Abstract
After briefly discussing endothelial glycocalyx and its role in vascular physiology and renal disease, this overview focuses on its degradation very early in the course of microbial sepsis. We describe our recently proposed mechanism for glycocalyx degradation induced by exocytosis of lysosome-related organelles and release of their cargo. Notably, an intermediate in nitric oxide synthesis, NG-hydroxy-l-arginine, shows efficacy in curtailing exocytosis of these organelles and improvement in animal survival. These data not only depict a novel mechanism responsible for very early glycocalyx degradation, but may also outline a potential preventive therapy. The second issue discussed in this article is related to the therapeutic acceleration of restoration of already degraded endothelial glycocalyx. Here, using as an example our recent findings obtained with sulodexide, we illustrate the importance of the expedited repair of degraded endothelial glycocalyx for the survival of animals with severe sepsis. These two focal points of the review on glycocalyx may not only have broader disease applicability, but they may also provide additional evidence to buttress the idea of the importance of endothelial glycocalyx and its maintenance and repair in the prevention and treatment of an array of renal and nonrenal diseases.
Keywords: endothelium, endotoxemia, lysosome, sulodexide
INTRODUCTION
Sepsis is defined as a systemic inflammatory syndrome induced by bacterial infection that can lead to multiorgan failure. It afflicts >750 000 people annually in the USA alone and has mortality rates of 28–50%. Even a few percentage points in improved survival would account for a large salutary net effect. One of the key molecular causes of Gram-negative septicemia is endotoxin consisting of lipopolysaccharides (LPSs) bound with high affinity to LPS-binding glycoprotein (LPS-LBP). Complex LPS-LBP is recognized by pattern recognition receptors, like its cognate receptor TLR4 and a coreceptor CD14 expressed on monocytes/macrophages and endothelial cells [1]. Gram-positive bacterial superantigens and bacterial wall products peptidoglycan and lipoteichoic acids induce sepsis which is clinically indistinguishable from Gram-negative bacteria due to the involvement of similar receptors/mediators (TLR2). Considering the systemic nature of septicemia, vascular endothelium represents the first line of exposure to bacterial endotoxins [2]. It responds to endotoxins with a complex system of ‘danger signals’, which are chronologically sequenced and spatially propagating [3]. Functionally, these waves of danger signaling tend to secure proper organismal responses, both pro- and anti-inflammatory.
VASCULATURE-RELATED COMPLICATIONS OF SEPSIS COMPRISE THE MAJOR PART OF CLINICAL MANIFESTATIONS
One of the hallmarks of circulation in severe sepsis accompanied by the decline in blood pressure is the redistribution of blood flow to vital organs, like the brain and heart, stealing it from other organs. In humans with sepsis and acute kidney injury (AKI), renal blood flow is reduced [4]. The dynamics of renal blood flow in less severe forms of sepsis are inconsistent. More important, however, is the impairment of renal microcirculation in sepsis [5, 6], of which endothelial glycocalyx (EG) is an early victim [7–9]. Its degradation is believed to contribute to the symptomatology of sepsis, as will be elaborated below.
EG: STRUCTURE AND FUNCTIONS
The endothelial cell surface is coated with a carbohydrate-rich layer with an average thickness of 0.1 to ∼ 2 µm and consisting of hyaluronic acid (HA) cords reaching 1 µm in length and heparan sulfate (HS) chains reaching 200 nm in length and comprising 50–90% of endothelial glycosaminoglycans, with an admixture of dermatan, keratan and chondroitin sulfates (reviewed in [10]). The high degree of sulfation of these components provides EG with a net negative charge. The membrane-tethered scaffold for these glycosaminoglycans consists of two families of proteoglycans: syndecans 1–4 (single membrane-spanning domain) and glypicans 1–6 (glycosylphosphatidylinositol-anchored). HA, on the other hand, binds to osteopontin receptor CD44 (Figure 1A). In addition, several glycoprotein families (selectins, integrins and immunoglobulins) are present in EG and their expression is induced by pro-inflammatory stimuli. EG provides a repository for diverse biologically active molecules, as it incorporates and interacts with extracellular superoxide dismutase (SOD), xanthine oxidoreductase, several interleukins [(ILs), including IL-2–IL-5, IL-7, IL-8 and IL-12], low-density lipoprotein (LDL) and LDL lipase, fibroblast growth factor, vascular endothelium growth factor, transforming growth factor β and several regulators of coagulation (such as antithrombin III, heparin cofactor II and tissue pathway factor inhibitor) [10].
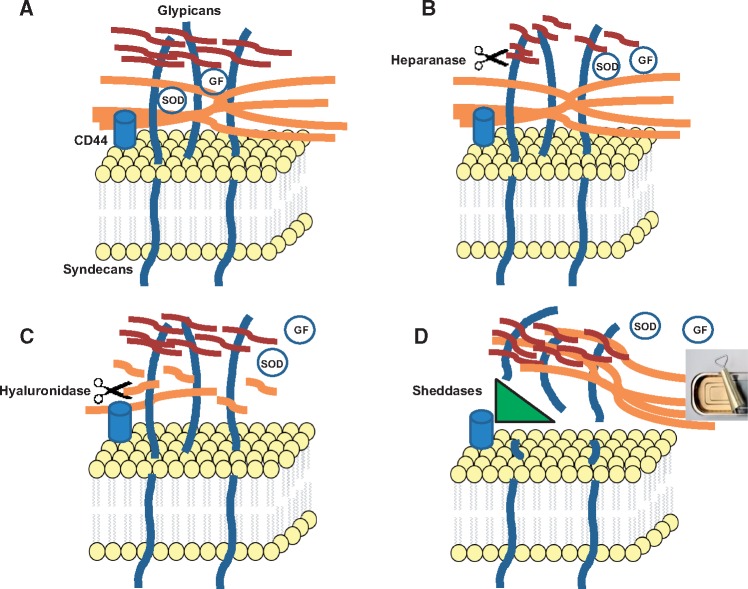
FIGURE 1.
Scenarios for degradation of EG. (A) A simplified schema of EG. HA (orange); heparan sulfate (red; dermatan and chondroitin sulfates are not shown for clarity). Note that diverse proteins are nested in EG, as those shown, as well as albumin and others (see text). GF, growth factors. (B) Heparanase-induced fragmentation of HS results in a partial degradation of EG. (C) Hyaluronidase-induced breakdown of HA chains results in a partial degradation of EG and an increase in permeability. (D) Sheddases cleave proteoglycans, syndecans and glypicans, resulting in peeling off of the entire EG structure. It should be noted that derangements in each component of EG lead in time to disorganization of other components. Hence, the schema depicts only the initial action of EG-degrading enzymes. Nonetheless, this latter scenario based on the action of sheddases results in a most severe and complete degradation of EG (akin to opening a can).
Due to its unique location, this structure provides a passive barrier to water and solute transport (regulation of vascular permeability) and to the interaction between circulating cells and endothelial cells (regulation of leukocyte trafficking). It also serves as a sensor of mechanical forces, such as shear stress and pressure, and shields cell surface receptors, preventing their hyperactivation [11, 12]. However, this structure is quite vulnerable and tends to disintegrate under the influence of various stressors, such as endotoxins, ischemia/hypoxia/reperfusion and oxidative stress, among others. It also leads to hyperactivation of plasma membrane receptors left exposed to respective unhindered ligands, with further activation of endothelial cells and propagation of danger signaling. The degradation of EG is also accompanied by compromised anticoagulant properties of this layer, increased endothelial permeability, reduced antioxidant barrier, enhanced transmigration of pro-inflammatory cells, impaired mechanotransduction and endothelial nitric oxide synthase activity.
Glycocalyx remains poorly studied due to difficulties in preserving its structure upon tissue fixation and the limited number of experimental tools for its visualization. Therefore, there is no confidently charted time course of damage and disintegration for EG. Some studies detect these changes at 6 h post-insult, others at 20 min [13, 14]. Yet, the precise chronology of the loss of EG is of critical significance, as it determines whether and to what extent the loss of glycocalyx is involved in the early pathogenic steps of systemic inflammatory response, its maintenance, or both. Our recent use of stochastic optical reconstruction microscopy (STORM) has made it possible to visualize this structure at high resolution and lend support to the idea that EG is degraded very early in the course of a sepsis model.
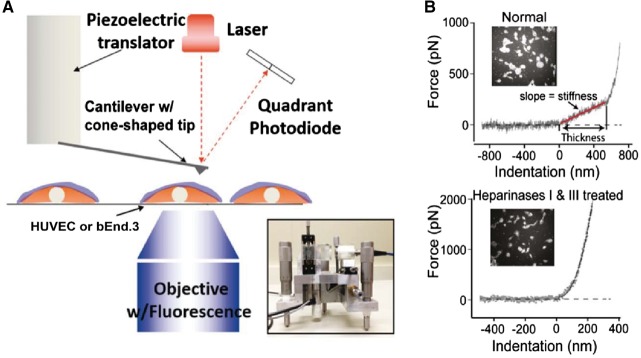
STORM employs organic dyes and fluorescent proteins as photoswitchable emitters to trade temporal resolution for a super spatial resolution (∼20 nm lateral and ∼50 nm axial at present), which is an order of magnitude greater than conventional confocal microscopy. In addition, atomic force microscopy (AFM), in accordance with the Oberleithner’s pioneering work [9], offers an ex vivo tool to study EG, as briefly detailed below. We have performed AFM nanoindentation on living human umbilical vein endothelial cells (HUVECs), as schematically detailed in Figure 2. Light microscopy was used to ensure that the AFM tip was located neither at the nuclear nor at the junctional region of cultured ECs. In some experiments, the cells were stained with wheat germ agglutinin-fluorescein isothiocyanate (FITC) to simultaneously monitor the intactness of EG (Figure 2B, insets). During the indentation scan, the AFM tip travels vertically towards the cell surface. Upon indentation of the EG, the AFM cantilever, serving as a soft spring, is deflected (boxed region in Figure 2B, upper panel). The cantilever deflection is measured and plotted as a function of the sample position along the z-axis. The resulting curve is transformed into a force-versus-indentation curve using the cantilever’s spring constant and the light lever sensitivity. The slope of a force indentation curve directly reflects the stiffness (expressed in pN/nm), which is necessary to indent the EG for a certain distance. The first slope indicates the stiffness (in this trace 0.30 pN/nm) of the very first layer encountered by the cantilever, which is the EG. The second nonlinear region indicates the stiffness of the plasma membrane. In agreement with studies from Oberleithner’s group [9, 15], we found that EG behaves as a linear spring, resulting in a single fixed stiffness value. The distance between the starting point of EG indentation and the starting point of the second slope (projected to the x-axis) corresponds to the thickness of the EG (in this trace ∼550 nm). On average, EG on HUVECs has a thickness of 651 ± 54 nm and a stiffness of 0.25 ± 0.07 pN/nm. Treatment of HUVECs with heparanases I and III eliminates the linear spring region of the force-indentation curve, indicating that the EG is degraded by enzymatic digestion.
FIGURE 2.
AFM nanoindentation. (A) A schematic diagram of the AFM assay. Insert: a picture of the AFM head used for nanoindentation. The blue layer on the cell surface indicates the ESG. (B) Quantification of the thickness and stiffness of EG by AFM nanoindentation.
VERY EARLY EVENTS IN SEPSIS: STRESS-INDUCED EXOCYTOSIS OF LYSOSOME-RELATED ORGANELLES AND PATCHY LOSS OF EG
Damage to and loss of EG have been observed in many diseases, including diabetes, ischemia, myocardial infarction, chronic infections, atherosclerosis and tumor metastases, among others [10, 11, 16, 17]. The disintegration of this structure predisposes to leukocyte adhesion, emigration and tissue infiltration by polymorphonuclear leukocytes, monocyte/macrophages and lymphocytes. Degradation of EG at the sites of exocytosis of lysosome-related organelles occurs very early in sepsis, as detailed below.
The mechanisms responsible for the loss of EG have not been exhaustively studied. Two scenarios have been proposed. Schmidt et al. [13] proposed that its shedding occurs as a result of liberation of heparanase. These studies, in our view, disclose one part of the problem, namely degradation of heparan sulfate moieties, which have been shown [18] to be dispensable for the maintenance of the barrier properties of EG. Annecke et al. [14] proposed that the loss of EG is initiated by the purine metabolites–induced degranulation of resident mast cells and release of tryptase β acting as a sheddase. The instantaneous dilution of tryptase β in the bloodstream would, however, reduce the impact of this factor on EG. Hence, though elegant and convincing, neither explanation appears to be overarching.
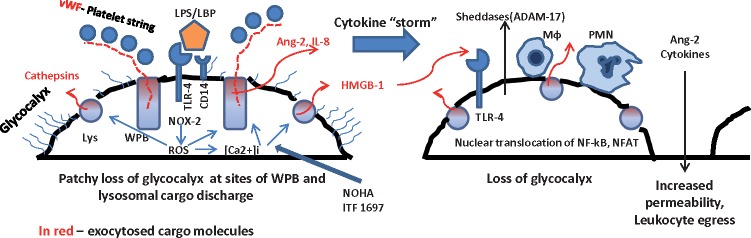
Among the earliest responses of endothelial cells to stressful activators are exocytosis of Weibel–Palade bodies (WPBs) and secretory lysosomes [19]. WPBs are rod-shaped organelles (0.2 × 2–3 µm in size) characteristic to endothelial cells and containing an array of proteins, peptides and cytokines, which can be released emergently on demand. Among these biologically active compounds are von Willebrand factor (vWF), P-selectin, IL-8, endothelin 1 and its converting enzyme, angiopoietin 2, fucosyltransferrase and others, which contribute to the induction of a pro-inflammatory milieu on the one hand, but also induce mobilization of stem cells on the other. A list of compounds known to induce exocytosis of WPBs includes thrombin, histamine, peptido-leukotrienes, complement components, superoxide anion, vascular endothelial growth factor (VEGF), sphingosine-1-phosphate, ceramide, purine nucleotides, serotonin, vasopressin, epinephrine and calcium ionophores [20–22]. The endothelial lysosomes contain acid/secretory sphingomyelinase, glycohydrolases, cathepsins, fucosidase, phosphatases and heparansulfate sulfatase, among others [23].
The mechanisms of exocytosis of lysosome-related organelles, such as WPBs and secretory lysosomes, and release of their lytic cargo have been partially elucidated. These ‘unconventional secretory pathways’ [24] include organellar carriers. WPBs or lysosomes dock to the plasma membrane via the soluble N-ethylmaleimide sensitive factor attachment protein receptor (SNARE) mechanism, undergo priming and Ca-dependent fusion with the plasma membrane to release their cargo [20]. N-ethylmaleimide sensitive factor (NSF) and its adaptor synaptosome-associated protein (α-SNAP) interact with SNARE proteins to prime organelles for fusion and/or recycling. This process can be inhibited by S-nitrosylation of NSF occurring as a result of nitric oxide action [25]. The b-helix-loop-helix transcription factor EB that specifically recognizes E-box sequence 5’-CANNTG-3’ [bHLH-leucine zipper transcription factor EB (TFEB)] increases the pool of lysosomes in the proximity of the plasma membrane and promotes their fusion by a calcium-dependent mechanism through activation of lysosomal non-selective cation channel mucolipin-1 (MCOLN 1) [26]. Another source of LPS-induced elevated calcium in endothelial cells is a stromal interaction molecule-1 (STIM-1)-mediated opening of Orai calcium channels and refilling of the endoplasmic reticulum. Blockade of STIM-1 or NOX-2 [an NADPH oxidase activated by toll-like receptor 4 (TLR4)] limits LPS-induced vascular inflammation [27]. This mechanism explains the inhibitory effect of preventing the elevation of intracellular calcium on exocytosis of lysosomes and WPB. Activation of TFEB has been shown to recruit lysosomal glycohydrolases β-hexosaminidase and β-galactosidase to the plasma membrane [28] and cleave oligosaccharide chains of glycosphingolipids. Matsushita et al. [29] developed fusion polypeptides composed of a 22-amino acid NSF, a regulator of exocytosis, and a carrier peptide derived from the human immunodeficiency virus transactivating regulatory protein domain and demonstrated that inhibition of WPB exocytosis decreased leukocyte trafficking and peritonitis [30]. N-ethylmaleimide itself is also a potent inhibitor of exocytosis. de Leeuw et al. [31] demonstrated that a small GTP-binding protein, Ral, is involved in exocytosis of WPBs and that expression of a dominant-negative Ral variant prevented it. More recently, Bertuglia et al. [32] explored a lysine-proline motif encountered in several biologically active small peptides and synthesized a glycine-(Nα-Et)lysine-proline-arginine (ITF 1697) peptide, with a biological half-life of 20–120 min, and demonstrated that it inhibits ischemia–reperfusion-induced exocytosis of WPBs and protected pulmonary microcirculation by preventing an increase in permeability, leukocyte and platelet adhesion, P-selectin and vWF secretion. This peptide has in its core the Lys-Pro-motif found in tuftsin (a γ-globulin-derived peptide stimulator of phagocytosis), a C-terminal region of α-melanocyte-stimulating hormone endowed with anti-inflammatory properties and an IL-1β-derived peptide antagonist of hyperalgesic effect of the parent molecule. We reported the use of ITF 1697 peptide in an ischemia–reperfusion model of renal injury, confirmed its inhibitory action on exocytosis of WPBs and demonstrated that the peptide salvages renal function and morphology after renal ischemic injury [33]. Importantly, prevention of exocytosis of lysosome-related organelles using nitric oxide intermediate donor NG-hydroxy-L-arginine (NOHA) results in improved survival in severe experimental sepsis [9].
Based on these studies, we have recently proposed an additional possible explanation for the stress-induced loss of EG, namely, that exocytosis of WPBs and secretory lysosomes containing proteolytic, hydrolytic and glycolytic enzymes is responsible for the initiation of EG degradation. We have also predicted that this exocytotic mechanism could be responsible for the shedding of syndecans and CD44, further degrading EG. Specifically, exocytosis of WPBs and lysosomes, both very early responses of endothelial cells to endotoxin and oxidative stress, result in the focal degradation of EG, as detected using STORM [9] (Figure 3 and schema in Figure 4). This very early event hair-triggers an avalanche of secondary pathological processes, such as attraction of leukocytes and platelets, thrombosis and increased vascular permeability. In addition, exocytosed cargo directly or indirectly (via attracted leukocytes and platelets) activate a coagulation cascade and sheddases, like disintegrin and metalloproteinase domain-containing-17 (ADAM-17), thus potentially leading to shedding of CD44 and syndecans, critical structural components of EG (Figure 1B–D). This catapults further propagation of EG loss and escalation of septic damage to the endothelium. The proof of this hypothesis was obtained through the demonstration of two key facts, namely, (i) that exocytosis of WPBs and lysosomes does indeed trigger the initial loss of EG and (ii) that maneuvers directed toward preservation of EG, such as peptide inhibitors of exocytosis, blockers of calcium transients, nitric oxide donors and/or hyaluronate-hydrogel-embedded stem cells, improve the course of sepsis and overall survival [9].
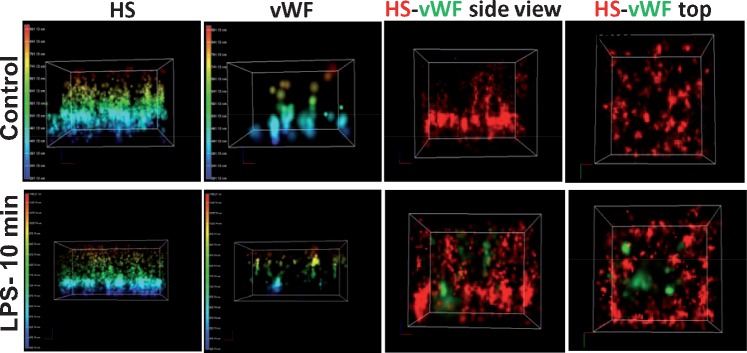
FIGURE 3.
Representative STORM-acquired images of anti-HS-labeled EG and vWF distribution in controls and 10 min after LPS. The panels show three-dimensional reconstructed images of cultured endothelial cells stained with antibodies against HS and vWF. Note that preparations were nonpermeabilized so that vWF readily detectable after LPS is located extracellularly. Images display the height of EG and detectability of vWF in controls and 10 min after application of LPS (left panels) and side or top views. Note the readily detectable appearance of vWF 10 min after LPS, where it encroaches into the EG. The sites of detectable vWF also appear to be depleted of HS.
FIGURE 4.
Schematic representation of a very early patchy loss of EG followed by the propagating innate immune response–driven global loss of EG. Exocytosed cargo molecules are represented in red.
EG DEGRADATION IN AKI AND DIABETIC NEPHROPATHY
The above mechanisms of EG degradation are ignited not only in the initiation of the systemic inflammatory response, like in sepsis, but they are active in a variety of pathological conditions. In AKI induced by ischemia–reperfusion and/or kidney transplantation, EG is impaired both in experimental animals and in humans [34–37]. Another most frequent condition associated with the degradation of EG is diabetic nephropathy. Deckert et al. [38] observed that the de novo synthesis of heparan sulfate was reduced in fibroblasts isolated from diabetes patients with albuminuria, but not from those without albuminuria or healthy control subjects, and formulated a hypothesis that the loss of EG is a prerequisite for developing diabetic nephropathy. Recently, upregulation of endothelin 1 in diabetes was incriminated in the induction of heparanase in podocytes, resulting in impairment of glomerular EG [39]. This is in agreement with studies by different investigators who have demonstrated the loss of glycocalyx integrity [40–43]. In addition to this, defective EG is seen in multiple other conditions characterized by endothelial dysfunction. We have recently observed J.W. Song, unpublished data) that mice with catalytically defective sirtuin 1 in endothelial cells alone, apart from being characterized by premature vascular senescence, impaired vasorelaxation and angiogenesis and a propensity towards fibrogenesis, also exhibit a 50% reduction in the global volume of EG. In fact, the mechanisms of EG loss, such as oxidative stress or pro-inflammatory cyto- and chemokines, are highly prevalent in diverse pathologic states. Under those circumstances, the loss of EG is not as fulminant as in sepsis, but much more insidious. This fact explains why preventive measures of EG degradation are insufficient and in clinical practice it would be more useful to obtain pharmacological tools to facilitate the restoration of EG.
In all the cases described above, exocytosis of lysosome-related organelles actuates the initial localized damage to the EG that exceeds the constitutive recycling of lysosomes and EG. The localized loss of EG should be reparable under normal conditions. Yet, the pro-inflammatory milieu and persistence of noxious stimuli aggravate the initial injury to EG and propagate it to the systemic circulation. These hypothetical scenarios are schematically depicted in Figure 4.
FACILITATING GLYCOCALYX RESTORATION
Natural restoration of EG is sluggish. It has previously been determined that after degradation of EG with either hyaluronidase, heparanase III or tumor necrosis factor α the restoration of hydrodynamically relevant EG in vivo requires ∼7 days [44]. Attempts to accelerate EG restoration have been entertained. Administration of antioxidants, like N-acetylcysteine [45], was used to prevent EG shedding during hyperglycemia. However, considering the fact that SOD is heavily intercalated in the EG, one is left wondering whether the effects of antioxidants are really preventive or rather do they act when EG has been degraded and it endogenous SOD barrier has been lost. An alternative strategy is represented by systemic use of high molecular weight HA. Another uses heparan sulfate or its analog, sulodexide (an 8:2 mixture of fast-moving heparin fraction and dermatan sulfate [46]) to improve EG in diabetes [47]. In in vitro studies we have tested the efficacy of various components of EG restoration and observed a marginal efficacy of HA, HS or inhibitors of their degradation and improved efficacy of a cocktail containing all of these compounds (not shown). However, superior efficacy in restoring EG was observed using sulodexide. Taking into account the organismal size of the HA pool (∼15 g, which is mostly contained on the cell surface and the extracellular matrix [48, 49]) and the rapidity of its exchange [50], it is difficult to imagine that HA availability could be a limiting factor for the rate of EG restoration. Yet, studies by Henry and Duling [48] have demonstrated that supplemental infusion of HA and chondroitin sulfate accelerates the restoration of EG. With the serum concentration of HS being >100 µg/ml in humans [51], it would require significant prior exhaustion of the pool of HS to account for the salutary effect of supplemental HS. These arguments should provoke the idea that the restoration of EG is dependent mostly on the activity of enzymes participating in its degradation. There are six different hyaluronidases [52] and their activity is induced in a number of diseases [53]. Heparanase activity is similarly induced by inflammatory conditions (reviewed in [54]), which accounts for the accumulation of HS fragments. The different scenarios of EG degradation are summarized in Figure 1B–D. While the degradation of HS and HA alone would appear to result in the incomplete erasure of EG (Figure 1B and C), the action of sheddases results in desquamation of the sheets of EG; these, perhaps, may be flapping when incompletely detached and float in the bloodstream when completely detached (Figure 1D).
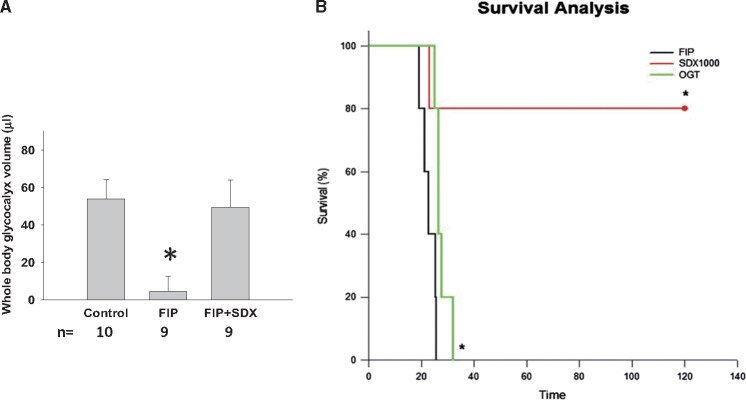
We hypothesize that superior results of EG restoration could be achieved by combining components or surrogates of EG with inhibitors of their respective degradation enzymes—hyaluronidase and heparanase. Such a prediction is supported by the rapid kinetics of the basal rate of degradation and sluggish restoration of EG components. For instance, the rate of hyaluronan synthase production of HA approximates 1 molecule/5 min [55] and the entire pool of HA in the body equals 15 g and is exchanged every 3 days [49]. Only a combination of components of EG with hyaluronidase + heparanase inhibitors could efficiently accelerate the restoration of EG and its key components. Unfortunately, there are no inhibitors approved for humans. There are multiple analogs of commercially available (−)-Epicatechin, a nonselective HA-ase inhibitor. It has been proposed that a polyketide originating from marine Streptomyces species, hyaluromycin, is a selective hyaluronidase inhibitor [56]. On the other hand, PI-88 has been introduced as a potent inhibitor of heparanase, although its availability to investigators seems to be quite limited [57]. This leaves sulodexide, which combines the properties of EG components with matrix metalloproteinase–inhibitory and heparanase-inhibitory activities, as the only currently available and approved compound for clinical use. Its uses in diabetic nephropathy have been described previously [58–60]. Below, we recount our own experience using sulodexide in mice with polymicrobial sepsis and loss of EG [61]. For these in vivo studies, we elected to use the indicators dilution technique, combining one fluorophore that percolates EG (dextran 40–Texas Red) with the fluorophore that does not percolate (dextran 500–FITC). We elected the soilage model of polymicrobial sepsis and showed that sulodexide treatment instituted after the induction of sepsis results in a significantly improved restoration of EG (Figure 5), both using the volume of distribution fluorophore dilution technique and en face aorta staining. In these experiments, sulodexide administered 2 h after induction of sepsis resulted in a dramatic restoration of total body glycocalyx 16 h later. Similar results were obtained using the LPS model of sepsis. This accelerated restoration of EG was associated with the amelioration of increased vascular permeability measured in pulmonary and peritoneal vascular beds. Additionally, our AFM nanoindentation data [61] confirmed that treatment of the damaged EG with sulodexide restored the thickness of EG.
FIGURE 5.
(A) Whole-body EG volume and (B) survival of mice with the soil model of sepsis (FIP) with and without treatment with sulodexide. *P < 0.05. FIP, fecal-induced peritonitis; SDX, sulodexide; OGT, heparanase inhibitor OGT 2115.
To evaluate the end effect of improved restoration of EG on survival, we video-monitored animal activity in cages and derived survival curves. There was a remarkable improvement in survival of septic mice treated with 40 mg/kg sulodexide compared with vehicle [hazard ratio 0.11 (95% confidence interval) 0.02–0.59; P = 0.015] as judged by Kaplan–Meyer survival curves (Figure 5). This effect was not secondary to any interference with the growth or virulence of bacterial flora, but was accompanied by the improved glycocalyx of neutrophils (not shown). This latter finding is in accord with the previously demonstrated interaction between the glycocalyx of endothelial cells and erythrocytes when one of these cellular partners has lost glycocalyx integrity [62]. The glycocalyx in neutrophils is lost in septic mice and, similar to the findings in endothelial cells, sulodexide treatment facilitates its restoration. These findings raise the possibility that damaged EG affects the glycocalyx of all circulating formed elements. This would result in the increased egress of leukocytes, as well as hemolysis and dysmorphic changes in erythrocytes, both phenomena actually observed in severe sepsis.
CONCLUSIONS
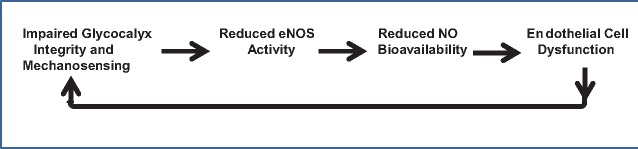
In summary, focusing on the integrity of EG, we set out to elucidate several tacit points related to the initiation and maintenance of sepsis, sepsis-associated renal failure or contributory to other renal diseases. We have provided evidence buttressed by the STORM-acquired images that implicates exocytosis of lysosome-related organelles in the very early occurring (within minutes) crater-like patchy loss of EG at the sites of release of their cargo. Parenthetically, considering the constitutive physiological exocytosis of these organelles, clearly EG is being perpetually remodeled. The distinction from sepsis, however, consists of the ongoing oxidant stress and pro-inflammatory environment leading to the spike in exocytosis and EG degradation. An intermediate of nitric oxide synthesis, NG-hydroxy-l-arginine, is able to contain exocytosis and ameliorate EG degradation in sepsis. This alone is accompanied by the improved survival of mice with lethal sepsis. The second EG-related issue, namely, therapeutic acceleration of its recovery after a massive degradation, was addressed here using a compound, sulodexide, that is clinically approved for human use. It has been previously used to restore EG in diabetic nephropathy with variable success. In the case of severe sepsis, however, we observe accelerated restoration in the whole-body EG volume and a remarkable improvement in the animals’ survival after administration of sulodexide. These two subjects may not only have a broader disease applicability, but they also provide additional evidence to buttress the idea of the importance of EG and its maintenance and repair in the prevention and treatment of vascular complications of renal and nonrenal diseases. Specifically, studies of EG and endothelial dysfunction prompt us to speculate on the progressive nature of both, when they coexist: damage to EG leads to uncoupling of endothelial nitric oxide synthase and a reduction in bioavailable nitric oxide with development or aggravation of endothelial dysfunction, which in turn provides a positive feedback to continuing degradation of EG, as depicted in Figure 6. This speculative sequence has significant indirect support, which surely will be expanded in the years to come.
FIGURE 6.
A hypothetical view of the events linking and perpetuating the pathology of EG and endothelial dysfunction.
FUNDING
The studies were supported in part by National Institutes of Health grants DK54602, DK052783, DK45462 (M.S.G.) and NIH SC1CA153325 (BMF); the New York Trust Fund (M.S.G.); the Dr. Werner Jackstaedt Foundation (M.L.); the ‘ILJIN’ Faculty Research Assistance Program of Yonsei University College of Medicine for 2013 (6-2013-0068) and the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning (NRF-2013R1A1A1010863) (J.W.S.).
REFERENCES
- 1. Aird W. The role of the endothelium in severe sepsis and multiorgan dysfunction syndrome. Blood 2003; 101: 3765–3777 [DOI] [PubMed] [Google Scholar]
- 2. Morrison D, Ulevitch R.. The effect of bacterial endotoxins on host mediation systems: a review. Am J Pathol 1978; 93: 526–617 [PMC free article] [PubMed] [Google Scholar]
- 3. Ratliff B, Rabadi M, Vasko R. et al. Messengers without borders: mediators of systemic inflammatory response in acute kidney injury. J Am Soc Nephrol 2013; 24: 529–536 [DOI] [PubMed] [Google Scholar]
- 4. Prowle J, Molan M, Hornsey E. et al. Measurement of renal blood flow by phase-contrast magnetic resonance imaging during septic acute kidney injury. A pilot investigation . Crit Care Med 2012; 40: 1768–1776 [DOI] [PubMed] [Google Scholar]
- 5. Wu L, Tiwari M, Messer K. et al. Peritubular capillary dysfunction and renal tubular epithelial cell stress following lipopolysaccharide administration in mice. Am J Physiopl Renal 2006; 292: F2621–FF268 [DOI] [PubMed] [Google Scholar]
- 6. Seely K, Holtoff J, Burns S. et al. Hemodynamic changes in the kidney in a pediatric rat model of sepsis-induced acute kidney injury. Am J Physiol Renal Physiol 2011; 301: F209–F217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chelazzi C, Villa G, Mancinelli P. et al. Glycocalyx and sepsis-induced alterations in vascular permeability. Crit Care 2015; 19: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wiesinger A, Peters W, Chappell D. et al. Nanomechanics of the endothelial glycocalyx in experimental sepsis. PloS One 2013; 8: e80905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zullo J, Fan J, Azar T. et al. Exocytosis of endothelial lysosome-related organelles hair-triggers a patchy loss of glycocalyx at the onset of sepsis. Am J Pathol 2016; 186: 248–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reitsma S, Slaaf D, Vink H. et al. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch 2007; 454: 345–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fu BM, Tarbell JM.. Mechano-sensing and transduction by endothelial surface glycocalyx: composition, structure and function. Wiley Interdiscip Rev Syst Biol Med 2013; 5: 381–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Becker B, Chappell D, Jacob M.. Endothelial glycocalyx and coronary vascular permeability: the fringe benefit. Basic Res Cardiol 2010; 105: 687–701 [DOI] [PubMed] [Google Scholar]
- 13. Schmidt E, Yang Y, Janssen W. et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med 2012; 18: 1217–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Annecke T, Fischer J, Hartmann H. et al. Shedding of the coronary endothelial glycocalyx: effects of hypoxia/reoxygenation vs ischemia/reperfusion. Br J Anaesth 2011; 107: 679–686 [DOI] [PubMed] [Google Scholar]
- 15. Fels J, Jeggle P, Liashkovich I. et al. Nanomechanics of vascular endothelium. Cell Tissue Res 2014; 355: 727–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cai B, Fan J, Zeng M. et al. Adhesion of malignant mammary tumor cell MDA-MB-231 to microvessel wall increases microvascular permeability via degradation of endothelial surface glycocalyx. J Appl Physiol 2012; 13: 1141–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chappell D, Heindl B, Jacob M. et al. Sevoflurane reduces leukocyte and platelet adhesion after ischemia-reperfusion by protecting the endothelial glycocalyx. Anesthesiology 2011; 115: 483–491 [DOI] [PubMed] [Google Scholar]
- 18. Zeng Y, Ebong EE, Fu BM. et al. The structural stability of the endothelial glycocalyx after enzymatic removal of glycosaminoglycans. PLoS One 2012; 7: e43168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuo MC, Patschan D, Patschan S. et al. Ischemia-induced exocytosis of Weibel-Palade bodies mobilizes stem cells. J Am Soc Nephrol 2008; 19: 2321–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lowenstein C, Morrell C, Yamakuchi M.. Regulation of Weibel-Palade body exocytosis. Trends Cardiovasc Med 2005; 15: 302–308 [DOI] [PubMed] [Google Scholar]
- 21. Rondaij M, Bierings R, Kragt A. et al. Dynamics and plasticity of Weibel-Palade bodies in endothelial cells. Atheroscl Thromb Vasc Biol 2006; 26: 1002–1007 [DOI] [PubMed] [Google Scholar]
- 22. Goligorsky MS, Patschan D, Kuo MC.. Weibel-Palade bodies—sentinels of acute stress. Nat Rev Nephrol 2009; 5: 423–426 [DOI] [PubMed] [Google Scholar]
- 23. Simons K, Gruenberg J.. Jamming the endosomal system: lipid rafts and lysosomal storage diseases. Trends Cell Biol 2000; 10: 459–462 [DOI] [PubMed] [Google Scholar]
- 24. Zhang M, Schekman R.. Unconventional secretion, unconventional solutions. Science 2013; 340: 559–561 [DOI] [PubMed] [Google Scholar]
- 25. Matsushita K, Morrell C, Camblen B. et al. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell 2003; 115: 139–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Medina D, Fraldi A, Bouche V. et al. Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev Cell 2011; 21: 421–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gandhirajan R, Meng S, Chandramurthy H. et al. Blockade of NOX2 and STIM1 signaling limits LPS-induced vascular inflammation. J Clin Invest 2013; 123: 887–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Magini A, Polchi A, Urbanelli L. et al. TFEB activation promotes the recruitment of lysosomal glycohydrolases β-hexosaminidase and β-galactosidase to the plasma membrane. Biochem Biophys Res Commun 2013; 440: 251–257 [DOI] [PubMed] [Google Scholar]
- 29. Matsushita K, Morrell C, Loewenstein C.. A novel class of fusion polypeptides inhibits exocytosis. Mol Pharmacol 2005; 67: 1137–1144 [DOI] [PubMed] [Google Scholar]
- 30. Morrell C, Matsushita K, Lowenstein C.. A novel inhibitor of N-ethyl-maleimide-sensitive factor decreases leukocyte trafficking and peritonitis. J Pharmacol Exp Ther 2005; 314: 155–161 [DOI] [PubMed] [Google Scholar]
- 31. de Leeuw H, Fernandez-Borja M, Reits E. et al. Small GTP-binding protein Ral modulates regulated exocytosis of von Willebrand factor by endothelial cells. Arterioscler Thromb Vasc Biol 2001; 21: 899–904 [DOI] [PubMed] [Google Scholar]
- 32. Bertuglia S, Ichimura H, Fossati G. et al. ITF 1697, a stable Lys-Pro-containing peptide, inhibits Weibel-Palade body exocytosis induced by ischemia/reperfusion and pressure elevation. Mol Med 2007; 13: 615–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yasuda K, Vasko R, Hayek P. et al. Functional consequences of inhibiting exocytosis of Weibel-Palade bodies in acute renal ischemia. Am J Physiol Renal Physiol 2012; 302: F713–F721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chappell D, Jacob M, Hofmann-Kiefer K. et al. Anthithrombin reduces shedding of the endothelial glycocalyx following ischemia/reperfusion. Cardiovasc Res 2009; 83: 388–396 [DOI] [PubMed] [Google Scholar]
- 35. Mulivor A, Lipowsky H.. Inflammation and ischemia-induced shedding of venular glycocalyx. Am J Physiol Heart Circ Physiol 2004; 286: H1672–H1680 [DOI] [PubMed] [Google Scholar]
- 36. Platta S, Linden J, Duling B.. Rapid modification of glycocalyx caused by ischemia-reperfusion is inhibited by adenosine 2A2 receptor activation. Am J Physiol Heart 2003; 284: H2360–H2367 [DOI] [PubMed] [Google Scholar]
- 37. Shoeijs M, Vink H, Voesten N. et al. Acute ischemic injury to the renal microvasculature in human kidney transplantation. Am J Physiol Renal Physiol 2010; 299: F1134–F1140 [DOI] [PubMed] [Google Scholar]
- 38. Deckert T, Horowitz I, Kofoed-Enevoldsen A. et al. Possible genetic defects in regulation of glycosaminoglycans in patients with diabetic nephropathy. Diabetes 1991; 40: 764–770 [DOI] [PubMed] [Google Scholar]
- 39. Garsen M, Lenoir O, Rops A. et al. Endothelin-1 induces proteinuria by heparanase-mediated disruption of the glomerular glycocalyx. J Am Soc Nephrol 2016; 27: 3545–3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Krankel N, Adams V, Linke A. et al. Hyperglycemia reduces survival and impairs function of circulating blood-derived progenitor cells. Arterioscler Thromb Vasc Biol 2005; 25: 698–703 [DOI] [PubMed] [Google Scholar]
- 41. Myrup B, Hansen P, Jensen T. et al. Effect of low-dose heparin on urinary albumin excretion in insulin-dependent diabetes mellitus. Lancet 1995; 345: 421–422 [DOI] [PubMed] [Google Scholar]
- 42. Nieuwdorp M, Meuwese MC, Vink H. et al. The endothelial glycocalyx: a potential barrier between health and vascular disease. Curr Opin Lipidol 2005; 16: 507–511 [DOI] [PubMed] [Google Scholar]
- 43. Nieuwdorp M, Mooij HL, Kroon J. et al. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes 2006; 55: 1127–1132 [DOI] [PubMed] [Google Scholar]
- 44. Potter D, Jiang J, Damiano E.. The recovery time course of the endothelial cell glycocalyx in vivo and its implications in vitro. Circ Res 2009; 104: 1318–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van den Berg B, Nieuwdrop M, Stroes E. et al. Glycocalyx and endothelial (dys)function: from mice to men. Pharmacol Rep 2006; 58(Suppl 58): 75–80 [PubMed] [Google Scholar]
- 46. Coccheri S, Mannello F.. Development and use of sulodexide in vascular diseases: implications for treatment. Drug Des Devel Ther 2014; 8: 49–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Broekhuizen L, Lemkes B, Mooij H. et al. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia 2010; 53: 2646–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Henry C, Duling B.. Permeation of the luminal capillary glycocalyx is determined by hyaluronan. Am J Physiol 1999; 277: H508–H514 [DOI] [PubMed] [Google Scholar]
- 49. Lennon F, Singleton P.. Hyaluronan regulation of vascular integrity. Am J Cardiovasc Dis 2011; 1: 200–213 [PMC free article] [PubMed] [Google Scholar]
- 50. Fraser J, Laurent T, Laurent U.. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med 1997; 242: 27–33 [DOI] [PubMed] [Google Scholar]
- 51. Yokoyama H, Sato K, Okudaira M. et al. Serum and urinary concentration of heparan sulfate in patients with diabetic nephropathy. Kidney Int 1999; 56: 650–658 [DOI] [PubMed] [Google Scholar]
- 52. Stern R, Asari A, Sugahara K.. Hyaluronan fragments: an information-rich system. Eur J Cell Biol 2006; 85: 699–715 [DOI] [PubMed] [Google Scholar]
- 53. Girish K, Kemparaju K.. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci 2007; 80: 1921–19437 [DOI] [PubMed] [Google Scholar]
- 54. Dane M, van der Vlag J, Vink H. et al. A microscopic view of the renal endothelial glycocalyx. Am J Physiol Renal Physiol 2015; 308: F956–F966 [DOI] [PubMed] [Google Scholar]
- 55. Itano N, Sawai T, Yoshida M. et al. Three isoforms of mammalian hyaluronan synthasees have distinct enzymatic properties. J Biol Chem 1999; 274: 25085–25092 [DOI] [PubMed] [Google Scholar]
- 56. Harunari E, Imada C, Igarashi Y. et al. Hyaluromycin, a new hyaluronidase inhibitor of polyketide origin from marine Streptomyces sp. Mar Drugs 2014; 12: 491–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ziolkovski A, Popp S, Freeman C. et al. Heparan sulfate and heparanase play key roles in mouse β-cell survival and autoimmune diabetes. J Clin Invest 2012; 122: 132–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Salmon A, Satchell S.. Endothelial glycocalyx dysfunction in disease: albuminuria and increased microvascular permeability. J Pathol 2012; 226: 562–574 [DOI] [PubMed] [Google Scholar]
- 59. Masola V, Zaza D, Gambaro G.. Sulodexide and glycosaminoglycans in the progression of renal disease. Nephrol Dial Transplant 2014; 29: 174–179 [DOI] [PubMed] [Google Scholar]
- 60. Padberg J, Weisinger A, di Marco G. et al. Damage of the endothelial glycocalyx in chronic kidney disease. Atherosclerosis 2014; 234: 335–343 [DOI] [PubMed] [Google Scholar]
- 61. Song JW, Zullo JA, Liveris D. et al. Therapeutic restoration of endothelial glycocalyx in sepsis. J Pharmacol Exp Ther 2017; 361: 115–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Oberleithner H. Vascular endothelium leaves “fingerprints” on the surface of erythrocytes. Pflugers Arch 2013; 465: 1451–1458 [DOI] [PubMed] [Google Scholar]