Abstract
Amphibian metamorphosis is driven by thyroid hormone (TH). We used prometamorphic tadpoles and a cell line of the African clawed frog (Xenopus laevis) to examine immediate effects of dioxin exposure on TH. Gene expression patterns suggest cross-talk between the thyroid hormone receptor (TR) and aryl hydrocarbon receptor (AHR) signaling pathways. In XLK-WG cells, expression of Cytochrome P450 1A6 (cyp1A6), an AHR target, was induced 1000-fold by 100 nM TCDD (2, 3, 7, 8 tetrachlorodibenzo-p-dioxin). Krüppel-Like Factor 9 (klf9), the first gene induced in a cascade of TH responses tied to metamorphosis, was upregulated over 5-fold by 50 nM triiodothyronine (T3) and 2-fold by dioxin. Co-exposure to T3 and TCDD boosted both responses, further inducing cyp1A6 by 75% and klf9 about 60%. Additional canonical targets of each receptor, including trβa and trβb (TR) and udpgt1a (AHR) responded similarly. Induction of TH targets by TCDD in XLK-WG cells predicts that exposure could speed metamorphosis. We tested this hypothesis in two remodeling events: tail resorption and hind limb growth. Resorption of ex vivo cultured tails was accelerated by 10 nM T3, while a modest increase in resorption by 100 nM TCDD lacked statistical significance. Hind limbs doubled in length over four days following 1 nM T3 treatment, but limb length was unaffected by 100 nM TCDD. TCDD co-exposure reduced the T3 effect by nearly 40%, despite TCDD induction of klf9 in whole tadpoles, alone or with T3. These results suggest that tissue-specific TCDD effects limit or reverse the increased metamorphosis rate predicted by klf9 induction.
Keywords: dioxin, aryl hydrocarbon receptor, thyroid, metamorphosis, frog
Dioxin-like compounds (DLCs), including chlorinated dioxins and furans and planar polychlorinated biphenyls (PCBs), exert diverse toxic effects in vertebrates, such as developmental defects, immune system alterations, endocrine disruption, cancer, and death (Sany et al., 2015a, b). The aryl hydrocarbon receptor (AHR), a ligand-activated transcription factor, binds DLCs and, as a heterodimer with the ARNT protein, mediates their biological effects by altering expression of numerous genes (Gasiewicz and Henry, 2012). Prominent gene targets comprise the AHR gene battery: Phase I detoxification enzymes like Cytochrome P450s of family I (CYP1s) and Phase II enzymes such as glutathione-S-transferases and UDP glucuronosyl transferases (UGTs; Nebert et al., 2000). 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), a high-affinity AHR agonist, represents the most toxic DLC in most species and the prototype ligand for toxicological studies.
The thyroid hormone (TH) system is among the endocrine pathways disrupted by DLCs (Crofton, 2008). Secreted under control of the hypothalamus-pituitary-thyroid axis, thyroxine (T4) circulates in complex with serum binding proteins, including transthyretin (TTR), albumin, and thyroxine-binding globulin (TBG). At target tissues, deiodinases convert T4 to triiodothyronine (T3). T3 has high affinity for thyroid receptor alpha (TRα), which is constitutively bound to thyroid responsive elements (TREs) as a dimer with retinoid X receptor (RXR). T3 binding converts this complex from a transcriptional repressor to an activator, triggering expression of a target gene cascade starting with Krüppel-like factor 9 (klf9; Bagamasbad et al., 2015; Furlow and Kanamori, 2002) and trβs (Kanamori and Brown, 1992). These transcription factors subsequently upregulate additional gene targets. TH is cleared by deiodinases and liver sulfatases and UGTs (Crofton, 2008; Visser, 1996).
Each thyroid pathway step is theoretically subject to contaminant disruption (reviewed in Crofton, 2008; Patrick, 2009). In rodents, DLCs are associated with reduced serum T4 (Potter et al., 1986), the result of liver UGT1 activity induced via AHR (Hood and Klaassen, 2000; Nishimura et al., 2005; Roth et al., 1988). TCDD exposure also triggers elevated TSH, thyroid hyperplasia, and tumorigenesis, likely feedback responses to T4 insufficiency (Huff et al., 1991; Nishimura et al., 2003, 2005; Sewall et al., 1995). Hydroxylated PCBs can compete with T4 in TTR binding, decreasing TH bioavailability; however, the poorly metabolized dioxins and furans do not affect TH binding to serum proteins (Browuer et al., 1990; Lans et al., 1993, 1994).
During frog metamorphosis tadpoles undergo extensive morphological alterations, including tail resorption, limb growth, and remodeling of intestine, central nervous system, respiratory system, skeleton, and skin (Dodd and Dodd, 1976). Initiated by TH (Brown et al., 1995; Tata, 1998; Wong et al., 1995), metamorphosis is used as a model to study TH activity during development (Brown and Cai, 2007; Buchholz et al., 2006; Tata, 2006) and the effects of thyroid disruptors (Miyata and Ose, 2012).
Few studies address the immediate and direct effects of DLCs on metamorphosis, especially at the molecular level. Gutleb et al. (2000, 2007) exposed embryos of Xenopus laevis (African clawed frog) to planar PCBs (PCB-77 or PCB-126) according to the FETAX regimen (through NF 46; ASTM, 2012), measuring modest declines in the percentage of metamorphosed animals ∼2 months later. Similarly, Collier et al. (2008) observed delays in completion of metamorphosis by X. laevis and Pseudacris tristeriata (western chorus frog) following early TCDD exposure. In these studies, a direct mechanistic link between negative DLC effects on metamorphosis and disruption of the TH system is not straightforward to discern. The onset of TH secretion occurs much later than the period of exposure, around NF 50 (Furlow and Neff 2006), while TCDD is rapidly eliminated from tadpoles (Jung and Walker, 1997; Philips et al., 2006). It is thus possible that the negative effect of DLC exposure on metamorphosis was secondary to early life stage toxicity. However, TRs are expressed as early as NF 39 (Kawahara et al., 1991), potentially enabling dysregulated expression of their target genes prior to the production of endogenous TH.
Induction of ugt1 and subsequent reduction of serum T4 in rodents predicts TCDD exposure during later, thyroid-active frog life stages could slow metamorphosis through direct molecular mechanisms concomitant with exposure. However, Rosenshield et al. (1999), treating Rana pipiens (leopard frog) with PCB 126 from early embryogenesis through metamorphosis, observed greater ‘metamorphic success’ at most exposure levels, although 50 µg/ml, the highest concentration, reduced the proportion of completely metamorphosed tadpoles. This pattern did not hold for R. clamitans (green frog); concentrations below 50 µg/ml did not affect metamorphosis (Rosenshield et al., 1999).
In this study, we sought to resolve these contradictions and uncertainties, testing the hypothesis that TCDD directly and immediately disrupts thyroid signaling and metamorphosis. We measured molecular endpoints in XLK-WG, one of few widely available X. laevis cell lines and the only one that has been well characterized with respect to AHR signaling (Freeburg et al., 2017; Iwamoto et al., 2012; Laub et al., 2010). In addition, we measured both molecular and morphological traits in prometamorphic tadpole tissues, delaying TCDD and/or T3 exposure until the outset of metamorphosis. We report an intriguing interaction between T3 and TCDD in the induction of TR and AHR target genes and complex effects on resorption of cultured tails and hind-limb growth in animals.
MATERIALS AND METHODS
Chemicals
Dimethyl sulfoxide (DMSO) and Triiodothyronine (T3) were purchased from Sigma Aldrich (St. Louis, Missouri). 2,3,7,8-tetrachlorodibezo-p-dioxin (TCDD) was obtained from Ultra Scientific (North Kingstown, Rhode Island). 6-formylindolo[3,2-b]carbazole (FICZ) was purchased from Enzo Life Sciences (Farmingdale, New York). TR-antagonist 1-850 and the AHR antagonist StemRegenin1 (SR1) were purchased from Millipore (Billerica, Massachussetts).
Cell culture
XLK-WG, an X. laevis cell line derived from kidney epithelium, was obtained from the American Type Culture Collection (ATCC; Manassas, Virginia) and cultured as directed in RPMI-1640 medium plus 20% fetal bovine serum (FBS) at 29°C with humidified air and 5% CO2. One of 2 commercially available X. laevis cell lines, XLK-WG has been used in several previous studies of AHR signaling in frogs. These cells express AHR1α (ahr1.L), AHR1β (ahr1.S), cyp1A6 (cyp1a1.S), cyp1A7 (cyp1a1.L), and ARNT, and they are responsive to both TCDD and FICZ (Freeburg et al., 2017; Iwamoto et al., 2012; Laub et al., 2010). Their ready availability, rapid growth and passage characteristics, and demonstrated utility for studies of the AHR pathway make them a uniquely well-suited cell culture model for studies of frog AHR signaling. Also, recent characterization of AHR-deficient rats revealed disruptions of kidney development (Harrill et al., 2013), underscoring the importance of this organ in AHR biology and DLC toxicology.
XLK-WG cells were grown to ∼90% confluence and exposed to 100 nM TCDD in the presence or absence of 50 nM T3 in DMSO vehicle (0.25%). 100 nM TCDD exposures lasted 24 h, while 1 nM FICZ exposure was 3 h. Controls were exposed to DMSO vehicle alone. To test the role of serum binding proteins in T3/TCDD co-exposures, TCDD experiments were repeated using RPMI-1640 medium without FBS. To verify that changes in gene expression were dependent on AHR and TR activity, cells were co-exposed to TR antagonist 1-850 (Schapira et al., 2003) or AHR antagonist SR-1 (Boitano et al., 2010) in DMSO as indicated in figure legend. Effects of candidate endogenous AHR agonist 6-formylindolo[3,2b]carbazole (FICZ; 1 nM) were also examined in conjunction with T3. Exposure times were limited to 3 h to maximize the magnitude of the response, which diminishes with longer exposure due to the metabolism of the compound by induced enzymes (Laub et al., 2010; Wincent et al., 2009).
Animal care
Xenopus laevis tadpoles (Nieuwkoop and Faber stage 52–54) were obtained from NASCO (Fort Atkinson, Wisconsin). Tadpoles were kept at room temperature in charcoal-filtered tap water treated with AmQuel Plus (Kordon, Haywood, California) to remove any residual chloramine. Tadpoles were maintained on a 12 L:12 D photoperiod and were fed frog brittle (NASCO) ad libitum. Protocols for use and handling of animals were approved by the Kenyon College Institutional Animal Care and Use Committee.
Tissue explant culture and exposures
Tail explant culture was performed as described by Bonett et al. (2010). Tadpoles were treated with 100 µg/ml oxytetracycline in aquarium water for 24 h before dissections were carried out under sterile conditions. Tadpoles were sacrificed by anesthetic overdose in 2% MS-222 (Tricaine methanesulfonate, Argent Labs, Redmond, Washington; buffered with NaHCO3) and submerged in 70% ethanol to sterilize the epidermis. Tails were severed posterior to the anus, and moved to sterile 6-well tissue culture dishes containing 2 ml of ice cold tissue culture medium. Tails were cultured in high glucose Dulbecco’s Modified Eagle’s Medium (Gibco) diluted 1:1.5 to be isotonic to amphibian tissues. Tails were maintained at 25°C with 5% CO2 and gentle rotation (50 rpm). Stock solutions of T3 and TCDD were prepared in DMSO. Final concentrations were 10 nM for T3 (Bonett et al., 2010) and 100 nM for TCDD. All treatments received an equal amount of DMSO vehicle (0.12%). Treatments were renewed every 12 h for the duration of the experiment.
Tails were photographed daily for 7 days with an Olympus SZX10 stereomicroscope and Olympus Q-Color 3 camera. ImageJ (Schneider et al., 2012) was used to trace the perimeter of each tail and calculate the area. A Mixed Effects Model (including 2-way ANOVA with repeated measures) was produced in R-studio to statistically analyze the rate of tail resorption over the 7-day period.
Hind limb growth analysis
NF stage 52–54 tadpoles (NASCO, Fort Atkinson, Wisconsin) were maintained at a density of 100 tadpoles/25 l FETAX solution (ASTM, 2012). Tadpoles were incubated at 23°C for 4 days under the following exposure conditions: 0.25% DMSO, 1 nM T3, 100 nM TCDD, or co-treatment with 1 nM T3+ 100 nM TCDD. T3 concentrations were 10-fold lower than in tail explant studies in an effort to avoid saturating the response and enable greater opportunity to observe any additive effect of TCDD treatment.
Tadpoles were anesthetized in iced FETAX buffer for imaging. Left hind limb images were captured using a microscope and digital camera (Olympus SZX10 and Olympus Q-Color 3), and length measured using ImageJ. After 4 days of treatment, tadpoles were sacrificed in a lethal dose of NaHCO3-buffered MS-222, and left hind limbs were once again imaged. Statistical analysis was performed with R Studio using one-way ANOVA with Tukey‘s multiple comparisons test.
RNA extraction from whole tadpoles
Tadpoles were maintained and exposed as described above. After 24 h of exposure, tadpoles were sacrificed in a lethal dose of NaHCO3-buffered MS-222. Whole tadpoles were ground with STAT-60 reagent (Tel-Test, Inc., Friendswood, Texas), 50 mg of tissue per 500 µL of STAT-60. RNA was isolated and treated with DNase using the Direct-zol RNA MiniPrep kit (ZYMO Research, Irvine, California).
Quantitative RT-PCR
cDNA was synthesized from total RNA derived from XLK-WG cells or whole tadpoles using a Taqman reverse transcription kit with random hexamer primers (Applied Biosystems). Reaction conditions for reverse transcriptase were: 25°C for 10 min, 48°C for 30 min, and 95°C for 5 min. Target genes and endogenous control (β-actin) were amplified using Power SYBR Green Maser Mix (Applied Biosystems) on a 7500 Real Time PCR System (Applied Biosystems) using cDNA derived from 20 ng of total RNA. Reaction conditions for PCR were: 50°C for 2 min, 95°C for 10 min, and 50 cycles of: 95°C for 15 s, 60°C for 1 min. Parallel reactions used cDNA derived from reactions that omitted the addition of reverse transcriptase. Additional control reactions entirely omitted the addition of template. Together, these controls ensured PCR products were derived only from mRNA. Sequences of custom PCR primers (synthesized by Eurofins MWG Operon, Huntsville, Alabama) are listed in Table 1. Relative expression of each transcript was determined by the ΔΔCt method on ABI 7500 Sequence Detection System v 1.4 software (Applied Biosystems). Statistical significance of expression difference was assessed in R Studio using a mixed effects model with one-way ANOVA and Tukey’s test for individual contrasts, as indicated in figure legends.
Table 1.
qPCR Primer Sequences
| Transcripta | Forward | Reverse |
|---|---|---|
| β-actin (actb.L) | 5′-CGAGCCGCATAGAAAGGAGA-3′ | 5′-TGTGATTCTGAGGGTTGGACG-3′ |
| cyp1A6 (cyp1a.L) | 5′-GCTTGGTTGGTGATGGGAAG-3′ | 5′-TCTGCGTCGAGCTCTCCAC-3′ |
| klf9 (klf9.L) | 5′-GCCGCTGTTTTGTGTCTTTG-3′ | 5′-CTGCGCCCCTCCTGTGT-3′ |
| trβa (thrb.L) | 5′-GTGCCAAGAATGTCGCTTCAA-3′ | 5′-TTGTTGTCATCCAAAACCAAGTCT-3′ |
| trβb (thrb.S) | 5′-TTGGCCAAAACTGCTGATGA-3′ | 5′-GCTGGCTGGCATGCT-3′ |
| udpgt-1a (ugt1a6.S) | 5′-GAACATTCAGCGTTTGTCATCTCT-3′ | 5′-CCACCCAGTGCACAGCAA-3′ |
Commonly recognized gene name with designation under newest nomenclature guidelines (Amaya et al., 2013) in parentheses.
RESULTS
Induction of TR and AHR Target Genes in XLK-WG Cells
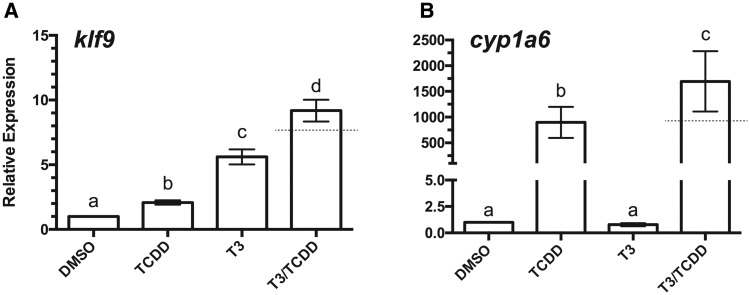
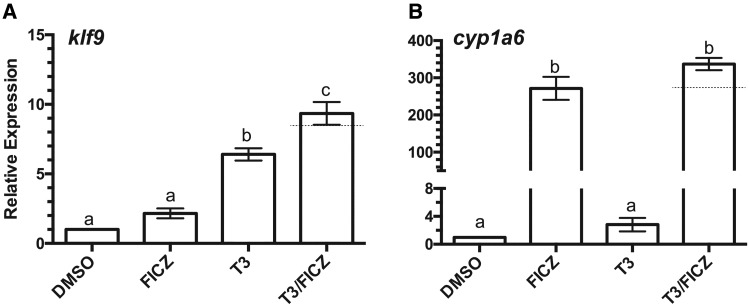
XLK-WG cells have been used extensively by our group to characterize the activity of multiple X. laevis AHRs (Freeburg et al., 2017) and associated inducible CYP1s (Iwamoto et al., 2012) in conjunction with different agonists (Freeburg et al., 2017; Laub et al., 2010). The initial goal of this study was to establish the TH responsiveness of this cell line. klf9, among the first genes transcriptionally induced by TRα, was upregulated by T3. Unexpectedly, klf9 was also induced by TCDD and to an even greater degree by treatment of cells with both compounds (Figure 1A). As demonstrated previously (Freeburg et al., 2017; Laub et al., 2010), cyp1a6 was dramatically induced by TCDD. While T3 exposure had no independent effect on cyp1a6, it augmented the effect of TCDD (Figure 1B). Taken together, these results demonstrate that the XLK-WG cell line is TH-responsive and that T3 and TCDD signaling pathways can interact to affect expression of their respective target genes.
Figure 1.
Induction of klf9 and cyp1a6 mRNAs by T3 and TCDD. XLK-WG cells were incubated in 0.25% DMSO, 100 nM TCDD, 50 nM T3, or both TCDD and T3 for 24 h. mRNA abundance was measured by qPCR with β-actin as endogenous control. Error bars represent standard error of the mean. A dotted line indicates the calculated additive response. Statistical significance of differences between treatment groups was assessed by one-way ANOVA with Tukey’s test for individual contrasts and by a linear mixed model in R, as described in Materials and Methods. Values sharing a letter designation are not significantly different. n = 13 per treatment group.
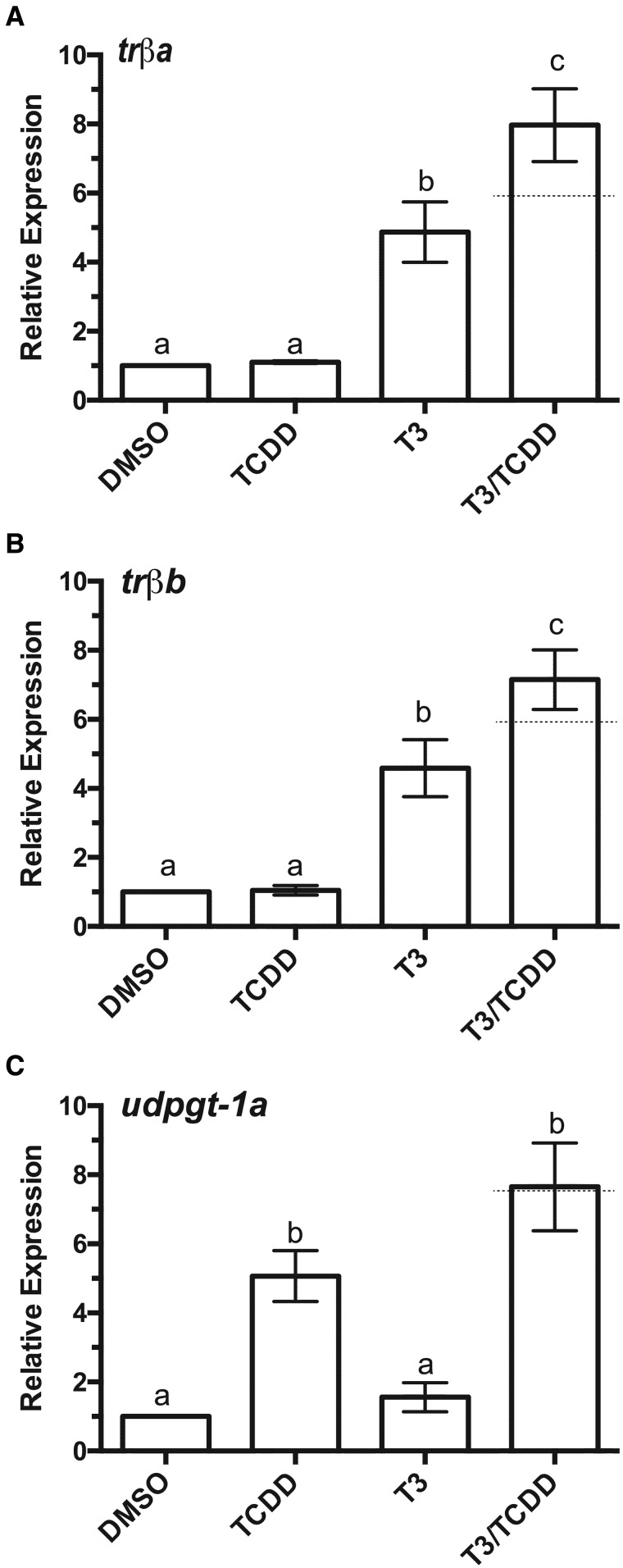
We next sought to determine the extent to which other known TR- and AHR-responsive genes are induced by each compound. trβa and trβb, targets of the ligand-bound TRα, were both induced by T3 (Figs. 2A and 2B). Unlike klf9, we observed no induction by TCDD alone, but co-exposure to T3 and TCDD boosted expression of both transcripts 60%–65% relative to T3 only (Figs. 2A and 2B). UGTs (uridine 5′-diphosphate glucuronosyl transferases), are phase II detoxification enzymes, orthologs of which are well known as AHR targets in other vertebrates (Nebert et al., 2000). Like cyp1a6, udpgt-1a was readily induced by TCDD but not by T3. Co-treatment with both compounds boosted transcript abundance about 60%, although this increase was not statistically significant (Figure 2C;n = 3). Preliminary experiments revealed that induction of all target genes increased with increasing concentration of TCDD (Supplementary Figure 1).
Figure 2.
Induction of additional TR and AHR target mRNAs by T3 and TCDD. (A) trβa, (B) trβb, (C) udpgt-1a. XLK-WG cells were incubated in 0.25% DMSO, 100 nM TCDD, 50 nM T3, or both TCDD and T3 for 24 h. mRNA abundance was measured by qPCR with β-actin as endogenous control. Error bars represent standard error of the mean. A dotted line indicates the calculated additive response. Statistical significance of differences between treatment groups was assessed by one-way ANOVA with Tukey’s test for individual contrasts. Values sharing a letter designation are not significantly different. n = 3 per treatment group.
The enhanced induction of klf9 and cyp1a6 by T3/TCDD co-treatment suggests the intriguing possibility of an interaction between the respective receptor-mediated signaling pathways, TR and AHR. However, it is possible that the co-treatment effect related to competition for serum-binding globulins (albumin, TTR, or TBG) in the cell culture media. If the abundance of such proteins were limiting, then the addition of one compound could displace the other, increasing its availability in culture and ultimately triggering its partitioning into the lipid-rich cellular fraction of the largely aqueous environment. In this scenario, the absence of serum in the culture medium should attenuate the apparent induction of mRNA expression resulting from co-treatment. We tested this hypothesis by conducting the exposures in serum-free media. klf9 expression increased over 9-fold following T3 exposure. Co-treatment with both T3 and TCDD further increased transcript abundance ∼65%, while the apparent 4-fold induction by TCDD alone lacked statistical significance (Figure 3A). The expression of cyp1a6 exhibited a nearly identical response as in the experiments with serum-supplemented media (Figure 3B). These results suggest that the effects of T3/TCDD co-exposure on the expression of these 2 target genes were not merely an artifact of cell culture with serum-containing media.
Figure 3.
Induction of klf9 and cyp1a6 mRNAs by T3 and TCDD in serum-free culture media. XLK-WG cells were grown in serum-free RPMI-1640 media. Exposure conditions and mRNA expression analysis were as described for Figure 1. Error bars represent standard error of the mean. A dotted line indicates the calculated additive response. Statistical significance of differences between treatment groups was assessed by one-way ANOVA with Tukey’s test for individual contrasts. Values sharing a letter designation are not significantly different. n = 3 per treatment group.
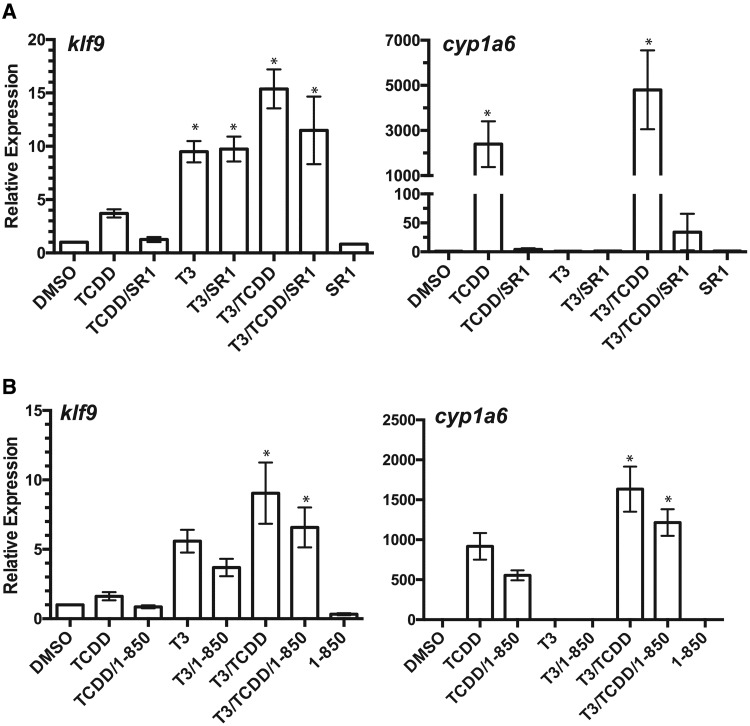
We also examined the effect of AHR and TR antagonists on induction of target genes by TCDD and T3. AHR antagonist SR-1 (Boitano et al., 2010) reduced TCDD induction of klf9 by 66% and cyp1a6 more than 99% and diminished the augmentation of transcript abundance in co-exposed cells, 25% for klf9 and more than 99% for cyp1a6 (Figure 4A). The potency and efficacy of TR antagonist 1–850 appeared much lower than reported for repression of TR-responsive CAT reporter gene activity in HeLa cells (Schapira et al., 2003). Nonetheless, 1–850 reduced T3 induction of klf9 by 34% and 27% in cells co-treated with TCDD (Figure 4B). 1–850 exerted a similar effect on cyp1a6 induction in cells treated with both T3 and TCDD (Figure 4B). Although these substantial differences were not statistically significant in all cases, they were consistently observed in all replicate experiments, providing evidence that the interactive effects of the 2 compounds on target gene expression involve ligand activation of both AHR and TR.
Figure 4.
TR and AHR antagonists reduce induction of klf9 and cyp1a6. (A) TR antagonist 1–850 (100 µM, n = 4). (B) AHR antagonists SR1 (1 µM, n = 3). XLK-WG cells were treated as described for Figure 3 in the presence or absence of the indicated receptor antagonists. Expression of the indicated transcripts was determined by qPCR as described. Statistical significance of differences between treatment groups was assessed by one-way ANOVA with Tukey’s test for individual contrasts. Significant differences between treatment groups and DMSO controls are indicated by an asterisk.
The apparent interaction between T3 and TCDD signaling pathways prompted the examination of FICZ, a tryptophan photoproduct and oxidation product (Smirnova et al., 2016) that is a well-characterized candidate endogenous agonist for the AHR (Wincent et al., 2009). FICZ is much more potent agonist than TCDD for Xenopus AHRs (Freeburg et al., 2017; Laub et al., 2010; Odio et al., 2013). Exposure times were limited to 3 h to maximize the magnitude of the response, which diminishes with longer exposure due to the metabolism of the compound by induced enzymes (Laub et al., 2010; Wincent et al., 2009). Under these conditions, FICZ augmented T3-induced klf9 mRNA expression about 50%, although it had a minimal effect alone (Figure 5A). The augmentation of T3-induced klf9 expression (as well as that of trα and trβ) increased in concentration-responsive fashion to FICZ (Supplementary Figure 2). These results implicate endogenous AHR signaling for a role in the regulation of klf9 expression. Conversely, T3 triggered no additional expression of FICZ-induced cyp1a6 (Figure 5B).
Figure 5.
Induction of klf9 and cyp1a6 mRNAs by T3 and FICZ. XLK-WG cells were incubated in 0.25% DMSO, 1 nM FICZ, 50 nM T3, or both FICZ and T3 for 3 h. mRNA abundance was measured by qPCR with β-actin as endogenous control. Error bars represent standard error of the mean. A dotted line indicates the calculated additive response. Statistical significance of differences between treatment groups was assessed by one-way ANOVA with Tukey’s test for individual contrasts. Values sharing a letter designation are not significantly different. n = 3 per treatment group.
Gene Expression and Morphological Changes in Vivo and Ex Vivo
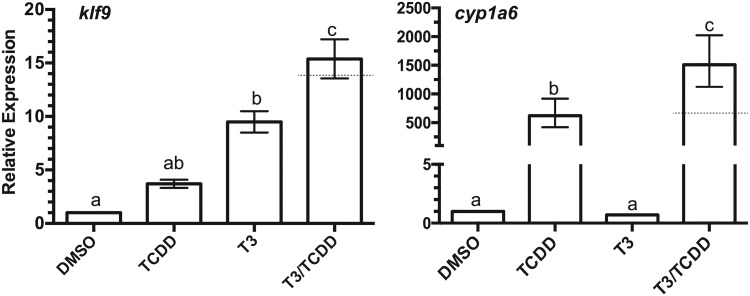
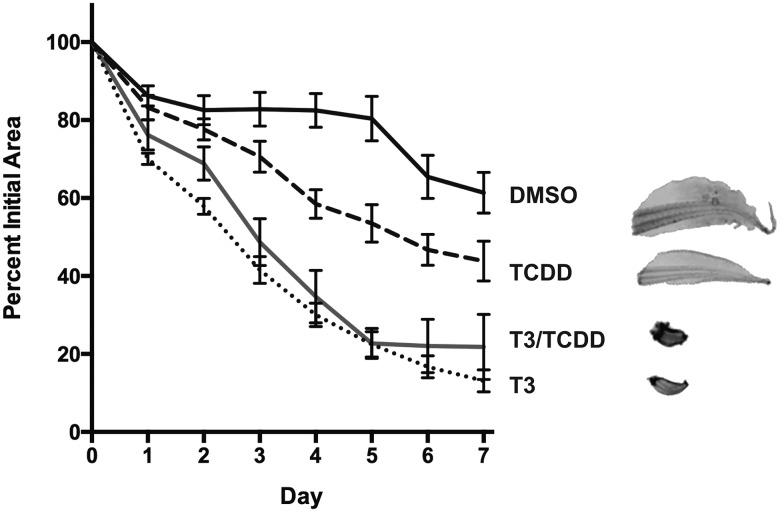
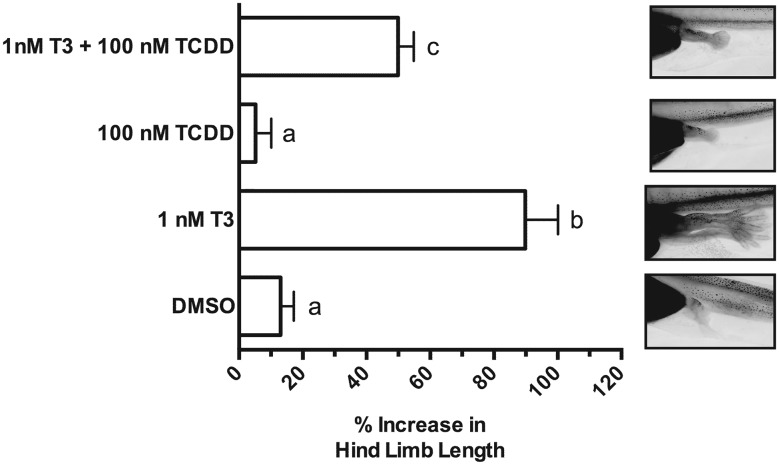
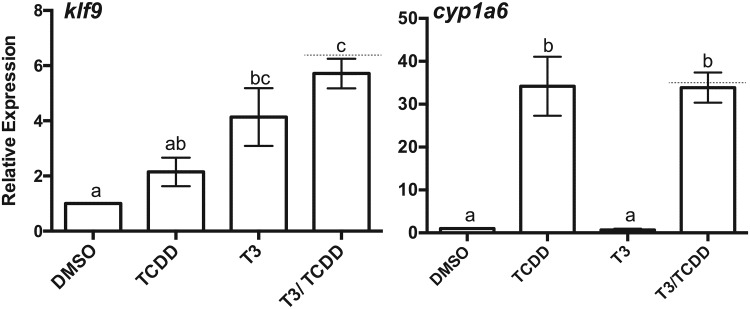
The induction of klf9, trβa, and trβb mRNAs by AHR agonists suggests that TCDD exposure could potentially speed developmental events related to tadpole metamorphosis. We examined this possibility in excised tissues and in intact prometamorphic tadpoles in the range of NF 51–54, coincident with the beginning of TH secretion and the onset of metamorphosis. Excised tadpole tails were cultured and exposed to 100 nM TCDD, 10 nM T3, or both compounds over 7 days with twice daily renewal of the exposure medium. T3 exposure resulted in a steady decline in tail size (area), mimicking T3-driven tail resorption during metamorphosis (Figure 6). At these concentrations, co-exposure with T3 and TCDD had no discernible additional effect on the resorption rate or ultimate size of the cultured tail. Tails exposed to TCDD alone experienced size reduction at a seemingly faster rate than those exposed to DMSO vehicle, especially at days 4 and 5. The mean percent of remaining area, ∼25% less than controls at the conclusion of the experiment (day 7), did not differ significantly (p = .079), nor was there a statistically significant interaction between time and these 2 treatments (2-way ANOVA with repeated measures). Examination of hind limb growth in intact tadpoles revealed no evidence of stimulation of metamorphosis by TCDD. Rather, T3-stimulated limb growth was diminished, reaching only about one-half the length in co-exposed animals (Figure 7). klf9 expression in whole tadpoles tracked the pattern seen in XLK-WG cells, with the highest mean mRNA induction in co-exposed tadpoles. TCDD induced cyp1a6 expression around 30-fold; T3 had no apparent effect on cyp1a6 expression regardless of TCDD exposure (Figure 8). Alterations in tail and hind limb morphology did not track directly with predictions from klf9 expression patterns in either XLK-WG cells or in whole, prometamorphic tadpoles. Although varying time and concentration of exposures could alter these responses, it is likely that this discrepancy points to more complex, tissue-specific molecular and cellular responses to both compounds.
Figure 6.
Effects of T3 and TCDD on regression of tadpole tail explants. Tails were harvested from NF 52–54 tadpoles and cultured for 1 week. Tissue was exposed to 0.12% DMSO, 100 nM TCDD, 10 nM T3, or both TCDD and T3, with renewal every 12 h. Tails were imaged and area was calculated daily using ImageJ. Percent initial tail area was compared using a mixed effects model and one-way ANOVA. n = 9 per treatment group.
Figure 7.
Effect of T3 and TCDD on growth of hind limb during early tadpole metamorphosis. Tadpoles were subjected to static exposure to DMSO vehicle (0.25%), 1 nM T3, 100 nM TCDD, or both T3 and TCDD for 24 h. Hind limb images were captured under identical magnification and analyzed using ImageJ. Exposures began between NF51 and NF54. Error bars represent standard error of the mean. One-way ANOVA with Tukey’s test for individual contrasts. Values sharing a letter designation are not significantly different. n = 51–55 tadpoles per treatment group.
Figure 8.
Induction of klf9 and cyp1a6 mRNAs in tadpoles following T3 and TCDD exposure. Tadpoles (NF 51–54) were maintained at a density of 4 animals/liter of FETAX solution and exposed to 0.25% DMSO, 100 nM TCDD, 1 nM T3, or both T3 and TCDD for 24 h. Total RNA was extracted from individual tadpoles and mRNA expression assessed by qPCR with β-actin as the endogenous control. Error bars represent standard error of the mean. A dotted line indicates the calculated additive response. One-way ANOVA with Tukey’s test for individual contrasts. Values sharing a letter designation are not significantly different. n = 3–6 tadpoles per treatment group.
DISCUSSION
Combining TCDD exposure at the outset of X. laevis metamorphosis with molecular endpoints in tadpoles and in cultured cells, this study sought to assess the effect of DLCs on this thyroid-driven process at morphological and molecular levels. Frogs are generally regarded as insensitive to dioxin-induced lethality (Beatty et al., 1976; Jung and Walker, 1997). Tadpoles eliminate TCDD rapidly (Jung and Walker, 1997; Philips et al., 2006), and frog AHRs exhibit low binding affinity for TCDD (Lavine et al., 2005; Odio et al., 2013). Lethality and sublethal responses to DLCs have nonetheless been reported for various species exposed to TCDD or planar PCBs, especially at high concentrations or body burdens (e.g., Collier et al., 2008; Dell’Orto et al., 1998; Gutleb et al., 2000, 2007; Rosenshield et al., 1999; Sakamoto et al., 1995, 1997). Consequently, we used 100 nM TCDD in these studies, both in cell/tissue culture and in vivo exposures. This concentration is less than the EC50 for cyp1a6 induction in XLK-WG cells (Laub et al., 2010).
T3 concentrations were chosen to be consistent with various TH-responsive endpoints in a range of previously published studies, typically between 5 and 50 nM for cultured cells (e.g., Bagamasbad et al., 2015; Furlow and Kanamori, 2002; Furlow et al., 2004) and up to 100 nM in tail culture (e.g., Bonett et al., 2010). In our hands, 10 nM T3 elicited a greater tail resorption response than observed by Bonett et al. (2010). In subsequent hind leg growth assays, we reduced the T3 concentration to 1 nM in an effort to avoid saturating the response and enable greater opportunity to observe any additive effect of TCDD treatment.
We detected a unique interaction between the AHR and TR signaling pathways—the reciprocal or cooperative upregulation of each receptor’s prototypical target gene. In some instances, klf9, trβa, trβb, or cyp1a6 induction in cells co-treated with T3 and TCDD appeared to exceed the calculated additive response (Bagamasbad et al., 2015), suggesting synergism (Figs. 1, 2A, 2B, 3, and 5B). However, there was no evidence for T3:FICZ synergism in klf9 induction in cultured cells or T3:TCDD synergism in the induction of either gene in intact tadpoles (Figs. 5A and 8).
Previous studies in heterologous systems also suggest the possibility of a functional interaction between TR and AHR signaling pathways. T3 augmented AHR-dependent luciferase reporter gene induction in stably transfected HepG2 cells (Vrzal et al., 2017), while TCDD augmented TR-dependent reporter gene expression in stably transfected HeLaTR cells (Yamada-Okabe et al., 2004). Furthermore, ‘cross-talk’ with other nuclear receptor systems has been previously observed for both AHR and TR for endogenous genes. AHR and estrogen receptor alpha (ERα) can exhibit positive or negative transcriptional interactions (reviewed in Matthews and Gustafsson, 2006). Direct protein:protein contacts between the two receptors (Beischlag and Perdew, 2005; Ohtake et al., 2003) can mediate estradiol-dependent activation (Matthews et al., 2005; Ohtake et al., 2003) or repression (Beischlag and Perdew, 2005) of cyp1a1 and/or cyp1b1 transcription, depending on cell type. Similarly, glucocorticoids and TH synergistically induce expression of klf9 in frog and mouse cells (Bagamasbad et al., 2012, 2015) and coordinately affect expression of hundreds of other genes in X. tropicalis tadpoles (Kulkarni and Buchholz, 2012). Synergistic klf9 transcription is mediated via protein:DNA interactions within the klf9 synergy module (KSM), an extraordinarily well-conserved ∼140 bp sequence located 5–8 kb upstream of the transcriptional start site (Bagamasbad et al., 2012, 2015). The KSM contains numerous functional transcription factor binding sites, including a glucocorticoid/mineralocorticoid responsive element plus a TRE (TR + 4). In addition, the KSM comprises part of a long non-coding RNA or enhancer RNA that is bi-directionally transcribed (Bagamasbad et al., 2015). The KSM does not contain canonical xenobiotic response elements (XREs) to support AHR binding, although several of these are predicted by MATINSPECTOR (Quandt et al., 1995) elsewhere upstream of the promoter. No evidence yet exists for a role of the KSM in TCDD-induced klf9 expression, but this intriguing sequence element adds a layer of complexity to co-operativity in nuclear receptor-induced transcription that remains to be fully elucidated. Notably, the TRE overlaps with a predicted NFκB binding sequence. Known interactions between AHR and NFκB subunits (reviewed in Tian, 2009; Vogel and Matsumura, 2009) suggest a possible mechanism of KSM involvement in AHR-mediated klf9 induction.
Since UGTs metabolize TH, their inducibility by AHR agonists raises the issue of compatibility between TR signaling and AHR signaling involving endogenous ligands. Enhanced induction of klf9 by AHR agonists suggests a potential mechanism of compensation, facilitating high level expression of thyroid responsive genes even in the face of UGT-mediated reduction of serum TH that could result from AHR activity. This hypothesis bears resemblance to cross-talk between AHR and the hypoxia-inducible factor. Hypoxia-inducible factor 1 alpha (HIF1α) and AHR potentially compete for a limiting pool of ARNT (a.k.a. HIF1β), the heterodimeric partner for both transcription factors, which would limit the activity of either transcription factor in cells exposed simultaneously to hypoxia and dioxin. In an apparent compensatory mechanism, the erythropoietin gene (epo), a major HIF1 target, is also directly upregulated by AHR through a promoter-proximal XRE (Chan et al., 1999).
While it is clear that TCDD exposure alters TH-directed gene expression, the morphological effects on the developing tadpole were more complicated than gene expression data predicted, varying with tissue type. Recent studies of X. tropicalis frogs lacking functional TRα highlight the complexity of temporal and site-specific coordination of tissue remodeling events during metamorphosis and their susceptibility to alteration by disruption of the TR signaling system (Choi et al., 2015, 2017; Wen et al., 2017). TRα knockout tadpoles initiated limb growth earlier than wild-type counterparts and grew faster during metamorphosis, while the degree of intestinal shrinking and folding was reduced, and tail resorption time was unaltered (Choi et al., 2017; Wen et al., 2017). However, the response to exogenous T3 was diminished, both in the induction of TR target genes and in the rate of gill resorption and hind limb development (Choi et al., 2015). These studies demonstrate the in vivo importance of TRα’s dual function as both transcriptional repressor and ligand-dependent activator and highlight the potential for disruption of the thyroid system by xenobiotics both before and after the onset of TH secretion. While our own results point toward cross-talk between TR and AHR signaling pathways following DLC exposure at the outset of metamorphosis, they do not exclude the possibility of a DLC effect on TR function during earlier developmental stages that subsequently alters the course of metamorphosis.
In these experiments, TCDD exposure likely exceeded environmentally relevant levels, even in highly polluted environments. It is not clear that DLC contamination is a significant driver of changes in amphibian ecology. Nonetheless, these studies in Xenopus cells and tadpoles enabled the initial molecular characterization of novel TCDD effects on thyroid signaling while preventing overly simplistic extrapolation of exposure biomarker expression data in cultured cells to the organismal level. They also highlight the utility of the prometamorphic tadpole as a model for thyroid disruption, one that allows precisely timed exposures for the characterization of specific windows of vulnerability for disruption of this developmental process. Molecular features of the TH system are exceptionally well conserved between frogs and humans, displaying strikingly analogous roles during development (Buchholz, 2015; Sachs and Buchholz, 2017), and despite the relatively low affinity of amphibian AHRs for TCDD, the number and evolutionary history of Xenopus AHR genes resembles humans more closely than any other non-mammalian vertebrate group (Hahn et al., 2017; Lavine et al., 2005). Thus, the interaction of AHR and TR signaling pathways revealed here is likely to be relevant to the mechanisms of DLC toxicity in humans.
FUNDING
This study was supported by the National Institute of Environmental Health Sciences (R15 ES011130), a Newton Chun Award from Kenyon College, and the Kenyon College Summer Science Scholars program.
Supplementary Material
ACKNOWLEDGMENTS
We thank Andrew Kerkhoff, Chris Gillen, and Natalie Wright (Kenyon College) for advice on statistical analysis and Dan Buchholz (University of Cincinnati) for technical advice.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
REFERENCES
- Amaya E., Baker J., Blitz I., Frank D., Gilchrist M. J., Guille J., Harland R. M., Horb M. E., Khokha M. K., Ogino H., et al. (2013). Xenbase Gene Nomenclature Guidelines (RRID: SCR_003280) Available at: http://www.xenbase.org/gene/static/geneNomenclature.jsp, last accessed October 12, 2017.
- ASTM (American Society of Testing and Materials) (2012). Standard guide for conducting the frog embryo teratogenesis assay–Xenopus (FETAX).Annual Book of ASTM Standards, Vol. 11.06, pp. 826–836. ASTM, Philadelphia, PA. [Google Scholar]
- Bagamasbad P., Ziera T., Borden S. A., Bonett R. M., Rozeboom A. M., Seasholtz A., Denver R. J. (2012). Molecular basis for glucocorticoid induction of the Kruppel-like factor 9 gene in hippocampal neurons. Endocrinology 153, 5334–5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagamasbad P. D., Bonett R. M., Sachs L., Buisine N., Raj S., Knoedler J. R., Kyono Y., Ruan Y., Ruan X., Denver R. J. (2015). Deciphering the regulatory logic of an ancient, ultraconserved nuclear receptor enhancer module. Mol. Endocrinol. 29, 856–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty P. W., Holscher M. A., Neal R. A. (1976). Toxicity of 2,3,7,8-tetrachloridibenzo-p-dioxin in larval and adult forms of Rana catespeiana. Bull. Environ. Contam. Toxicol. 16, 578–581. [DOI] [PubMed] [Google Scholar]
- Beischlag T. V., Perdew G. H. (2005). ER alpha-AHR-ARNT protein-protein interactions mediate estradiol-dependent transrepression of dioxin-inducible gene transcription. J. Biol. Chem. 280, 21607–21611. [DOI] [PubMed] [Google Scholar]
- Boitano A. E., Wang J., Romeo R., Bouchez L. C., Parker A. E., Sutton S. E., Walker J. R., Flaveny C. A., Perdew G. H., Denison M. S., et al. (2010). Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science 329, 1345–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonett R. M., Hoopfer E. D., Denver R. J. (2010). Molecular mechanisms of corticosteroid synergy with thyroid hormone during tadpole metamorphosis. Gen. Comp. Endocrinol. 168, 209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browuer A., Klasson-Wehler E., Bokdam M., Morse D. C., Traag W. A. (1990). Competitive-inhibition of thyroxine binding to transthyretin by monohydroxy metabolites of 3,4,3',4'-tetrachlorobiphenyl. Chemosphere 20, 1257–1262. [Google Scholar]
- Brown D. D., Cai L. Q. (2007). Amphibian metamorphosis. Dev. Biol. 306, 20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. D., Wang Z., Kanamori A., Eliceiri B., Furlow J. D., Schwartzman R. (1995). Amphibian metamorphosis: a complex program of gene expression changes controlled by the thyroid hormone. Rec. Prog. Horm. Res. 50, 309–315. [DOI] [PubMed] [Google Scholar]
- Buchholz D. R. (2015). More similar than you think: frog metamorphosis as a model of human perinatal endocrinology. Dev. Biol. 408, 188–195. [DOI] [PubMed] [Google Scholar]
- Buchholz D. R., Paul B. D., Fu L., Shi Y. B. (2006). Molecular and developmental analyses of thyroid hormone receptor function in Xenopus laevis, the African clawed frog. Gen. Comp. Endocr. 145, 1–19. [DOI] [PubMed] [Google Scholar]
- Chan W. K., Yao G., Gu Y.-Z., Bradfield C. A. (1999). Cross-talk between the aryl hydrocarbon receptor and hypoxia inducible factor signaling pathways. Demonstration of competition and compensation. J. Biol. Chem. 274, 12115–12123. [DOI] [PubMed] [Google Scholar]
- Choi J., Ishizuya-Oka A., Buchholz D. R. (2017). Growth, development, and intestinal remodeling occurs in the absence of thyroid hormone receptor alpha in tadpoles of Xenopus tropicalis. Endocrinology 158, 1623–1633. [DOI] [PubMed] [Google Scholar]
- Choi J. Y., Suzuki K. T., Sakuma T., Shewade L., Yamamoto T., Buchholz D. R. (2015). Unliganded thyroid hormone receptor alpha regulates developmental timing via gene repression in Xenopus tropicalis. Endocrinology 156, 735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier A., Orr L., Morris J., Blank J. (2008). The effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on the mortality and growth of two amphibian species (Xenopus laevis and Pseudacris triseriata). Int. J. Environ. Res. Public Health 5, 368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofton K. M. (2008). Thyroid disrupting chemicals: mechanisms and mixtures. Int. J. Androl. 31, 209–223. [DOI] [PubMed] [Google Scholar]
- Dell'Orto N., Cantelli D., Urani C. (1998). Cellular targets in response to dioxin exposure. Chemosphere 37, 2809–2821. [DOI] [PubMed] [Google Scholar]
- Dodd M. H. I., Dodd J. M. (1976). The biology of metamorphosis In Physiology of the Amphibia (Lofts B., Ed.), Vol. 2, pp. 467–599. Academic Press, New York. [Google Scholar]
- Freeburg S. H., Engelbrecht E., Powell W. H. (2017). Subfunctionalization of paralogous aryl hydrocarbon receptors from the frog Xenopus laevis: distinct target genes and differential responses to specific agonists in a single cell type. Toxicol. Sci. 155, 337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlow J. D., Kanamori A. (2002). The transcription factor basic transcription element-binding protein 1 is a direct thyroid hormone response gene in the frog Xenopus laevis. Endocrinology 143, 3295–3305. [DOI] [PubMed] [Google Scholar]
- Furlow J. D., Neff E. S. (2006). A developmental switch induced by thyroid hormone: Xenopus laevis metamorphosis. Trends Endocrinol Metab17, 38–45. [DOI] [PubMed] [Google Scholar]
- Furlow J. D., Yang H. Y., Hsu M., Lim W., Ermio D. J., Chiellini G., Scanlan T. S. (2004). Induction of larval tissue resorption in Xenopus laevis tadpoles by the thyroid hormone receptor agonist GC-1. J. Biol. Chem. 279, 26555–26562. [DOI] [PubMed] [Google Scholar]
- Gasiewicz T. A., Henry E. C. (2012). History of research on the AHR. In The AH Receptor in Biology and Toxicology (Pohjanvirta R., Ed.). pp. 3–32. Wiley, Hoboken, NJ. [Google Scholar]
- Gutleb A. C., Appelman J., Bronkhorst M., van den Berg J. H., Murk A. J. (2000). Effects of oral exposure to polychlorinated biphenyls (PCBs) on the development and metamorphosis of two amphibian species (Xenopus laevis and Rana temporaria). Sci. Total Environ. 262, 147–157. [DOI] [PubMed] [Google Scholar]
- Gutleb A. C., Mossink L., Schriks M., van den Berg H. J., Murk A. J. (2007). Delayed effects of environmentally relevant concentrations of 3,3',4,4'-tetrachlorobiphenyl (PCB-77) and non-polar sediment extracts detected in the prolonged-FETAX. Sci. Total Environ. 381, 307–315. [DOI] [PubMed] [Google Scholar]
- Hahn M. E., Karchner S. I., Merson R. R. (2017). Diversity as opportunity: insights from 600 million years of AHR evolution. Curr. Opin. Toxicol. 2, 58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrill J. A., Hukkanen R. R., Lawson M., Martin G., Gilger B., Soldatow V., Lecluyse E. L., Budinsky R. A., Rowlands J. C., Thomas R. S. (2013). Knockout of the aryl hydrocarbon receptor results in distinct hepatic and renal phenotypes in rats and mice. Toxicol. Appl. Pharmacol. 272, 503–518. [DOI] [PubMed] [Google Scholar]
- Hood A., Klaassen C. D. (2000). Differential effects of microsomal enzyme inducers on in vitro thyroxine (T(4)) and triiodothyronine (T(3)) glucuronidation. Toxicol. Sci. 55, 78–84. [DOI] [PubMed] [Google Scholar]
- Huff J. E., Salmon A. G., Hooper N. K., Zeise L. (1991). Long-term carcinogenesis studies on 2,3,7,8-tetrachlorodibenzo-p-dioxin and hexachlorodibenzo-p-dioxins. Cell. Biol. Toxicol. 7, 67–94. [DOI] [PubMed] [Google Scholar]
- Iwamoto D. V., Kurylo C. M., Schorling K. M., Powell W. H. (2012). Induction of cytochrome P450 family 1 mRNAs and activities in a cell line from the frog Xenopus laevis. Aquat. Toxicol. 114–115, 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung R. E., Walker M. K. (1997). Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on development of anuran amphibians. Environ. Toxicol. Chem. 16, 230–240. [Google Scholar]
- Kanamori A., Brown D. D. (1992). The regulation of thyroid hormone receptor beta genes by thyroid hormone in Xenopus laevis. J. Biol. Chem. 267, 739–745. [PubMed] [Google Scholar]
- Kawahara A., Baker B. S., Tata J. R. (1991). Developmental and regional expression of thyroid-hormone receptor genes during Xenopus metamorphosis. Development 112, 933–943. [DOI] [PubMed] [Google Scholar]
- Kulkarni S. S., Buchholz D. R. (2012). Beyond synergy: corticosterone and thyroid hormone have numerous interaction effects on gene regulation in Xenopus tropicalis tadpoles. Endocrinology 153, 5309–5324. [DOI] [PubMed] [Google Scholar]
- Lans M. C., Klasson-Wehler E., Willemsen M., Meussen E., Safe S., Brouwer A. (1993). Structure-dependent, competitive interaction of hydroxy-polychlorobiphenyls, hydroxy-dibenzo-p-dioxins and hydroxy-dibenzofurans with human transthyretin. Chem.-Biol. Interact. 88, 7–21. [DOI] [PubMed] [Google Scholar]
- Lans M. C., Spiertz C., Brouwer A., Koeman J. H. (1994). Different competition of thyroxine binding to transthyretin and thyroxine-binding globulin by hydroxy-PCBs, PCDDs and PCDFs. Eur. J. Pharmacol.-Environ. Toxicol. 270, 129–136. [DOI] [PubMed] [Google Scholar]
- Laub L. B., Jones B. D., Powell W. H. (2010). Responsiveness of a Xenopus laevis cell line to the aryl hydrocarbon receptor ligands 6-formylindolo[3,2-b]carbazole (FICZ) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Chem. Biol. Interact. 183, 202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine J. A., Rowatt A. J., Klimova T., Whitington A. J., Dengler E., Beck C., Powell W. H. (2005). Aryl hydrocarbon receptors in the frog Xenopus laevis: two AhR1 paralogs exhibit low affinity for 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Toxicol. Sci. 88, 60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J., Wihlen B., Thomsen J., Gustafsson J. A. (2005). Aryl hydrocarbon receptor-mediated transcription: ligand-dependent recruitment of estrogen receptor alpha to 2,3,7,8-tetrachlorodibenzo-p-dioxin-responsive promoters. Mol. Cell. Biol. 25, 5317–5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J., Gustafsson J. A. (2006). Estrogen receptor and aryl hydrocarbon receptor signaling pathways. Nucl. Recept. Signal. 4, e016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata K., Ose K. (2012). Thyroid hormone-disrupting effects and the amphibian metamorphosis assay. J. Toxicol. Pathol. 25, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert D. W., Roe A. L., Dieter M. Z., Solis W. A., Yang Y., Dalton T. P. (2000). Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem. Pharmacol. 59, 65–85. [DOI] [PubMed] [Google Scholar]
- Nishimura N., Yonemoto J., Miyabara Y., Sato M., Tohyama C. (2003). Rat thyroid hyperplasia induced by gestational and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Endocrinology 144, 2075–2083. [DOI] [PubMed] [Google Scholar]
- Nishimura N., Yonemoto J., Nishimura H., Ikushiro S., Tohyama C. (2005). Disruption of thyroid hormone homeostasis at weaning of Holtzman rats by lactational but not in utero exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 85, 607–614. [DOI] [PubMed] [Google Scholar]
- Odio C., Holzman S. A., Denison M. S., Fraccalvieri D., Bonati L., Franks D. G., Hahn M. E., Powell W. H. (2013). Specific ligand binding domain residues confer low dioxin responsiveness to AHR1β of Xenopus laevis. Biochemistry 52, 1746–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake F., Takeyama K., Matsumoto T., Kitagawa H., Yamamoto Y., Nohara K., Tohyama C., Krust A., Mimura J., Chambon P., et al. (2003). Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature 423, 545–550. [DOI] [PubMed] [Google Scholar]
- Patrick L. (2009). Thyroid disruption: mechanism and clinical implications in human health. Altern. Med. Rev. 14, 326–346. [PubMed] [Google Scholar]
- Philips B. H., Susman T. C., Powell W. H. (2006). Developmental differences in elimination of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) during Xenopus laevis development. Mar. Environ. Res. 62, S34–S37. [DOI] [PubMed] [Google Scholar]
- Potter C. L., Moore R. W., Inhorn S. L., Hagen T. C., Peterson R. E. (1986). Thyroid status and thermogenesis in rats treated with 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol 84, 45–55. [DOI] [PubMed] [Google Scholar]
- Quandt K., Frech K., Karas H., Wingender E., Werner T. (1995). Matind and MatInspector—new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res. 23, 4878–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenshield M. L., Jofre M. B., Karasov W. H. (1999). Effects of polychlorinated biphenyl 126 on green frog (Rana clamitans) and leopard frog (Rana pipiens) hatching success, development, and metamorphosis. Environ. Toxicol. Chem. 18, 2478–2486. [Google Scholar]
- Roth W., Voorman R., Aust S. D. (1988). Activity of thyroid hormone-inducible enzymes following treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Appl. Pharmacol. 92, 65–74. [DOI] [PubMed] [Google Scholar]
- Sachs L. M., Buchholz D. R. (2017). Frogs model man: In vivo thyroid hormone signaling during development. Genesis 55. [DOI] [PubMed] [Google Scholar]
- Sakamoto M. K., Mima S., Tanimura T. (1995). A morphological study of liver lesions in Xenopus larvae exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) with special reference to apoptosis of hepatocytes. J. Environ. Pathol. Toxicol. Oncol. 14, 69–82. [PubMed] [Google Scholar]
- Sakamoto M. K., Mima S., Takahashi K. P., Tanimura T. (1997). Apoptotic cell death of erythrocytes in Xenopus larvae exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Pathol. 25, 398–402. [DOI] [PubMed] [Google Scholar]
- Sany S. B. T., Hashim R., Rezayi M., Rahman M. A., Razavizadeh B. B. M., Abouzari-lotf E., Karlen D. J. (2015a). Integrated ecological risk assessment of dioxin compounds. Environ. Sci. Pollut. Res. 22, 11193–11208. [DOI] [PubMed] [Google Scholar]
- Sany S. B. T., Hashim R., Salleh A., Rezayi M., Karlen D. J., Razavizadeh B. B. M., Abouzari-lotf E. (2015b). Dioxin risk assessment: mechanisms of action and possible toxicity in human health. Environ. Sci. Pollut. Res. 22, 19434–19450. [DOI] [PubMed] [Google Scholar]
- Schapira M., Raaka B. M., Das S., Fan L., Totrov M., Zhou Z., Wilson S. R., Abagyan R., Samuels H. H. (2003). Discovery of diverse thyroid hormone receptor antagonists by high-throughput docking. Proc. Natl Acad. Sci. U. S. A. 100, 7354–7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C. A., Rasband W. S., Eliceiri K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewall C. H., Flagler N., Vanden Heuvel J. P., Clark G. C., Tritscher A. M., Maronpot R. M., Lucier G. W. (1995). Alterations in thyroid function in female Sprague-Dawley rats following chronic treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Appl. Pharmacol. 132, 237–244. [DOI] [PubMed] [Google Scholar]
- Smirnova A., Wincent E., Vikstrom Bergander L., Alsberg T., Bergman J., Rannug A., Rannug U. (2016). Evidence for new light-independent pathways for generation of the endogenous aryl hydrocarbon receptor agonist FICZ. Chem. Res. Toxicol. 29, 75–86. [DOI] [PubMed] [Google Scholar]
- Tata J. R. (1998). Amphibian metamorphosis as a model for studying the developmental actions of thyroid hormone. Ann. Endocrinol.-Paris 59, 433–442. [PubMed] [Google Scholar]
- Tata J. R. (2006). Amphibian metamorphosis as a model for the developmental actions of thyroid hormone. Mol. Cell. Endocrinol. 246, 10-20.. [DOI] [PubMed] [Google Scholar]
- Tian Y. A. (2009). Ah receptor and NF-kappa B interplay on the stage of epigenome. Biochem. Pharmacol. 77, 670–680. [DOI] [PubMed] [Google Scholar]
- Visser T. J. (1996). Pathways of thyroid hormone metabolism. Acta Med. Aust. 23, 10–16. [PubMed] [Google Scholar]
- Vogel C. F. A., Matsumura F. (2009). A new cross-talk between the aryl hydrocarbon receptor and RelB, a member of the NF-kappa B family. Biochem. Pharmacol. 77, 734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrzal R., Vrzalova A., Grycova A., Dvorak Z. (2017). Activated thyroid hormone receptor modulates dioxin-inducible aryl hydrocarbon receptor-mediated CYP1A1 induction in human hepatocytes but not in human hepatocarcinoma HepG2 cells. Toxicol. Lett. 275, 77–82. [DOI] [PubMed] [Google Scholar]
- Wen L., Shibata Y., Su D., Fu L. Z., Luu N., Shi Y. B. (2017). Thyroid hormone receptor a controls developmental timing and regulates the rate and coordination of tissue-specific metamorphosis in Xenopus tropicalis. Endocrinology 158, 1985–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wincent E., Amini N., Luecke S., Glatt H., Bergman J., Crescenzi C., Rannug A., Rannug U. (2009). The suggested physiologic aryl hydrocarbon receptor activator and cytochrome P4501 substrate 6-formylindolo[3,2-b]carbazole is present in humans. J. Biol. Chem. 284, 2690–2696. [DOI] [PubMed] [Google Scholar]
- Wong J. M., Wolffe A., Shi Y. B. (1995). Transcription regulation of Xenopus tr-beta-a gene by thyroid-hormone receptor and effect of chromatin assembly. J. Cell. Biochem. 9, 2696.. [DOI] [PubMed] [Google Scholar]
- Yamada-Okabe T., Aono T., Sakai H., Kashima Y., Yamada-Okabe H. (2004). 2,3,7,8-tetrachlorodibenzo-p-dioxin augments the modulation of gene expression mediated by the thyroid hormone receptor. Toxicol. Appl. Pharmacol. 194, 201–210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.