Abstract
In a previous study, the oral administration of an Aloe vera whole leaf extract induced dose-related mucosal and goblet cell hyperplasia in the rat colon after 13 weeks and colon cancer after 2 years. The primary goal of this study was to determine whether or not the administration of aloin, a component of the Aloe vera plant leaf, would replicate the pathophysiological effects that were observed in rats in the previous study with an Aloe vera whole leaf extract. Groups of 10 male F344/N rats were administered aloin at 0, 6.95, 13.9, 27.8, 55.7, 111, 223, and 446 mg/kg drinking water for 13 weeks. At the end of study, rat feces were collected, and the composition of fecal bacteria was investigated by next generation sequencing of the PCR-amplified V3/V4 region of the 16S rRNA gene. At necropsy, blood was collected by cardiac puncture and organs and sections of the large intestine were collected for histopathology. Aloin induced dose-related increased incidences and severities of mucosal and goblet cell hyperplasia that extended from the cecum to the rectum, with increased incidences and severities detected at aloin doses ≥55.7 mg/kg drinking water. Analysis of the 16S rRNA metagenomics sequencing data revealed marked shifts in the structure of the gut microbiota in aloin-treated rats at each taxonomic rank. This study highlights the similarities in effects observed for aloin and the Aloe vera whole leaf extract, and points to a potential mechanism of action to explain the observed pathological changes via modulation of the gut microbiota composition.
Keywords: aloe vera, aloin, gut microbiota, gastrointestinal, systems toxicology, food safety/nutrition, safety evaluation
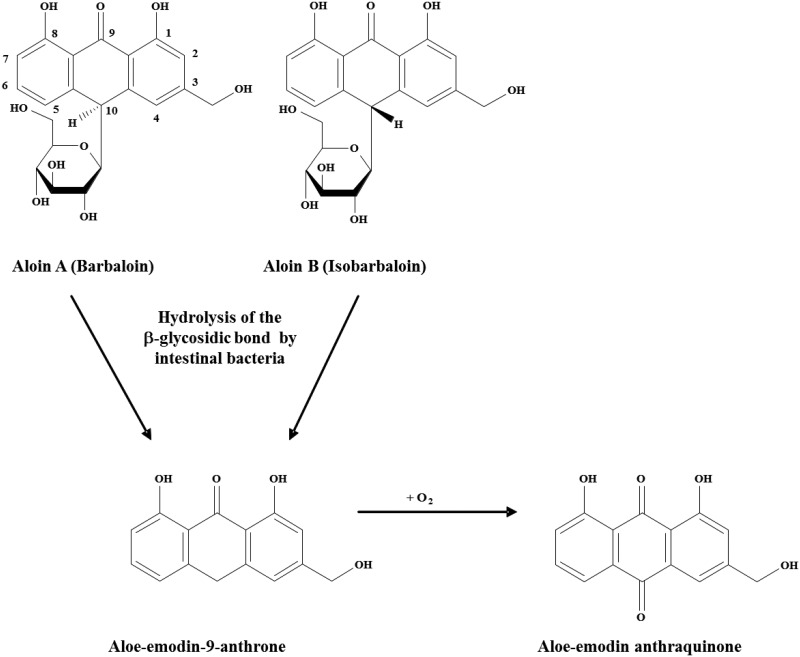
Aloe barbadensis Miller, commonly referred to as Aloe vera, is one of approximately 420 plant species of Aloe and is a popular herbal remedy and dietary supplement (Briggs, 1995; Grindlay and Reynolds, 1986; Klein and Penneys, 1988). Aloes have green fleshy leaves covered by a thick cuticle, an inner clear pulp, termed Aloe vera gel, and pericyclic tubules that store and transport Aloe vera latex along the outer margin of the leaf pulp (Viljoen et al., 2001). The main feature of the Aloe vera plant leaf is its high water content, ranging from 99.0% to 99.5% (Atherton, 1998); however, the remaining 0.5%–1.0% of the plant leaf is reported to contain over 75 potentially bioactive compounds, including phenolic compounds, such as aloin. The anthrone C-glycoside, aloin, is a major component of Aloe vera latex, and it exists as a mixture of diastereoisomers, aloin A and aloin B, also referred to as barbaloin and isobarbaloin (Groom and Reynolds, 1987; Haynes et al., 1970). The sugar moiety in aloin A and aloin B is d-glucose, and studies indicate that the C1 position of d-glucose is linked directly to the C10 position of the anthrone ring in a β-configuration (Smith and Smith, 1851). The β-(1–10) C–C bond is resistant to acid and alkali conditions and to the β-glycosidases of plants and most bacteria; however, certain human and animal intestinal bacteria species are able to cleave the β-C-glycosyl bond, although considerable variation in response occurs among animal species (humans > rabbits > guinea pig > rats ≫ mice) (Che et al., 1991; Franz and Grün, 1983; Hattori et al., 1988; Hay and Haynes, 1956; Joshi, 1998). Aloe vera latex possesses laxative properties, and aloin A and aloin B are the principal agents responsible for the cathartic activities of Aloe vera latex in humans and animals. The laxative activity is not due directly to aloin A and aloin B, but rather to the metabolism of these diastereoisomers by bacteria in the large intestine to a common metabolite, aloe-emodin-9-anthrone (Joshi, 1998; Mapp and McCarthy, 1970), and subsequent oxidation to aloe-emodin, a free anthraquinone (Figure 1). Confirmation of aloe-emodin-9-anthrone as the cathartic agent of Aloe vera latex was accomplished by the intra-cecal administration of aloin A to rats and the subsequent detection of aloe-emodin-9-anthrone in the large intestine, with accompanying diarrhea (Ishii et al., 1994; Westendorf et al., 1990).
Figure 1.
The metabolism of aloin. Chemical structure diagrams are shown for Aloe-vera latex-derived anthrone-C-glycosides, anthrones, and anthraquinones (Boudreau and Beland, 2006).
The gastrointestinal tract (GIT) microbiota plays a key role in host barrier defense mechanisms and in numerous metabolic, physiological, nutritional, and immunological processes (O'Hara and Shanahan, 2006). The GIT is adapted to bi-directional host–microbiota exchange, and this interaction occurs along the epithelial cell mucosal lining of the GIT (O'Hara and Shanahan, 2006). The composition of the gut microbiota reflects natural selection at the host and microbial levels that promotes mutual cooperation and stability within the complex environment of the GIT (O'Hara and Shanahan, 2006). The majority and widest diversity of bacteria in the GIT are found within the large intestine, where they contribute to the metabolism and fermentation of undigested food components, such as complex carbohydrates and fiber (Bäckhed et al., 2005; Conlon and Bird, 2015; Power et al., 2014). As in most mammals, the human gut microbiota is dominated by four phyla (Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria) that represent a relatively stable population of approximately 500 species of bacteria (The Human Microbiome Project Concortium, 2012; Walsh et al., 2014; Wells et al., 1987). Phylogenetic analysis of adult rat gut microbiota indicates that rat and human share similarities in their microbiota at the phylum level (Brooks et al., 2003; Manichanh et al., 2010); the dominant species of bacteria from the rat gut belong to the Firmicutes and Bacteroidetes phyla, and these phyla constitute the majority (60%–90%) of human phylotypes (Ley et al., 2008; The Human Microbiome Project Concortium, 2012). Results from the Human Microbiome Project have shown that thousands of different bacteria may collectively inhabit the human gut and confer a high degree of diversity between individuals; however, consistently lower variability in the bacterial composition of the gut was observed within the same individual over time (The Human Microbiome Project Concortium, 2012). Furthermore, there is a growing recognition that diet and other environmental factors can impact health and disease pathophysiology via modulation in the composition and metabolic activity of the gut microbiota (Lecomte et al., 2015; Nehra et al., 2016; Ojeda et al., 2016; Wong, 2014; Xu and Knight, 2015). Of all environmental factors examined to date, diet has the greatest known impact on the composition and metabolic activity of the gut microbiota (Ojeda et al., 2016; Xu and Knight, 2015).
In a previous 13-week study, an Aloe vera whole leaf extract was administered to F344/N rats and B6C3F1 mice at doses of 1%, 2%, and 3% by weight in their drinking water (Boudreau et al., 2013). The Aloe vera whole leaf extract contained all components of the Aloe vera plant leaf, namely the Aloe vera gel from the inner leaf pulp and the Aloe vera latex from the outer leaf. The contents of aloin A in the dosed water solutions were approximately 125, 250, and 375 mg/kg water for the 1%, 2%, and 3% (wt/wt) dose levels, respectively; aloin B was not measured. The results of the study revealed that animals administered the Aloe vera whole leaf extract had significantly decreased GIT transit times and dose-dependent increased incidences of mucosal and goblet cell hyperplasia in the large intestine, when compared to control animals. In a 2-year drinking water study, an Aloe vera whole leaf extract was administered to rats in their drinking water at doses of 0.5%, 1.0%, and 1.5% by weight. The study results showed that the Aloe vera whole leaf extract induced dose-related increased incidences of adenomas and carcinomas of the rat cecum and large intestine, with male rats showing higher incidences than female rats (Boudreau et al., 2013). The aloin A content was approximately 32, 66, and 98 mg/kg water in the 0.5%, 1.0%, and 1.5% dosed water solutions of Aloe vera whole leaf extract, respectively; aloin B was not measured.
The association between colorectal cancer risk in humans and anthraquinone laxative use remains controversial (Nusko et al., 2000; Willems et al., 2003). Some dihydroxy anthraquinones, such as aloe-emodin, have been shown to have tumor-promoting effects and tumorigenic activities in the large intestine of rodents (de Witt and Lemili, 1990; Mori et al., 1985). Paradoxically, anti-tumorigenic effects are equally well documented (Pan et al., 2013; Tabolacci et al., 2013). The Aloe vera whole leaf extract used in the aforementioned studies contained the Aloe gel from the inner leaf pulp as well as the Aloe vera latex from the outer leaf pulp. We hypothesized that aloin was the single-most important component of the Aloe vera whole leaf extract that could explain the pathology observed in the aforementioned studies and that a potential mechanism of action by aloin may be via modulation of microbiota in the GIT, thereby, altering the host-microbiota exchange. In this manuscript, we report the findings of a 13-week drinking water study to test the effects of aloin exposure on the pathophysiology of the GIT and modulation of the GIT microbiota in male F344/N rats.
MATERIALS AND METHODS
Test article procurement and characterization
Aloin [CAS 1415-73-2] was purchased as a single lot from Pure Chemistry Scientific, Inc. (Sugarland, Texas) and was manufactured by Shanghai Tongxiang Trading Co., Ltd (Shanghai, China). In order to confirm the identity and purity of the test material, reference standards of aloin A (Sigma Aldrich, St Louis, Missouri) and aloin B (Chengdu Biopurify Phytochemicals Ltd, Sichuan, China) were analyzed, along with the aloin test material, by ultra-high performance liquid chromatography coupled to a mass spectrometer (Quattro Premier XE, Waters, Milford, Massachusetts) and a photodiode array detector. The test material showed two major elution peaks, with a mean analytical composition of 55.4% aloin A and 43.3% aloin B. The absorption spectra of both peaks were similar (λmax 355 nm) and corresponded to those of the reference standards. The test material was also subjected to proton nuclear magnetic resonance analysis, using DMSO-d6 as the solvent. The spectrum of the test material was consistent with a 1:1 mixture of aloin A and aloin B. The purity of the test material was estimated to be 98.8%.
The pH of the Aloe vera plant leaf is reported to be 4.4–4.7 (Boudreau and Beland, 2006). Studies have shown that the stability of aloin is temperature- and pH-dependent and that aloin is readily oxidized to form 10-hydroxyaloins A and B, but a temperature of 4 °C or acidic conditions at pH 2–3 ensured excellent stability (Ding et al., 2014). In the present study, water acidified with 3 mM sodium citrate buffer served as the vehicle control, and the stability of aloin was confirmed in the vehicle control solution for a period of up to 10 days at a temperature of 4 °C. This vehicle was used in a previous study and had no discernable effects in rats when compared to water alone (Boudreau et al., 2016).
Dose selection
Due to agricultural practices and environmental conditions, the level of aloin in the Aloe vera plant is quite variable and may constitute 30% or more of the dry matter weight of the aloe latex (Joshi, 1998). The International Aloe Science Council (IASC), a self-regulating trade association that serves the aloe industry, has set a limit of 10 mg aloin/kg (10 ppm) for orally consumed products in their voluntary certification program, based on analyses conducted on solutions that contain 0.5% Aloe vera solids by weight (AHP, 2012; IASC, 2012). There remain a large number of Aloe vera whole leaf extract products outside of the IASC certification program that are readily obtained by consumers from a variety of sources, and the aloin content in these products may be quite variable. The most frequent recommendation for adult intake of Aloe vera whole leaf products on the various websites is 2–8 ounces (approximately 60–240 ml)/per serving and 1–3 servings per day. For IASC-certified Aloe vera products, a maximum intake of 720 ml would result in a daily aloin intake of approximately 7.2 mg.
The doses of aloin selected for study were 0, 6.95, 13.9, 27.8, 55.7, 111, 223, and 446 mg/kg drinking water. The 2-fold aloin dose increases were selected to include a low dose below the analytical maximum of 10 mg/kg for aloin established by the IASC for certified orally consumed products (IASC, 2012) and a high-dose equivalent to the aloin A content (250 mg/kg water) of the 2% (wt/wt) dose of the Aloe vera whole leaf extract (assuming a ratio of 55.4/43.3, aloin A/aloin B) that was used in the previously conducted 13-week study (Boudreau et al., 2013).
Preparation and analysis of dose formulations
Dosed water formulations were prepared on a weekly basis. All doses were prepared by serial dilutions of the high dose (446 mg aloin/kg drinking water), using the vehicle control as diluent. The vehicle control and dosed formulations were dispensed into sterile 250 ml high-density polyethylene rodent water bottles, encased in plastic wrap and stored at 4 °C.
Samples of control vehicle and each dose formulation were collected weekly from each formulation and certifications for each dose level were conducted. All samples analyzed were within an acceptability range of ±10% of the target.
Animals and animal maintenance
Male F344/N Nctr rats were selected for study because male rats were more sensitive than female rats to the pathological changes observed in the previous study of the Aloe vera whole leaf extract (Boudreau et al., 2013). Groups of ten F344 male rats, housed two rats per cage, were administered the aloin test material at concentrations of 0, 6.95, 13.9, 27.8, 55.7, 111, 223, and 446 mg/kg drinking water. The vehicle control and aloin dosed water bottles were replaced on a daily basis and served as the sole source of drinking water for a period of 13 weeks. Water and feed (irradiated NIH 41 rodent pelleted diet; Purina Mills, Richmond, New Jersey) were provided ad libitum. Cage water consumption was recorded daily, and individual body weights, cage feed consumptions, and clinical observations were recorded weekly. At the end of the study, the rats were fasted overnight, anesthetized with carbon dioxide, and blood was collected by cardiac puncture until exsanguination. Whole blood was collected in EDTA for complete blood counts and analyzed the same day on a Pentra 60C analyzer (ABX, Irvine, California). Blood samples for clinical chemistry were allowed to clot and centrifuged, and the serum removed and stored at −60 °C until analyzed on an ACE Alera clinical chemistry analyzer (Alfa Wassermann, West Caldwell, New Jersey) with Roche diagnostic reagents. Animals continued to receive the vehicle control or dosed water until being anesthetized.
Pathology
At necropsy, all organs and tissues were examined for grossly visible lesions, and gross findings were recorded. Organs were fixed in 10% neutral-buffered formalin, with the exception that the testis and eyes were placed in Modified Davidson’s fixative for histopathology. The GIT was removed in its entirety and placed in a petri dish containing physiological saline. The ileum, cecum, and cecal-colic junction were collected, the cecum and cecal-colic junction were opened and flattened, and the tissues were separately placed in cassettes and fixed in 10% neutral-buffered formalin. The large intestine was flushed with physiological saline and the ascending, transverse, and descending colon, and rectum were collected separately in cassettes and fixed in 10% neutral-buffered formalin. All tissues, including the GIT, were trimmed, processed, embedded in infiltrating media (Formula R, Surgipath Medical Industries, Inc., Richmond, Illinois), sectioned at approximately 5 μm, and stained with hematoxylin and eosin for microscopic examination.
Fecal collection, DNA extraction, PCR amplification, and next generation sequencing
Three days prior to necropsy, fresh fecal pellets were collected from individual rats. The rats were transferred individually to fresh sterilized and pre-labeled cages and feces were collected for 2 h before the rats were returned to their home cage. Fecal pellets were placed in pre-labeled microfuge tubes, immediately flash frozen in liquid nitrogen, and stored at −60 °C. For DNA extraction, approximately 1 g of fecal pellets from each rat of cage mates (2 rats/cage) was pooled to reduce inter-rat fecal community variability, and the pellets were ground to a fine powder in liquid nitrogen with a sterilized mortar and pestle. Pooling of fecal pellets of cage mates was not expected to be a confounding factor because microbial communities have been shown to stabilize after 7 days when fed a similar diet (Kalmokoff et al., 2015; Kalmokoff et al., 2013). Total genomic DNA from 250 mg of each pooled sample was extracted using a PowerSoil DNA Isolation kit (Mo BIO Laboratories, Inc., Carlsbad, California) in accordance with the protocol supplied by manufacturer. The purity and concentration of the DNA samples were assessed using a Nanodrop 1000 Spectrophoto-meter (ThermoScientific, Waltham, Massachusetts). The range of the 260/280 nm absorbance ratio was 1.8–2.0, confirming the purity of the DNA. The DNA samples were stored frozen at −20 °C before PCR amplification.
The forward primer (5’-CCTACGGGNGGCWGCAG-3’) and reverse primer (5’-TACHVGGGTATCTAATCC-3’) were used to amplify a region corresponding to the V3 and V4 regions of the 16S rRNA gene (Gu et al., 2013). This set of primers has been validated for quantification of predominant bacterial species in rodents by PCR (Yang et al., 2015). Barcode sequences to identify each fecal sample were attached between the adaptor sequence and the forward primer (Supplementary Table 1). Polymerase chain reactions were carried out using 25 μl reaction mixtures of 2× Kapa HiFi HotStart Ready mix (Kapa Biosystems, Boston, Massachusetts), 200 nM of each primer, and 5 ng of sample DNA. The amplification program consisted of an initial denaturation step at 95 °C for 3 min, followed by 25 cycles of 95 °C for 30 s (denaturing), 55 °C for 30 s (annealing), and 72 °C for 30 s (extension), and then a final extension step at 72 °C for 5 min. Aliquots (5 μl) of each PCR were run on agarose gels; as expected, a single PCR product of about 500 bp was observed in all PCRs. The remaining 45 µl of each PCR were pooled together into a single tube and stored at −20 °C for sequencing.
The 16S rRNA gene amplicons were sequenced on an Illumina MiSeq benchtop sequencer using 2× 300 bp paired-end reads (Illumina, San Diego, California) at the UAMS Sequencing Core Facility (Department of Microbiology/Immunology, University of Arkansas for Medical Sciences, Little Rock, Arkansas). FASTQ data files were processed using FASTQ Tool Kit application in Illumina’s BaseSpace environment. Raw FASTQ data files were demultiplexed and trimmed using the bcl2fastq2 v2.17 conversion software. Demultiplexed FASTQ data files were uploaded into Illumina’s BaseSpace Sequence Hub and processed using QIIME Preprocessing and Visualizations applications following the Illumina’s settings (Caporaso et al., 2010). In order to calculate downstream diversity measures (alpha and beta diversity indices, with Unifrac analysis), the 16S rRNA sequences with a mean sequence quality score >30 were assigned to operational taxonomic units (OTUs) based on sequence similarity. The OTUs were defined at ≥97% sequence homology and even rarefaction depth was set to 190 000 based on the distribution of sequences per sample in the OTU table. Taxonomic identities were assigned using reference databases, and all reads were classified to the lowest possible taxonomic rank.
Statistical methods
A repeated mixed model analysis of variance (ANOVA) in SAS (v9.3; SAS Institute Inc., Cary, North Carolina) was used to test the effect of aloin exposure on body weight, food consumption, and water intake. Weekly food consumption and averaged weekly water intake per animal per day were calculated for each cage by dividing the total by the number of animal-days. Week was the repeated measure. Dunnett’s comparison tests were used to analyze significant (P ≤ 0.05) differences between the treatment groups and the vehicle, and contrasts were constructed to test for dose-related trends.
An ANOVA was performed to determine the effect of treatment on hematology and clinical chemistry parameters using a nonparametric method with midranks (the average of left and right ranks were used for ties) and an unstructured covariance (Brunner et al., 2002). Pairwise comparisons of each treatment group to the vehicle group were performed with Bonferroni adjustments.
Histopathology of gross and study-defined tissues revealed a number of non-neoplastic lesions that were graded with severity score qualifiers: 1 (minimum), 2 (mild), 3 (moderate), or 4 (marked). Lesion incidence for each dose group was compared to the vehicle control group using the Fisher’s exact test, and the Cochran–Armitage test was used to test lesion incidences for linear trend effects (Armitage, 1955; Cochran, 1954). The Shirley–Williams test, utilizing the assumption of monotonic dose response, was used to compare mean lesion severity scores of each treatment group to the vehicle control, and the Jockheere–Terpstra test was used to determine dose-related trend effects (Shirley, 1977; Williams, 1986).
Microbiota data were analyzed with a generalized linear model ANOVA to evaluate the effect of aloin treatment on the relative abundances of bacteria at each taxonomy rank. For the sequence data, least square means and standard error of the means were determined for the vehicle control and each aloin treatment group. For each taxonomic rank, least square means were compared across treatments for any taxonomic rank represented at ≥1.0% of the relative abundance for any one treatment group. Dunnett’s pairwise comparison tests were used to analyze significant (P ≤ .05) differences between the treatment groups and the vehicle control group, and contrasts were constructed to test for linear dose-related trend effects.
All statistical analyses were conducted as two-sided tests in SAS, with the exception of non-neoplastic lesions, which were one-sided tests, at the 5% significance level.
Quality assurance
This study was conducted in accordance with FDA regulations for Good Laboratory Practices in Nonclinical Studies (eCFR, 2016), the OECD guidelines for testing chemicals in toxicity studies in rodents (OECD, 1998), and the NTP specifications for the conduct of studies in laboratory animals (NTP, 2011). The animal care and all experimental procedures were performed in accordance with an animal study protocol that was approved by the NCTR Institutional Animal Care and Use Committee.
RESULTS
Effects of Aloin Administration on the Survival and Growth of Rats
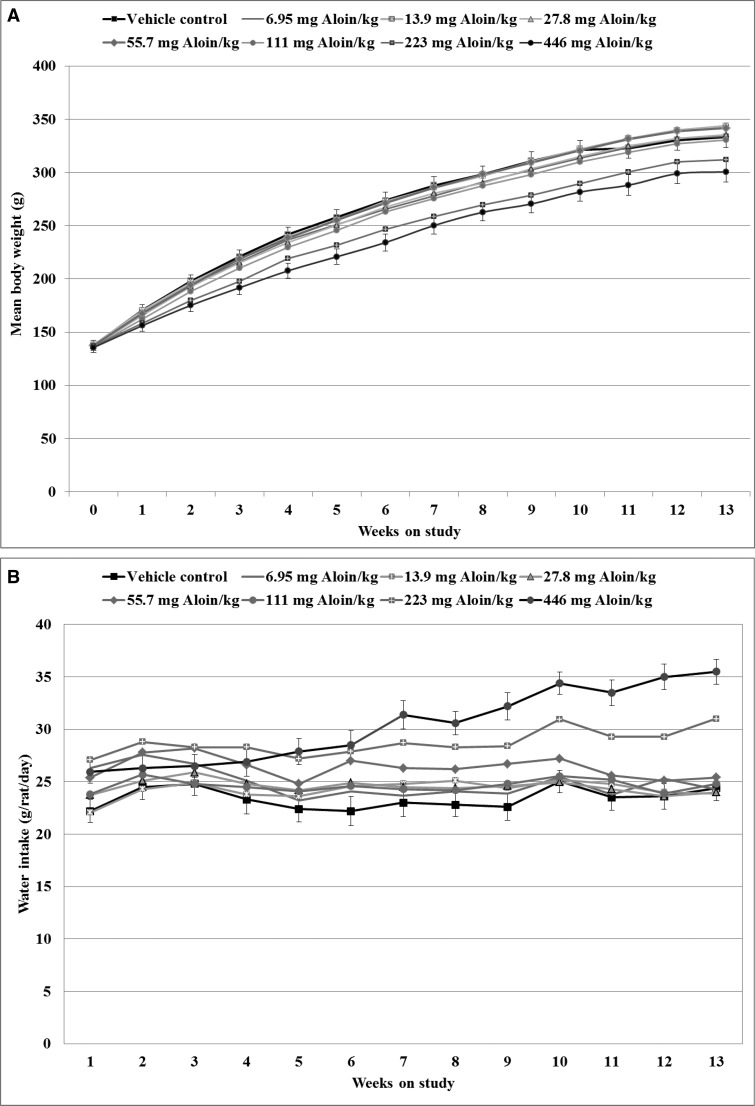
Two animals were removed as moribund prior to study termination, one rat from the vehicle control group with a diagnosis of hydronephrosis and one rat from the 446 mg aloin/kg drinking water group with an undetermined cause-of-death diagnosis. The body weight growth curves of male rats throughout the 13 week study (Figure 2A) indicate that body weights did not differ from vehicle controls at the start (week 0) of the dosing period; however, significant dose-related trend decreases in body weight were observed in male rats administered aloin beginning at week 1 of the study and continuing until study termination. In pairwise comparison tests to the vehicle controls, the body weights of animals exposed to aloin in the drinking water at 446 mg aloin/kg water were significantly lower beginning at week 2 of the study; final mean body weights relative to controls were 93% and 90% for the 223 and 446 mg aloin/kg dosed water formulation treatment groups. Mean body weights of aloin-treated rats never fell below 86% of the mean body weights of vehicle control animals at any time point measured throughout the study.
Figure 2.
Body weights and water consumption of male rats in the 13-week drinking water study of aloin. A, Growth curves of vehicle and aloin-treated rats plotted as least squares means in grams of body weight by week. B, Water consumption of vehicle and aloin-treated rats plotted as least squares means in grams per animal per day by week. Error bars (shown for the vehicle and 446 mg aloin/kg water dose groups) represent the standard error of the least squares means; n = 10, with the exception that n = 9 for the 446 mg aloin/kg water dose group beginning at week 7 and for the vehicle group beginning at week 12.
Impact of Aloin Administration on Water and Feed Consumption Patterns
Polydipsia was prevalent in male rats administered aloin, with significant dose-related increases in water intake observed at each week of the study, beginning at week 4 (Figure 2B). Similar findings were reported in the previous study with an Aloe vera whole leaf extract (Boudreau et al., 2013). Aloin-dosed water consumption by the rats was significantly higher than that of vehicle controls. In comparison tests with vehicle controls, the mean daily water consumption of the rats was significantly higher beginning at week 4 for the 223 mg aloin/kg water dose group and beginning at week 5 for the 446 mg aloin/kg water dose group. The mean water intakes of rats in the 223 and 446 mg aloin/kg water dose groups were 27.9 and 28.5 g/rat/day at week 6 compared to 22.2 g/rat/day for vehicle control rats at the same time points. At week 13, mean water intakes of rats in the 223 and 446 mg aloin/kg water dose groups were 31.6 and 36.3 g/rat/day (132% and 151%, respectively, of control values). Based upon the mean body weights and mean water intakes, the average consumption of aloin throughout the 13-week study was 0.7, 1.3, 2.6, 5.5, 10.7, 26.5, and 57.9 mg/kg body weight/day for the 6.95, 13.9, 27.8, 55.7, 111, 223, and 446 mg aloin/kg water dose groups, respectively.
Significant dose-related decreasing trends in the mean weekly feed consumption of aloin-treated animals were observed sporadically in the study; however, feed consumptions of aloin-treated animals were not significantly different from vehicle controls in pairwise comparisons tests (Supplementary Table 2).
Effects of Aloin Administration on Hematology and Clinical Chemistry
Hematology and clinical chemistry parameters recorded for rats exposed to the vehicle and aloin water formulations are provided in Supplementary Table 3. Sporadic differences in the hematological and biochemistry parameters were observed for the aloin treatment groups when compared to the vehicle controls. Reference values for the rat indicated that the hematology and clinical chemistry values generally were within the normal range for the species (CRL, 1984; Giknis and Cliffford, 2006), with the exception that white blood cell (WBC) counts and the absolute and relative percentage of WBC as neutrophils were significantly elevated in the two highest aloin dose groups of male rats (223 and 446 mg aloin/kg dosed water), while serum cholesterol and albumin were lower in the same aloin dose groups relative to control values (Supplementary Table 3). For the two highest aloin dose groups, both the WBC and neutrophil cell counts were higher and cholesterol values lower than reference values for the rat (CRL, 1984; Giknis and Cliffford, 2006). Elevated WBC and neutrophil counts were suggestive of a heightened acute immune response in these animals (Jones et al., 2016). WBC and neutrophil cell counts were similarly elevated and serum cholesterol and albumin were similarly lowered in the 13-week study of the Aloe vera whole leaf extract (Boudreau et al., 2013).
Pathological Changes in Rats Induced by Aloin Administration
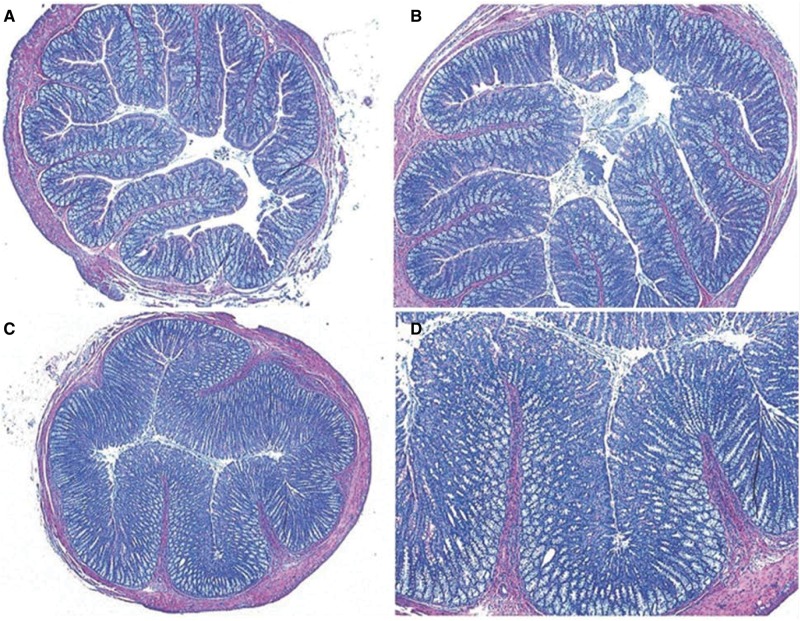
Microscopic evaluations found no incidences of neoplasia in vehicle control or aloin-treated rats. The prevalence and severity of non-neoplastic lesions that were evaluated by histopathology are summarized in Tables 1 and 2. The most prominent pathological change was dose-related mucosal and goblet cell hyperplasia, which extended from the cecum to the rectum of aloin treated rats (Table 1). This lesion was characterized by an increased number of both enterocytes and goblet cells resulting in a marked thickness of the intestinal mucosa (Figure 3). Mucosal hyperplasia was noted at aloin doses as low as 27.8 mg/kg dosed water, and incidences of mucosal hyperplasia were 100% in the colon of rats administered aloin at the 223 and 446 mg/kg water dose levels. Significant dose-related increased incidences and severities of mucosal and goblet cell hyperplasia were observed at each region of the large intestine and were most prominent in the ascending and transverse colon and lower in the descending colon and rectum. Similar findings were reported the previous study with an Aloe vera whole leaf extract (Boudreau et al., 2013). In comparison tests with the vehicle controls, the incidences and severity scores of mucosa and goblet cell hyperplasia were significantly higher in the ascending and transverse colon of rats administered aloin at doses ≥55.7 mg aloin/kg water and in the proximal and descending colon and rectum of rats administered aloin doses ≥ 111 mg/kg water.
Table 1.
Incidence and Severity of Mucosa and Goblet Cell Hyperplasia and Mesenteric Lymph Node Hyperplasia in the Alimentary System of Male Rats Administered Aloin in the Drinking Water for 13 Weeks
| Aloin Concentration (mg/kg) in Dosed Water Formulations |
||||||||
|---|---|---|---|---|---|---|---|---|
| 0 |
6.95 |
13.9 |
27.8 |
55.7 |
111 |
223 |
446 |
|
| Dose equivalency of Aloe vera whole leaf extract | 0 | 0.03% | 0.06% | 0.13% | 0.25% | 0.5% | 1.0% | 2.0% |
| Cecum | ||||||||
| Incidencea | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 2/10 (20%)a | 7/10 (70%) | 10/10 (100%) |
| P-valueb | <.001 | .237 | .002 | <.001 | ||||
| Severityc | — | — | — | — | — | 2.0 | 2.4 | 2.5 |
| P-valued | <.001 | .500 | .583 | .618 | .636 | .008 | <.001 | <.001 |
| Proximal colon | ||||||||
| Incidence | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 1/10 (10%) | 9/10 (90%) | 9/10 (90%) | 10/10 (100%) |
| P-value | <.001 | .500 | <.001 | <.001 | <.001 | |||
| Severity | — | — | — | — | 1.0 | 2.0 | 2.7 | 3.3 |
| P-value | <.001 | .500 | .583 | .618 | .072 | <.001 | <.001 | <.001 |
| Ascending colon | ||||||||
| Incidence | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 1/10 (10%) | 6/10 (60%) | 10/10 (100%) | 10/10 (100%) | 10/10 (100%) |
| P-value | <.001 | .500 | .005 | <.001 | <.001 | <.001 | ||
| Severity | — | — | — | 2.0 | 1.6 | 2.9 | 3.8 | 4.0 |
| P-value | <.001 | .500 | .583 | .098 | <.001 | <.001 | <.001 | <.001 |
| Transverse colon | ||||||||
| Incidence | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 1/10 (10%) | 6/10 (60%) | 10/10 (100%) | 10/10 (100%) | 10/10 (100%) |
| P-value | <.001 | .500 | .005 | <.001 | <.001 | <.001 | ||
| Severity | — | — | — | 2 | 1.5 | 2.6 | 3.3 | 3.9 |
| P-value | <.001 | .500 | .583 | .098 | <.001 | <.001 | <.001 | <.001 |
| Descending colon | ||||||||
| Incidence | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 1/10 (10%) | 10/10 (100.0%) | 10/10 (100.0%) | 10/10 (100.0%) |
| P-value | <.001 | .500 | <.001 | <.001 | <.001 | |||
| Severity | — | — | — | — | 2.0 | 2.3 | 2.6 | 3.6 |
| P-value | <.001 | .500 | .583 | .618 | .072 | <.001 | <.001 | <.001 |
| Rectum | ||||||||
| Incidence | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 6/10 (60%) | 10/10 (100%) | 10/10 (100%) |
| P-value | <.001 | .005 | <.001 | <.001 | ||||
| Severity | — | — | — | — | — | 1.6 | 1.9 | 2.9 |
| P-value | <.001 | .500 | .583 | .618 | .636 | <.001 | <.001 | <.001 |
| Mesenteric lymph node hyperplasia | ||||||||
| Incidence | 9/10 (90%) | 7/10 (70%) | 9/10 (90%) | 7/10 (70%) | 8/10 (80%) | 10/10 (100%) | 10/10 (100%) | 10/10 (100%) |
| P-value | .034 | .291N | .763N | .291N | .500N | .500 | .500 | .500 |
| Severity | 2.0 | 1.9 | 1.7 | 1.8 | 1.3 | 2.4 | 3.7 | 4.0 |
| P-value | <.001 | .878 | .891 | .911 | .975 | .146 | .003 | <.001 |
Incidence reported as the number of lesion bearing animals over the total number of animals examined by histopathology in the group.
P-values are based on the Fisher exact test for comparisons of aloin treatments to vehicle controls; P-values under vehicle control column are the results of the Cochran–Armitage test for linear trends. P-values ≤ .05 are shown in bold face type. An “N” following a P-value indicates a negative comparison result.
Severity scores are mean severity of affected animals. Non-neoplastic lesions were graded for severity as 1 (minimal), 2 (mild), 3 (moderate), and 4 (marked).
P-values are based on the Shirley–Williams test for comparisons of aloin treatments to vehicle controls; P-values under vehicle control column are the results of the Jonckheere–Terpstra test for linear trends. P-values ≤ .05 are shown in bold face type.
Table 2.
Incidence and Severity of Inflammation in the Cecum and Colon of Male Rats Administered Aloin in the Drinking Water for 13 Weeks
| Aloin Concentration (mg/kg) in Dosed Water Formulations |
||||||||
|---|---|---|---|---|---|---|---|---|
| 0 |
6.95 |
13.9 |
27.8 |
55.7 |
111 |
223 |
446 |
|
| Dose equivalency of Aloe vera whole leaf extract | 0 | 0.03% | 0.06% | 0.13% | 0.25% | 0.5% | 1.0% | 2.0% |
| Cecum | ||||||||
| Incidence | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 1/10 (10%) | 2/10 (20%) | 5/10 (50.0%) |
| P-valueb | <.001 | .500 | .237 | .016 | ||||
| Severityc | — | — | — | — | — | 1.0 | 2.0 | 1.8 |
| P-valued | <.001 | .500 | .583 | .618 | .636 | .053 | .016 | <.001 |
| Proximal colon | ||||||||
| Incidence | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 1/10 (0%) | 8/10 (80%) | 9/10 (90%) |
| P-value | <.001 | .500 | <.001 | <.001 | ||||
| Severity | — | — | — | — | — | 2.0 | 2.3 | 2.1 |
| P-Value | <.001 | .500 | .583 | .618 | .636 | .053 | <.001 | <.001 |
| Ascending colon | ||||||||
| Incidence | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 1/10 (10%) | 9/10 (90%) | 10/10 (100%) | 10/10 (100%) |
| P-Value | <.001 | 0.500 | <.001 | <.001 | <.001 | |||
| Severity | — | — | — | — | 1.0 | 1.6 | 3.3 | 3.1 |
| P-Valueb | <.001 | .500 | .583 | .618 | .072 | <.001 | <.001 | <.001 |
| Transverse colon | ||||||||
| Incidence | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 1/10 (0%) | 6/10 (60%) | 9/10 (90%) | 9/10 (90%) |
| P-Value | <.001 | .500 | .005 | <.001 | <.001 | |||
| Severity | — | — | — | — | 1.0 | 1.6 | 2.2 | 2.1 |
| P-Value | <.001 | .500 | .583 | .618 | .072 | <.001 | <.001 | <.001 |
| Descending colon | ||||||||
| Incidence | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 5/10 (50%) | 7/10 (70%) | 10/10 (100%) |
| P-Value | <.001 | .016 | .002 | <.001 | ||||
| Severity | — | — | — | — | — | 1.0 | 1.4 | 1.6 |
| P-Value | <.001 | .500 | .583 | .618 | .636 | <.001 | <.001 | <.001 |
| Rectum | ||||||||
| Incidence | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 4/10 (40%) | 5/10 (50%) | 8/10 (80%) |
| P-Value | <.001 | .043 | .016 | <.001 | ||||
| Severity | — | — | — | — | — | 1.2 | 1.8 | 2.3 |
| P-Value | <.001 | .500 | .583 | .618 | .636 | <.001 | <.001 | <.001 |
Incidence reported as the number of lesion bearing animals over the total number of animals examined by histopathology in the group.
P-Values are based on the Fisher exact test for comparisons of aloin treatments to vehicle controls; P-values under vehicle control column are the results of the Cochran–Armitage test for linear trends. P-values ≤ .05 are shown in bold face type.
Severity scores are mean severity of affected animals. Non-neoplastic lesions were graded for severity as 1 (minimal), 2 (mild), 3 (moderate), and 4 (marked).
P-Values are based on the Shirley-Williams test for comparisons of aloin treatments to vehicle controls; P-values under vehicle control column are the results of the Jonckheere–Terpstra test for linear trends. P-values ≤ .05 are shown in bold face type.
Figure 3.
Mucosal and goblet cell hyperplasia in the colons of rats in the 13-week drinking water study of aloin. Colon sections from rats administered the vehicle controls (A), magnification ×5 (original); B, 55.7 mg aloin/kg water dose group, magnification ×5 (original); C, 446 mg aloin/kg dose group, magnification ×2.5 (original); D, 446 mg aloin/kg water dose group, magnification ×5 (original).
Mesenteric lymph node hyperplasia was detected in both vehicle- and aloin-treated rats, with significant aloin dosed-related trend increases in the incidences and severities of this lesion being observed. Rats in the 223 and 446 mg aloin/kg water dose groups had significantly higher severity scores, when compared to that of the vehicle control group (Table 1). Dose-related increased incidences and severities of chronic-active inflammation of the large intestine were also noted in most of these same animals and were prominent even at the 55.7 mg aloin/kg water dose group (Table 2).
Impact of Aloin on Rat Fecal Bacterial Communities
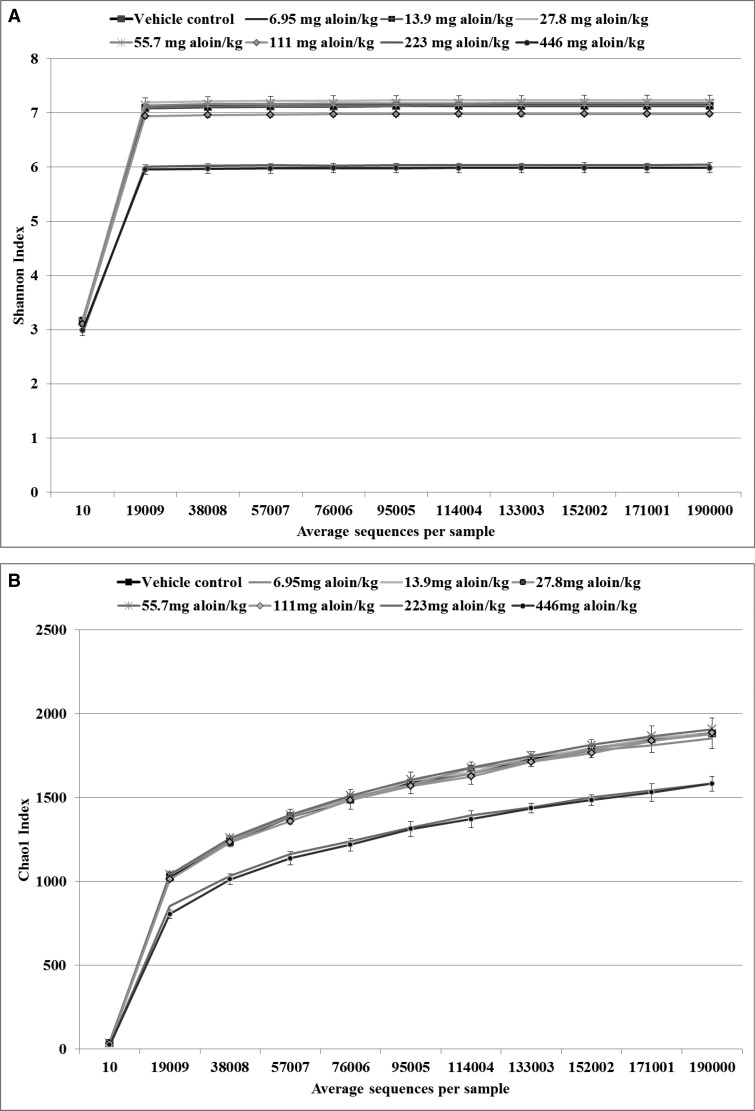
Fecal community DNA composition was determined by analyzing 16S rRNA gene content generated through next generation sequencing. The OTUs were defined at ≥97% sequence homology and the even rarefaction depth was set to 190 000 based on the distribution of sequences per sample in the OTU table. In total, 139 phylotypes were identified in the feces of rats in the eight treatment groups. The rarefaction table generated in QIIME was the basis for generating the alpha diversity (Shannon) and alpha richness (Chao1) plots (Figure 4). The rarefaction plots were used to describe the phyla communities and the curves represent community diversity versus sequencing depth for the number of different OTUs in the 40 samples analyzed. The Shannon index curves (Figure 4A) indicated that the majority of bacteria were identified in the samples at an average number of 190 000 sequences per sample, although the plots show that the diversity of communities was unevenly distributed between the vehicle control and the two highest dose groups of aloin (223 and 446 mg aloin/kg water), with greater diversity being shared between the vehicle control and doses of aloin below 223 mg/kg water. The plots of phylotype richness (Chao1) represented the number of different species in the bacterial communities. A greater number of phylotypes was shared between the vehicle and lower doses of aloin (6.95–55.7 mg aloin/kg water) than between the vehicle control and higher aloin dose groups (Figure 4B).
Figure 4.
Alpha diversity curves. The rarefaction table that was generated in Qiime was used to generate the alpha diversity curves. The diversity metrics reflect the diversity within a sample based on the abundance of various phylotypes within a bacterial community. A, Shannon phylotypes diversity curves by sequences per treatment (n = 5). B, Chao1phylotype richness curves by sequences per treatment (n = 5). Data suggest that the fecal microbiota profile in F-344 rats was considerable altered as a consequence of aloin treatment; fecal content richness and diversity were lower in rats administered aloin in the drinking water at doses of 223 and 446 mg/kg water.
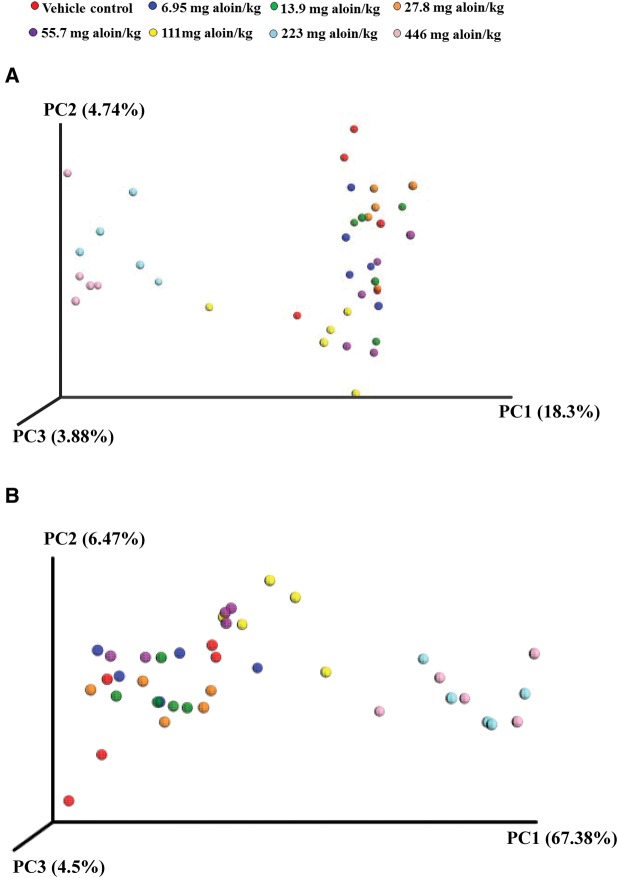
Beta diversity was used to compare the differences in the composition of bacteria between samples/treatments, and principal coordinate analysis plots (PCA) were generated by Emperor in QIIME (Figure 5). The PCA plots based on the relative abundance of OTUs revealed a separation of the high-dosed aloin treatment groups and the vehicle control and low-dosed aloin groups based on the first two principal coordinate scores, which accounted for 18.3% and 4.74% of the total variation (Figure 5A). PCA plots based on the weighted UniFrac metric confirmed the above findings. The maximum amount of variation (67.38%) could be explained by the first principal coordinate (PC1) and showed that treatment had the greatest effect on the distribution of bacterial sequences. Samples from the two highest dose groups of aloin segregated to the right of the plot, while the control and lower aloin dose group samples clustered together to the left of the plot, with the exception that the 111 mg aloin/kg water dose group samples clustered toward the middle of the plot (Figure 5B).
Figure 5.
Beta diversity plots. Principal coordinates analysis (PCA) scores plots. A, PCA plot based on unweighted UniFrac metric; B, PCA plot based on weighted uniFrac metric. Each color represents a treatment sample (n = 5).
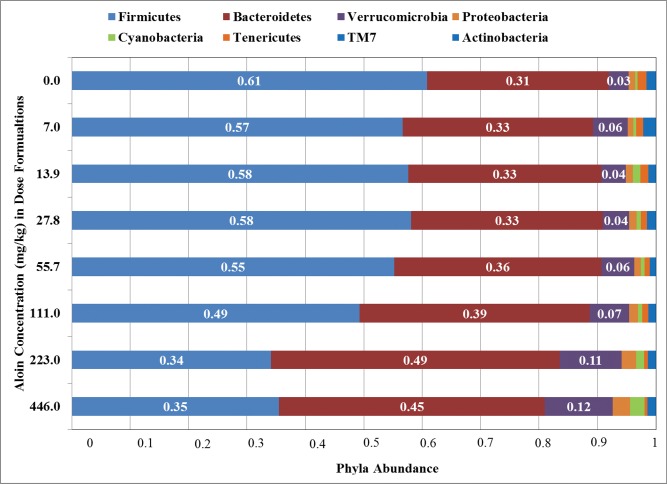
Changes in the bacterial population abundance were assessed in fecal samples of all groups of rats at the taxonomic ranks of phylum, class, order, family, and genus (Table 3). At each taxonomic rank, bacteria represented at ≤1.0% of the total rank abundance were eliminated from additional analyses. Sequences were distributed among eight phyla that were identified at ≥1.0% abundance in all fecal 16S rRNA samples (Figure 6). The majority of the sequences belonged to Bacteroidetes and Firmicutes, together accounting for up to 90% of the sequences, as well as phyla Verrucomicrobia, Tenericutes, Proteobacteria, Cyanobacteria, Actinobacteria, and TM7, each accounting for 1%–12% of the sequences. Significant linear dose trend effects were observed for each phylum (Table 3). The most remarkable shifts in phyla abundances were observed for Bacteroidetes, Firmicutes, and Verrucomicrobia. In pairwise comparison tests to the vehicle controls, the abundance of Bacteroidetes was significantly increased in rats by the daily consumption of drinking water containing aloin at doses of 111, 223, and 446 mg/kg water. The abundance of Bacteroidetes was approximately 31% in the vehicle control group and was approximately 39%, 49%, and 45% in rats administered aloin at 111, 223, and 446 mg/kg water formulation, respectively. Similarly, the abundance of Verrucomicrobia was significantly increased in groups of rats administered drinking water containing aloin at the 223 and 446 mg/kg water dose levels when compared to the vehicle control group. In contrast, the abundance of Firmicutes in the vehicle control group was significantly decreased in fecal 16S rRNA sequences of rats administered drinking water containing 111, 223, and 446 mg aloin/kg water (Figure 6 and Table 3).
Table 3.
Mean Abundance Sequence Counts by Taxonomic Rank in Fecal 16S rRNA Collected From Rats Exposed to Aloin in the Drinking Water for 13 Weeks
| Aloin Concentration (mg/kg) in Dose Formulations |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phylum | Class | Order | Family | Genus | 0 | 6.95 | 13.9 | 27.8 | 55.7 | 111 | 223 | 446 | |||||
| Percentage (%) of Total Sequence Reads per Samplea | |||||||||||||||||
| Actinobacteria | 0.32* | 0.31 | 0.20 | 0.25 | 0.24 | 0.62 | 0.87 | 1.30* | |||||||||
| Actinobacteria | 0.10* | 0.10 | 0.04 | 0.04 | 0.07 | 0.48 | 0.82* | 1.25* | |||||||||
| Bifidobacteriales | Bifidobacteriaceae | Bifidobacterium | 0.08* | 0.08 | 0.02 | 0.02 | 0.05 | 0.46 | 0.81* | 1.23* | |||||||
| Bacteroidetes | Bacteroidia | Bacteroidales | 31.14* | 32.57 | 33.09 | 32.80 | 35.50 | 39.44* | 49.45* | 45.46* | |||||||
| Bacteroidaceae | Bacteroides | 1.91 | 1.98 | 2.15 | 1.90 | 2.88 | 2.36 | 2.62 | 2.27 | ||||||||
| Odoribacteraceae | Odoribacter | 2.71* | 2.25 | 2.07 | 2.01 | 2.46 | 2.55 | 0.27* | 0.25* | ||||||||
| Porphyromonadaceae | Parabacteroides | 1.39* | 1.85 | 1.84 | 2.15 | 1.78 | 2.17 | 3.55* | 3.96* | ||||||||
| Prevotellaceae | Prevotella | 7.63* | 7.5 | 9.32 | 8.25 | 8.25 | 8.84 | 13.25* | 12.74* | ||||||||
| Rikenellaceae | 6.27* | 7.45 | 6.00 | 5.78 | 8.64 | 9.23 | 6.89 | 5.49 | |||||||||
| Unidentified | 5.89 | 6.99 | 5.57 | 5.62 | 8.58 | 9.12 | 6.76 | 5.41 | |||||||||
| S24-7 | Unidentified | 11.22* | 11.54 | 11.73 | 12.71 | 11.49 | 14.30 | 22.86* | 20.75* | ||||||||
| Cyanobacteria | 4C0d-2 | YS2 | Unidentified | Unidentified | 0.45* | 0.51 | 1.27 | 0.78 | 0.66 | 0.65 | 1.38 | 2.47* | |||||
| Firmicutes | 60.83* | 56.65 | 57.61 | 58.13 | 55.21 | 49.26* | 34.10* | 35.49* | |||||||||
| Bacilli | 6.61* | 5.33 | 3.92 | 4.77 | 5.04 | 7.43 | 3.27* | 4.25 | |||||||||
| Lactobacillales | 5.55* | 4.62 | 3.52 | 4.33 | 4.65 | 6.64 | 2.48* | 3.22* | |||||||||
| Lactobacillaceae | Lactobacillus | 5.53* | 4.61 | 3.51 | 4.32 | 4.63 | 6.62 | 2.47* | 3.21* | ||||||||
| Turicibacterales | Turicibacteraceae | Turicibacter | 1.06 | 0.71 | 0.40 | 0.43 | 0.39 | 0.78 | 0.79 | 1.03 | |||||||
| Clostridia | Clostridiales | 54.07* | 51.25 | 53.51 | 53.20 | 50.05 | 41.53* | 29.47* | 30.13* | ||||||||
| Clostridiaceae | 1.65* | 1.06 | 0.60 | 0.76 | 1.11 | 1.86 | 1.80 | 2.76 | |||||||||
| Unidentified | 0.84* | 0.65 | 0.28 | 0.45 | 0.75 | 1.19 | 0.96 | 1.6 | |||||||||
| Lachnospiraceae | 7.81* | 7.62 | 7.16 | 5.68 | 5.89 | 6.77 | 5.44 | 5.94 | |||||||||
| Blautia | 0.24* | 0.13 | 0.20 | 0.18 | 0.13 | 0.94 | 2.32* | 2.93* | |||||||||
| Coprococcus | 1.26 | 1.20 | 1.34 | 0.55 | 0.32 | 0.48 | 1.14 | 0.93 | |||||||||
| Ruminococcus | 0.63* | 1.07* | 0.88 | 0.80 | 0.73 | 0.52 | 0.17* | 0.17* | |||||||||
| Unidentified | 4.66* | 4.40 | 3.93 | 3.27 | 4.19 | 4.17 | 1.46* | 1.56* | |||||||||
| Ruminococcaceae | 22.19* | 18.95 | 21.69 | 24.07 | 17.87 | 13.77 | 13.7* | 13.48* | |||||||||
| Oscillospira | 5.20* | 5.51 | 6.15 | 6.60 | 6.64 | 4.00 | 1.45* | 1.04* | |||||||||
| Ruminococcus | 3.70* | 3.13 | 3.35 | 4.52 | 3.29 | 2.36 | 0.89* | 0.92* | |||||||||
| Unidentified | 13.3 | 10.31 | 12.20 | 12.95 | 7.94* | 7.40* | 11.37 | 11.52 | |||||||||
| Unidentified | Unidentified | 21.05* | 22.59 | 23.02 | 21.66 | 23.99 | 17.77 | 7.23* | 6.36* | ||||||||
| Erysipelotrichi | Erysipelotrichales | Erysipelotrichaeae | 0.15* | 0.06 | 0.18 | 0.16 | 0.12 | 0.31 | 1.36* | 1.11* | |||||||
| Proteobacteria | 1.09* | 0.94 | 1.23 | 1.28 | 1.14 | 1.57 | 2.55* | 2.96* | |||||||||
| Alphaproteobacteria | 0.41* | 0.36 | 0.59 | 0.71 | 0.57 | 0.59 | 1.05* | 1.62* | |||||||||
| RF32 | Unidentified | Unidentified | 0.40* | 0.35 | 0.57 | 0.70 | 0.55 | 0.58 | 1.04* | 1.61* | |||||||
| Betaproteobacteria | Burkholderiales | Alcaligenacea | Sutterella | 0.46* | 0.29 | 0.29 | 0.37 | 0.29 | 0.73 | 1.41* | 1.08 | ||||||
| Tenericutes | 1.51* | 1.19 | 1.38 | 1.01 | 0.86 | 1.09 | 0.61* | 0.53* | |||||||||
| Mollicutes | 1.49* | 1.16 | 1.33 | 0.96 | 0.83 | 0.99 | 0.56* | 0.36* | |||||||||
| RF39 | Unidentified | Unidentified | 1.37* | 1.06 | 1.30 | 0.94 | 0.81 | 0.85 | 0.53 | 0.27* | |||||||
| TM7 | TM7-3 | CW040 | F16 | Unidentified | 1.27* | 1.87 | 1.06 | 1.28 | 0.79 | 0.65 | 0.50 | 0.11 | |||||
| Verrucomicrobia | Verrucomicrobiae | Verrucomicrobiales | Verrucomicrobiaceae | Akkermansia | 3.39* | 5.95 | 4.17 | 4.47 | 5.58 | 6.71 | 10.55* | 11.68* | |||||
Values are least square means (n = 5) of sequence counts per sample for the phylotypes as a percent of 190 000 total sequence counts per sample (range 193 597–408 656; median: 308 142 counts). Under the 0 aloin dose column, an “*” indicates significant results of linear trend tests with increasing doses of aloin. Under the aloin dose treatment columns, an “*” indicates significant results of pairwise comparison tests with the vehicle control group. All statistical tests were two-sided and significant P-values were defined at a probability level of .05 or below (P ≤ .05). P-values ≤ .05 are shown in bold face type.
Figure 6.
Bar chart showing the relative abundance of microbe sequences in the 16S rRNA fecal microbiota of male F-344 rats administered aloin for 13 weeks, annotated to the phylum level by treatment (n = 5).
At the rank of class, Clostridia, Bacteroidia, and Verrucomicobiae accounted for approximately 85% of the total abundance across treatment groups. Most of the sequences from the phylum Firmicutes belonged to the class Clostridia. The abundances of Clostridia were significantly decreased in the 111, 223, and 446 mg aloin/kg water dose groups when compared to that of the vehicle control (Table 3). The phylum Bacteroidetes was primarily represented at the class level by Bacteroidia that was, on average, significantly increased by aloin administration. At the rank of family, Prevotellaceae and S24-7 were the principal members identified in the Bacteroidetes phylum to explain the increase in abundance induced by aloin administration, whereas Ruminococcaceae, Lachnispiraceae, and an as yet unidentified member were the principal families in the Firmicutes phylum to explain the decrease in abundance induced by aloin administration (Table 3).
DISCUSSION
The present study was conducted to evaluate the toxicological effects of aloin when administered to male F344/N rats in their drinking water for a period of 13 weeks. The primary goal of the study was to determine whether or not the administration of aloin in the drinking water of F344/N rats would replicate the pathophysiological effects that were observed previously in the large intestine of F344/N rats in a 13-week study of Aloe vera whole leaf extract. A secondary goal of the study was to determine whether or not the administration of aloin in the drinking water of male F344 rats for 13 weeks would modulate the microbiota populations in the large intestine.
For this study, doses of aloin were selected to include a low dose that was below the level of aloin (10 mg aloin/kg) used by the IASC in their product certification program and a high dose that was equivalent in aloin A content to a 2% (wt/wt) dose formulation of the Aloe vera whole leaf extract (∼250 mg aloin A/kg water) that was used in the previous 13-week study (Boudreau et al., 2013). In this study, the 446 mg aloin/kg dosed water formulation had an aloin A content of ∼250 mg/kg water. Lower doses equated to the aloin content in Aloe vera whole leaf extract doses of 1%, 0.5%, 0.25%, 0.13%, 0.06%, and 0.03% (wt/wt) that were used in the previous study (Boudreau et al., 2013).
Several parameters measured in rats from the present study of aloin followed similar patterns that were described in the previous study of the Aloe vera whole leaf extract. Polydipsia was prevalent in rats administered aloin and significant dose-related trend increases in water consumption were noted, beginning at week 4 of the study. The mean daily water intake of the 223 and 446 mg aloin/kg water groups was 28.7 and 30.4 g, respectively, compared to 23.4 g for the controls and equated to approximately 123% and 130% of the control group intake. In the previous study on Aloe vera whole leaf, mean water intakes of the 1% and 2% Aloe vera whole leaf groups were approximately 174% and 194% of control intake levels.
As in the previous study, histological evaluations found no incidence of neoplasms in the present study of aloin, and non-neoplastic lesions were primarily confined to the large intestine; the most prominent pathological lesion being mucosa and goblet cell hyperplasia, which was observed by microscopy throughout the entire length of the large intestine. Incidences and severities of mucosa and goblet cell hyperplasia were higher in the ascending colon and lower in the rectum. A significant dose-related response in the incidences and severities of these lesions was evident, and changes were observed even in the 27.8 mg aloin/kg water group of rats. Mucosa and goblet cell hyperplasia were prominent lesions observed in the previous 13-week study of Aloe vera whole leaf extract at doses of 1%, 2%, and 3% (Boudreau et al., 2013). In that study, the incidence of mucosa and goblet cell hyperplasia in male rats administered the 2% dose of Aloe vera whole leaf extract (equivalent to 446 mg aloin/kg water) was 100% in the cecum and ascending colon, with severity scores of 1.7 and 1.8, respectively. In the present study, aloin induced similar incidences at a dose (223 mg aloin/kg water) that was equivalent to the 1% Aloe vera whole leaf extract, with severity scores of 2.4 and 3.8 in the cecum and ascending colon, respectively. These data suggest that aloin was much more effective than the Aloe whole leaf extract in inducing these lesions. Even at an aloin dose of 27.8 mg/kg water, the severities of these lesions were greater than that observed with the 1% Aloe vera whole leaf extract—an aloin dose approximately 7.5-fold lower than the Aloe whole leaf extract. The administration of aloin also induced dose-related increased incidences and severities of chronic-active inflammation in the large intestine, a lesion not observed in the previous 13-week study of an Aloe whole leaf extract.
There is mounting evidence that the gut microbiota is altered in people with chronic-active inflammatory conditions resulting in chronic diseases, including diarrhea, gastritis, inflammatory bowel disease, and colorectal cancer and that diet plays an important role in shaping the structure of the microbiota in the large intestine and in influencing the function of microbiota community by serving as a source of material for microbiota metabolism (Dolan and Chang, 2017; Rupa and Mine, 2012). In the healthy colon, microbiota are regulated by the mucosal immune system that is aimed at maintaining a symbiotic relationship between the intestinal microbiota and the host; however, when bacterial dysbiosis occurs in the colon, the host may experience inflammation, a loss of barrier function, and an increased risk for colon cancer (Arthur and Jobin, 2011).
In this study, next generation sequencing analysis of the 16S rRNA gene from feces was used to investigate the microbiota structure of the aloin- and vehicle-treated rats, and the results indicated that the administration of aloin in the drinking water of rats induced dysbiosis of fecal microbiota. At the rank of phylum, the most predominant phyla associated with the rat colonic microbiota were Firmicutes and Bacteroidetes; these two phyla represent approximately 90% of the gut bacteria in humans. The ratio of Firmicutes to Bacteroidetes (F/B), which generally represents 57.2% and 32.0% of human colonic microbiota, respectively, has been used to express the degree of overall gut microbiota balance, and shifts in this ratio have been linked to undesirable health conditions (Borges-Canha et al., 2015). Wu et al. (2013), for example, observed that the fecal microbiota of patients with colorectal cancer not only had an increase in Bacteroides, which belong to the Bacteroidetes phylum, but also had a positive correlation between the abundance of Bacteroides species and colorectal cancer disease status.
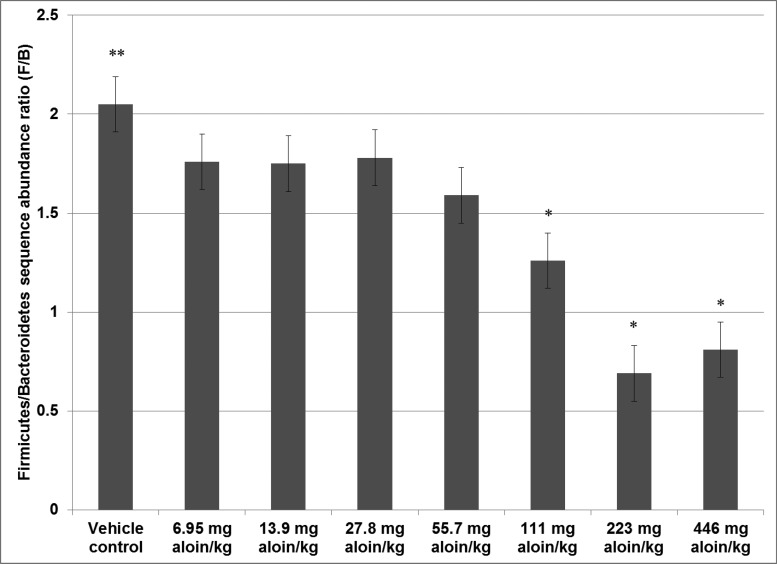
In the current study, the F/B ratio for rats in the vehicle control group was 2.1 and administration of aloin in the drinking water of male rats induced significant decreasing linear dose shifts in the contributions of these two phyla; the F/B ratio was 1.8 in rats administered the low dose (6.95 mg aloin/kg water) and 0.8 in rats administered the high dose (446 mg aloin/kg water group) treatment (Figure 7).
Figure 7.
Bar chart showing the Firmicutes to Bacteroidetes phlyla ratio. Values are least squares means (n = 5). Comparisons to vehicle that are significant at the 0.05 level or below (P ≤ .05) are indicated by an asterisk (*) above the bar; (**) above the vehicle control bar indicates significant linear dose trend effects at the 0.001 level or below (P ≤ .001).
Colonic fermentation is the anaerobic process by which carbohydrates and proteins are metabolized by intestinal microflora (Alles et al., 1999). Fermentation of carbohydrates by bacteria mainly produces gases and short-chain fatty acids, such as acetate, butyrate, and propionate. Short-chain fatty acids, especially butyrate, nourish the colonic epithelium and may protect against inflammation, ulcerative colitis, and colon cancer through their ability to promote differentiation and select cells with damaged DNA for apoptosis (Cummings, 1998; Cummings and Bingham, 1998). Butyrate-producing bacteria in the colon are predominately members of the Clostridiales order (Levine et al., 2013), and significant decreasing dose-related trends in the abundance of Clostridiales were observed by the administration of aloin (Table 3). In fecal samples collected from patients with colorectal cancer and healthy controls, increased relative abundances of microbes in the families of Fusobacteriaceae, Bacteroidaceae, and Prevotellaceae, as well as decreased relative abundances of butyrate-producing bacteria from the Ruminococcaceae and Lachnospiraceae families, have been associated with malignancy (Ericsson et al., 2015; Sobhani et al., 2011). The relative abundance of Bacteroidaceae family was not significantly altered by aloin administration; however, the Pevotellaceae family showed significant dose-related increasing trends, and the Ruminococcaceae and Lachnospiraceae families showed significant dose-related decreasing trends from the administration of aloin. The effect of aloin on the microbiota was not evaluated in the previously conducted 13-week or 2-year studies of an Aloe vera whole leaf extract, so any role of microbiota dysbiosis in the carcinogenesis in the colon of rats in the 2-year study would be pure conjecture at this time.
Plant sources of anthraquinones appear primarily as mixtures of O- or C-glycosides of anthrones and anthraquinones; aloin is an anthrone C-glycoside and is a major component of Aloe vera latex (Manitto et al., 1990). Due to the β-glycosidic bond between the d-glucose moiety and the anthrone ring structure, as well as the hydrophilic nature of the compound, aloin is protected after oral ingestion from acid hydrolysis in the stomach and enzymatic digestion in the small intestine. Aloin reaches the large intestine in undigested form, where bacteria in the GIT metabolize aloin to release glucose and aloe-emodin-9-anthrone, which is subsequently oxidized to aloe-emodin (Hattori et al., 1988). Mutagenic and genotoxic activities in bacteria and eukaryotic cells have been shown for some anthraquinones, in particular for hydroxyanthraquinones and dihydroxyanthraquinones. Aloe-emodin, a dihydroxyanthraquinone, exhibited dose-related mutagenicity and cell-transforming activities in rat hepatocyte DNA-repair induction assays and in assays to determine the malignant transformation of C3H/M mouse fibroblasts (Westendorf et al., 1990). Aloe-emodin and other dihydroxyanthraquinones have also exhibited tumor-promoting activities. One area that has not been addressed is the potential of aloin to induce effects in the GIT, where the concentrations of aloin metabolites may be higher and where the microbial environment may actively participate in the metabolism of these compounds to aloe-emodin-9-anthrone, which is subsequently oxidized to aloe-emodin.
In the previous 13-week study of an Aloe vera whole leaf extract, the lowest concentration administered was 1% by weight, and the aloin concentration in this formulation was approximately 223 mg aloin/kg water (Boudreau et al., 2013). In the current study, the administration of aloin at doses as low as 27.8 mg/kg of dosed water was shown to induce mucosa and goblet cell hyperplasia in the rat colon—a concentration of aloin that is only 2.7-fold higher than the maximum concentration set for aloin in Aloe vera products certified by the IASC for human consumption (IASC, 2012).
Recent findings suggest that the loss of normal balance in the populations of bacteria in the colon results in a subclinical pro-inflammatory state that increases DNA mutations and increases the risk of colon cancer (Borges-Canha et al., 2015). The administration of aloin in the dosed water formulations induced significant dose-related inflammation in the large intestine of rats and dysbiosis of fecal microbiota. Inflammation in the large intestine of rats was not noted in the previous study of Aloe vera whole leaf extract; however, hematological values showed a 2-fold increase in neutrophil counts and a 4-fold increase in the percentage of WBC when compared to control values (Boudreau et al., 2013). Furthermore, mutational analysis of adenomas and carcinomas collected from rats administered the Aloe vera whole leaf extract for 2-years showed that several pathways, with important roles in inflammation, were altered, including MAPK, TGF-β, and WNT (Pandiri et al., 2011). The microbiota profile in feces was not evaluated in the previous study; however, in vitro findings showed that the Aloe vera whole leaf extract had differential effects on the growth of representative fecal bacterial species and altered their production of short-chain fatty acids (Pogribna et al., 2008).
Although the correlation between aloin and the Aloe vera whole leaf extract is very strong for the pathophysiology of the rat colon, the exact mechanism by which colon cancer was induced in rats exposed to the Aloe vera whole leaf remains uncertain. Microbial dysbiosis is an attractive mechanism that correlates well with observations in human colorectal cancer (Arthur and Jobin, 2011); however, intestinal inflammation and immune responses may have contributed to alterations in the microbial community and our studies did not evaluate the interplay of these parameters at various time points throughout the study. Additional studies are needed to elucidate the mechanism by which aloin and Aloe vera whole leaf extract induce colon cancer in rats and to determine the relevance of these mechanisms for humans ingesting Aloe vera preparations.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr Luísa Camacho for providing expertise and guidance on DNA extraction and polymerase chain reaction (PCR) amplifications and Jasyl Nichols for performing the fecal DNA extractions and PCR amplifications.
FUNDING
This work was supported by an Interagency Agreement (IAG) between the US Food and Drug Administration/National Center for Toxicological Research, US Department of Health and Human Services and the National Institute of Environmental Health Sciences/National Toxicology Program, National Institutes of Health (FDA IAG #224-12-003/NIEHS IAG #AES12013).
CONFLICT OF INTEREST
The authors report no conflict of interest. The opinions and conclusions expressed in this paper are those of the authors and do not necessarily reflect the views of the U.S. Food and Drug Administration.
REFERENCES
- AHP (2012). Aloe Vera Leaf, Aloe Vera Leaf Juice, Aloe Vera Inner Leaf Juice, Aloe vera (L.) Burm.f. Standards of Identity, Analysis, and Quality Control In Aloe Vera Leaf (R. Upton, R. H. DAyu, Eds.), p. 50 American Herbal Pharmacopoeia, Scotts Valley, CA. [Google Scholar]
- Alles M. S., Hartemink R., Meyboom S., Harryvan J. L., Van Laere K. M. J., Nagengast F. M., Hautvast J. G. A. J. (1999). Effect of transgalactooligosaccharides on the composition of the human intestinal microflora and on putative risk markers for colon cancer. Am. J. Clin. Nutr. 69, 980–991. [DOI] [PubMed] [Google Scholar]
- Armitage P. (1955). Tests for linear trends in proportions and frequencies. Biometrics 11, 375–386. [Google Scholar]
- Arthur J. C., Jobin C. (2011). The struggle within: Microbial influences on colorectal cancer. Inflamm. Bowel Dis. 17, 396–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton P. (1998). Aloe vera: Magic or medicine? Nurs. Stand. 12, 49–52, 54. [DOI] [PubMed] [Google Scholar]
- Bäckhed F., Ley R. E., Sonnenburg J. L., Peterson D. A., Gordon J. I. (2005). Host-bacterial mutualism in the human intestine. Science 307, 1915–1920. [DOI] [PubMed] [Google Scholar]
- Borges-Canha M., Portela-Cidade J. P., Dinis-Ribeiro M., Leite-Moreira A. F., Pimentel-Nunes P. (2015). Role of colonic microbiota in colorectal carcinogenesis: A systematic review. Rev. Esp. Enferm. Dig. 107, 659–671. [DOI] [PubMed] [Google Scholar]
- Boudreau M. D., Beland F. A. (2006). An evaluation of the biological and toxicological properties of Aloe barbadensis (Miller), Aloe vera. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 24, 103–154. [DOI] [PubMed] [Google Scholar]
- Boudreau M. D., Imam M. S., Paredes A. M., Bryant M. S., Cunningham C. K., Felton R. P., Jones M. Y., Davis K. J., Olson G. R. (2016). Differential effects of silver nanoparticles and silver ions on tissue accumulation, distribution, and toxicity in the Sprague-Dawley rat following daily oral gavage administration for 13 weeks. Toxicol. Sci. 150, 131–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau M. D., Mellick P. W., Olson G. R., Felton R. P., Thorn B. T., Beland F. A. (2013). Clear evidence of carcinogenic activity by a whole-leaf extract of Aloe barbadensis Miller (Aloe vera) in F344/N rats. Toxicol Sci. 131, 26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs C. (1995). Herbal medicine: Aloe. CPJ 128, 48–50. [Google Scholar]
- Brooks S. P. J., McAllister M., Sandoz M., Kalmokoff M. L. (2003). Culture-independent phylogenetic analysis of the faecal flora of the rat. Can. J. Microbiol. 49, 589–601. [DOI] [PubMed] [Google Scholar]
- Brunner E., Domhof S., Langer F. (2002). Nonparametric Analysis of Longitudinal Data in Factorial Experiments. John Wiley & Sons, New York, NY. [Google Scholar]
- Caporaso J. G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F. D., Costello E. K., Fierer N., Gonzalez Peña A., Goodrich J. K., Gordon J. I., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che Q.-M., Akao T., Hattori M., Kobashi K., Namba T. (1991). Isolation of a human intestinal bacterium capable of transforming barbaloin to aloe-emodin anthrone. Planta Med. 57, 15–19. [DOI] [PubMed] [Google Scholar]
- Cochran W. G. (1954). Some methods for strengthening the common χ2 tests. Biometrics 10, 417–451. [Google Scholar]
- Conlon M. A., Bird A. R. (2015). The impact of diet and lifestyle on gut microbiota and human health. Nutrients 7, 17–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRL (1984). Baseline Hematology and Clinical Chemistry Values for Charles River Fisher 344 Rats-CDF® (F-344)CrlBR as a Function of Sex and Age. January 1984 edition, Charles River Laboratories, p. 7.
- Cummings J. H. (1998). Dietary carbohydrates and the colonic microflora. Curr. Opin. Clin. Nutr. Metab. Care 1, 409–414. [DOI] [PubMed] [Google Scholar]
- Cummings J. H., Bingham S. A. (1998). Diet and the prevention of cancer. BMJ 317, 1636–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Witt P., Lemili L. (1990). The metabolism of anthranoid laxatives. Hepatogastroenterology 37, 601–605. [PubMed] [Google Scholar]
- Ding W.-J., Wu X.-F., Zhong J.-S., Wan J.-Z. (2014). Effects of temperature, pH and light on the stability of aloin A and characterisation of its major degradation products. Int. J. Food Sci. Technol. 49, 1773–1779. [Google Scholar]
- Dolan K. T., Chang E. B. (2017). Diet, gut microbes, and the pathogenesis of inflammatory bowel diseases. Mol. Nutr. Food Res. 61. doi: 10.1002/mnfr.201600129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- eCFR. (2016). Electronic Code of Federal Regulations 21, Part 58. Good Laboratory Practice for Nonclinical Laboratory Studies.
- Ericsson A. C., Akter S., Hanson M. M., Busi S. B., Parker T. W., Schehr R. J., Hankins M. A., Ahner C. E., Davis J. W., Franklin C. L., et al. (2015). Differential susceptibility to colorectal cancer due to naturally occurring gut microbiota. Oncotarget 6, 33689–33704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz G., Grün M. (1983). Chemistry, occurence and biosynthesis of C-glycosyl compounds in plants. Planta Med. 47, 131–140. [DOI] [PubMed] [Google Scholar]
- Giknis M. L. A., Cliffford C. B. (2006) Clinical Laboratory Parameters for Crl:CD (SD) rats. March 2006 edition, Charles River Laboratories, p. 14.
- Grindlay D., Reynolds T. (1986). The Aloe vera phenomenon: A review of the properties and modern uses of the leaf parenchyma gel. J. Ethnopharmacol. 16, 117–151. [DOI] [PubMed] [Google Scholar]
- Groom Q. J., Reynolds T. (1987). Barbaloin in Aloe species. Planta Med. 52, 345–348. [DOI] [PubMed] [Google Scholar]
- Gu S., Chen D., Zhang J.-N., Lv X., Wang K., Duan L.-P., Nie Y., Wu X.-L. (2013). Bacterial community mapping of the mouse gastrointestinal tract. PLoS One 8, e74957.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Kanda T., Shu Y.-Z., Akao T., Kobashi K., Namba T. (1988). Metabolism of barbaloin by intestinal bacteria. Chem. Pharm. Bull. (Tokyo) 36, 4462–4466. [DOI] [PubMed] [Google Scholar]
- Hay J. E., Haynes L. J. (1956). The aloins. Part I. The structure of barbaloin. J. Chem. Soc. 3141–3147. [Google Scholar]
- Haynes L. J., Holdsworth D. K., Russell R. (1970). C-Glycosyl compounds. Part VI. Aloesin, a C-glucosylchromone from Aloe sp. J. Chem. Soc. 2581–2586. [Google Scholar]
- IASC. (2012). Certification Program Policies & Operational Procedures. Rev. March 12, 2012; amended July 1, 2016.
- Ishii Y., Tanizawa H., Takino Y. (1994). Studies of aloe. V. Mechanism of cathartic effect. (4). Biol. Pharm. Bull. 17, 651–653. [DOI] [PubMed] [Google Scholar]
- Jones H. R., Robb C. T., Perretti M., Rossi A. G. (2016). The role of neutrophils in inflammation resolution. Semin. Immunol. 28, 137–145. [DOI] [PubMed] [Google Scholar]
- Joshi S. P. (1998). Chemical constituents and biological activity of Aloe barbadensis – a review. J. Med. Arom. Plant Sci. 20, 768–773. [Google Scholar]
- Kalmokoff M., Franklin J., Petronella N., Green J., Brooks S. P. J. (2015). Phylum level change in the cecal and fecal gut communities of rats fed diets containing different fermentable substrates supports a role for nitrogen as a factor contributing to community structure. Nutrients 7, 3279–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmokoff M., Zwicker B., O'Hara M., Matias F., Green J., Shastri P., Green-Johnson J., Brooks S. P. J. (2013). Temporal change in the gut community of rats fed high amylose cornstarch is driven by endogenous urea rather than strictly on carbohydrate availability. J. Appl. Microbiol. 114, 1516–1528. [DOI] [PubMed] [Google Scholar]
- Klein A. D., Penneys N. S. (1988). Aloe vera. J. Am. Acad. Dermatol. 18, 714–720. [DOI] [PubMed] [Google Scholar]
- Lecomte V., Kaakoush N. O., Maloney C. A., Raipuria M., Huinao K. D., Mitchell H. M., Morris M. J. (2015). Changes in gut microbiota in rats fed a high fat diet correlate with obesity-associated metabolic parameters. PLoS One 10, e0126931.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine U. Y., Looft T., Allen H. K., Stanton T. B. (2013). Butyrate-producing bacteria, including mucin degraders, from the swine intestinal tract. Appl. Environ. Microbiol. 79, 3879–3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley R. E., Hamady M., Lozupone C., Turnbaugh P. J., Ramey R. R., Bircher J. S., Schlegel M. L., Tucker T. A., Schrenzel M. D., Knight R., et al. (2008). Evolution of mammals and their gut microbes. Science 320, 1647–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manichanh C., Reeder J., Gibert P., Varela E., Llopis M., Antolin M., Guigo R., Knight R., Guarner F. (2010). Reshaping the gut microbiome with bacterial transplantation and antibiotic intake. Genome Res. 20, 1411–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manitto P., Monti D., Speranza G. (1990). Studies on aloe. Part 6. Conformation and absolute configuration of aloins A and B and related 10-C-glucosyl-9-anthrones. J. Chem. Soc. Perkin Trans. 1, 1297–1300. [Google Scholar]
- Mapp R. K., McCarthy T. J. (1970). The assessment of purgative principles in aloes. Planta Med. 18, 361–365. [DOI] [PubMed] [Google Scholar]
- Mori H., Sugie S., Niwa K., Takahashi M., Kawai K. (1985). Induction of intestinal tumours in rats by chrysazin. Br. J. Cancer 52, 781–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehra V., Allen J. M., Mailing L. J., Kashyap P. C., Woods J. A. (2016). Gut mircobiota: Modulation of host physiology in obesity. Physiology 31, 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTP. (2011). Specifications for the Conduct of Studies to Evaluate the Toxic and Carcinogenic Potential of Chemical, Biological and Physical Agents in Laboratory Animals for the National Toxicology Program (NTP).
- Nusko G., Schneider B., Schneider I., Wittekind Ch., Hahn E. G. (2000). Anthranoid laxative use is not a risk factor for colorectal neoplasia: Results of a prospective case control study. Gut 46, 651–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara A. M., Shanahan F. (2006). The gut flora as a forgotten organ. EMBO Rep. 7, 688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD (1998). OECD Guidelines for the Testing of Chemicals, Section 4. Health Effects. Test No. 408: Repeated Dose 90-Day Oral Toxicity Study in Rodents.
- Ojeda P., Bobe A., Dolan K., Leone V., Martinez K. (2016). Nutritional modulation of gut microbiota – the impact on metabolic disease pathophysiology. J. Nutr. Biochem. 28, 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Q., Pan H., Lou H., Xu Y., Tian L. (2013). Inhibition of the angiogenesis and growth of Aloin in human colorectal cancer in vitro and in vivo. Cancer Cell Int 13, 69.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandiri A. R., Sills R. C., Hoenerhoff M. J., Peddada S. D., Ton T.-V. T., Hong H.-H. L., Flake G. P., Malarkey D. E., Olson G. R., Pogribny I. P., et al. (2011). Aloe vera non-decolorized whole leaf extract-induced large intestinal tumors in F344 rats share similar molecular pathways with human sporadic colorectal tumors. Toxicol. Pathol. 39, 1065–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogribna M., Freeman J. P., Paine D., Boudreau M. D. (2008). Effect of Aloe vera whole leaf extract on short chain fatty acids production by Bacteroides fragilis, Bifidobacterium infantis and Eubacterium limosum. Lett. Appl. Microbiol. 46, 575–580. [DOI] [PubMed] [Google Scholar]
- Power S. E., O'Toole P. W., Stanton C., Ross R. P., Fitzgerald G. F. (2014). Intestinal microbiota, diet and health. Br. J. Nutr. 111, 387–402. [DOI] [PubMed] [Google Scholar]
- Rupa P., Mine Y. (2012). Recent advances in the role of probiotics in human inflammation and gut health. J. Agric. Food Chem. 60, 8249–8256. [DOI] [PubMed] [Google Scholar]
- Shirley E. (1977). A non-parametric equivalent of Williams' test for contrasting increasing dose levels of a treatment. Biometrics 33, 386–389. [PubMed] [Google Scholar]
- Smith T., Smith H. (1851). On aloin: The cathartic principles of aloes. Month. J. Med. Sci. 12, 127–131. [Google Scholar]
- Sobhani I., Tap J., Roudot-Thoraval F., Roperch J. P., Letulle S., Langella P., Corthier G., Tran Van Nhieu J., Furet J. P. (2011). Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS One 6, e16393.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabolacci C., Rossi S., Lentini A., Provenzano B., Turcano L., Facchiano F., Beninati S. (2013). Aloin enhances cisplatin antineoplastic activity in B16-F10 melanoma cells by transglutaminase-induced differentiation. Amino Acids 44, 293–300. [DOI] [PubMed] [Google Scholar]
- The Human Microbiome Project Concortium. (2012). Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viljoen A. M., Van Wyk B.-E., Newton L. E. (2001). The occurrence and taxonomic distribution of the anthrones aloin, aloinoside and microdontin in Aloe. Biochem. Syst. Ecol. 29, 53–67. [DOI] [PubMed] [Google Scholar]
- Walsh C. J., Guinane C. M., O'Toole P. W., Cotter P. D. (2014). Beneficial modulation of the gut microbiota. FEBS Lett. 588, 4120–4130. [DOI] [PubMed] [Google Scholar]
- Wells C. L., Maddaus M. A., Reynolds C. M., Jechorek R. P., Simmons R. L. (1987). Role of anaerobic flora in the translocation of aerobic and facultatively anaerobic intestinal bacteria. Infect Immun. 55, 2689–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorf J., Marquardt H., Poginsky B., Dominiak M., Schmidt J., Marquardt H. (1990). Genotoxicity of naturally occurring hydroxyanthraquinones. Mutat. Res. 240, 1–12. [DOI] [PubMed] [Google Scholar]
- Willems M., van Buuren H. R., de Krijger R. (2003). Anthranoid self-medication causing rapid development of melanosis coli. Neth. J. Med. 61, 22–24. [PubMed] [Google Scholar]
- Williams D. A. (1986). A note on Shirley's nonparametric test for comparing several dose levels with a zero-dose control. Biometrics 42, 183–186. [PubMed] [Google Scholar]
- Wong J. M. W. (2014). Gut microbiota and cardiometabolic outcomes: Influence of dietary patterns and their associated components. Am. J. Clin. Nutr. 100(Suppl), 369S–377S. [DOI] [PubMed] [Google Scholar]
- Wu N., Yang X., Zhang R., Li J., Xiao X., Hu Y., Chen Y., Yang F., Lu N., Wang Z., et al. (2013). Dysbiosis signature of fecal microbiota in colorectal cancer patients. Microb. Ecol. 66, 462–470. [DOI] [PubMed] [Google Scholar]
- Xu Z., Knight R. (2015). Dietary effects on human gut microbiome diversity. Br. J. Nutr. 113, S1–S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.-W., Chen M.-K., Yang B.-Y., Huang X.-J., Zhang X.-R., He L.-Q., Zhang J., Hua Z.-C. (2015). Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in mouse feces. Appl. Environ. Microbiol. 81, 6749–6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.