Abstract
We have developed an animal model of amodiaquine-induced liver injury that has characteristics very similar to idiosyncratic drug-induced liver injury (IDILI) in humans by impairing immune tolerance using a PD1−/− mouse and cotreatment with anti-CTLA-4. In order to test the usefulness of this model as a general model for human IDILI risk, pairs of drugs with similar structures were tested, one of which is associated with a relatively high risk of IDILI and the other not. One such pair is troglitazone and pioglitazone; troglitazone has caused fatal cases of IDILI while pioglitazone is quite safe. Another pair is tolcapone and entacapone; tolcapone can cause serious IDILI; in contrast, although entacapone has been reported to cause liver injury, it is relatively safe. PD1−/− mice treated with anti-CTLA-4 and troglitazone or tolcapone displayed liver injury as determined by ALT levels and histology, while pioglitazone and entacapone showed less signs of liver injury. One possible mechanism by which drugs could induce an immune response leading to IDILI is by causing the release of danger-associated molecular pattern molecules that activate inflammasomes. We found that the supernatants from incubations of troglitazone, tolcapone, or entacapone with hepatocytes were also able to activate inflammasomes in macrophages, while the supernatant from pioglitazone incubations did not. These results are consistent with an immune mechanism for troglitazone- and tolcapone-induced IDILI and add to the evidence that this may be a general model for IDILI.
Keywords: idiosyncratic drug-induced liver injury, inflammasome, danger associated molecular pattern molecules, adverse drug reactions, immunotoxicology, liver, systems toxicology
Idiosyncratic drug-induced liver injury (IDILI) remains a significant issue in healthcare and drug development (Regev, 2014). Identifying IDILI risk early on in drug development is critical to minimize human exposure to drugs that cause liver injury and to reduce the cost and time involved in drug development. It is unlikely that specific biomarkers will be discovered without a better mechanistic understanding of IDILI. Valid animal models of IDILI would make controlled mechanistic experiments possible, but idiosyncratic reactions are also idiosyncratic in animals (Ng et al., 2012). Unfortunately, until recently animal models of IDILI had characteristics very different from the characteristics of IDILI in humans; therefore, the mechanisms are presumably different. Specifically, the toxicity was acute rather than delayed in onset, and it usually required concentrations of the drug much higher than therapeutic concentrations. The inflammagen model sought to induce an immune response to drugs by cotreatment with lipopolysaccharide (LPS). However, the injury occurred within hours, and the histology was similar to that of injury caused by higher doses of LPS rather than typical of IDILI (Luyendyk et al., 2003). Our attempts to develop animal models similar to IDILI in humans by activating the immune system with agents such as LPS have failed, and this is consistent with the clinical observation that inflammatory conditions such as ulcerative colitis do not appear to increase the risk of IDILI.
The dominant immune response in the liver is immune tolerance, and most IDILI in humans resolves despite continued treatment with the drug. This suggests that the resolution of injury involves immune tolerance. Based on this hypothesis new strategies were used to develop animal models of IDILI. In one case immune tolerance was impaired with an antibody to deplete myeloid-derived suppressor cells (MDSCs). Combined with 2 exposures to halothane, this produced liver injury similar to the injury in patients who had been anesthetized with halothane on multiple occasions (Chakraborty et al., 2015). In contrast we impaired immune tolerance by checkpoint inhibition; specifically, we used PD-1−/− mice and cotreatment with anti-CTLA-4. This produced a model of amodiaquine-induced liver injury very similar to IDILI in humans (Metushi et al. 2015). This impaired immune tolerance model was also able to unmask the IDILI potential of isoniazid and nevirapine (NVP), drugs also known to cause IDILI in humans (Mak and Uetrecht, 2015a).
In this study, we tested whether this model could differentiate drugs that cause IDILI from those that do not. One such pair was troglitazone and pioglitazone. Both are thiazolidinedione drugs used to treat diabetes, and where troglitazone causes liver injury in humans and pioglitazone injury is quite rare (Senior, 2014). Another pair of drugs with similar structures is tolcapone and entacapone, both are nitrocatechols used to treat Parkinson’s disease. Although entacapone can cause mild liver injury, only tolcapone is associated with a significant risk of liver failure (Borges, 2003). Both troglitazone and tolcapone have characteristics that suggest their drug-induced liver injury is immune mediated.
We have also recently reported an in vitro test of the ability of a drug to induce an immune response that could lead to an idiosyncratic drug reaction (Weston and Uetrecht, 2014). Specifically, inflammasome activation in macrophages appears to be a major mechanism by which agents can initiate an immune response and is measured by release of IL-1β. THP-1 macrophages were treated with chemically reactive drugs known to cause skin rashes and were distinguished from safe chemically similar drugs by comparing IL-1β secretion. However, most drugs that cause IDILI probably require reactive metabolite formation in the liver, and THP-1 cells have little cytochrome P450 activity. Hepatocytes are the site of most reactive metabolite formation, but they have less inflammasome activity. However, it has been suggested that reactive metabolite formation by hepatocytes leads to the release of danger-associated molecular pattern molecules (DAMPs) that are responsible for activation of antigen presenting cells. We recently demonstrated that, although NVP does not directly activate macrophages, the supernatant from the incubation of NVP with cultured hepatocytes did activate THP-1 cells (Kato and Uetrecht, 2017). Oda et al. (2016) used a similar strategy to test a large number of drugs with mixed results; however, they did not try to determine a dose response relationship that included the therapeutic concentration of the drug. In this study, we also tested the ability of drugs to cause the release of DAMPs that could activate macrophages using the same 2 pairs of drugs that we used in the in vivo studies.
MATERIALS AND METHODS
Animals and drug treatments
Female C57BL/6 mice (8–10 weeks of age) were purchased from Charles River Laboratories (QC, Canada). Female PD1−/− mice between 10 and 12 weeks of age were bred and housed as previously described (Metushi et al., 2015). Troglitazone and pioglitazone (Daiichi Sankyo Co., Ltd, Tokyo, Japan) were thoroughly mixed with rodent meal at a concentration of 0.2% (w/w) and then provided to the mice ad libitum. The troglitazone dose resulted in blood levels similar to the therapeutic Cmax of troglitazone in humans, while the pioglitazone dose resulted in blood levels about 20× the therapeutic Cmax of pioglitazone in humans (Supplementary Material 1). Tolcapone (BOC Sciences, Shirley, New York) and entacapone (Orion Pharma, Espoo, Finland) were also thoroughly mixed with rodent meal at a concentration of 0.25% (w/w) and then provided to the mice ad libitum. The tolcapone dose resulted in blood levels about 2× the therapeutic Cmax of tolcapone in humans, while this entacapone dose resulted in blood levels similar to the therapeutic Cmax in humans (Supplementary Material 1). Mice were split into 3 groups with 3 mice per drug group; a control C57BL/6 mouse group (Control), a group of C57BL/6 mice treated with troglitazone, pioglitazone, tolcapone, or entacapone (Troglitazone, Pioglitazone, Tolcapone, or Entacapone), and a group of PD1−/− mice treated with anti-CTLA-4 (clone 9D9; Bristol-Myers Squibb, Redwood City, California) and troglitazone, pioglitazone, tolcapone, or entacapone (PD1/anti-CTLA-4/Troglitazone, PD1/anti-CTLA-4/Pioglitazone, PD1/anti-CTLA-4/Tolcapone, or PD1/anti-CTLA-4/Entacapone). The anti-CTLA-4 antibody was administered as previously described in Metushi et al. (2015). All animal protocols used in this study were approved by the University of Toronto Animal Care Committee and conducted in an animal facility accredited by the Canadian Council on Animal Care.
ALT and drug blood levels
Blood was collected as previously described (Metushi et al., 2015). Serum ALT levels (Thermo Scientific; Middletown, Virginia) were measured as described by the manufacturer to determine the extent liver injury. Serum was also saved for analysis of serum drug concentrations, which were measured as previously described with modifications (Lobach and Uetrecht, 2014). Troglitazone and pioglitazone were used as their respective internal standards. Tolcapone and entacapone were also used as their respective internal standards. The mobile phase consisted of 80: 20; methanol: 1 mM ammonium formate (0.1% formic acid, v/v). A PE Sciex API 3000 quadrupole mass spectrometer with an electrospray ionization source (Sciex, Concord, Ontario, Canada) was used in negative mode, and an AQUA 5 μm C18 125 A 150 × 2 mm column (Phenomenex, Torrance, California) was employed. The ions monitored for troglitazone and pioglitazone were 440/397 and 355/312, respectively. The ions monitored for tolcapone and entacapone were 304/287 and 272/255, respectively.
Histology
Liver and spleen samples were excised and placed in 10% neutral buffered formalin solution (Sigma; Ottawa, Ontario, Canada). The samples were paraffin-embedded, sectioned to 4 µm, stained with H&E and then scanned (CFIBCR Histology/Microscopy Core Unit; Toronto, Ontario, Canada).
Isolation of mononuclear cells and flow cytometry
Mononuclear cells were isolated from livers and spleens, stained with antibodies, and then phenotyped by flow cytometry by a previously described method (Mak and Uetrecht, 2015b). Mononuclear cells were stained for macrophages (M1 and M2), MDSCs, CD8 T cells, CD4 T cells, Th17 Cells, Treg cells, NK cells, NKT cells, and B cells. Macrophages were characterized as CD11b + F4/80+ and further characterized as M1 (CD11b + F4/80+ and iNOS+ or IL-12+) or M2 (CD11b + F4/80+ and Arg1+ or IL-10+). MDSC were characterized as CD11b + Gr1+, CD4 T-cells as CD3 + CD4+, CD8 T-cells as CD3 + CD8+, Th17 cells as CD4 + IL-17+, Treg cells as CD4 + FOXP3+, NK cells as NK1.1 + CD3-, NKT cells as NK1.1 + CD3+, and B-cells as CD45R+.
Cell culture
FLC-4 cells (JCRB0435; Hasumura et al., 1988) were obtained from Health Science Research Resources Bank (Osaka, Japan). Cryopreserved mouse (C57BL/6) hepatocytes were purchased from Triangle Research Labs (Research Triangle Park, North Carolina). THP-1 cells (JCRB0112) were obtained from Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan). J774A.1 cells were purchased from American Type Culture Collection (Manassas, Virgina). FLC-4 and J774A.1 cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM, 4.5 g glucose/l; Thermo Fisher Scientific, Waltham, Massachusetts), and THP-1 cells were cultured in Roswell Park Memorial Institute medium (Thermo Fisher Scientific), all supplemented with 10% fetal bovine serum (Sigma) at 37 °C with 5% CO2. Mouse hepatocytes were cultured in Hepatocyte Plating Medium (Triangle Research Labs) at 37 °C with 5% CO2.
FLC-4 or mouse hepatocyte cell suspensions (100 µl at 1.5 × 104 cells/ml) and the same volume of medium containing troglitazone, pioglitazone, tolcapone, or entacapone were applied to a Prime Surface 96 U plate (S-BIO; New Hampshire, USA) for 3D culture. The cells in 96 U plate were cultured at 37 °C with 5% CO2 for 7 days.
THP-1 cells (4 × 105 cells/ml) were differentiated in medium containing phorbol 12-myristate 13-acetate (50 ng/ml; Sigma) for 3 days on 24-well multiplate. After differentiation, each well was washed by PBS (Sigma), medium (1 ml) was added on each well, and incubated for 24 h at 37 °C with 5% CO2. Then the medium was aspirated, culture medium including one of the drugs or supernatant of FLC-4 cells was added, and incubated at 37 °C with 5% CO2 for 18 h.
J774A.1 cells (4 × 105 cells/ml) were pre-incubated overnight at 37 °C with 5% CO2 and then stimulated with 500 ng/ml of LPS (Sigma) for 6 h on 24-well multiplate. After LPS stimulation, each well was washed by PBS (Sigma), medium (1 ml) was added on each well, and incubated for 1 h at 37 °C with 5% CO2. Then the medium was aspirated, culture medium including one of the drugs or supernatant of mouse hepatocytes was added, and incubated at 37 °C with 5% CO2 for 18 h.
The troglitazone, pioglitazone, tolcapone, and entacapone concentrations were set within the therapeutic range and showed no effect on cell viability. Benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (ZVAD, 10 μg/ml; InvivoGen, California) was used to inhibit caspase activity.
Measurement of IL-1β concentration in culture medium
Culture medium of differentiated THP-1 and J774A.1 cells was collected, transferred to a tube and stored at −80 °C until analysis. IL-1β was measured in each culture medium sample using IL-1β ELISA kits (Thermo Fisher Scientific).
Caspase-1 activity of differentiated THP-1 cells
Caspase-1 activity was measured using the Caspase-Glo 1 Inflammasome Assay (Promega Corporation, Wisconsin). Caspase-Glo reagent was added to each well and then incubated for 1 h at room temperature. Supernatant was transferred to 96-well white-plate and luminescence was then measured with a plate reader.
Statistical analysis
Mean ± SEM values were calculated for each experimental group. Statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, California). Data were analyzed using a 2-way analysis of variance (ANOVA) or a 1-way ANOVA. A p value < .05 was considered significant (*p < .05; **p < .01; ***p < .001).
RESULTS
PD1−/− Mice Treated With anti-CTLA-4 Plus a Drug Was Able to Distinguish Between Drugs Known to Cause Liver Injury in Humans and Safe Drugs
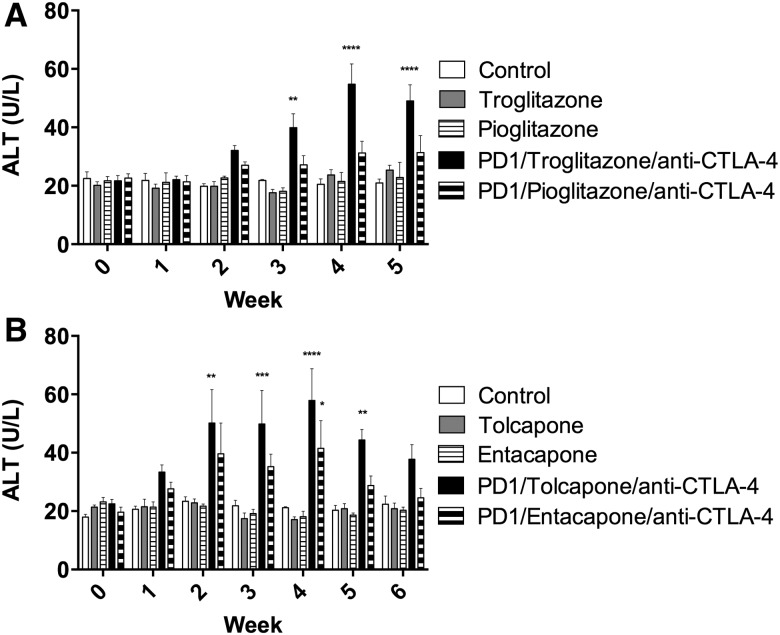
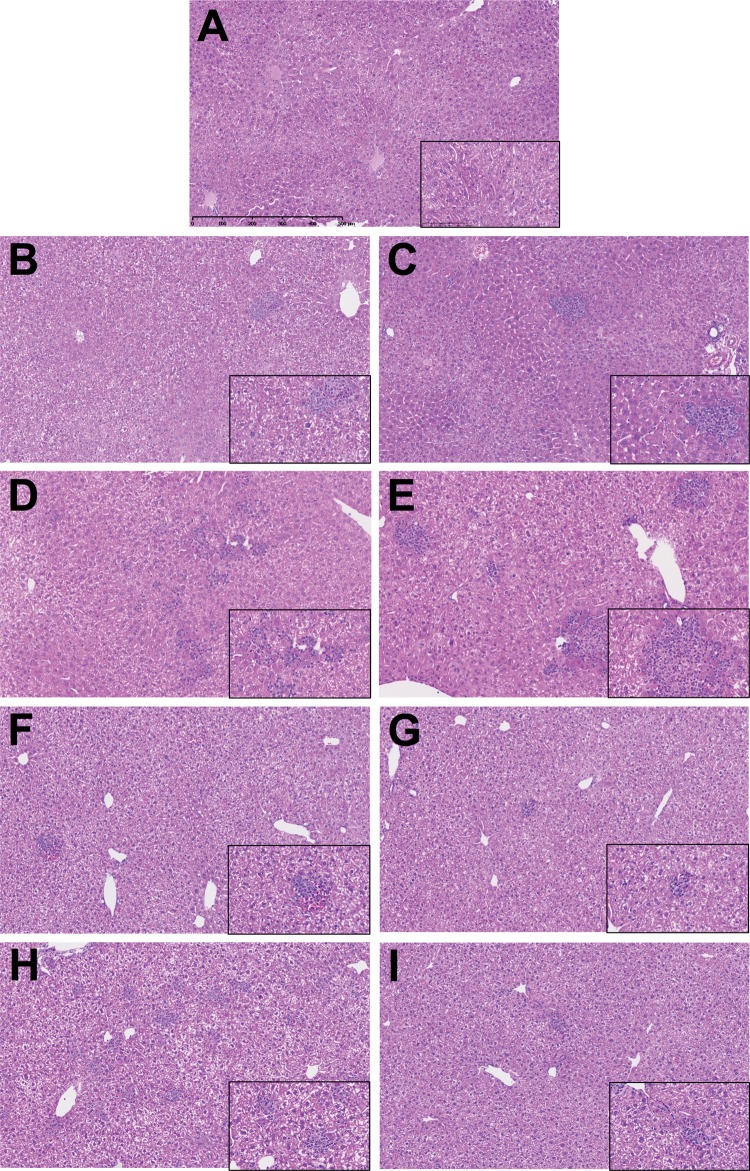
Wild type mice treated with troglitazone, pioglitazone, tolcapone, or entacapone alone showed no increase in ALT (Figure 1). PD1−/− mice treated with anti-CTLA-4 and either troglitazone or tolcapone showed a delayed onset increase in ALT that was significant compared with control (Figure 1). In contrast, PD1−/− mice treated with anti-CTLA-4 and pioglitazone showed no significant increase in ALT, while PD1−/− mice treated with anti-CTLA-4 and entacapone showed an increase in ALT that was less than with tolcapone (Figure 1). The spleens appeared normal and histologically similar among the groups (data not shown). Livers of control untreated mice were histologically unremarkable with rare small focal infiltrates, some of which were normal hematopoiesis (Figure 2A). A few small focal inflammatory infiltrates were found in livers of wild type mice treated with troglitazone, pioglitazone, tolcapone or entacapone alone (Figs. 2B–E). By comparison, PD1−/− mice treated with anti-CTLA-4 and troglitazone had numerous necroinflammatory foci characterized by macrophage infiltrates associated with hepatocellular necrosis and neutrophils (Figure 2F). PD1−/− mice treated with anti-CTLA-4 and pioglitazone also had similar necroinflammatory foci (Figure 2G). Livers of PD1−/− mice treated with anti-CTLA-4 and tolcapone had larger numbers of focal infiltrates characterized by macrophages with fewer necrotic hepatocytes and neutrophils (Figure 2H). Livers of PD1−/− mice treated with anti-CTLA-4 and entacapone only showed a few small mixed cell foci (Figure 2I). A table of detailed counts of foci is provided in Supplemental Material 2.
Figure 1.
Treatment of PD-1−/− mice with troglitazone, tolcapone, or entacapone leads to a delayed onset increase in ALT. (A) Control, Control C57BL/6 mice; Troglitazone, C57BL/6 mice treated with troglitazone only (n = 3); Pioglitazone, C57BL/6 mice treated with pioglitazone only (n = 3); PD1/Troglitazone/anti-CTLA-4, PD1−/− mice treated with troglitazone and anti-CTLA-4 (n = 3); PD1/Pioglitazone/anti-CTLA-4, PD1−/− mice treated with pioglitazone and anti-CTLA-4 (n = 3). (B) Control, Control C57BL/6 mice; Tolcapone, C57BL/6 mice treated with tolcapone only (n = 3); Entacapone, C57BL/6 mice treated with entacapone only (n = 3); PD1/Tolcapone/anti-CTLA-4, PD1−/− mice treated with tolcapone and anti-CTLA-4 (n = 3); and PD1/Entacapone/anti-CTLA-4, PD1−/− mice treated with entacapone and anti-CTLA-4 (n = 3). ALT for PD1−/− mice treated with anti-CTLA-4 and troglitazone were also significantly greater than troglitazone alone or pioglitazone alone at weeks 4 and 5. ALT for PD1−/− mice treated with anti-CTLA-4 and tolcapone were significantly greater than tolcapone alone or entacapone alone at weeks 2–5. Values represent the mean ± SE. Analyzed for statistical significance by 2-way ANOVA. p < .05 was considered significant (*p < .05;**p < .01; ***p < .001; ****p < .0001).
Figure 2.
Representative H + E stained liver sections, all at 10× with 40× inset, with scale in (A). (A) Control untreated C57BL/6 mice; (B) Troglitazone, C57BL/6 mice treated with troglitazone only; (C) Pioglitazone, C57BL/6 mice treated with pioglitazone only; (D) PD1/Troglitazone/anti-CTLA-4, PD1−/− mice treated with troglitazone and anti-CTLA-4; (E) PD1/Pioglitazone/anti-CTLA-4, PD1−/− mice treated with pioglitazone and anti-CTLA-4; (F) Tolcapone, C57BL/6 mice treated with tolcapone only; (G) Entacapone, C57BL/6 mice treated with entacapone only; (H) PD1/Tolcapone/anti-CTLA-4 = PD1−/− mice treated with tolcapone and anti-CTLA-4; and (I) PD1/Entacapone/anti-CTLA-4, PD1−/− mice treated with entacapone and anti-CTLA-4.
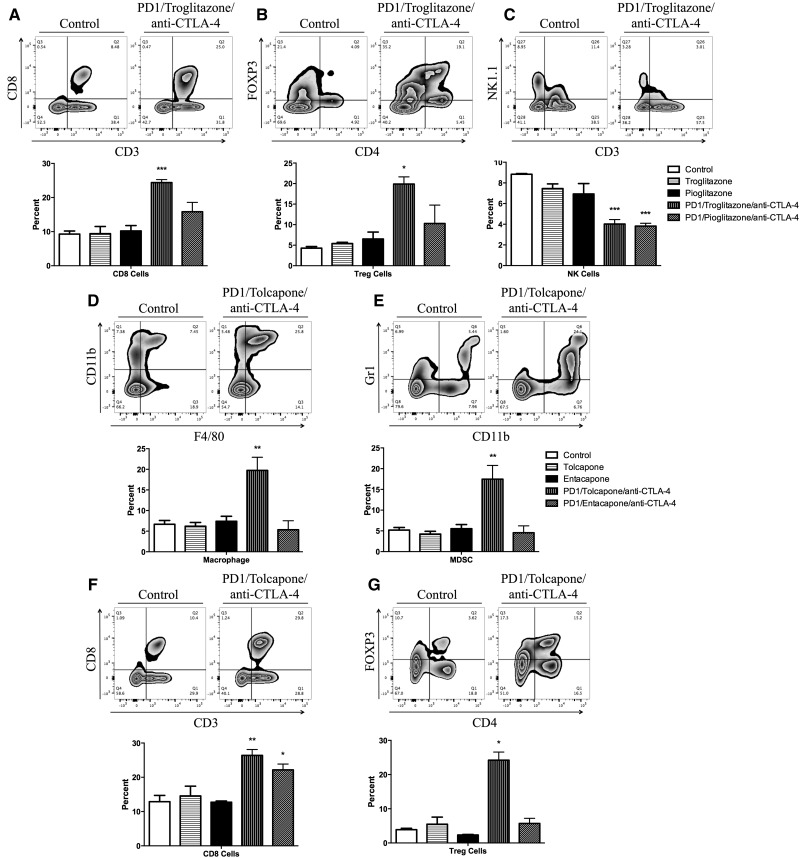
Troglitazone- and Tolcapone-Induced Liver Injury Was Characterized by an Increase in Infiltrating CD8+ T Cells and Treg Cells Into the Liver
Liver and spleen mononuclear cells were isolated and characterized by flow cytometry to determine the immune cells associated with the liver injury. There were no significant changes in the percentage of immune cells in the spleen across groups (data not shown). PD1−/− mice treated with anti-CTLA-4 and troglitazone showed a significant increased percentage of CD8 T cells and Treg cells in the liver compared with control (Figs. 3A and 3B). Additionally, PD1−/− mice treated with anti-CTLA-4 and troglitazone or pioglitazone showed a significantly decrease in the percentage of NK cells in the liver (Figure 3C). PD1−/− mice treated with anti-CTLA-4 and tolcapone showed a significantly increased percentage of macrophages, MDSCs, CD8 T cells, and Treg cells in the liver compared with control (Figs. 3D–G). In contrast to tolcapone, there was no change in MDSCs or Tregs in entacapone-treated PD1−/− mice, and the increase in CD8 T cells was less than in tolcapone-treated mice.
Figure 3.
Flow cytometry analysis of mononuclear leukocytes in the liver. A representative zebra plot and the average of 3 animals is displayed. Mononuclear cells with significant differences among the treatment groups compared with control are displayed (when the difference was not significant the data are not shown). (A) CD8 T cells; (B) Treg cells; (C) NK Cells. Control, Control C57BL/6 mice; Troglitazone, C57BL/6 mice treated with troglitazone only; Pioglitazone, C57BL/6 mice treated with pioglitazone only; PD1/Troglitazone/anti-CTLA-4, PD1−/− mice treated with troglitazone and anti-CTLA-4; PD1/Pioglitazone/anti-CTLA-4, PD1−/− mice treated with pioglitazone and anti-CTLA-4. (D) Macrophages; (E) MDSCs; (F) CD8 T cells; (G) Treg cells. Control, Control C57BL/6 mice; Tolcapone, C57BL/6 mice treated with tolcapone only (n = 3); Entacapone, C57BL/6 mice treated with entacapone only; PD1/Tolcapone/anti-CTLA-4, PD1−/− mice treated with tolcapone and anti-CTLA-4; and PD1/Entacapone/anti-CTLA-4, PD1−/− mice treated with entacapone and anti-CTLA-4. Values represent the mean ± SE. Analyzed for statistical significance by 1-way ANOVA. p < .05 was considered significant (*p < .05; **p < .01; ***p < .001).
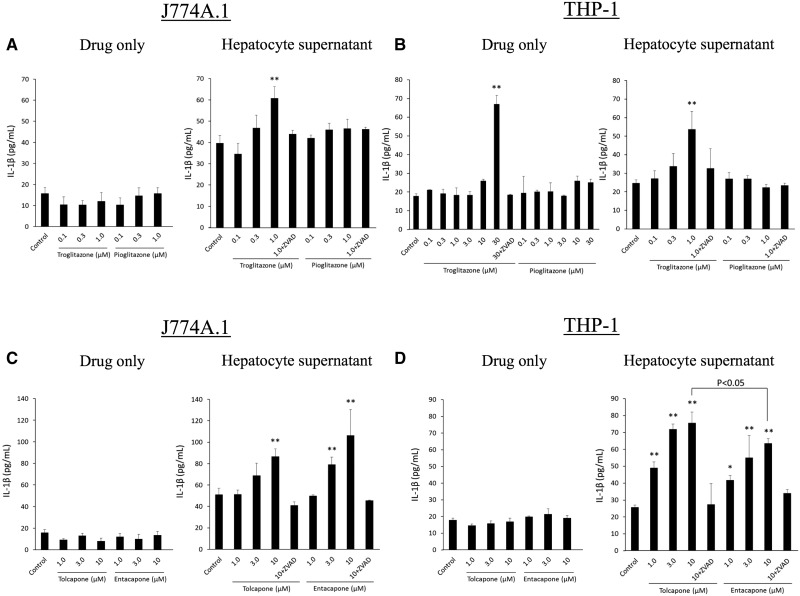
Troglitazone and Tolcapone Induced Increased IL-1β Production and Caspase-1 Activity in Human and Mouse Macrophages
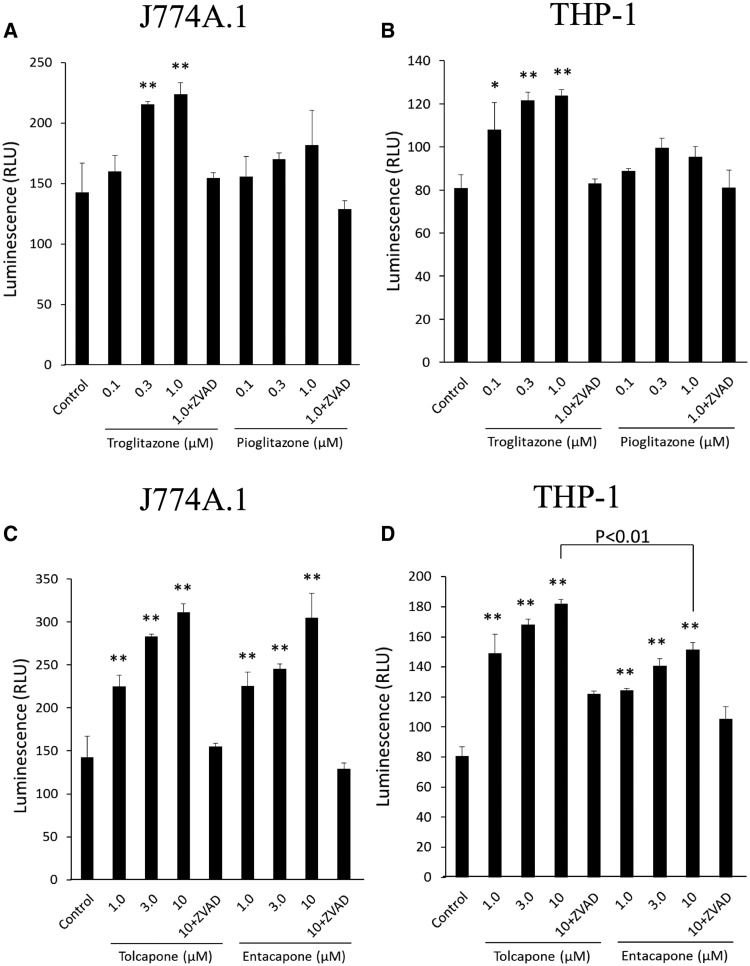
IL-1β production by macrophages and caspase-1 activity were measured to determine the ability of troglitazone, pioglitazone, tolcapone, and entacapone to trigger an immune response. FLC-4 cells, a human hepatocyte cell line, or C57BL/6 hepatocytes were incubated with troglitazone, pioglitazone, tolcapone, or entacapone for 7 days. The resulting supernatant was then incubated with human (THP-1 cells) or mouse (J774A.1 cells) macrophages, respectively. IL-1β production from J774A.1 and differentiated THP-1 cells was not changed when control medium containing pioglitazone, tolcapone, or entacapone was added (Figs. 4A and 4B). However, IL-1β production by differentiated THP-1 cells was significantly increased when control medium containing a concentration of troglitazone greater than Cmax (30 μM) was added (Figure 4B). Using a higher concentration of the other drugs did not elicit a response. In contrast, IL-1β production by differentiated THP-1 or J774A.1 cells was significantly increased when hepatocyte supernatant from troglitazone or tolcapone incubations was added, and this production was inhibited by ZVAD (Figs. 4A–D). Conversely, IL-1β production was not changed when hepatocyte supernatant containing pioglitazone was added (Figs. 4A and 4B). IL-1β production from differentiated THP-1 cells was also significantly increased when hepatocyte supernatant containing entacapone was added, although the highest added tolcapone concentration induced IL-1β production significantly more than with entacapone (Figure 4D). IL-1β production from differentiated J774A.1 cells treated with hepatocyte supernatant containing entacapone was similarly elevated (Figure 4C).
Figure 4.
Incubation of J774A.1 or THP-1 macrophages with drugs except the highest concentration of troglitazone does not lead to the release of IL-1ß, but incubation of these cells with supernatants from incubations of hepatocytes with troglitazone, tolcapone, or entacapone does. (A) J774A.1 cells treated with troglitazone or pioglitazone directly, or with hepatocyte supernatant from hepatocytes previously treated with troglitazone or pioglitazone. (B) THP-1 cells treated with troglitazone or pioglitazone directly, or with hepatocyte supernatant from hepatocytes previously treated with troglitazone or pioglitazone. (C) J774A.1 cells with tolcapone or entacapone directly, or with hepatocyte supernatant from hepatocytes previously treated with tolcapone or entacapone. (D) THP-1 cells treated with tolcapone or entacapone directly, or with hepatocyte supernatant from hepatocytes previously treated with tolcapone or entacapone. Values represent the mean ± SE. Analyzed for statistical significance by 1-way ANOVA. p < .05 was considered significant (*p < .05; **p < .01).
Caspase-1 activity of differentiated THP-1 and J774A.1 cells was also significantly increased when the supernatant from hepatocytes incubated with troglitazone was added, and this activation was inhibited by ZVAD (Figs. 5A and 5B). Caspase-1 activity was not changed when hepatocyte supernatant containing pioglitazone was added to the cells (Figs. 5A and 5B). As with the IL-1β production, caspase-1 activity was significantly increased in both differentiated THP-1 and J774A.1 cells in response to the addition of hepatocyte supernatant containing tolcapone or entacapone (Figs. 5C and 5D). Again, similar to IL-1β production, the caspase-1 activity in differentiated THP-1 cells induced by the highest added tolcapone concentration was significantly greater than with entacapone (Figs. 5D).
Figure 5.
Incubation of J774A.1 or THP-1 cells with the supernatants from hepatocytes incubated with troglitazone, tolcapone, or entacapone but not pioglitazone leads to activation of caspase-1 activity. (A) J774A.1 cells treated with hepatocyte supernatant from hepatocytes previously treated with troglitazone or pioglitazone. (B) THP-1 cells treated with hepatocyte supernatant from hepatocytes previously treated with troglitazone or pioglitazone. (C) J774A.1 cells treated with hepatocyte supernatant from hepatocytes previously treated with tolcapone or entacapone. (D) THP-1 cells treated with hepatocyte supernatant from hepatocytes previously treated with tolcapone or entacapone. Values represent the mean ± SE. Analyzed for statistical significance by 1-way ANOVA. p < .05 was considered significant (*p < .05; **p < .01).
DISCUSSION
In the current set of experiments we sought to extend the impaired immune tolerance model to see if it could differentiate similar drugs, one that is associated with a significant risk of IDILI and the other being relatively safe. The pairs we chose were troglitazone/pioglitazone and tolcapone/entacapone.
It has been proposed that the IDILI caused by troglitazone is due to inhibition of the bile salt export protein (Morgan et al., 2010) and that IDILI caused by tolcapone is due to uncoupling oxidative phosphorylation (Haasio et al., 2002). However, the clinical characteristics of the IDILI caused by troglitazone and tolcapone are similar to other IDILI that is known to be immune mediated. In fact clinical evidence suggests that most IDILI is immune mediated, and in many patients the liver injury resolves despite continued treatment, presumably due to immune tolerance (Uetrecht, 2007). Therefore, the observation that impairing immune tolerance unmasked the potential of these 2 drugs to cause IDILI (Figure 1) suggests that they are also immune mediated. Although the liver injury with these drugs was not as serious as with AQ, there was an increase in liver injury in animals with impaired immune tolerance. All 4 drugs caused at least a small increase in CD8+ T cells (Figure 3). CD8+ T cells are cytotoxic cells that could be involved in causing the liver injury from these mice. CTLA-4 alone has been reported to cause a small increase in CD8+ T cells; therefore, the smaller increases in the pioglitazone- and entacapone-treated mice could be due to the CTLA-4 antibody alone. Troglitazone and tolcapone also increased Treg cells (CD4 + FOXP3+); in addition, tolcapone also increased MDSCs (Gr-1 + CD11b+), which are antiinflammatory and are presumably a compensatory mechanism that limits the liver damage (Figure 3). This also suggests that immune tolerance is the dominant immune response to these drugs. However, even though entacapone did cause mild injury in this model, in contrast to tolcapone, it did not cause any increase in Tregs or MDSCs. Thus it is possible that the compensatory increase in Tregs and MDSCs is a better predictor of severe liver injury in humans than simply the mild liver injury seen in the PD1−/− mice. Even if the ultimate liver injury is immune mediated, inhibition of the bile salt export pump or mitochondrial injury could play a role in induction of an immune response by causing the release of DAMPs as discussed below.
The dominant hypotheses for how a drug or reactive metabolite may induce an immune response leading to an idiosyncratic drug reaction are the hapten and the danger hypotheses (Uetrecht, 2007). In general, drugs alone do not induce a strong immune response; therefore, they need to be bound to endogenous proteins (hapten hypothesis). Additionally signal 2, which is produced by costimulatory molecules on antigen presenting cells is required to induce an immune response (danger hypothesis) (Matzinger, 1994). Therefore, reactive metabolites of drugs could bind to endogenous proteins and cause cell damage to generate danger signals that activate inflammasomes (Bettigole and Glimcher, 2015). Activation of the inflammasome results in release of IL-1β. IL-1β is a strong proinflammatory molecule capable of promoting an immune response (Guo et al., 2015; Heymann and Tacke, 2016). This experiment differs compared with the previously described inflammasome experiment (Weston and Uetrecht, 2014), which involved chemically reactive drugs. The drugs studied in these experiments presumably require bioactivation by cytochromes P450; therefore, the drugs were first incubated with hepatocytes, and the supernatant from these incubations were evaluated for their ability to activate inflammasomes. It is known that stressed hepatocytes have the potential to release DAMPs to alert the immune system (Szabo and Petrasek, 2015). In this experiment, the supernatants from hepatocytes incubated with troglitazone, tolcapone, or entacapone were found to activate differentiated THP-1 and J774A.1 cells resulting in an increase in the release of IL-1β and caspase-1 activation. In contrast, hepatocyte supernatant containing pioglitazone did not activate differentiated THP-1 or J774A.1 cells (Figs. 4 and 5). In humans, tolcapone has a longer elimination half-life, a longer duration of action, and a better bioavailability than entacapone (Forsberg et al., 2003). Therefore plasma concentrations of tolcapone are significantly higher (about 10-fold higher) than that of entacapone, but we used the same concentrations in vitro, which may have overestimated the risk of entacapone. This may also be one reason for the difference in risk of hepatotoxicity between tolcapone and entacapone in humans; however, it is unlikely to completely explain the difference in risk. Given these results, inflammasome activation may represent the basis for an in vitro assay to determine IDILI risk, but entacapone represents a false positive, at least for the risk of liver failure. On the other hand, IL-1β production was significantly increased in THP-1 cells by troglitazone itself (30 μM). It has been reported that troglitazone facilitates caspase-8 and -9 activities by increasing the enzymatic activity of protein-tyrosine phosphatase-1β on human glioma cells (Akasaki et al., 2006). This causes down-regulation of FADD-like IL-1β-converting enzyme-inhibitory protein and leads to increase IL-1β production. Therefore in the current study, the increase in IL-1β production by addition of troglitazone itself may involve the same mechanism. Inflammasome activation by DAMPs released by hepatocytes may represent the mechanism by which reactive metabolites can induce an immune response that in some patients leads to IDILI, but there may be other mechanisms by which drugs directly activate inflammasomes.
In summary, the impaired immune tolerance animal model has shown potential as a general model of IDILI. Additionally the in vitro inflammasome model provided one possible mechanism by which drugs can initiate an immune response that can lead to IDILI. These studies add to our understanding of the basic mechanisms of IDILI. However, the immune response is exceedingly complex, and different drugs may initiate an immune response by different mechanisms. Therefore, many other drugs need to be studied, and no method is likely to perfectly predict IDILI risk. Nevertheless, with a better understanding of the range of mechanisms that can lead to IDILI it may be possible to better predict the IDILI potential of drug candidates.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
FUNDING
This work was supported by grants from the Canadian Institutes of Health Research (12323131).
Supplementary Material
ACKNOWLEDGMENTS
Ryuji Kato is a trainee from the Japanese Society of Clinical Pharmacology and Therapeutics. We would like to thank Daiichi Sankyo Co., Ltd for providing the troglitazone and pioglitazone, as well as Orion Pharma for providing the entacapone. We would like to thank Tiffany Cho for her assistance in isolating liver and spleen mononuclear cells and Dr M.A. Hayes for reviewing histology sections.
REFERENCES
- Akasaki Y., Liu G., Matundan H. H., Ng H., Yuan X., Zeng Z., Black K. L., Yu J. S. (2006). A peroxisome proliferator-activated receptor-gamma agonist, troglitazone, facilitates caspase-8 and -9 activities by increasing the enzymatic activity of protein-tyrosine phosphatase-1B on human glioma cells. J. Biol. Chem. 281, 6165–6174. [DOI] [PubMed] [Google Scholar]
- Bettigole S. E., Glimcher L. H. (2015). Endoplasmic reticulum stress in immunity. Annu. Rev. Immunol. 33, 107–138.http://dx.doi.org/10.1146/annurev-immunol-032414-112116 [DOI] [PubMed] [Google Scholar]
- Borges N. (2003). Tolcapone-related liver dysfunction: Implications for use in Parkinson’s disease therapy. Drug Saf. 26, 743–747. [DOI] [PubMed] [Google Scholar]
- Chakraborty M., Fullerton A. M., Semple K., Chea L. S., Proctor W. R., Bourdi M., Kleiner D. E., Zeng X., Ryan P. M., Dagur P. K., et al. (2015). Drug-induced allergic hepatitis develops in mice when myeloid-derived suppressor cells are depleted prior to halothane treatment. Hepatology 62, 546–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg M., Lehtonen M., Heikkinen M., Sovalainen J., Järvinen T., Männistö P. T. (2003). Pharmacokinetics and pharmacodynamics of entacapone and tolcapone after acute and repeated administration: A comparative study in the rat. J. Pharmacol. Exp. Ther. 304, 498–506. [DOI] [PubMed] [Google Scholar]
- Guo H., Callaway J. B., Ting J. P. (2015). Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 21, 677–687.http://dx.doi.org/10.1038/nm.3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haasio K., Nissinen E., Sopanen L., Heinonen E. H. (2002). Different toxicological profile of two COMT inhibitors in vivo: The role of uncoupling effects. J. Neural. Transm. 109, 1391–1401. [DOI] [PubMed] [Google Scholar]
- Hasumura S., Sujino H., Nagamori S., Kameda H. (1988). Establishment and characterization of a human hepatocellular carcinoma cell line JHH-4. Hum. Cell 1, 98–100. [PubMed] [Google Scholar]
- Heymann F., Tacke F. (2016). Immunology in the liver—from homeostasis to disease. Nat. Rev. Gastroenterol. Hepatol. 13, 88–110. [DOI] [PubMed] [Google Scholar]
- Kato R., Uetrecht J. (2017). Supernatant from hepatocyte cultures with drugs that cause idiosyncratic liver injury activates macrophage inflammasomes. Chem. Res. Toxicol. 30, 1327–1332. [DOI] [PubMed] [Google Scholar]
- Lobach A. R., Uetrecht J. (2014). Clozapine promotes the proliferation of granulocyte progenitors in the bone marrow leading to increased granulopoiesis and neutrophilia in rats. Chem. Res. Toxicol. 27, 1109–1119.http://dx.doi.org/10.1021/tx500184c [DOI] [PubMed] [Google Scholar]
- Luyendyk J. P., Maddox J. F., Cosma G. N., Ganey P. E., Cockerell G. L., Roth R. A. (2003). Ranitidine treatment during a modest inflammatory response precipitates idiosyncrasy-like liver injury in rats. J. Pharmacol. Exp. Ther. 307, 9–16. [DOI] [PubMed] [Google Scholar]
- Mak A., Uetrecht J. (2015a). The combination of anti-CTLA-4 and PD1–/– mice unmasks the potential of isoniazid and nevirapine to cause liver injury. Chem. Res. Toxicol. 28, 2287–2291. [DOI] [PubMed] [Google Scholar]
- Mak A., Uetrecht J. (2015b). The role of CD8 T Cells in amodiaquine-induced liver injury in PD1–/– mice cotreated with anti-CTLA-4. Chem. Res. Toxicol. 28, 1567–1573. [DOI] [PubMed] [Google Scholar]
- Matzinger P. (1994). Tolerance, danger, and the extended family. Annu. Rev. Immunol. 12, 991–1045.http://dx.doi.org/10.1146/annurev.iy.12.040194.005015 [DOI] [PubMed] [Google Scholar]
- Metushi I. G., Hayes M. A., Uetrecht J. (2015). Treatment of PD-1−/− mice with amodiaquine and anti-CTLA4 leads to liver injury similar to idiosyncratic liver injury in patients. Hepatology 61, 1332–1342. [DOI] [PubMed] [Google Scholar]
- Morgan R. E., Trauner M., van Staden C. J., Lee P. H., Ramachandran B., Eschenberg M., Afshari C. A., Qualls C. W., Lightfoot-Dunn R., Hamadeh H. K. (2010). Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol. Sci. 118, 485–500. [DOI] [PubMed] [Google Scholar]
- Ng W., Lobach A. R. M., Zhu X., Chen X., Liu F., Metushi I. G., Sharma A., Li J., Cai P., Ip J., et al. (2012). Animal Models of Idiosyncratic Drug Reactions. Adv. Pharmacol. 63, 81–135. [DOI] [PubMed] [Google Scholar]
- Oda S., Matsuo K., Nakajima A., Yokoi T. (2016). A novel cell-based assay for the evaluation of immune- and inflammatory-related gene expression as biomarkers for the risk assessment of drug-induced liver injury. Toxicol. Lett. 241, 60–70.http://dx.doi.org/10.1016/j.toxlet.2015.10.029 [DOI] [PubMed] [Google Scholar]
- Regev A. (2014). Drug-induced liver injury and drug development: Industry perspective. Semin. Liver Dis. 34, 227–239.http://dx.doi.org/10.1055/s-0034-1375962 [DOI] [PubMed] [Google Scholar]
- Senior J. R. (2014). Evolution of the food and drug administration approach to liver safety assessment for new drugs: Current status and challenges. Drug Saf 37, 9–17.http://dx.doi.org/10.1007/s40264-014-0182-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G., Petrasek J. (2015). Inflammasome activation and function in liver disease. Nat. Rev. Gastroenterol. Hepatol. 12, 387–400.http://dx.doi.org/10.1038/nrgastro.2015.94 [DOI] [PubMed] [Google Scholar]
- Uetrecht J. (2007). Idiosyncratic drug reactions: Current understanding. Annu. Rev. Pharmacol. Toxicol. 47, 513–539.http://dx.doi.org/10.1146/annurev.pharmtox.47.120505.105150 [DOI] [PubMed] [Google Scholar]
- Weston J. K., Uetrecht J. (2014). Activation of inflammasomes by agents causing idiosyncratic skin reactions: A possible biomarker. Chem. Res. Toxicol. 27, 949–951.http://dx.doi.org/10.1021/tx5001333 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.