042 potentially relevant studies, seven trials met the inclusion criteria. Data were obtained for six of them and were included in the final meta-analysis.7–12 The major characteristics of the included trials are shown in Supplementary material online, Table S1, the inclusion and exclusion criteria are reported in Supplementary material online, Table S2, the risk of bias in Supplementary material online, Table S3, the definitions of clinical endpoints in Supplementary material online, Table S4, and the clinical angiographic and procedural characteristics stratified by DAPT duration in Supplementary material online, Table S5. Among 11 473 randomized patients 4758 (41.5%) presented with ACS and 6714 (58.5%) had stable CAD. In one patient the clinical presentation was not defined. The majority of ACS patients (67.0%) had biomarker-negative unstable angina. Baseline characteristics of patients with and without ACS stratified by different DAPT durations are reported in Supplementary material online, Tables S6–8.
Clinical outcomes in the intention-to-treat population
At 1-year follow up, patients with ACS compared with stable CAD had significantly higher composite rates of MI or definite/probable ST (HR = 1.50, 95%CI 1.12–2.00; P = 0.006), and cardiac death, MI, or definite/probable ST, but similar rates of major bleeding.
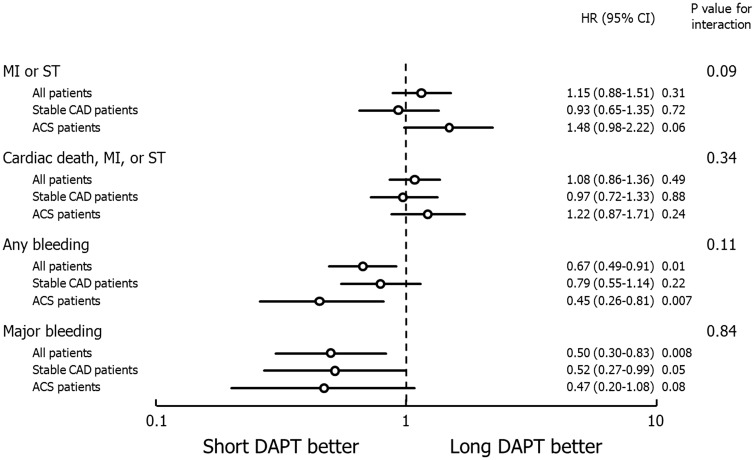
In the entire study population ≤6-month DAPT was associated with similar rates of composite MI or definite/probable ST compared with 1-year DAPT (HR 1.15, 95%CI 0.88–1.51; P = 0.31) with a borderline interaction between DAPT duration and clinical presentation (Pinteraction = 0.09) (Table 1, Figure 1). Specifically there were 57 MI or ST events among 2383 ACS patients treated with short DAPT and 39 such events among 2375 ACS patients treated with 1-year DAPT (HR 1.48, 95%CI 0.98–2.22; P = 0.059). Conversely, there were 55 MI or ST events among 3347 patients with stable CAD treated with short DAPT and 59 such events among 3367 stable patients treated with 1-year DAPT (HR 0.93, 95%CI 0.65–1.35; P = 0.72).
Table 1.
Clinical outcomes and interaction analysis of short-term (≤6-month) versus long-term (1-year) dual antiplatelet therapy in patients stratified by clinical presentation
| Estimated risk of short versus long DAPTa | Hazard ratio (95%CI) | Interaction p | |
|---|---|---|---|
| MI or definite/probable ST | 0.09 | ||
| All patients | 1.98% vs. 1.74% | 1.15 (0.88–1.51) | |
| Stable CAD | 1.67% vs. 1.79% | 0.93 (0.65–1.35) | |
| ACS | 2.43% vs. 1.67% | 1.48 (0.98–2.22) | |
| Cardiac death | 0.48 | ||
| All patients | 1.03% vs. 1.19% | 0.86 (0.61–1.23) | |
| Stable CAD | 1.01% vs. 1.03% | 0.97 (0.60–1.57) | |
| ACS | 1.07% vs. 1.41% | 0.75 (0.45–1.27) | |
| Non-cardiac death | 0.82 | ||
| All patients | 0.70% vs. 0.74% | 0.95 (0.61–1.47) | |
| Stable CAD | 0.52% vs. 0.58% | 0.89 (0.47–1.72) | |
| ACS | 0.95% vs. 0.97% | 0.99 (0.55–1.80) | |
| All-cause death | 0.77 | ||
| All patients | 1.71% vs. 1.92% | 0.89 (0.67–1.17) | |
| Stable CAD | 1.49% vs. 1.60% | 0.92 (0.63–1.36) | |
| ACS | 2.02% vs. 2.37% | 0.85 (0.58–1.26) | |
| MI | 0.14 | ||
| All patients | 1.79% vs. 1.63% | 1.10 (0.83–1.47) | |
| Stable CAD | 1.51% vs. 1.66% | 0.91 (0.62–1.34) | |
| ACS | 2.17% vs. 1.18% | 1.39 (0.91–2.13) | |
| Stroke | 0.11 | ||
| All patients | 0.46% vs. 0.50% | 0.92 (0.55–1.59) | |
| Stable CAD | 0.34% vs. 0.55% | 0.61 (0.29–1.30) | |
| ACS | 0.64% vs. 0.43% | 1.49 (0.67–3.33) | |
| Definite/probable ST | 0.27 | ||
| All patients | 0.49% vs. 0.42% | 1.17 (0.68–2.02) | |
| Stable CAD | 0.36% vs. 0.42% | 0.86 (0.40–1.86) | |
| ACS | 0.68% vs. 0.43% | 1.59 (0.72–3.51) | |
| Any bleeding | 0.11 | ||
| All patients | 1.19% vs. 1.78% | 0.67 (0.49–0.91) | |
| Stable CAD | 1.51% vs. 1.91% | 0.79 (0.55–1.14) | |
| ACS | 0.73% vs. 1.60% | 0.45 (0.26–0.81) | |
| Major bleeding | 0.84 | ||
| All patients | 0.39% vs. 0.78% | 0.50 (0.30–0.83) | |
| Stable CAD | 0.42% vs. 0.82% | 0.52 (0.27–0.99) | |
| ACS | 0.34% vs. 0.73% | 0.47 (0.20–1.08) | |
| Target vessel revascularization | 0.07 | ||
| All patients | 3.36% vs. 2.97% | 1.14 (0.92–1.41) | |
| Stable CAD | 2.91% vs. 3.07% | 0.96 (0.72–1.27) | |
| ACS | 3.98% vs. 2.83% | 1.42 (1.04–1.95) | |
| Cardiac death, MI, or definite/probable ST | 0.34 | ||
| All patients | 2.74% vs. 2.55% | 1.08 (0.86–1.36) | |
| Stable CAD | 2.42% vs. 2.48% | 0.97 (0.72–1.33) | |
| ACS | 3.20% vs. 2.65% | 1.22 (0.87–1.71) | |
| Cardiac death or MI | 0.55 | ||
| All patients | 2.64% vs. 2.48% | 1.07 (0.85–1.35) | |
| Stable CAD | 2.36% vs. 2.36% | 1.00 (0.73–1.37) | |
| ACS | 3.03% vs. 2.65% | 1.16 (0.82–1.63) | |
| Cardiac death, MI, or stroke | 0.35 | ||
| All patients | 2.92% vs. 2.82% | 1.04 (0.84–1.30) | |
| Stable CAD | 2.61% vs. 2.76% | 0.95 (0.71–1.27) | |
| ACS | 3.37% vs. 2.91% | 1.17 (0.85–1.62) | |
| Cardiac death, MI, stroke, or major bleeding | 0.33 | ||
| All patients | 3.38% vs. 3.50% | 0.97 (0.79–1.18) | |
| Stable CAD | 3.06% vs. 3.44% | 0.88 (0.68–1.16) | |
| ACS | 3.84% vs. 3.59% | 1.08 (0.80–1.45) | |
| All-cause death or MI | 0.72 | ||
| All patients | 2.65% vs. 2.69% | 0.99 (0.79–1.24) | |
| Stable CAD | 2.31% vs. 2.24% | 1.03 (0.75–1.42) | |
| ACS | 3.13% vs. 3.32% | 0.94 (0.69–1.31) | |
| All-cause death, MI, or stroke | 0.73 | ||
| All patients | 2.93% vs. 2.97% | 0.99 (0.80–1.23) | |
| Stable CAD | 2.53% vs. 2.46% | 1.03 (0.76–1.40) | |
| ACS | 3.51% vs. 3.71% | 0.95 (0.71–1.29) | |
| All-cause death, MI, stroke, or major bleeding | 0.73 | ||
| All patients | 3.13% vs. 3.39% | 0.93 (0.76–1.14) | |
| Stable CAD | 2.74% vs. 2.85% | 0.96 (0.72–1.28) | |
| ACS | 3.69% vs. 4.14% | 0.89 (0.67–1.29) |
CAD, coronary artery disease; DAPT, dual antiplatelet therapy; ACS, acute coronary MI, myocardial infarction; ST, stent thrombosis, HR, hazard ratio; CI, confidence interval.
Determined by Kaplan–Meier analysis.
Figure 1.
Main clinical outcomes and interaction analysis between dual antiplatelet therapy (DAPT) duration and clinical presentation in the intention-to-treat population. MI, myocardial infarction; ST, definite/probable stent thrombosis; CAD, coronary artery disease; ACS, acute coronary syndrome; HR, hazard ratio; CI, confidence interval.
Lesser than or equal to six-month DAPT duration was associated with lower 1-year rates of major bleeding (HR 0.50, 95%CI 0.30–0.83; P = 0.008) and any bleeding (HR 0.67, 95%CI 0.49–0.91, P = 0.01) compared with prolonged DAPT, with no interaction between DAPT duration and clinical presentation (Figure 1). Additional clinical outcomes and their hazard function curves are shown in Table 1 and Figure 2. Of note, all-cause mortality was not significantly different with short vs. long DAPT in both patients with stable CAD and ACS. Similar results were apparent in sensitivity analyses using parametric survival models as well as Royston Parmar models (Supplementary material online, Table S9).
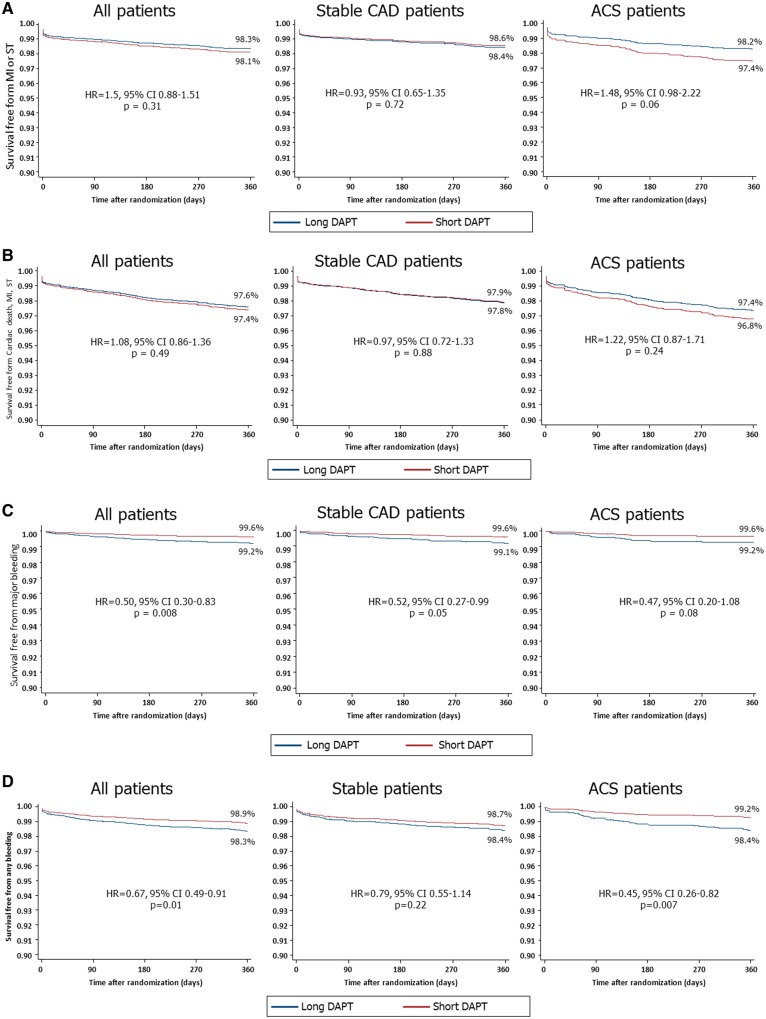
Figure 2.
Cumulative hazard function curves determined by Cox regression analyses in the overall population and in patients with or without acute coronary syndrome (ACS) showing the 1-year risk of (A) myocardial infarction (MI) or definite/probable stent thrombosis (ST); (B) cardiac death, MI or ST; (C) major bleeding; and (D) any bleeding with ≤6-month versus 1-year dual antiplatelet therapy (DAPT).
Landmark analysis in the per-protocol population
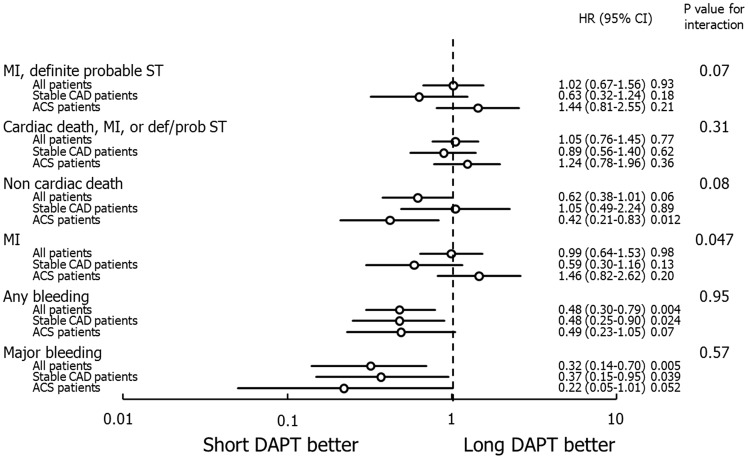
The per protocol population included 11 070 patients, including 4567 (41.3%) with ACS and 6502 (58.7%) with stable CAD. Their baseline characteristics stratified by DAPT duration are presented in Supplementary material online, Tables S10–12. For the entire randomized per-protocol population beginning after the time-point of DAPT discontinuation, short DAPT was associated with similar rates of composite MI or ST as prolonged DAPT (HR 1.02, 95%CI 0.67–1.56, P = 0.77), with a borderline interaction favoring prolonged DAPT in ACS but not stable CAD (Pinteraction = 0.07) (Supplementary material online, Table S13 and Figure 3). Significant interactions between DAPT duration and clinical presentation were present for MI and TVR, with prolonged DAPT favored in ACS patients and shorter DAPT favoured in stable CAD patients. Conversely, ≤6-month DAPT was associated with lower 1-year rates of major bleeding (HR 0.32, 95%CI 0.14–0.70; P = 0.005) and any bleeding (HR 0.48, 95%CI 0.30–0.79, P = 0.004) compared with prolonged DAPT, with no interaction between DAPT duration and clinical presentation (Supplementary material online, Table S13). Finally, patients treated with short vs. long DAPT had a trend toward lower rates of non-cardiac mortality (HR 0.62, 95%CI 0.38–1.01; P = 0.058), with a non-significant interaction between DAPT duration and clinical presentation (Pinteraction = 0.08).
Figure 3.
Major clinical outcomes and interaction analysis between dual antiplatelet therapy (DAPT) duration and clinical presentation in the per-protocol population in the landmark period between DAPT discontinuation and 1 year. Short DAPT indicates 3 or 6-month DAPT. Abbreviations as in Figure 1.
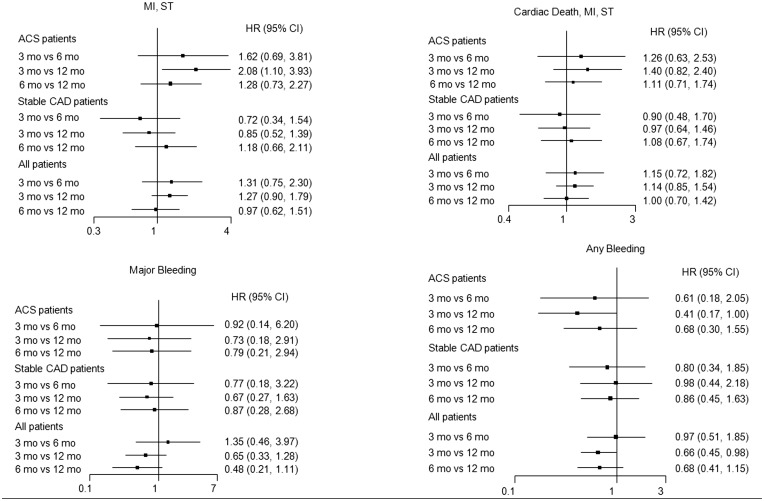
Three-months vs. six-months vs. Twelve-months of Dual antiplatelet therapy
The evidence network is shown in Supplementary material online, Figure S2 and the results in Figure 4. All trials included in the meta-analysis were sufficiently similar in all respects other than the treatment assignment, holding the transitivity assumption of network meta-analysis. Compared with ACS patients treated with 12-month DAPT, those treated with 3-month DAPT (HR 2.08, 95%CI 1.10–3.93) but not 6-month DAPT (HR 1.28, 95%CI 0.73–2.27) had higher composite MI or ST rates. Conversely, there was no significant difference in the risk of MI or ST in stable patients treated with 3-month, 6-month, or 12-month DAPT. Finally, treatment with 3-month and 6-month DAPT was associated with lower rates of major bleeding and any bleeding compared with 12-month DAPT, independent of clinical presentation.
Figure 4.
Forest plot illustrating major clinical outcomes with 3-month versus 6-month versus 1-year DAPT in the entire population and in patients with or without acute coronary syndromes. (ACS). Abbreviations as in Figure 1.
Additional analyses
A significant interaction was apparent between DAPT duration, clinical presentation and history of prior MI, such that 1-year DAPT significantly reduced the risk of MI or ST compared with short DAPT only in patients with ACS and a history of prior MI, whereas no significant difference between the two DAPT strategies was apparent in the other clinical strata (Supplementary material online, Table S14). No statistical heterogeneity was present in any pair-wise analyses for the main outcome measures (Supplementary material online, Table S15).
Discussion
The principal findings of the present patient-level pooled meta-analysis including six RCTs and 11 473 patients are: (1) Overall, a strategy of short-term DAPT (3 or 6 months) after DES implantation is associated with similar 1-year composite rates of MI or definite/probable ST compared with 1-year DAPT, with a borderline interaction between DAPT duration and clinical presentation; (2) Similar results were seen in the per-protocol population in the landmark period between DAPT discontinuation and 1-year follow-up; (3) In patients with ACS, 3-month but not 6-month DAPT was associated with higher rates of MI or ST compared with 1-year DAPT, whereas in stable CAD no such difference was apparent; (4) Patients with both ACS and stable CAD treated with short DAPT (either 3 or 6 months) had lower rates of bleeding compared with patients treated with 1-year DAPT; and (5) Although by intention-to-treat all-cause mortality was not significantly different with short vs. long DAPT, a trend towards increased non-cardiac mortality with prolonged compared with short DAPT was present in the per-protocol landmark period between DAPT discontinuation and 1-year follow-up, with no interaction present between DAPT duration and clinical presentation in either analysis.
Several RCTs have recently challenged the notion that 1-year (or longer) DAPT is necessary after contemporary DES implantation, suggesting that 6-month or even 3-month DAPT may be as effective and safer.7–13 In stable CAD, European and US guidelines state 6-month DAPT may be reasonable after second generation DES in stable CAD.14,15 The optimal DAPT duration in patients with ACS is also controversial. There is general consensus that in such patients DAPT should be administered for at least1 year,16 but the evidence supporting this recommendation relies on a single randomized trial (CURE) performed nearly 2 decades ago when ACS patients were treated conservatively, and before DES.17 In this regard, second generation DES may be less thrombogenic than bare metal stents.18 In CURE, a reduction in the risk of cardiovascular death, MI or stroke favoring DAPT with clopidogrel compared with aspirin monotherapy was already apparent at 30 days, confounding interpretation of the utility of DAPT thereafter. Unfortunately, a second randomization at a later time period was not performed, which might have addressed this issue. Moreover, landmark analysis from CURE demonstrated that nearly all of the benefit of DAPT occurred within 3 months after randomization.19 Specifically, DAPT compared with aspirin monotherapy prevented 20 cardiovascular events/1000 patients treated during the first 3 months, vs. only two initial cardiovascular events/1000 patients treated between 3 and 12 months. In addition, among 2658 patients undergoing PCI, no significant difference in any ischaemic endpoint was apparent between aspirin monotherapy versus DAPT in a landmark period between 1 month and 1 year.20
In the present patient-level pooled meta-analysis including 11 473 patients, 4758 of whom had ACS, we found no significant difference in the risk of MI or definite/probable stent thrombosis between ≤6-month DAPT versus 1-year DAPT in the entire randomized population. However, a trend was apparent suggesting a significant benefit of 1-year DAPT vs. shorter DAPT in ACS patients, and by network meta-analysis 3-month DAPT, but not 6-month DAPT was associated with significantly higher rates of MI or ST in ACS. In contrast, no significant difference in the risk of MI or ST was apparent in patients with stable CAD treated with 3, 6, or 12 months DAPT.
These findings contrast with the DAPT trial, which suggested higher rates of ST and MI in patients treated with 1-year DAPT compared with 2.5-year DAPT after DES, irrespective of clinical presentation.21,22 The difference in DAPT duration between treatment arms was 18 months in the DAPT trial, but only 6–9 months in our meta-analysis. It is possible that a benefit may appear with DAPT continuation longer than 1 year. Moreover, in the DAPT trial, the benefit of prolonged DAPT was accentuated in patients with MI at presentation,22 consistent with the results of the present study. Our analysis confirms that prolonging DAPT carries a substantial risk of major bleeding and any bleeding. These findings are of clinical relevance considering the association between bleeding and mortality.23 In addition, a meta-regression analysis including eight RCTs has recently found a significant association between all bleeding and non-cardiovascular mortality, but not between ST and cardiovascular mortality, suggesting that these endpoints may weigh differently on the risk of mortality.24 In this regard, we observed a borderline increase in non-cardiac mortality with prolonged DAPT compared with aspirin monotherapy in the landmark period between DAPT discontinuation and 1-year.
These data suggest that the optimal DAPT duration after DES placement should be tailored in individual patients after carefully balancing the risks of ischaemic versus bleeding events. The DAPT score was developed to identify patients with a greater risk of ischemia than bleeding who may benefit the most from prolonging DAPT.21 Limitations of this score include its modest discrimination power, absence of ticagrelor treatment, applicability only to the 12–30-month period after DES, and lack of availability of several important variables predicting bleeding.22 The DAPT score also needs external validation in a trial of different DAPT durations using contemporary DES. Further studies are thus warranted to investigate the utility of the DAPT score.
The majority of enrolled patients with ACS were biomarker negative, and few had acute STEMI, representing a relatively low-risk cohort. However, 1-year DAPT significantly reduced the risk of MI or ST compared with short DAPT in patients with ACS and prior MI, whereas no significant difference was apparent in other clinical strata. These findings are consistent with the results of the PEGASUS trial, which showed significantly lower rates of ischaemic events with long-term DAPT with aspirin and ticagrelor compared with aspirin alone in patients with prior MI 1–3 years earlier,25 and with a recent meta-analysis reporting benefit of long-term DAPT for secondary prevention in patients with previous MI.26
Other limitations should be acknowledged. The analysis in patients with prior MI is underpowered and therefore should be interpreted with caution. A significant proportion of implanted DES was first generation devices, in particular fast-release zotarolimus-eluting stents, which are no longer used. However, as second generation DES are safer than first generation DES,18 the balance between risk and benefit would likely favour shorter DAPT duration to an even greater degree with contemporary DES. Although we observed no significant difference in the risk of ischaemic events between 3-month versus 1-year DAPT in patients with stable CAD, further studies are required to establish the generalizability of this finding. All trials included in the meta-analysis were open-label, potentially introducing bias. Definitions of some clinical endpoints slightly differed across trials, potentially introducing effect modifiers. All patients were treated with clopidogrel as adjunctive therapy to aspirin. It remains undetermined how the more potent antiplatelet agents prasugrel and ticagrelor might affect the risk-benefit balance of prolonged DAPT in ACS. Comparison between 3-month versus 6-month DAPT is based on indirect evidence only, and therefore should be interpreted with caution. Data from the large ISAR SAFE trial were not provided by the principal investigator, and therefore could not be included in the meta-analysis.13 The lack of a significant difference in the risk of ischaemic endpoints between 6-month and 1-year DAPT in ACS patients should be interpreted with caution, given the low risk nature of these patients (67.0% unstable angina), and the trend toward greater ischaemic events with 6-month DAPT. Thus 1-year mandatory DAPT would be a prudent minimum in high-risk ACS patients.
Considering the small number of studies included in the meta-analysis, we did not use common methods for evaluating publication bias such as Egger test or the funnel plot.
In conclusion, in the present meta-analysis including 11 473 patients treated with DES, the absolute and relative benefit of different DAPT durations varied according to clinical presentation. In patients with ACS, 3-month DAPT was associated with increased ischaemic risk, whereas 3-month DAPT appeared safe in stable CAD. Prolonged DAPT increases bleeding regarding of clinical presentation. Further study is required to identify the optimal duration of DAPT after DES in individual patients at varying level of ischaemic and bleeding risk.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This study was supported by the British Heart Foundation and the NIHR Bristol Cardiovascular Biomedical Research Unit (UB and GDA contribution).
Conflict of interest: T.P. has received speaker fee from Abbott Vascular and research grant from Eli Lilly. G.B.-Z. has consulted for Bayer Pharma, and Novartis, has lectured for Abbott Vascular, Astra Zeneca, DirectFlow Medical, and St. Jude Medical, and has received career grant support from Medtronic. P.G. has received speaker fees from Abbott and Cardiovascular System Inc. D.L.B. discloses the following relationships: Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Vice-Chair), VA CART Research and Publications Committee (Chair); Research Funding: Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Forest Laboratories, Ischemix, Medtronic, Pfizer, Roche, Sanofi Aventis, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, St. Jude Medical; Trustee: American College of Cardiology; Unfunded Research: FlowCo, PLx Pharma, Takeda. M.-C.M. has received minor lecture fees from GE, Abott and Terumo. The other authors have no conflicts to disclose.
Supplementary Material
References
- 1. Palmerini T, Stone GW.. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: conceptual evolution based on emerging evidence. Eur Heart J 2016;37:353–364. [DOI] [PubMed] [Google Scholar]
- 2. Kukreja N, Onuma Y, Garcia-Garcia HM, Daemen J, van Domburg R, Serruys PW.. The risk of stent thrombosis in patients with acute coronary syndromes treated with bare-metal and drug-eluting stents. JACC Cardiovasc Interv 2009;2:534–541. [DOI] [PubMed] [Google Scholar]
- 3. Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, Tierney JF.. Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD Statement. JAMA 2015;313:1657–1665. [DOI] [PubMed] [Google Scholar]
- 4. Royston P, Lambert P.. Flexible Parametric Survival Analysis Using STATA: Beyond the Cox Model. College Station, Texas: STATA PRESS; 2011. [Google Scholar]
- 5. Klein J, Moeschberger M, Survival Analysis. Techniques for censored and truncated data. 2nd ed. New York: Springer Publishers; 2003. [Google Scholar]
- 6. Biondi-Zoccai G. Network Meta-Analysis: Evidence Synthesis with Mixed Treatment Comparison. Hauppauge, NY: Nova Science Publishers; 2014. [Google Scholar]
- 7. Colombo A, Chieffo A, Frasheri A, Garbo R, Masotti-Centol M, Salvatella N, Oteo Dominguez JF, Steffanon L, Tarantini G, Presbitero P, Menozzi A, Pucci E, Mauri J, Cesana BM, Giustino G, Sardella G.. Second-generation drug-eluting stent implantation followed by 6- versus 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol 2014;64:2086–2097. [DOI] [PubMed] [Google Scholar]
- 8. Feres F, Costa RA, Abizaid A, Leon MB, Marin-Neto JA, Botelho RV, King SB III, Negoita M, Liu M, de Paula JE, Mangione JA, Meireles GX, Castello HJ Jr, Nicolela EL Jr, Perin MA, Devito FS, Labrunie A, Salvadori D Jr, Gusmao M, Staico R, Costa JR Jr, de Castro JP, Abizaid AS, Bhatt DL.. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA 2013;310:2510–2522. [DOI] [PubMed] [Google Scholar]
- 9. Gilard M, Barragan P, Noryani AA, Noor HA, Majwal T, Hovasse T, Castellant P, Schneeberger M, Maillard L, Bressolette E, Wojcik J, Delarche N, Blanchard D, Jouve B, Ormezzano O, Paganelli F, Levy G, Sainsous J, Carrie D, Furber A, Berland J, Darremont O, Le Breton H, Lyuycx-Bore A, Gommeaux A, Cassat C, Kermarrec A, Cazaux P, Druelles P, Dauphin R, Armengaud J, Dupouy P, Champagnac D, Ohlmann P, Endresen K, Benamer H, Kiss RG, Ungi I, Boschat J, Morice MC.. 6- versus 24-month dual antiplatelet therapy after implantation of drug-eluting stents in patients nonresistant to aspirin: the randomized, multicenter ITALIC trial. J Am Coll Cardiol 2014;65:777–786. [DOI] [PubMed] [Google Scholar]
- 10. Gwon HC, Hahn JY, Park KW, Song YB, Chae IH, Lim DS, Han KR, Choi JH, Choi SH, Kang HJ, Koo BK, Ahn T, Yoon JH, Jeong MH, Hong TJ, Chung WY, Choi YJ, Hur SH, Kwon HM, Jeon DW, Kim BO, Park SH, Lee NH, Jeon HK, Jang Y, Kim HS.. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus versus cypher to reduce late loss after stenting (EXCELLENT) randomized, multicenter study. Circulation 2012;125:505–513. [DOI] [PubMed] [Google Scholar]
- 11. Kim BK, Hong MK, Shin DH, Nam CM, Kim JS, Ko YG, Choi D, Kang TS, Park BE, Kang WC, Lee SH, Yoon JH, Hong BK, Kwon HM, Jang Y.. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol 2012;60:1340–1348. [DOI] [PubMed] [Google Scholar]
- 12. Valgimigli M, Campo G, Monti M, Vranckx P, Percoco G, Tumscitz C, Castriota F, Colombo F, Tebaldi M, Fuca G, Kubbajeh M, Cangiano E, Minarelli M, Scalone A, Cavazza C, Frangione A, Borghesi M, Marchesini J, Parrinello G, Ferrari R.. Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation 2012;125:2015–2026. [DOI] [PubMed] [Google Scholar]
- 13. Schulz-Schupke S, Byrne RA, Ten Berg JM, Neumann FJ, Han Y, Adriaenssens T, Tolg R, Seyfarth M, Maeng M, Zrenner B, Jacobshagen C, Mudra H, von Hodenberg E, Wohrle J, Angiolillo DJ, von Merzljak B, Rifatov N, Kufner S, Morath T, Feuchtenberger A, Ibrahim T, Janssen PW, Valina C, Li Y, Desmet W, Abdel-Wahab M, Tiroch K, Hengstenberg C, Bernlochner I, Fischer M, Schunkert H, Laugwitz KL, Schomig A, Mehilli J, Kastrati A.. ISAR-SAFE: a randomized, double-blind, placebo-controlled trial of 6 vs. 12 months of clopidogrel therapy after drug-eluting stenting. Eur Heart J 2015;36:1252–1263. [DOI] [PubMed] [Google Scholar]
- 14. Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, Mehran R, Mukherjee D, Newby LK, O'gara PT, Sabatine MS, Smith PK, Smith SC. Jr.,. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American college of Cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol 2016;68:1082–1115. [DOI] [PubMed] [Google Scholar]
- 15. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A.. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541–2619. [DOI] [PubMed] [Google Scholar]
- 16. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S.. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 17. Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK.. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494–502. [DOI] [PubMed] [Google Scholar]
- 18. Palmerini T, Biondi-Zoccai G, Della Riva D, Stettler C, Sangiorgi D, D'ascenzo F, Kimura T, Briguori C, Sabate M, Kim HS, De Waha A, Kedhi E, Smits PC, Kaiser C, Sardella G, Marullo A, Kirtane AJ, Leon MB, Stone GW.. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet 2012;379:1393–1402. [DOI] [PubMed] [Google Scholar]
- 19. Yusuf S, Mehta SR, Zhao F, Gersh BJ, Commerford PJ, Blumenthal M, Budaj A, Wittlinger T, Fox KA.. Early and late effects of clopidogrel in patients with acute coronary syndromes. Circulation 2003;107:966–972. [DOI] [PubMed] [Google Scholar]
- 20. Eriksson P. Long-term clopidogrel therapy after percutaneous coronary intervention in PCI-CURE and CREDO: the “Emperor's New Clothes” revisited. Eur Heart J 2004;25:720–722. [DOI] [PubMed] [Google Scholar]
- 21. Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, Normand SL, Braunwald E, Wiviott SD, Cohen DJ, Holmes DR Jr, Krucoff MW, Hermiller J, Dauerman HL, Simon DI, Kandzari DE, Garratt KN, Lee DP, Pow TK, Ver Lee P, Rinaldi MJ, Massaro JM.. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014;371:2155–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yeh RW, Kereiakes DJ, Steg PG, Windecker S, Rinaldi MJ, Gershlick AH, Cutlip DE, Cohen DJ, Tanguay JF, Jacobs A, Wiviott SD, Massaro JM, Iancu AC, Mauri L.. Benefits and risks of extended duration dual antiplatelet therapy after PCI in patients with and without acute myocardial infarction. J Am Coll Cardiol 2015;65:2211–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mehran R, Pocock SJ, Nikolsky E, Clayton T, Dangas GD, Kirtane AJ, Parise H, Fahy M, Manoukian SV, Feit F, Ohman ME, Witzenbichler B, Guagliumi G, Lansky AJ, Stone GW.. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol 2010;55:2556–2566. [DOI] [PubMed] [Google Scholar]
- 24. Capodanno D, Gargiulo G, Buccheri S, Giacoppo D, Capranzano P, Tamburino C.. Meta-analyses of dual antiplatelet therapy following drug-eluting stent implantation: do bleeding and stent thrombosis weigh similar on mortality? J Am Coll Cardiol 2015;66:1639–1640. [DOI] [PubMed] [Google Scholar]
- 25. Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K, Bengtsson O, Oude Ophuis T, Budaj A, Theroux P, Ruda M, Hamm C, Goto S, Spinar J, Nicolau JC, Kiss RG, Murphy SA, Wiviott SD, Held P, Braunwald E, Sabatine MS.. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015;372:1791–1800. [DOI] [PubMed] [Google Scholar]
- 26. Udell JA, Bonaca MP, Collet JP, Lincoff AM, Kereiakes DJ, Costa F, Lee CW, Mauri L, Valgimigli M, Park SJ, Montalescot G, Sabatine MS, Braunwald E, Bhatt DL.. Long-term dual antiplatelet therapy for secondary prevention of cardiovascular events in the subgroup of patients with previous myocardial infarction: a collaborative meta-analysis of randomized trials. Eur Heart J 2015;37:390–399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.