Abstract
Background
The Kidney Donor Risk Index (KDRI) is a quantitative evaluation of the quality of donor organs and is implemented in the US allocation system. This single-centre study investigates whether the implementation of the KDRI in our decision-making process to accept or decline an offered deceased donor kidney, increases our acceptance rate.
Methods
From April 2015 until December 2016, we prospectively calculated the KDRI for all deceased donor kidney offers allocated by Eurotransplant to our centre. The number of the transplanted versus declined kidney offers during the study period were compared to a historical set of donor kidney offers.
Results
After implementation of the KDRI, 26.1% (75/288) of all offered donor kidneys were transplanted, compared with 20.7% (136/657) in the previous period (P < 0.001). The median KDRI of all transplanted donor kidneys during the second period was 0.97 [Kidney Donor Profile Index (KDPI) 47%], a value significantly higher than the median KDRI of 0.85 (KDPI 34%) during the first period (P = 0.047). A total of 68% of patients for whom a first-offered donor kidney was declined during this period were transplanted after a median waiting time of 386 days, mostly with a lower KDRI donor kidney.
Conclusions
Implementing the KDRI in our decision-making process increased the transplantation rate by 26%. The KDRI can be a supportive tool when considering whether to accept or decline a deceased donor kidney offer. More data are needed to validate this score in other European centres.
Keywords: graft survival, Kidney Donor Profile Index (KDPI), Kidney Donor Risk Index (KDRI), kidney transplantation
INTRODUCTION
Long-term survival is better for patients who receive a kidney transplant, compared with those who remain on dialysis [1]. However, the number of patients on the transplant waiting list still exceeds the number of suitable deceased donor kidneys. To expand the kidney donor pool, the dichotomous standard criteria donor (SCD) and extended criteria donor (ECD) classification was introduced in 2002 [2]. This led to a dilemma for the clinician whether to accept an ECD kidney, associated with a relative risk of allograft loss >1.7 compared with a kidney recovered from a SCD, or to let the patient remain on dialysis knowing the mortality risk while waiting for the next offer.
In 2009, Rao et al. developed the Kidney Donor Risk Index (KDRI) as a decision-making tool for the clinician [3]. This is a continuous risk score, based on the association of 10 donor characteristics with graft survival. The 10 factors include age, height, weight, ethnicity, history of hypertension and/or diabetes, cause of death, serum creatinine, hepatitis C virus (HCV) serology and donation after cardiac death (DCD). The association between these 10 donor characteristics and graft survival was determined by using a multivariable Cox proportional hazard regression model on 69.440 adult recipients of ABO blood type-compatible, first-time, deceased donor kidney-only transplants from 1995 to 2005.
The KDRI gives an estimate of the relative risk of post-transplant kidney graft failure for a particular deceased donor compared with the median donor. Nowadays, the median donor (50th percentile) is set equal to the median donor of all deceased donors in the USA from whom a kidney was recovered for the purpose of transplantation during the prior calendar year. The median donor has thus, by definition, a KDRI of 1. Higher values are associated with higher estimated risk of graft failure. For example, a donor kidney with a KDRI of 1.28 has an estimated risk of graft failure that is 1.28-fold that of the median donor. With an increase in KDRI there is a gradual decrease in graft survival [3]. Based on the KDRI, the Kidney Donor Profile Index (KDPI) was determined. The KDPI is a numerical mapping of the KDRI that expresses the quality of a particular deceased donor kidney relative to other donor kidneys. For example, a donor kidney with a KDPI of 90% has a KDRI >90% of all recovered donor kidneys during the previous calendar year. The KDRI/KDPI calculator is freely accessible on the web (http://optn.transplant.hrsa.gov/resources/allocationcalculators.asp?index=81) [4] allowing its calculation for each donor offer. The KDRI/KDPI scores are already routinely used in the USA. Indeed, the 20% best-recovered organs (those with the 20% lowest KDRI/KDPI) are preferentially allocated to the recipients with the estimated highest longevity in order to maximize the number of life-years gained by transplantation.
Of note, several donor characteristics that are thought to be associated with post-transplant graft loss, such as female donor sex or smoking history, were not significant predictors of graft loss in the Rao analysis. These characteristics are sometimes put forward as a reason to decline a donor within our transplant unit. Therefore, we hypothesized that calculating and basing our decision to accept or decline a donor on the KDRI/KDPI, instead of on other, less-validated parameters, might help us to increase the acceptance rate of high-quality donor kidneys.
Previously, our group retrospectively calculated the KDRI for all offered deceased donor kidneys in our centre (Antwerp University Hospital) from January 2010 until December 2013. This study showed that the KDRI/KDPI of all transplanted donor kidneys was quite low, suggesting that we might decline too many offers. Only 20.7% of all deceased donor kidneys offered to one of our patients on the transplant waiting list were transplanted.
In the present study, we prospectively investigated whether implementation of the KDRI in our decision-making process to accept or decline an offered deceased donor kidney has helped us to evaluate the suitability of a deceased donor kidney for a recipient in order to increase the acceptance rate of high-quality donor kidneys. In cases where we decided not to transplant an offered deceased donor kidney, we identified the cause in a pre-set list of reasons.
MATERIALS AND METHODS
A prospective single-centre study was performed at the Antwerp University Hospital. All single-offered deceased donor kidneys between April 2015 and December 2016 from Eurotransplant to one of the patients (age >18 years) on the transplant waiting list were included. Kidneys offered in combination with another organ were excluded.
Besides the data provided by Eurotransplant, the KDRI/KDPI was calculated at the moment of an offer. This allowed the transplant physicians of our centre to immediately implement the KDRI/KDPI in their decision-making process to accept or decline an offered deceased donor kidney.
All 10 donor characteristics need to be available to calculate the KDRI. However, for the parameters ‘history of hypertension’ and ‘diabetes status’, it is possible to fill in ‘unknown’. In these cases, the calculator assumes that the donor has the same chance of having this condition as a randomly selected donor: 31% and 10%, respectively. If the HCV status is unknown, the calculator will assume the donor is negative for HCV. Missing data in any of the other fields makes the calculation of the KDRI impossible. Eurotransplant provides these donor characteristics at the moment of an offer, except ‘ethnicity’ for ethical reasons. The ethnicity only influences the KDRI in case the donor is Black or African American. We decided not to fill in ‘Black or African American’ in the field ‘ethnicity’, because the prevalence of this race is low in the Eurotransplant zone (<5%).
To calculate the KDRI, we used the online KDRI calculator of the Organ Procurement and Transplantation Network (OPTN) [4], which does not include non-donor factors. The reference population was all deceased donors in the USA with a kidney recovered for the purpose of transplantation during the prior calendar year (2014–15). For each offer to our centre, the clinician filled out a form where he took note of the KDRI/KDPI and whether or not the donor kidney was accepted and transplanted. When the clinician decided to decline an offered donor kidney, he noted on a predefined list the reason(s) why.
The transplantation rate of this study was compared with the transplantation rate observed from January 2010 until December 2013. During this period, 20.7% of single-deceased donor kidneys (136 out of 657 offered organs) were transplanted. The KDRI/KDPI was calculated retrospectively for all 657 offers during this period, by using the KDRI calculator of the OPTN 2015.
During the prospective trial, we also analysed if there was a difference in recipients’ characteristics such as age, dialysis vintage, number of previous grafts and virtual panel-reactive antibodies (vPRA), for those who received a low-KDRI donor kidney compared with those who received a higher KDRI donor kidney. Therefore, we divided the recipients into two groups according to the KDRI of the donor. One group consisted of the recipients who received a donor kidney with a KDRI lower than the median of all transplanted donor kidneys in our centre during the prospective study, whereas the other group consisted of the ones who received a donor kidney with a KDRI higher than the median.
To investigate whether implementation of the KDRI had an impact on our reasoning, we compared how often donor parameters included in the KDRI were evoked as a reason to decline an offer both before and after the implementation of the KDRI. In addition, smoking behaviour of the donor as a reason was also investigated.
Statistical analysis
Power analysis (power = 80%, significance = 0.05) revealed that 247 deceased donor kidney offers needed to be included to see a significant increase in transplantation rate from 20.7% (of 657 deceased donor kidneys offered during the first study period) to 30%. Normality of the parameters was tested with QQ-plots and the Shapiro–Wilk test. Since all parameters turned out to be not-normally distributed, we used the Mann–Whitney U-test to compare parameters between the two groups and the Wilcoxon test to compare parameters within one group. For binary or categorical correlations, the chi-square test was used. To analyse if implementation of the KDRI resulted in a significantly higher transplantation rate, we performed a binary logistic regression with transplantation rate as outcome and the KDRI and study period as predictors. The median waiting time until transplantation after a first-declined kidney donor offer was calculated using the Kaplan–Meier curve. All statistical analyses were performed using SPSS software, version 23.
This study protocol was approved by the ethics committee of the Antwerp University Hospital.
RESULTS
From 1 January 2010 until 31 December 2013, 657 single-deceased donor kidneys were offered to one of the patients of the transplant waiting list of the Antwerp University Hospital, for which the KDRI was retrospectively calculated. Thus, this value was not taken into account in the decision to accept or decline a kidney offer. The donor characteristics of these offers are summarized in Table 1. From 1 April 2015 until 31 December 2016, 287 single-deceased donor kidneys were offered, for which the KDRI was prospectively calculated and included in the decision-making of acceptance or rejection of the offer. These characteristics are summarized in Table 2.
Table 1.
Donor characteristics of all offered deceased donor kidneys that were either transplanted or not transplanted, from 1 January 2010 until 31 December 2013
| Donor factor | Median (Q1–Q3) or percentage |
P-value | |
|---|---|---|---|
| Transplanted (n = 136) (20.7%) | Not transplanted (n = 521) (79.3%) | ||
| Age (years) | 44 (31–54) | 55 (47–60) | <0.001 |
| Length (cm) | 175 (168–183) | 175 (165–180) | 0.101 |
| Weight (kg) | 75 (66–85) | 80 (70–90) | 0.28 |
| Creatinine (mg/dL) | 0.69 (0.60–0.89) | 0.80 (0.62–1.1) | <0.001 |
| Diabetes (%) | No: 85.3 | No: 72.7 | 0.04 |
| Yes: 2.2 | Yes: 9.2 | ||
| Unknown: 12.5 | Unknown: 18 | ||
| Hypertension (%) | No: 69.1 | No: 46.3 | <0.001 |
| Yes: 19.1 | Yes: 37.6 | ||
| Unknown: 11.8 | Unknown: 16.1 | ||
| ECD (%) | 12.5 | 35.7 | <0.001 |
| DCD (%) | 17.6 | 28.6 | 0.010 |
| Positive hepatitis C serology (%) | Negative: 100 | Negative: 98.8 | 0.354 |
| Positive: 0 | Positive: 0 | ||
| Unknown: 0 | Unknown: 1.2 | ||
| CVA (%) | 14 | 21.5 | 0.050 |
| KDPI (%) | 34 (18–58) | 65 (47–79) | <0.001 |
| KDRI | 0.85 (0.72–1.08) | 1.17 (0.96–1.35) | <0.001 |
Table 2.
Donor characteristics of all offered deceased donor kidneys that were either transplanted or not transplanted, from 1 April 2015 until 31 December 2016
| Donor factor | Median (Q1–Q3) or percentage |
P-value | |
|---|---|---|---|
| Transplanted (n = 75) (26.1%) | Not transplanted (n = 212) (73.9%) | ||
| Age (years) | 50 (39–54) | 57 (48–63) | <0.001 |
| Length (cm) | 172 (165–180) | 171 (162–180) | 0.182 |
| Weight (kg) | 80 (70–90) | 78 (67–90) | 0.712 |
| Creatinine (mg/dL) | 0.64 (0.53–0.94) | 0.82 (0.61–1.18) | 0.001 |
| Diabetes (%) | No: 77.3 | No: 72.3 | 0.06 |
| Yes: 0 | Yes: 11.7 | ||
| Unknown: 22.7 | Unknown: 16.0 | ||
| Hypertension (%) | No: 64.0 | No: 46.9 | 0.023 |
| Yes: 21.3 | Yes: 37.6 | ||
| Unknown: 14.7 | Unknown: 15.5 | ||
| ECD (%) | 8.1 | 47.8 | <0.001 |
| DCD (%) | 12.0 | 50.7 | 0.022 |
| Positive hepatitis C serology (%) | Negative: 98.7 | Negative: 100 | 0.261 |
| Positive: 0 | Positive: 0 | ||
| Unknown: 1.3 | Unknown: 0 | ||
| CVA (%) | 29.3 | 37.6 | 0.210 |
| KDPI (%) | 47 (33–58) | 74 (55–87) | <0.001 |
| KDRI | 0.97 (0.84–1.07) | 1.26 (1.05–1.49) | <0.001 |
Comparing both groups, there was a statistically significant difference between the transplanted and not transplanted donor kidneys for age, serum creatinine, the presence of arterial hypertension or diabetes, the number of DCDs and ECDs, and the KDRI/KDPI.
The transplantation rate increased from 20.7% (136/657) in the retrospective control group to 26.1% (75/287) when the KDRI was implemented in the decision algorithm (P < 0.001). This corresponds to an absolute increase of 5.4% of accepted donor kidneys and a relative increase of 26%. Moreover, the median KDRI of all transplanted donor kidneys during the prospective trial was 0.97 (KDPI 47%), a value significantly higher than the median KDRI of 0.85 (KDPI 34%) during the retrospective trial (P = 0.047). Of all offered deceased donor kidneys with a KDRI <1 (KDPI 50%), 38.7% (92/238) were transplanted during the retrospective trial, compared with 50.5% (47/93) from 1 April 2015 until 31 December 2016 (P = 0.020).
By comparing recipients who received a donor kidney with a KDRI lower than the median of all transplanted donor kidneys in our centre (KDRI <0.97) with those who received a donor kidney higher than the median during the prospective trial, we did not find a statistical significant difference concerning age (median: 51.5 and 55.0 years, respectively; P = 0.159), dialysis vintage (median: 1039 and 857 days, respectively; P = 0.291), number of previous grafts (median: both 0 previous grafts; P = 0.369) and vPRA (median: both 0%; P = 0.277).
Table 3 shows the comparison before and after the implementation of the KDRI of the reasons evoked to decline a donor kidney. Interestingly, donor age, history of hypertension, DCD and cerebrovascular accident (CVA) as a cause of death, all parameters included in the KDRI, were significantly less often evoked as a reason to decline an offer during the prospective study. Furthermore, smoking was also less frequently cited.
Table 3.
Comparison before and after the implementation of the KDRI of the reasons evoked to decline a donor kidney
| Donor factor | Retrospective study | Prospective study | P-value |
|---|---|---|---|
| KDRI | 0/206 (0%) | 76/212 (35.8%) | < 0.001 |
| Age | 72/206 (35.0%) | 29/212 (13.7%) | < 0.001 |
| History of hypertension | 47/206 (22.8%) | 12/212 (5.7%) | < 0.001 |
| History of diabetes | 17/206 (8.3%) | 10/212 (2.4%) | 0.166 |
| High creatinine | 43/206 (20.9%) | 35/212 (16.5%) | 0.261 |
| DCD | 40/206 (19.4%) | 21/212 (9.9%) | 0.008 |
| CVA as the cause of death | 9/206 (4.4%) | 0/212 (0%) | 0.002 |
| Smokinga | 38/206 (18.4%) | 19/212 (9.0%) | 0.006 |
For the retrospective study, the detailed reasons as reported above were available for 206 donor kidneys.
Besides smoking, all other parameters are included in the KDRI.
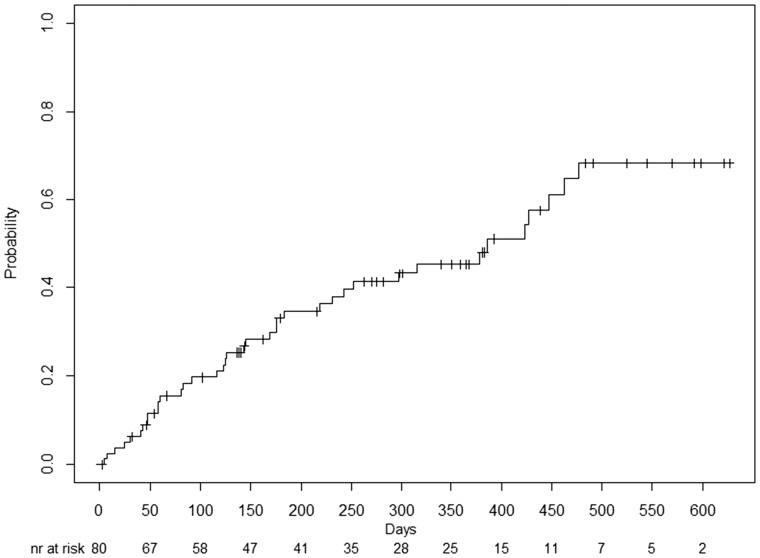
A total of 68% of patients (n = 38) were transplanted after the decline of a first offer in the period from 1 April 2015 until 31 December 2016. The median time to transplantation was 386 days (95% confidence interval 265–507 days). Figure 1 shows the cumulative incidence plot of transplantation after a first-declined donor kidney during this period. The KDRI of this first-declined donor kidney (1.29; KDPI 75%) was significantly higher than the KDRI of the transplanted donor kidney (0.97; KDPI: 47%). Of these groups, 82% (n = 31) indeed received a kidney with a lower KDRI (median 0.97; KDPI 47%), compared with a median KDRI of 1.33 (KDPI 78%) for the first-declined donor kidney. In 18% (n = 7), the transplant candidate received a higher KDRI donor kidney after a first-declined donor kidney (median: 0.98; KDPI 48%), compared with a median KDRI of 0.82 (KDPI 30%) for the first-declined offered donor kidney. Immunological reasons and acute kidney injury (AKI) in combination with proteinuria were the main reasons to decline these low-KDRI donor kidneys. Immunological reasons (n = 2) consisted of an insufficient HLA matching. In both cases, there was no match in HLA-DR antigens. DR. AKI was reported as a reason to decline the offer in two cases. Of note, AKI is not necessarily associated with a high KDRI/KDPI. Indeed, when creatinine increases >1.5 mg/dL, the KDPI increases only 1% per 1 mg/dL rise in creatinine. Therefore, we still were reluctant to accept offered deceased donor kidneys with AKI even with a low KDRI, especially in combination with proteinuria, which could suggest an underlying kidney disease.
FIGURE 1.
Cumulative incidence plot of transplantation after a first-declined kidney during the period from 1 April 2015 until 31 December 2016.
DISCUSSION
The present study demonstrates that the introduction of the KDRI in our decision-making process to accept or decline an offered deceased donor kidney was associated with a relative increase in our transplantation rate by 26%. Additionally, we transplanted a significantly higher percentage of all offered deceased donor kidneys with a KDRI <1, compared with the retrospective trial. While this association does not prove causality, we also observed that factors included in the KDRI such as donor age, history of hypertension, DCD and CVA as a cause of death were significantly less frequently evoked as reasons to decline an offer after the implementation of the KDRI in our decision-making process. The same occurred with smoking, which was not retained as a significant factor influencing outcomes in the Rao pivotal study. This suggests that KDRI implementation was indeed instrumental in increasing our transplantation rate. Additionally, investigating KDRI is a fruitful exercise for each transplant centre. This audit provides a rationale to discuss and possibly optimize the allocation policy within the medico-surgical team.
How do our data compare with those reported in the literature? Although we succeeded in expanding our donor pool, the median KDRI of the transplanted kidneys in our centre was nevertheless still <1 (0.97) during the prospective trial. As a corollary, a substantial proportion (74%) of all offered deceased donor kidneys was still declined. Further, in our prospective study trial, only one (1.3%) patient received a donor kidney with a KDPI >85%, as compared to 9.7% of patients in the USA in 2012 [5]. Recent data show that the outcome of high-KDPI donor kidneys has become better over the years, with a 5-year graft survival of 56% for donor kidneys with a KDPI >85% [5]. Further, Massie et al. showed a long-term survival benefit in recipients who underwent a kidney transplantation with high-KDPI (70–80%) kidney compared with recipients who remain wait listed while waiting for a lower KDPI kidney [6]. However, this improved survival occurred only when the recipient was older than 50 years or when the waiting time was >33 months [6].
Therefore, it seems that we still are too critical in accepting offered deceased donor kidneys. However, it is difficult to compare our data with the reports above, since no data regarding the KDRI are available from European countries. In this respect, it is important to note that survival on dialysis is generally better in Europe as compared with the USA. This might explain the reluctance of some European centres and/or countries to accept high-KDRI donors. For example, the 5-year survival rate for wait-listed patients in Europe who started dialysis in the period from 2004 to 2008 was 54% [7], higher than the reported 40% for patients who started dialysis in 2007 in the USA. Additionally, the median waiting time in the USA for a newly wait-listed candidate in 2009 was 3.6 years [8]. In Belgium, the median waiting time is <3 years. This obviously also influences our propensity to accept only the best kidney offers. Along this line, we demonstrated that the vast majority of transplant candidates for whom a first-offered deceased donor kidney was rejected, finally received with a short delay a donor kidney with a statistically significantly lower KDRI.
The limitations of our study include a small sample size of a single centre and the fact that the KDRI is based on the donor population of the USA and is not yet validated in Europe. We also presumed that all donors were Caucasian because Eurotransplant does not provide any information about the race. This assumption is unlikely to bias our data as <5% of the Eurotransplant population is Black. Finally, our study does not provide data about patient and graft survival. While this was not the aim of the study, it seems important to have European data that would validate the association between KDRI and long-term graft survival.
In summary, implementing the KDRI in our decision-making process increased the relative transplantation rate of our centre by 26%. The KDRI seems to be a helpful tool when considering whether to accept or decline a deceased donor kidney offer.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Wolfe RA, Ashby VB, Milford EL. et al. Comparison of mortality in all patients on dialysis, all patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999; 341: 1725–1730 [DOI] [PubMed] [Google Scholar]
- 2. Port FK, Bragg-Gresham JL, Metzger RA. et al. Donor characteristics associated with reduced graft survival: an approach to expanding the pool of kidney donors. Transplantation 2002; 74: 1281–1286 [DOI] [PubMed] [Google Scholar]
- 3. Rao PS, Schaubel DE, Guidinger MK. et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation 2009; 88: 231–236 [DOI] [PubMed] [Google Scholar]
- 4.Online KDRI calculator of the OPTN. https://optn.transplant.hrsa.gov/resources/allocation-calculators/kdpi-calculator/
- 5. Rege A, Irish B, Castleberry A. et al. Trends in usage and outcomes for expanded criteria donor kidney transplantation in the United States characterized by kidney donor profile index. Cureus 2016; 8: e887 DOI 10.7759/cureus.887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Massie AB, Luo X, Chow EKH. et al. Survival benefit of primary deceased donor transplantation with high-KDPI kidneys. Am J Transplant 2014; 14: 2310–2316 [DOI] [PubMed] [Google Scholar]
- 7. ERA-EDTA Registry: ERA-EDTA Registry Annual Report 2013. Amsterdam, The Netherlands: Academic Medical Center, Department of Medical Informatics, 2015
- 8. Saran R, Li Y, Robinson B et al. USDRS 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2015; 66: Svii, S1–S305 [DOI] [PMC free article] [PubMed]