Introduction
The original Riva-Rocci method to measure blood pressure (BP) using a cuff at the upper arm assumed the pressure obtained by this technique was a good proxy for central aortic BP.1,2 The clinical (prognostic) importance of brachial cuff BP is undeniable for both the assessment of cardiovascular risk associated with elevated BP and the benefits of treatment-induced BP reduction.3 However, it is also generally appreciated that peripheral artery systolic BP (SBP; brachial or radial artery) may be an inaccurate substitute for central SBP.4 This has been reported in human studies using intra-arterial catheterization of peripheral and central arteries.5–8 There may also be a discrepancy between peripheral and central BP responses to vasoactive drugs.9 These findings are corroborated in larger studies using non-invasive central aortic BP methods,10–13 and, while yet to be fully adopted in clinical practice, an independent prognostic value of central BP has been demonstrated.14–16 Altogether, there is a growing interest among clinicians towards improving risk estimates by using devices that provide more accurate measures of central aortic BP than those provided by current brachial cuff BP methods.
Many non-invasive devices have been developed that purport to estimate central BP from different peripheral artery sites (e.g. radial, brachial, carotid arteries) using different principles of recording the pressure or surrogate signals (e.g. applanation tonometry, oscillometry, ultrasound, or magnetic resonance imaging) and different calibration methods to derive central BP. Since upper arm cuff-based devices to estimate central BP are more clinically appealing, in recent years several companies have developed such devices using a variety of techniques (e.g. oscillometric sub-diastolic or supra-systolic waveform analysis with generalized transfer functions), which employ a variety of signal processing steps to estimate central BP from peripheral signals.17,18 Yet, with no standardized guidelines,17 the accuracy testing of these new devices (as well as the preceding devices) has not been undertaken in a uniform fashion with comparable protocols, emphasizing the need for guidance in this field.19–22 An international task force was convened to address this situation.
Task force aims
To identify issues that need to be addressed and reach consensus relating to methods for assessing and reporting the accuracy of central BP devices.
To provide recommendations regarding appropriate protocols to assess and report the evaluation of accuracy (validation) of central BP devices.
Task force process
Initiation of the task force emanated from issues raised at an organized debate relating to central BP at the ARTERY Society Meeting, Maastricht, The Netherlands in October 2014. Task force members were invited to provide feedback on a draft document of intent prior to the first meeting at the ESH Meeting, Milan, Italy, June 2015. At this meeting, terms of reference, principal issues, and topics were refined. A second meeting to discuss outstanding issues was held at the 2015 ARTERY Society meeting, Krakow, Poland, with communication of upgraded documents to members for input, and with disagreements settled by majority consensus.
Identified issues and recommendations (a glossary of terms is provided in Table 1):
Table 1.
Glossary of terms
| Intra-arterial (invasive) blood pressure | Direct measurement of blood pressure within the artery using an in-dwelling catheter-based pressure transducer |
| Peripheral (non-invasive) blood pressure | Blood pressure at a site distal from the aorta. This most often refers to brachial or radial artery blood pressure, but for the purpose of this paper also includes carotid blood pressure even though local derivation is regarded as a surrogate of central blood pressure |
| Central (aortic) blood pressure | Blood pressure in the proximal ascending aorta |
| Systolic blood pressure amplification | The increase in systolic blood pressure from proximal to peripheral arterial vessels (e.g. aorta-to-brachial, or brachial-to-radial arteries) |
| Transfer function | Signal processing step to estimate central blood pressure waveforms from peripherally recorded waveforms |
| Calibration | Process of scaling a waveform using units of pressure |
(1) Disparity of non-invasive central BP devices as to what is being measured. Most central BP devices claim to produce an estimated central BP relative to cuff brachial BP with brachial SBP usually higher than central SBP. This can be achieved by a variety of techniques including:
(a) Brachial forearm or radial applanation tonometry and a peripheral waveform-aorta transfer function23 or the radial waveform second systolic peak.24
(b) Local derivation of carotid BP on the assumption that with appropriate waveform calibration (i.e. mean arterial pressure [MAP] and diastolic BP [DBP]) carotid BP represents central aortic BP. This can be achieved by non-invasive carotid artery tonometry or ultrasound/magnetic resonance imaging of the carotid diameter/area waveform.25–27 (similar techniques are also being applied on aortic distension waveforms).28 However, for practical applications, the radial or brachial artery waveform is more readily recorded where waveforms are registered with more consistent reproducibility and repeatability, and are less prone to noise introduced by incorrect vessel applanation or respiration as can occur with carotid waveforms.
(c) Calculated central BP through transfer function and modified calibration of peripheral pressure waveforms.26,29,30
Methods (b) or (c) may produce central SBP estimates higher than brachial cuff SBP (discussed within issue 2).
(i) Recommendations: Device manufacturers should clearly state the purported measurement function of their device. While recognizing wide variety in devices, these can be broadly categorized into two types based on their function:
Type I—device purports to give an estimate of central BP relative to measured brachial BP (i.e. relatively accurate pressure difference between central and peripheral sites).
Type II—device purports to estimate the intra-arterial central BP (i.e. relatively accurate absolute central BP value despite inaccuracy at the peripheral site).
Both function types may be available within a single device. A summary of differences between device types in comparison to intra-arterial brachial and central aortic BP are presented in Figure 1.
Figure 1.
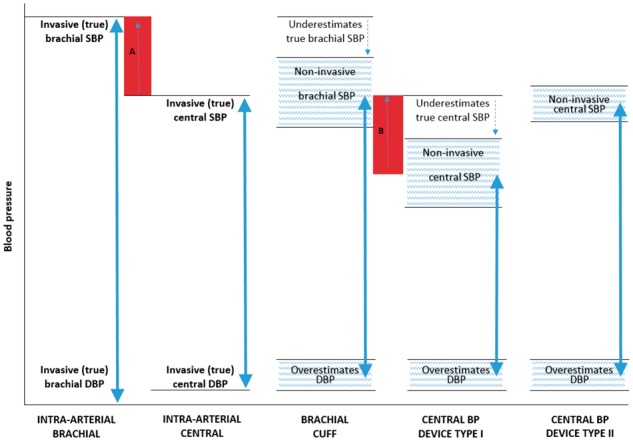
Illustration of the differences in systolic (SBP) and diastolic (DBP) blood pressure (BP) between intra-arterial brachial and central BP, brachial cuff BP, and non-invasive central BP devices Types I and II (BP ranges of different methods represented by the double arrows). Red shaded area A, represents the true (intra-arterial) level of central-to-brachial SBP amplification, and red shaded area B represents the non-invasive estimated central-to-brachial SBP amplification (A and B may be similar in magnitude). The non-invasive central SBP estimated using central BP device Type II may be higher than non-invasive brachial cuff SBP, but this is due to underestimation of true (intra-arterial) brachial SBP with the cuff device and, therefore, does not reflect physiological amplification. The hatched areas denote that there will be a degree of variability in estimated BP between devices.
(2) Calibration of peripheral artery signals using brachial cuff BP. Accuracy of non-invasive central BP methods depends on the accuracy of methods used to calibrate the peripheral artery (radial, brachial, or carotid) waveform. Central BP estimations can be acquired with reasonable accuracy if peripheral waveforms are calibrated with invasive BP.23,30,31 However, brachial cuff BP is commonly used for calibration purposes and this introduces error32,33 as a consequence of the recognized underestimation of intra-arterial brachial SBP together with overestimation of intra-arterial brachial DBP.34–36 Therefore, calibrating the waveform to brachial cuff SBP and DBP is likely to result in underestimation of central SBP and central pulse pressure, but overestimation of central DBP relative to intra-arterial central DBP. Furthermore, amplification of SBP from brachial to radial arteries may compound the error in underestimation of central SBP and central pulse pressure when radial artery waveforms are calibrated using brachial SBP and DBP.37–40 The magnitude of calibration-induced error may often exceed 10 mmHg for each of SBP, pulse pressure, and DBP, irrespective of brachial cuff BP methods (e.g. auscultation or oscillometry).41
Despite these issues, calibration of peripheral waveforms with brachial SBP and DBP still results in the estimation of a central SBP that is relative to, and usually lower than, the measured brachial cuff SBP (device Type I). This information is important to know because brachial cuff SBP is used clinically and the proportional difference with central SBP (degree of SBP amplification) may be relevant to hypertension management decisions, including monitoring the effects of drug treatment in which responses may differ between the brachial artery and the aorta.9,12 Different calibration modes have been successfully employed in an attempt to derive more accurate estimations of central SBP (device Type II);8,29,30,42 however, in doing so, the potentially confusing impression may be given that central SBP is substantially higher than brachial SBP (reverse amplification), which is likely to be non-physiological under normal conditions and arises from the combination of underestimated brachial SBP by cuff, together with more accurate (higher) central SBP estimation. Nonetheless, random scatter or measurement error could also contribute to reverse amplification, particularly among older arterial ageing phenotypes where the difference between central and brachial SBP may be small. Although improved accuracy of central SBP using a Type II device is desirable in terms of better risk stratification related to BP, determination of the true degree of aorta-to-brachial SBP amplification will be lost unless a recalibrated estimation of brachial SBP is provided, along with the details of how this is derived. Notwithstanding the complex interaction of calibration and wave propagation phenomena relating central and peripheral SBP, it still needs to be determined if the recalibrated brachial SBP derived by this process is an accurate estimate of intra-arterial brachial SBP.
Data from meta-analysis indicate that using MAP and DBP could be a preferred calibration option to provide a relatively more accurate non-invasive estimation of central SBP.43 Several methods may be used to derive MAP, including by calculation from potentially inaccurate brachial cuff BP [e.g. DBP + 1/3 (or 40%) pulse pressure,44 or from integration of the pressure waveforms calibrated to cuff BP], or estimation from the peak oscillometric signal,45 but information regarding the accuracy of these approaches is limited. Central BP indices derived from oscillometric MAP and DBP calibrated peripheral waveforms show stronger associations with hypertension-related end organ disease and outcomes than either brachial BP or central BP derived by calibration using brachial oscillometric SBP and DBP.46–50 These data come from independent investigators that have used the same device,46–50 and it remains to be clarified if the findings may be more widely generalizable or if this is a device-specific phenomenon. The observation that much of the inaccuracy in estimated central BP lies with poor calibration from inaccurate brachial cuff BP, implies that better BP risk stratification might be achieved with more accurate brachial cuff BP per se. Indeed, calls have been made for more rigorous brachial BP accuracy criteria.51,52
(ii) Recommendations. To achieve accurate non-invasive assessment of true central BP, more accurate non-invasive estimates of intra-arterial brachial BP are needed. Establishing more rigorous accuracy criteria for brachial BP is desirable. Current evidence suggests that calibration with MAP and DBP may provide a more accurate assessment of central BP than calibration with SBP and DBP, although further validation is required across cohorts with different characteristics (e.g. age, sex, levels of BP).
(3) Disparity in validation standards. Multiple reference standards and calibration methods have been used among previous ‘validation’ studies, often including:
Comparison of estimated central BP (by non-invasive device) with invasive central BP (by fluid filled or micromanometer-tipped intra-arterial catheter), and calibration of the non-invasive waveforms with invasive MAP and DBP on the assumption of minimal difference in MAP and DBP between central to peripheral large arteries (i.e. 1–3 mmHg). This assesses accuracy of the mathematical process of transforming peripheral into central pressure per se and not the accuracy of the device as used in clinical practice.
Comparison of estimated central BP with invasive central BP, and calibration of the non-invasive waveforms with non-invasive BP (typically brachial SBP and DBP or MAP and DBP). This assesses accuracy of the central BP estimation as may be used in clinical practice.
Comparison of estimated central BP from one non-invasive device with another non-invasive device as the reference standard (usually the SphygmoCor device),17 and calibration of both devices with the same non-invasive brachial BP, to assess inter-device concordance.
(3) Recommendations. The reference standard against which device accuracy of central BP estimation is gauged should be intra-arterial catheter in the ascending aorta [expanded details within section entitled ‘Invasive (intra-arterial) central BP reference standard’]. The calibration mode may vary depending on the operating principles of the device, but in all cases, details of the calibration method developed by the manufacturer should be provided (see Issues 1 and 2). If the brachial BP waveform undergoes recalibration to produce a ‘new’ brachial BP (as per two Type II devices already using this method),29,42 then the recalibrated brachial BP values (and the method to derive them) should also be provided so that the level of estimated aorta-to-brachial SBP amplification can be gauged.
(4) Limitations in performing invasive validation studies. The accepted central BP reference standard is intra-arterial measurement by catheter,4,53 but this method still requires careful handling by experienced operators to avoid measurement error. The technique is only routinely and necessarily performed in selected clinical populations (i.e. patients with suspected coronary artery disease or children with congenital heart disease), thus rendering data potentially non-generalizable to other patient populations or healthy people in whom non-invasive central BP devices may be clinically applied. Validation against the invasive standard among people for whom central artery catheterization is not clinically indicated is a matter for review by ethical boards.
(4) Recommendations. Whilst acknowledging that an intra-arterial validation standard is less practical, currently there are no non-invasive alternatives. In any case, with appropriate sample size it should still be possible to recruit participants from the catheterization laboratory of different age, sex, BP range, heart rate range, body habitus (e.g. body mass index, arm size), and disease status (e.g. +/− coronary artery disease, +/− diabetes). In future, it may be reasonable to use non-invasive central BP devices as reference standards for comparison among different subject populations. The acceptance criteria for such devices are yet to be determined, but may include appropriate validation by comparison with the intra-arterial standard, as well as proved clinical prognostic value. Currently such a device is not available. A summary of issues and recommendations is provided in Table 2.
Table 2.
Summary of issues in the assessment and reporting of central blood pressure (BP) monitors and recommendations
| Issue | Recommendation |
|---|---|
| 1. Disparity of non-invasive central BP devices as to what is being measured | Device manufacturers should clearly state the purported measurement function of their device. These can be broadly categorized into two types based on function: Type I—estimates central BP relative to measured brachial BP; Type II—estimates intra-arterial central BP |
| Both function types may be available within a single device | |
| 2. Calibration of peripheral artery signals using brachial cuff BP | To achieve accurate non-invasive assessment of true central BP, more accurate non-invasive estimates of intra-arterial brachial BP are needed. Establishing more rigorous accuracy criteria for brachial BP is desirable. Current evidence suggests that calibration with MAP and DBP may provide a more accurate assessment of central BP than calibration with SBP and DBP |
| 3. Disparity in validation standards | The reference standard against which device accuracy of central BP estimation is gauged should be intra-arterial catheter in the ascending aorta. Details of the calibration method should be provided. If the brachial BP waveform undergoes recalibration to produce a ‘new’ brachial BP, then the recalibrated brachial BP values (and the method to derive them) should also be provided so that the level of estimated aorta-to-brachial systolic BP amplification can be gauged |
| 4. Limitations in performing invasive validation studies | In future, it may be reasonable to use non-invasive central BP devices as reference standards, but the acceptance criteria for this are yet to be determined |
Validation protocol requirements
Several scientific bodies have developed validation protocols for non-invasive peripheral BP monitors,54–59 yet they differ on procedural features such as sample size and selection criteria, number of assessment phases, acceptable margin of error, BP range and pass/fail criteria.52 A ‘universal’ brachial BP validation protocol is under development through collaboration of the American Association for the Advancement of Medical Instrumentation (AAMI), the International Organization for Standardisation (ISO), and the ESH Working Group on Blood Pressure Monitoring and Cardiovascular Variability, and projected to be in effect in 2018. This harmonized protocol is expected to inform many aspects of central BP validation protocols that equally apply to brachial BP (e.g. age, gender, BP range), but an internationally accepted central BP protocol directed by regulatory authorities is still required, as distinct from the forthcoming brachial BP protocol.
Recommendations below focus on central BP specific protocol requirements, with some relevant features drawn from existing validation guidelines.54–56 For unambiguous interpretation of requirements, facets of the protocol have been listed as proposed in the revised Australian Code for the Responsible Conduct of Research, in terms of ‘must’, ‘should’ and ‘may’. ‘Must’ indicates a necessary component for highest quality, ‘should’ indicates a strong recommendation, but may not be the only way that the component can be achieved, and ‘may’ is used to provide further guidance.60 Protocol requirements are summarised in Table 3 as a pro-forma guide for investigators. Less attention is given to protocol features equally relevant to brachial BP (i.e. sample characteristics, results reporting and pass criteria) but some proposed direction is also provided based on existing guidelines54–56 for interim guidance (and to highlight outstanding issues) prior to development of an accepted international central BP validation protocol. A list of issues in need of resolution in the future development of such a protocol is provided in Table 4.
Table 3.
Summary of central blood pressure (BP) device validation protocol components and requirements
| Protocol section | Protocol item | Protocol requirement | Protocol undertaken (circle yes/no….comment) |
|---|---|---|---|
| Study setting | Isolated room without disturbing influences | Should | YES NO…………………………………………… |
| Non-invasive central BP device measurement standards | List manufacturer, model, software version, operating principles, signal processing step/s, calibration processes | Must | YES NO…………………………………………… |
| Time for BP measures; time points of brachial BP and central BP; cuff deflation speed | Should | YES NO…………………………………………… | |
| Define and use appropriate cuff size | Must | YES NO…………………………………………… | |
| Dimensions of inflatable bladder for all cuff sizes available; process to determine cuff size | Should | YES NO…………………………………………… | |
| Process of familiarization with equipment | Should | YES NO…………………………………………… | |
| Separate validation studies for additional or optional features or functions | Must | YES NO…………………………………………… | |
| Process/s of quality control; process used to delineate acceptable quality; number of unacceptable readings; reason/s for exclusion | Must | YES NO…………………………………………… | |
| Invasive (intra-arterial) central BP reference standard | Micromanometer-tipped catheter used if minor inflection points to be identified | Should | YES NO…………………………………………… |
| Full description of catheter; frequency response and handling procedures | Must | YES NO…………………………………………… | |
| Performance comparison of fluid filled catheter with micromanometer-tipped catheter | May | YES NO…………………………………………… | |
| Data acquisition at rest | Period of undisturbed rest; medications used | Should | YES NO…………………………………………… |
| No talking. Free from acute hemodynamic interventions | Must | YES NO…………………………………………… | |
| Test device compared with reference over time-period matching the test device deflation cycle; recorded under stable conditions | Must | YES NO…………………………………………… | |
| Complete description of protocol; time interval between test device and reference measures | Must | YES NO…………………………………………… | |
| Data acquisition at BP intervention | Hemodynamic change from resting state | May | YES NO…………………………………………… |
| Description of the intervention procedure | Must | YES NO…………………………………………… |
SBP, systolic BP; DBP, diastolic BP. Complete details of protocol components and requirements are contained within the body text. Must, necessary component for highest quality; Should, strong recommendation, but probably not the only way that the component can be achieved; May, further guidance required.60
Table 4.
Summary list of issues for consideration in development of an internationally accepted central blood pressure (BP) validation protocol
| Validation protocol features | Comments |
|---|---|
| Reference method | |
| Non-invasive reference standard | What criteria needed to satisfy for an acceptable non-invasive alternative to the invasive method which restricts study sample characteristics? |
| Error | |
| Minimum standard | What is the magnitude of the minimum acceptable error and its frequency based on the invasive reference standard? |
| Study sample | |
| Definition of general population sample | Which populations should be considered as special as there may be different device measurement accuracy from the general population, and therefore require separate validation? |
| Minimum sample size for a general population study | Based on the reference method for an acceptable statistical risk of false positive and negative results |
| Sample size for validations in special groups | To be defined after a successful study in the general population has been completed |
| Sex and age distribution | Representation of males and females, adolescents, young and middle aged adults and elderly |
| BP and heart rate range criteria | Based on reference central BP measurements and heart rate during the procedure? |
| Cuff size | Minimum number of subjects investigated per different cuff size, or number of different cuffs to be studied in a single study? |
| Exclusion criteria | On the basis of increased reference BP variation within individual validation procedures or clinical conditions |
| Procedural | |
| Number of measurements | Procedure for the number of reference and test BP measurements in a validation session |
| Comparison with reference | How to compare when operating characteristics differ between reference (i.e. beat-to-beat) and non-invasive test devices (i.e. averaging over seconds to minutes) and influence of respiratory variation and arrhythmias? |
| Reporting | |
| Data and pass criteria | What data, statistics and study features to be reported? What pass/fail criteria? |
Study setting. The isolated room should be without disturbing influences of excessive ambient noise from monitoring devices.
Non-invasive central BP device measurement standards. The manufacturer, model, software version, and description of operating principles, including the signal processing step/s (averaging, filtering) and complete calibration processes (including methods to derive calibration points such as SBP, DBP, and MAP) must be provided. The time taken to complete the measurement of BP, including the time points at which brachial BP and central BP are estimated and the cuff deflation speed should be reported. This is relevant to the timing of non-invasive BP measurements with respect to intra-arterial monitoring because there may be substantial beat-to-beat variation in intra-arterial BP that may not be captured using a non-invasive BP device.56 The appropriate cuff size must be defined and used. The dimensions of the inflatable bladder for all cuff sizes available with the device should be reported, as well as the process to determine appropriate cuff size (e.g. measurement of upper arm and/or fitting within prescribed range indicated on the cuff). A process of familiarization with the equipment, involving test measurements before starting the validation study should be performed and reported. If there are additional or optional features or functions, separate validation studies must be performed. Any process used to gauge quality control of waveform or BP measures, and the process used to delineate acceptable quality for analysis must be reported. For example if runs of bigeminy, trigeminy, atrial fibrillation, or isolated premature beats and the following compensatory beats have been removed from analysis.54 The number of readings deemed unacceptable must be reported, together with the reason/s for exclusion.
Invasive (intra-arterial) central BP reference standard. Micromanometer-tipped catheters are the preferred instruments to use, but meticulously handled fluid-filled catheters may also be acceptable for accurately measuring intra-arterial BP.61–63 For measurement of waveform features, in which minor inflection points need to be identified (i.e. augmented pressure), high frequency, micromanometer-tipped catheters with high-frequency acquisition systems should be used as signal-dampening will alter waveform features. A full description of the type, make and model of catheter, the frequency response and handling procedures must be provided. This should include: the process to determine frequency response; catheter French size; tubes length and number of taps and connectors, flushing protocol, sensor/s position on the catheter; how the manifold position was maintained at heart level (for fluid-filled devices where hydrostatic pressure may influence BP data); calibration/zeroing steps performed together with details of additional equipment used for this process where relevant (note: zero drift may still be a cause of imprecision when using micromanometer-tipped catheters); details of how positioning in the central aorta was confirmed in each case (e.g. fluoroscopy); sampling rate at which waveforms were recorded and all waveform data processing steps together with details of equipment/software used for this purpose; process by which waveform period for comparison with non-invasive central BP device was confirmed (e.g. marking relevant time points). As the recording unit and A/D converter also have their frequency characteristics, the frequency response of the overall acquisition system from fluid-filled catheter or micromanometer-tip to the end-recording unit (graph-plotter or digital data recorded) should be provided. Procedures such as the ‘pop test’ may be used to evaluate the overall acquisition system natural frequency and damping coefficient.62 Alternatively, the performance of the fluid filled catheter systems may be assessed by comparison with micromanometer-tipped catheter (including challenging the fluid filled system across different heart rates).
Data acquisition at rest. By necessity, subjects will be supine during catheterization. Before recording resting values, the subject should be allowed a period of undisturbed rest (e.g. without talking or movement for at least 5 min54,64) and must be free from the acute effects of interventions causing hemodynamic changes (e.g. vasoactive drugs,65 contrast dye66). There must be no talking whilst BP measures are being recorded. Medications used during the procedure should be reported. The non-invasive central BP values must be compared with the intra-arterial central BP (reference) values averaged over a time-period matching the deflation cycle of the non-invasive device and recorded under stable conditions, ideally simultaneously, or as contemporaneous as possible. If simultaneous measurement is not possible, a complete description of the protocol and the interval between intra-arterial and non-invasive BP measures must be provided, and ideally with the order between measures randomised. The time difference between measures should be in the immediate vicinity and without possibility of disturbing influences such as subject positional changes, drugs, or other interventions. The average intra-arterial monitoring period will vary between devices being tested due to different operating characteristics (e.g. range 10 s to 1 min) and should be reported. Comparison of single beat intra-arterial BP with non-invasive central BP is not acceptable due to potential for selective bias, but also because of the aforementioned issues of beat-to-beat variation of intra-arterial BP, which can be influenced by respiration (2–4 mmHg lower BP with inspiration) under normal circumstances, but with greater differences (>10 mmHg lower BP with inspiration) in the presence of cardiac or respiratory abnormalities such as constrictive pericarditis or severe pulmonary disease.53 Due to limits around the amount of time that can be dedicated to validation studies within the clinical laboratory, it may not be feasible to undertake repeat measures for comparison, but whenever possible this is preferred, with three measures being optimal.54
Data acquisition at BP intervention. Validation testing under hemodynamic conditions involving a change from the resting state may be undertaken. 24 hour ambulatory BP monitoring is used as an adjunct to clinical BP to determine underlying BP control and devices with ambulatory central BP capacity are available.47,67,68 Testing the performance of non-invasive central BP monitors under ambulatory conditions is not feasible using intra-aortic monitoring, but in any case this may not necessarily be required since subjects undertaking 24 hour ambulatory BP are instructed to avoid exercise and temporarily stop moving or talking, to relax the arm and breathe normally during device operation.69 Thus, the main objective for validation testing of ambulatory BP monitors is to determine device performance under conditions of a change in BP and heart rate from the stable resting state. To this end, a variety of standardized interventions causing a statistically significant (P < 0.05) hemodynamic change for BP and heart rate, may be acceptable, for example administering a standard dose of glyceryl trinitrate,9 table tilting, isometric hand grip exercise, or supine cycling.31 Once the hemodynamic change has been initiated, performance of the non-invasive BP test device can be tested against the intra-arterial standard, as described for the resting state. Description of the intervention procedure must be reported. Care should be taken to avoid testing during phases of large and acutely variable hemodynamic shifts such as may occur with bolus administration of vasoactive drugs, contrast dye or in the early phase of table tilting.
Sample characteristics. A sample size of at least n = 85 adults is proposed based on brachial BP validation protocols and the requirement to detect a mean difference of 5 mmHg [standard deviation (SD) of the difference 8 mmHg] with an estimated power of >99% (two-sided alpha of 5%), as currently proposed by the AAMI standard. Nevertheless, invasive BP measures during clinical procedures face additional constraints that can increase BP variability, such as selective patient characteristics and limited time for repeat measurements. Thus, a definitive sample size based on robust statistical methods is still needed. If devices are to be used in paediatric age groups, then wherever possible, accuracy should be tested separately in those groups and not extrapolated from adults. Participants should have a sex distribution of at least 30% male and female and in sinus rhythm unless the device is being tested for accuracy during arrhythmias.55 In keeping with all other brachial cuff BP validation guidelines, devices should be tested over a range of BP. An indicative range for invasive central SBP may be ≤100 mmHg (≥5% of readings), ≥140 mmHg (≥20% of readings), and ≥160 mmHg (≥5% of readings), and the indicative range for invasive central DBP may be ≤60 mmHg (≥5% of readings), ≥85 mmHg (≥20% of readings), and ≥100 mmHg (≥5% of readings).54 Device accuracy should also be tested across a range of heart rates (i.e. 60–100 b.p.m.), because heart rate influences aortic stiffness and SBP amplification.70,71 Exact criteria for BP and heart rate ranges needs to be resolved. Unless testing device performance in specific cardiac or respiratory diseases, it should be noted that subjects with the following conditions have a higher likelihood of measurement error due to abnormal haemodynamics: severe valvular stenosis or regurgitation, severely impaired left ventricular systolic function, atrial fibrillation, constrictive pericarditis, pericardial tamponade, restrictive cardiomyopathy or severe pulmonary disease.
Statistical requirements. Beyond the reporting of details already mentioned, description of subjects must be presented and should include basic demographics (age, sex, ethnicity, body mass index), medications and clinical conditions including outcome of coronary catheterization procedure. Comparison between non-invasive and reference BP’s must report mean difference, SD of the mean difference, and limits of agreement (LOA), illustrated by modified Bland–Altman plots72 in which the mean of measurements is replaced by the reference catheter measurement. Scatter plots of the measures obtained with the non-invasive device (on Y axis) vs. the reference method (on X axis), with the line of equality, may also be provided for descriptive purposes. Non-uniformity of SD across the range of measurement or evidence of non-constant bias (e.g. increasing difference between measures with increasing values) must be visually checked on the Bland–Altman plots. An increase in variability of the differences as the magnitude of the measurement increases can be dealt with by log transformation of both measurements before analysis and the LOA derived from log transformed data should be reported after back-transformation (and thus expressed as ratios of the actual measurements). When log transformations do not solve the problem of a relationship between the difference and the mean, regression approaches or non-parametric approaches can be used instead, but with preference for the latter (for details see72). Absolute BP differences from the reference should be presented as a clinically meaningful illustration of the results but without a pass/fail criteria.54 The proposed pass criteria is if the device has a mean difference of ≤5 mmHg with SD ≤8 mmHg compared with the reference, based on the magnitude of minimum tolerable error and frequency,54 but also recognizing this is a feature requiring resolution in future guidelines.
Conclusions and future directions
A major reason for producing this document to improve device validity has been the ongoing controversy over whether central BP adds prognostic value to that from routine brachial cuff BP. A recent Framingham paper found no additional value,73 while two systematic reviews not including those data came to opposite conclusions.14,74 For unfamiliar readers, an accompanying editorial addresses the issues.75 A number of perceived deficits relating to both brachial and central BP measurement have been brought to attention in this current paper, and accordingly some points of intent require additional explanation. Firstly, despite the premise of clinical brachial BP measurement being based on essentially inaccurate cuff measures, brachial BP is still important and regarded as the clinical standard. This document should not be interpreted as challenging the clinical utility of brachial BP measurement, nor its value in hypertension management. Similarly, this document does not seek to undermine the potential clinical use of currently available non-invasive central BP devices that have not undergone the validation procedures recommended in this document, but have already proved to provide measurement of physiological (e.g. vascular ageing)76 or prognostic significance. Nevertheless, with the advent of ‘precision medicine’, clinical decisions are expected to be refined and improved by using more accurate BP monitors into the future, whether brachial or central BP, and this is a key research need. Additional guidance on central BP validation protocols is keenly awaited from regulatory authorities.
Acknowledgements
The task force is grateful for the contribution by the European Society of Hypertension (ESH) Working Group on Arterial Structure and Function, and the ESH Working Group on Blood Pressure Monitoring and Cardiovascular Variability.
Funding
Completion of this work by JS was made possible through funding from the High Blood Pressure Research Council of Australia Franco-Australian Exchange Program, the Menzies Institute for Medical Research and a Mobility Scholarship from L'Institut Servier.
Conflict of interest: A.A. has received equipment for research from BPLab and AtCor Medical; J.B. has received equipment for research projects from AtCor Medical; C.C. has received funding and equipment for research projects from Omron Corporation and Microlife Corporation; P.C. and King’s College London have an interest in Centron Diagnostics; J.C. has received equipment and travel grants from I.E.M. GmbH, Philips Healthcare; I.F., M.R., R.R.T., L.V.B., and I.W.—none; A.H. has received loans of equipment from ALOKA/Hitachi Medical Systems, AtCor Medical, Finapres Medical Systems, I.E.M. GmbH, Philips Healthcare, Pulsecor Ltd, SpaceLabs/Dolby and USCOM; B.M.D. has received equipment from AtCor Medical and I.E.M. GmbH, Philips Healthcare; S.M. has received revenue from ALAM Medical, AtCor Medical, Omron and Esaote; T.G.P. has received equipment from I.E.M. GmbH; G.P. has conducted validation studies for various manufacturers and is a member of the advisory board of Medaval; J.P. has received equipment for research projects from Fukuda Denshi; A.P. has received funding and equipment for research projects from AtCor Medical and I.E.M. GmbH; P.S. has received equipment for research projects from Fukuda Denshi; G.S. has conducted validation studies for various manufacturers; advised manufacturers on device development, and is deputy chairman of the advisory board of Medaval; G.S. has received funding and equipment for research projects from AtCor Medical and I.E.M. GmbH, Philips Healthcare; J.S. has received funding and equipment for research projects from AtCor Medical, I.E.M. GmbH, Philips Healthcare and Pulsecor. C.V. has received equipment and honoraria from AtCor Medical and Omron; J.W. has received funding and equipment for research projects from Omron; T.W. has received an unrestricted grant for a multicentre study as well as lecture fees from I.E.M. GmbH, Germany.
References
- 1. Booth J. A short history of blood pressure measurement. Proc R Soc Med 1977;70:793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karamanou M, Papaioannou TG, Tsoucalas G, Tousoulis D, Stefanadis C, Androutsos G.. Blood pressure measurement: lessons learned from our ancestors. Curr Pharm Des 2015;21:700–704. [DOI] [PubMed] [Google Scholar]
- 3. Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 4. Avolio AP, Van Bortel LM, Boutouyrie P, Cockcroft JR, McEniery CM, Protogerou AD, Roman MJ, Safar ME, Segers P, Smulyan H. Role of pulse pressure amplification in arterial hypertension: experts’ opinion and review of the data. Hypertension 2009;54:375–383. [DOI] [PubMed] [Google Scholar]
- 5. Kroeker EJ, Wood EH.. Comparison of simultaneously recorded central and peripheral arterial pressure pulses during rest, exercise and tilted position in man. Circ Res 1955;3:623–632. [DOI] [PubMed] [Google Scholar]
- 6. Kroeker EJ, Wood EH.. Beat-to-beat alterations in relationship of simultaneously recorded central and peripheral arterial pressure pulses during Valsalva maneuver and prolonged expiration in man. J Appl Physiol 1956;8:483–494. [DOI] [PubMed] [Google Scholar]
- 7. Rowell LB, Brengelmann GL, Blackmon JR, Bruce RA, Murray JA.. Disparities between aortic and peripheral pulse pressures induced by upright exercise and vasomotor changes in man. Circulation 1968;37:954–964. [DOI] [PubMed] [Google Scholar]
- 8. Cheng HM, Sung SH, Shih YT, Chuang SY, Yu WC, Chen CH.. Measurement of central aortic pulse pressure: noninvasive brachial cuff-based estimation by a transfer function vs. a novel pulse wave analysis method. Am J Hypertens 2012;25:1162–1169. [DOI] [PubMed] [Google Scholar]
- 9. Kelly RP, Gibbs HH, O’rourke MF, Daley JE, Mang K, Morgan JJ, Avolio AP. Nitroglycerin has more favourable effects on left ventricular afterload than apparent from measurement of pressure in a peripheral artery. Eur Heart J 1990;11:138–144. [DOI] [PubMed] [Google Scholar]
- 10. McEniery CM, Yasmin McDonnell B, Munnery M, Wallace SM, Rowe CV, Cockcroft JR, Wilkinson IB. Central pressure: variability and impact of cardiovascular risk factors. The Anglo-Cardiff Collaborative Trial II. Hypertension 2008;6:1476–1482. [DOI] [PubMed] [Google Scholar]
- 11. Sharman JE, Stowasser M, Fassett RG, Marwick TH, Franklin SS.. Central blood pressure measurement may improve risk stratification. J Hum Hypertens 2008;22:838–844. [DOI] [PubMed] [Google Scholar]
- 12. Protogerou AD, Papaioannou TG, Lekakis JP, Blacher J, Safar ME.. The effect of antihypertensive drugs on central blood pressure beyond peripheral blood pressure. Part I: (Patho)-physiology, rationale and perspective on pulse pressure amplification. Curr Pharm Des 2009;15:267–271. [DOI] [PubMed] [Google Scholar]
- 13. Herbert A, Cruickshank JK, Laurent S, Boutouyrie P.. Establishing reference values for central blood pressure and its amplification in a general healthy population and according to cardiovascular risk factors. Eur Heart J 2014;35:3122–3133. [DOI] [PubMed] [Google Scholar]
- 14. Vlachopoulos C, Aznaouridis K, O’rourke MF, Safar ME, Baou K, Stefanadis C.. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J 2010;15:1865–1871. [DOI] [PubMed] [Google Scholar]
- 15. Cheng H-M, Chuang S-Y, Sung S-H, Yu W-C, Pearson A, Lakatta EG, Pan W-H, Chen C-H. Derivation and validation of diagnostic thresholds for central blood pressure measurements based on long-term cardiovascular risks. J Am Coll Cardiol 2013;62:1780–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang CM, Wang KL, Cheng HM, Chuang SY, Sung SH, Yu WC, Ting CT, Lakatta EG, Yin FC, Chou P, Chen CH. Central versus ambulatory blood pressure in the prediction of all-cause and cardiovascular mortalities. J Hypertens 2011;29:454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Millasseau S, Agnoletti D.. Non-invasive estimation of aortic blood pressures: a close look at current devices and methods. Curr Pharm Des 2015;21:709–718. [DOI] [PubMed] [Google Scholar]
- 18. McEniery CM, Cockcroft JR, Roman MJ, Franklin SS, Wilkinson IB.. Central blood pressure: current evidence and clinical importance. Eur Heart J 2014;35:1719–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Papaioannou TG, Protogerou A, Stefanadis C.. Comparison between Mobil-O-Graph and the SphygmoCor device for central systolic blood pressure estimation: consensus is required for ‘validation protocols’. Blood Press Monit 2012;17:259–260. [DOI] [PubMed] [Google Scholar]
- 20. Sharman JE. Central pressure should be used in clinical practice. Artery Res 2015;9:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mitchell GF. Central pressure should not be used in clinical practice. Artery Res 2015;9:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Agnoletti D, Millasseau S, Topouchian J, Safar ME, Blacher J.. Comparison of central blood pressure devices on the basis of a modified protocol of the European Society of Hypertension: application to the Centron cBP301. Blood Press Monit 2014;19:103–108. [DOI] [PubMed] [Google Scholar]
- 23. Chen CH, Nevo E, Fetics B, Pak PH, Yin FC, Maughan WL, Kass DA. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation 1997;95:1827–1836. [DOI] [PubMed] [Google Scholar]
- 24. Pauca AL, Kon ND, O’rourke MF.. The second peak of the radial artery pressure wave represents aortic systolic pressure in hypertensive and elderly patients. Br J Anaesth 2004;92:651–657. [DOI] [PubMed] [Google Scholar]
- 25. Pereira T, Maldonado J, Coutinho R, Cardoso E, Laranjeiro M, Andrade I, Conde J. Invasive validation of the complior analyse in the assessment of central artery pressure curves: a methodological study. Blood Press Monit 2014;19:280–287. [DOI] [PubMed] [Google Scholar]
- 26. Van Bortel LM, Balkestein EJ, van der Heijden-Spek JJ, Vanmolkot FH, Staessen JA, Kragten JA, Vredeveld JW, Safar ME, Struijker Boudier HA, Hoeks AP. Non-invasive assessment of local arterial pulse pressure: comparison of applanation tonometry and echo-tracking. J Hypertens 2001;19:1037–1044. [DOI] [PubMed] [Google Scholar]
- 27. Salvi P, Lio G, Labat C, Ricci E, Pannier B, Benetos A.. Validation of a new non-invasive portable tonometer for determining arterial pressure wave and pulse wave velocity: the PulsePen device. J Hypertens 2004;22:2285–2293. [DOI] [PubMed] [Google Scholar]
- 28. Quail MA, Steeden JA, Knight D, Segers P, Taylor AM, Muthurangu V.. Development and validation of a novel method to derive central aortic systolic pressure from the MR aortic distension curve. J Magn Reson Imaging 2014;40:1064–1070. [DOI] [PubMed] [Google Scholar]
- 29. Takazawa K, Kobayashi H, Shindo N, Tanaka N, Yamashina A.. Relationship between radial and central arterial pulse wave and evaluation of central aortic pressure using the radial arterial pulse wave. Hypertens Res 2007;30:219–228. [DOI] [PubMed] [Google Scholar]
- 30. Weber T, Wassertheurer S, Rammer M, Maurer E, Hametner B, Mayer CC, Kropf J, Eber B. Validation of a brachial cuff-based method for estimating central systolic blood pressure. Hypertension 2011;58:825–832. [DOI] [PubMed] [Google Scholar]
- 31. Sharman JE, Lim R, Qasem AM, Coombes JS, Burgess MI, Franco J, Garrahy P, Wilkinson IB, Marwick TH. Validation of a generalized transfer function to noninvasively derive central blood pressure during exercise. Hypertension 2006;47:1203–1208. [DOI] [PubMed] [Google Scholar]
- 32. Hope SA, Meredith IT, Cameron JD.. Effect of non-invasive calibration of radial waveforms on error in transfer-function-derived central aortic waveform characteristics. Clin Sci (Lond) 2004;107:205–211. [DOI] [PubMed] [Google Scholar]
- 33. Shih YT, Cheng HM, Sung SH, Hu WC, Chen CH.. Quantification of the calibration error in the transfer function-derived central aortic blood pressures. Am J Hypertens 2011;24:1312–1317. [DOI] [PubMed] [Google Scholar]
- 34. Cheng HM, Sung SH, Shih YT, Chuang SY, Yu WC, Chen CH.. Measurement accuracy of a stand-alone oscillometric central blood pressure monitor: a validation report for Microlife WatchBP Office Central. Am J Hypertens 2013;26:42–50. [DOI] [PubMed] [Google Scholar]
- 35. Kobayashi H, Kinou M, Takazawa K.. Correlation between the brachial blood pressure values obtained using the cuff method and the central blood pressure values obtained invasively. Intern Med 2013;52:1675–1680. [DOI] [PubMed] [Google Scholar]
- 36. Hunyor SN, Flynn JM, Cochineas C.. Comparison of performance of various sphygmomanometers with intra-arterial blood-pressure readings. Br Med J 1978;2:159–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Picone DS, Climie R, Ahuja KD, Keske MA, Sharman JE.. Brachial-to-radial-systolic-blood-pressure-amplification: implications of age and estimated central blood pressure from radial tonometry. J Hypertens 2015;33:1876–1883. [DOI] [PubMed] [Google Scholar]
- 38. Segers P, Mahieu D, Kips J, Rietzschel E, De Buyzere M, De Bacquer D, Bekaert S, De Backer G, Gillebert T, Verdonck P, Van Bortel L. Amplification of the pressure pulse in the upper limb in healthy, middle-aged men and women. Hypertension 2009;54:414–420. [DOI] [PubMed] [Google Scholar]
- 39. Xu J, Wu Y, Su H, Hu W, Li J, Wang W, Liu X, Cheng X. The value of a blood pressure determination method using a novel non-invasive blood pressure device against the invasive catheter measurement. PLoS One 2014;9:e100287.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Verbeke F, Segers P, Heireman S, Vanholder R, Verdonck P, Van Bortel LM.. Noninvasive assessment of local pulse pressure: importance of brachial-to-radial pressure amplification. Hypertension 2005;46:244–248. [DOI] [PubMed] [Google Scholar]
- 41. Cheng HM, Lang D, Tufanaru C, Pearson A.. Measurement accuracy of non-invasively obtained central blood pressure by applanation tonometry: a systematic review and meta-analysis. Int J Cardiol 2013;167:1867–1876. [DOI] [PubMed] [Google Scholar]
- 42. Ding FH, Fan WX, Zhang RY, Zhang Q, Li Y, Wang JG.. Validation of the noninvasive assessment of central blood pressure by the SphygmoCor and Omron devices against the invasive catheter measurement. Am J Hypertens 2011;24:1306–1311. [DOI] [PubMed] [Google Scholar]
- 43. Papaioannou TG, Karageorgopoulou TD, Sergentanis TN, Protogerou AD, Psaltopoulou T, Sharman JE, Weber T, Blacher J, Daskalopoulou SS, Wassertheurer S, Khir AW, Vlachopoulos C, Stergiopulos N, Stefanadis C, Nichols WW, Tousoulis D. Accuracy of commercial devices and methods for noninvasive estimation of aortic systolic blood pressure a systematic review and meta-analysis of invasive validation studies. J Hypertens 2016;34:1237–1248. [DOI] [PubMed] [Google Scholar]
- 44. Bos WJ, Verrij E, Vincent HH, Westerhof BE, Parati G, van Montfrans GA.. How to assess mean blood pressure properly at the brachial artery level. J Hypertens 2007;25:751–755. [DOI] [PubMed] [Google Scholar]
- 45. Smulyan H, Sheehe PR, Safar ME., Marwick TH, Sharman JE. A preliminary evaluation of the mean arterial pressure as measured by cuff oscillometry. Am J Hypertens 2008;21:166–171. [DOI] [PubMed] [Google Scholar]
- 46. Negishi K, Yang H, Wang Y, Nolan MT, Negishi T, Pathan F, Marwick TH, Sharman JE. Importance of calibration method in central blood pressure for cardiac structural abnormalities. Am J Hypertens 2016;29:1070–1076. [DOI] [PubMed] [Google Scholar]
- 47. Protogerou AD, Argyris AA, Papaioannou TG, Kollias GE, Konstantonis GD, Nasothimiou E, Achimastos A, Blacher J, Safar ME, Sfikakis PP. Left-ventricular hypertrophy is associated better with 24-h aortic pressure than 24-h brachial pressure in hypertensive patients: the SAFAR study. J Hypertens 2014;32:1805–1814. [DOI] [PubMed] [Google Scholar]
- 48. Zhang Y, Kollias G, Argyris AA, Papaioannou TG, Tountas C, Konstantonis GD, Achimastos A, Blacher J, Safar ME, Sfikakis PP, Protogerou AD. Association of left ventricular diastolic dysfunction with 24-h aortic ambulatory blood pressure: the SAFAR study. J Hum Hypertens 2015;29:442–448. [DOI] [PubMed] [Google Scholar]
- 49. Nakagomi A, Okada S, Shoji T, Kobayashi Y.. Aortic pulsatility assessed by an oscillometric method is associated with coronary atherosclerosis in elderly people. Blood Press 2016;1–8. [DOI] [PubMed] [Google Scholar]
- 50. Wassertheurer S, Baulmann J.. Assessment of systolic aortic pressure and its association to all cause mortality critically depends on waveform calibration. J Hypertens 2015;33:1884–1888. [DOI] [PubMed] [Google Scholar]
- 51. Jones DW, Appel LJ, Sheps SG, Roccella EJ, Lenfant C.. Measuring blood pressure accurately: new and persistent challenges. JAMA 2003;289:1027–1030. [DOI] [PubMed] [Google Scholar]
- 52. Beime B, Deutsch C, Gomez T, Zwingers T, Mengden T, Bramlage P.. Validation protocols for blood pressure-measuring devices: status quo and development needs. Blood Press Monit. [DOI] [PubMed] [Google Scholar]
- 53. Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, Morgenstern BZ. Human blood pressure determination by sphygmomanometry. Circulation 1993;88:2460–2470. [DOI] [PubMed] [Google Scholar]
- 54. American National Standard Non-invasive sphygmomanometers - Part 2: Clinical validation of automated measurement type. ANSI/AAMI/ISO 81060-2:2009. Association for the Advancement of Medical Instrumentation, Arlington, Virginia: AAMI; 2009.
- 55. O’Brien E, Atkins N, Stergiou G, Karpettas N, Parati G, Asmar R. European Society of Hypertension International Protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit 2010;15:23–38. [DOI] [PubMed] [Google Scholar]
- 56. O’Brien E, Petrie J, Littler W, de Swiet M, Padfield P, Altman DG, Imai Y, Wang J, Mengden T, Shennan A. The British Hypertension Society protocol for the evaluation of blood pressure measuring devices. J Hypertens 1993;11 (Suppl 2):S43–S62. [DOI] [PubMed] [Google Scholar]
- 57. O’Brien E, Pickering T, Asmar R, Myers M, Parati G, Staessen J, Mengden T, Imai Y, Waeber B, Palatini P, Gerin W. Working Group on Blood Pressure Monitoring of the European Society of Hypertension International Protocol for validation of blood pressure measuring devices in adults. Blood Press Monit 2002;7:3–17. [DOI] [PubMed] [Google Scholar]
- 58. Tholl U, Anlauf M.. Conscientious evaluation of measuring accuracy. Hypertension League provides approval seals for automatic blood pressure units. MMW Fortschritte Der Medizin 1999;141:45.. [PubMed] [Google Scholar]
- 59. White WB, Berson AS, Robbins C, Jamieson MJ, Prisant LM, Roccella E, Sheps SG. National standard for measurement of resting and ambulatory blood pressures with automated sphygmomanometers. Hypertension 1993;21:504–509. [DOI] [PubMed] [Google Scholar]
- 60. Review of the Australian Code for the Responsible Conduct of Research (2007). National Health and Medical Research Council, the Australian Research Council and Universities Australia; 2015.
- 61. Gardner RM. Direct blood pressure measurement–dynamic response requirements. Anesthesiology 1981;54:227–236. [DOI] [PubMed] [Google Scholar]
- 62. Avolio AP, Butlin M, Winter D, Cardiology: blood pressure In: Webster JG, ed. The Physiological Measurement Handbook. CRC Press; 2014. p17–22. [Google Scholar]
- 63. Webster JG, Clark JW, Measurement of system response In: Webster JG, ed. Medical Instrumentation: Application and Design. 2nd ed. New York: John Wiley; 1995. [Google Scholar]
- 64. Ogden E, Shock NW, Heck K.. Rate of stabilisation of systolic blood-pressure following adoption of the supine posture. Q J Exp Physiol Cogn Med Sci 1938;28:341–348. [Google Scholar]
- 65. Wilkinson IB, Hall IR, MacCallum H, Mackenzie IS, McEniery CM, van der Arend BJ, Shu YE, MacKay LS, Webb DJ, Cockcroft JR. Pulse-wave analysis: clinical evaluation of a noninvasive, widely applicable method for assessing endothelial function. Arterioscler Thromb Vasc Biol 2002;22:147–152. [DOI] [PubMed] [Google Scholar]
- 66. Morcos SK, Dawson P, Pearson JD, Jeremy JY, Davenport AP, Yates MS, Tirone P, Cipolla P, de Haen C, Muschick P, Krause W, Refsum H, Emery CJ, Liss P, Nygren A, Haylor J, Pugh ND, Karlsson JO. The haemodynamic effects of iodinated water soluble radiographic contrast media: a review. Eur J Radiol 1998;29:31–46. [DOI] [PubMed] [Google Scholar]
- 67. Williams B, Lacy P, Baschiera F, Brunel P, Dusing R.. Novel description of the 24-hour circadian rhythms of brachial versus central aortic blood pressure and the impact of blood pressure treatment in a randomized controlled clinical trial: the Ambulatory Central Aortic Pressure (AmCAP) Study. Hypertension 2013;61:1168–1176. [DOI] [PubMed] [Google Scholar]
- 68. Jankowski P, Bednarek A, Olszanecka A, Windak A, Kawecka-Jaszcz K, Czarnecka D.. Twenty-four-hour profile of central blood pressure and central-to-peripheral systolic pressure amplification. Am J Hypertens 2013;26:27–33. [DOI] [PubMed] [Google Scholar]
- 69. Head GA, McGrath BP, Mihailidou AS, Nelson MR, Schlaich MP, Stowasser M, Mangoni AA, Cowley D, Brown MA, Ruta LA, Wilson A. Ambulatory blood pressure monitoring in Australia: 2011 consensus position statement. J Hypertens 2012;30:253–266. [DOI] [PubMed] [Google Scholar]
- 70. Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ.. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol 2000;525:263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tan I, Spronck B, Delhaas T, Reesink K, Kiat H, Barin E, Butlin M, Avolio A. Quantification of heart rate dependency of aortic pulse wave velocity. J Hypertens 2016;34 (Suppl 2):e57. [Google Scholar]
- 72. Bland JM, Altman DG.. Measuring agreement in method comparison studies. Stat Methods Med Res 1999;8:135–160. [DOI] [PubMed] [Google Scholar]
- 73. Mitchell GF, Hwang SJ, Larson MG, Hamburg NM, Benjamin EJ, Vasan RS, Levy D, Vita JA. Transfer function-derived central pressure and cardiovascular disease events: the Framingham Heart Study. J Hypertens 2016;34:1528–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kollias A, Lagou S, Zeniodi ME, Boubouchairopoulou N, Stergiou GS.. Association of central versus brachial blood pressure with target-organ damage: systematic review and meta-analysis. Hypertension 2015;67:183–190. [DOI] [PubMed] [Google Scholar]
- 75. Laurent S, Sharman JE, Boutouyrie P.. Central versus peripheral blood pressure: finding a solution. J Hypertens 2016;34:1497–1499. [DOI] [PubMed] [Google Scholar]
- 76. McEniery CM, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR.. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: The Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol 2005;46:1753–1760. [DOI] [PubMed] [Google Scholar]


