Abstract
We aim to investigate the cardioprotective effects of L-carnitine (LC) on cardiac function during ischemia and reperfusion (I/R) and contractile function of single cardiomyocyte. C57BL/6 J mice were randomly assigned to 5 groups: sham group; vehicle group, LC preconditioning group, LC preconditioning + LY294002 (a PI3K/Akt signaling pathway inhibitor) group (LC + LY), and LY294002 group (LY). The sham group was exposed to the open heart operation but not I/R, the other groups received 45 min ischemia/48 h reperfusion. At the end of reperfusion, echocardiography was performed on every mouse. In order to determine whether LC’s cardioprotection could act directly at the level of cardiomyocytes, we also tested its effects on isolated cardiomyocytes under hypoxia condition. The expressions of p-PI3K, PI3K, Akt, p-Akt, Bax and Bcl-2 proteins were detected by immunoblotting. The results showed that LC preconditioning remarkably improved cardiac function after I/R, but the cardioprotective effect of LC was significantly weakened after the application of LY294002. We also found that LC could directly improve the contractile function of cardiomyocytes under hypoxia condition. The immunoblotting results showed that LC administration restrained myocardial apoptosis as evidenced by decreasing the level of Bax expression, increasing the levels of phosphorylation of Akt, PI3K, and Bcl-2 protein expression, but these were blocked by LYC94002. Thus, the cardioprotective effects of LC against myocardial ischemic damage and its effect on single cardiomyocyte under hypoxia may be associated with the PI3K/Akt signaling pathway.
Keywords: myocardial ischemia reperfusion, Akt, L-carnitine
Myocardial ischemia-reperfusion injury (MIRI) refers to although the ischemic myocardium restore its blood after reperfusion, the heart function cannot be restored as we expected, otherwise, its metabolic disorders and structural damage may further aggravated in a certain period of time (Schulze et al., 2003). Reducing MIRI is an urgent problem to be solved in the prevention and treatment of coronary heart disease.
These studies think that main mechanisms about the MIRI may related to cardiomyocyte apoptosis, free radicals increase, intracellular calcium overload and energy metabolism disorder. Apoptosis is not only vital in normal physiological processes but also in pathological processes. Oxidative stress and apoptosis are closely linked together in the pathological process of ischemia and reperfusion (I/R) injury (Dirnagl et al., 1999). 3-Phosphatidylinositol kinase-silk/threonine kinase (PI3K/Akt) is an important cell survival signaling pathway, plays an important role in the regulation of myocardial cell growth, survival and proliferation (Cantley, 2002; Fresno Vara et al., 2004; Li et al., 2016; Terragni et al., 2008). The phosphorylation of AKt (P-AKT) normally initiated by combining PI3K with its substrates, and activated AKt phosphorylates regulate the downstream targets and produces positive effects against I/R induced apoptosis (Abe et al., 2010; Wang et al., 2009; Xu et al., 2008). Matsui et al. (2001) transcribed the activated Akt into the rat myocardium by vivo gene transfer method, they found after I/R, the myocardial infarction decreased by 64% in Akt transgenic rats compared with the control group, and the cardiac systolic/diastolic function was significant improved.
L-carnitine (LC) is quaternary ammonium compound biosynthesized from amino acids lysine and methionine, it is required for the transfer of long-chain fatty acids into the mitochondrial matrix before they can undergo β-oxidation, resulting in ATP formation (Oyanagi et al., 2011). In addition, LC modulates the intra-mitochondrial acyl-CoA/CoA ratio to remove toxic compounds before they have a chance to accumulate in the mitochondria. Some previous studies have shown that LC exerts a protective effect against I/R injury. Najafi (2013) found that LC improved post-ischemic hemodynamic functions and reduced the extent of myocardial infarct size. Another research found that LC cardioplegia solution could improve cardiac function in patients undergoing heart valve replacement operation and alleviate CPB-mediated apoptosis of cardiac muscle cells (Xiang et al., 2005). The possible cardioprotective mechanisms of LC may related to the inhibition of mitochondrial membrane permeability transition, a decrease of oxidative stress, and the prevention of pro-apoptotic protein expression (Furuno et al., 2001; Hagen et al., 2002; Kalinec et al., 2005; Tousson et al., 2016). However, the detailed antiapoptotic mechanism of LC remains poorly understood. Therefore, the present study was set to evaluate the protective effect of LC on cardiac function during the MIRI and its possible mechanisms, and we also want to investigate if the cardioprotection of LC can act directly on the cardiomyocytes under hypoxia condition.
MATERIALS AND METHODS
I/R model
C57BL/6J mice (12 weeks) were from Chareles River. For I/R model, mice were anesthetized with 4% isoflurance and 100% O2, quickly intubated and ventilated with a respirator (Harvard apparatus, Holliston, Massachusetts) (Di Minno et al., 2013). Surgery was performed under a stereomicroscope. After a left lateral thoracotomy, the left anterior descending coronary artery was occluded just underneath the left atrium for 45 min with an 8–0 nylon suture. A small piece of gauze was used to prevent arterial injury. At the conclusion of the induced ischemia, the ligature was removed and the tissue was reperfused for 48 h before euthanizing. Following reperfusion, the chest was closed in layers. A drop of lidocaine was put on the suture site and the mouse was slowly weaned off the ventilator as it resumed spontaneous breathing. The mouse was returned to its cage and kept warm and medicated for pain (buprenorphine). ECGs confirmed the hallmark ischemic ST-segment elevation during coronary occlusion (AD Instruments, Colorado Springs, Colorado). All animal protocols in this study were approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Centre and conform to the NIH Guide for the care and use of laboratory animals.
Experimental group and drug administration
Mice were randomly divided into 5 groups: Sham operation group (Sham): the mice received thoracotomy, but only threading without ligating left coronal anterior descending branch. Vehicle group (Vehicle): ligate left coronary artery anterior descending, after 45 min ischemia, release of ligation line and reperfusion for 48 h. LC treatment group (LC): ischemia 45 min, then intraperitoneal (IP) injection LC (1umol/g) 20 min before reperfusion (Kuwajima et al., 1999), reperfusion 48 h, sham and vehicle group IP the same dose of the saline. LC combined with LY294002 group (LC + LY): ischemia 45 min, before the 5 min of LC pretreatment, IP PI3K/Akt signaling pathway inhibitor LY294002 (100ug, blocking PI3K) (Fan et al., 2017; Wang et al., 2016a), then LC preconditioning treatment was given at 20 min before reperfusion, continue reperfusion for 48 h. LY294002 preconditioning treatment group (LY): ischemia 45 min, IP LY294002 at the 15 min before reperfusion, then reperfusion for 48 h.
Echocardiography
In order to detect the heart function, transthoracic echocardiography (Echo) was performed on all the mice at the beginning of the experiment and the end of reperfusion. Following anesthesia with 1.5%–2.0% isoflurane, using a high-resolution imaging system for small animals (14.0 MHz, Sequoia 512; Acuson, München, Germany), equipped with a high-frequency ultrasound probe (GE-i13L). All hair was removed before the echo examination. Parasternal long-axis and short-axis views were acquired. The LV fractional shortening (FS) and LV ejection fraction (EF) were calculated as follows: FS (%) = [(LVIDd-LVAWd)/LVIDd] ×100; EF (%) = [(LVIDd3–LVAWd3)/LVIDd3] ×100 (Din et al., 2014; Liu et al., 2014).
TUNEL assay
In order to show cell apoptosis directly, TUNEL staining was performed with a Cell Death Detection kit (Roche, Mannheim, Germany) (Tong et al., 2013; Yang et al., 2016). To reveal total nuclei, the same slides were stained with DAPI (1 μg/ml) in PBS plus 0.5% 1,4-diazabicyclo [2,2,2] octane. α-Actininand TUNEL-stained cell slides were mounted with DAPI solution and observed with fluorescent microscope, and also record the Immunofluorescence images.
Isolation of cardiomyocytes
Cardiomyocytes were enzymatically isolated as previously described (Chen et al., 2014b; Ren et al., 2008). Briefly, mice were given 100 units of heparin (Sagent Pharmaceuticals, Schaumbrug, IL) for anticoagulation, then anesthetized with 4% isoflurane and 100% O2. The hearts were excised and fastened onto cardiomyocyte perfusion apparatus (Radnoti, Monrovia, CA). Hearts were perfused at 37 °C with a Ca2+-free Krebs-Henseleit based (KHB) buffer (pH 7.4) containing: 0.6 mM KH2PO4, 0.6 mM Na2HPO4, 10 mM HEPES, 14.7 mM KCl, 1.7 mM MgSO4, 120.3 mM NaCl, 4.6 mM NaHCO3, 30 mM taurine, 10 mM glucose, and 10 mM 2,3-butanedione monoxime. The buffer was oxygenated with 95% O2/5%CO2. After several minutes of stabilization, the hearts were digested with fresh buffer containing 0.067 mg/ml Liberase Blendzyme 4 (Roche, Indianapolis, IN) for 15 min without 2, 3-butanedione monoxime. After digestion, hearts were removed and minced to disperse the cardiomyocytes in fresh Ca2+-free-KHB buffer. Extracellular Ca2+ was added incrementally to 1 mM. Only rod-shaped myocytes with clear edges were selected for pharmacological testing and cell contractility studies.
Hypoxia treatment
Isolated mouse cardiomyocytes were separated into 2 treatment groups (normal and hypoxic). Hypoxic cells were exposed to 2 mM NaCN for 10 min (Kondo et al., 1998; Rodriguez-Sinovas et al., 2006; Yamamoto et al., 2009), then with or without 5 mM LC (Oyanagi et al., 2015). Normal cells were placed into 37 °C incubator under normal atmospheric oxygen levels during the same period, and also with or without 5 mM LC.
Cardiomyocyte shortening/relaxation measurement
The isolated cardiomyocytes were randomly assigned to 4 groups: normoxia plus vehicle group, normoxia plus LC group, hypoxia plus vehicle group and hypoxia plus LC group. All cardiomyocytes were assessed using the SsftEdge MyoCam system (IonOptix Corporation, Milton, Massachusetts) (Chen et al., 2014a). Cardiomyocytes were placed in a chamber mounted on the stage of an inverted microscope (Olympus, IX-70, Center Valley, Pennsylvania) and incubated at 25 °C in a buffer containing (in mM): 131 NaCl, 4 KCl,1 CaCl2,1 MgCl2,10 glucose, and 10 HEPES, at pH 7.4. The cells were stimulated with suprathreshold voltage at a frequency of 0.5 Hz, using a pair of platinum wires placed on opposite sides of the chamber and connected to a stimulator (FHC Inc., Brunswick, Nebraska). Images of the myocytes being studied were displayed on a computer monitor using an IonOptix MyoCam camera. IonOptix SoftEdge software was used to capture changes in sarcomere length during contraction and relaxation. Sarcomere shortening and lengthening were assessed using the following indices: peak shortening (PS) amplitude during contraction indicative of peak ventricular contractility; and maximal velocities of shortening/relengthening, maximal slope (derivative) of shortening and relengthening phases, indicative of the maximal velocities of ventricular pressure increase/decrease.
Intracellular Ca2+ transient measurements
Isolated cardiomyocytes were loaded with Fura-2/AM (2 μM) for 20 min, and florescence measurements were recorded with a dual-excitation fluorescence photomultiplier system (IonOptix). Myocytes were placed on an Olympus (St Louis, Missouri) IX-70inverted microscope and imaged through a 40× Fluor oil objective. Cells were illuminated by either 340 or 380 nm excitation, while being stimulated to contract at 0.5 Hz. Fluorescence emission was detected at 480 and 520 nm by a photomultiplier tube (Ren et al., 2008) and high speed sampling using the interpolated numerator method.
Immunoblotting analysis
Immunoblotting was performed as previously described (Tong et al., 2013; Wang et al., 2013). Rabbit antibodies p-PI3K (Tyr458), p-Akt (Ser473) and GAPDH were purchased from Cell Signaling (Danvers, Massachusetts). Bcl2 and Bax were from Abcam (Abcam). Akt antibody was purchased from Santa Cruz (Santa Cruz, California).
Statistical analysis
Data are expressed as means ± SE. Statistical significance was analyzed with either a Student’s t test or 1-way ANOVA where appropriate. For multi-pairwise comparisons the Tukey post hoc test was performed to measure individual group differences of interest. The F and α significance levels of 0.1, 0.05 and 0.01 are indicated.
RESULTS
LC Improves Cardiac Function During I/R
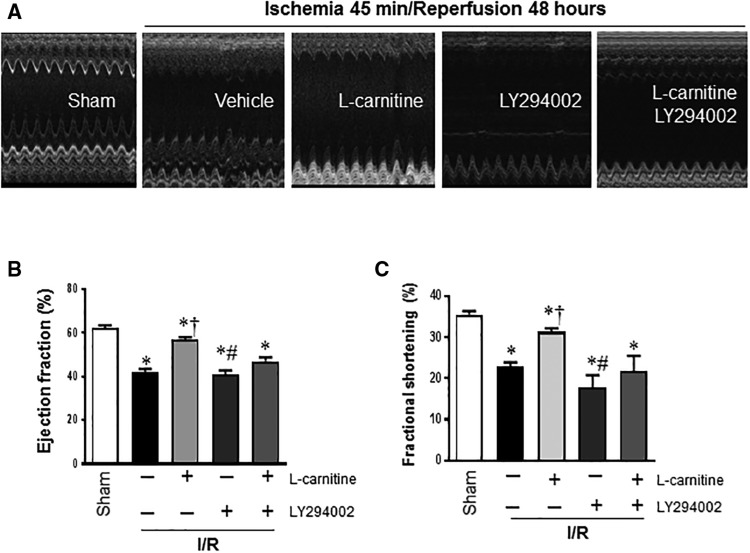
At the beginning of the experiment and the 48 h after the reperfusion, ECHO was performed to supervise the cardiac function of the mice. We found that there was no significant difference on EF and FS among the 5 groups at the beginning, but I/R challenge significantly reduced the LV FS and EF in vehicle group comparing to the sham group (FS 0.23 ± 0.02 versus 0.36 ± 0.03, EF 0.42 ± 0.03 versus 0.64 ± 0.03, respectively; p < .01 for both) (Figs. 1B and C). Mice in LC group showed improvement both in FS and EF compared with the vehicle group (FS 0.31 ± 0.02 vs 0.23 ± 0.03, EF 0.57 ± 0.03 vs 0.42 ± 0.03, respectively; p < .01 for both) (Figs. 1B and C). However, in the LC + LY and LY groups, EF and FS decreased significantly compared with LC group (p < .01 for both).
Figure 1.
Pre-administration of LC during the I/R improved cardiac function after 48 h of reperfusion. A, The representative Echo of the sham, vehicle, LC, LC + LY, and the LY group. B and C, I/R decreased the left ventricular EF and FS in vehicle group, LC administration improved the EF and FS, but it was blocked in the LC + LY and LY group. Values are means ± SE, n = 4–5, *p < .01 versus Sham; †p < .05 versus Vehicle; #p < .01 versus LC I/R.
LC Reduced Cardiomyocytes Apoptosis During I/R
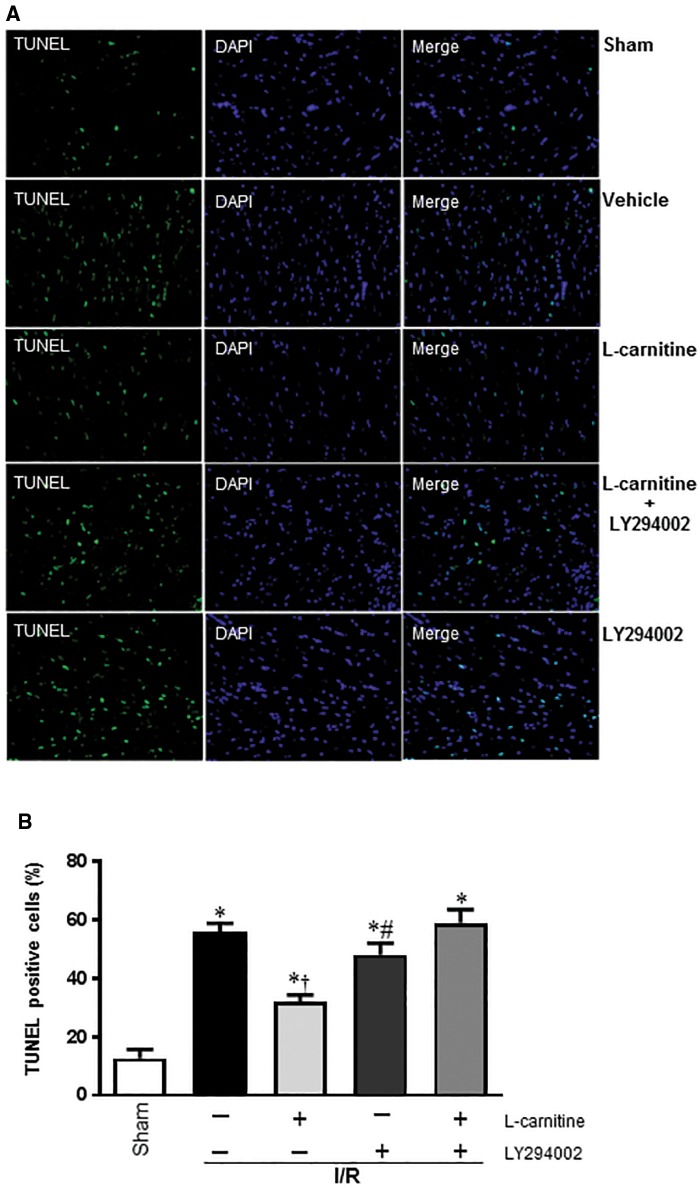
The protective effect of LC against the I/R-induced apoptosis in cardiomyocytes was examined using TUNEL stain (Figure 2A). By measuring the percentage of TUNEL-labeled cells, we can see the pretreatment of LC before reperfusion markedly decreased the number of apoptotic cells, but when we used the PI3K/Akt signaling pathway inhibitor (LY294002), we found the number of apoptotic cells significantly increased (Figure 2B).
Figure 2.
A, Presence of apoptosis was confirmed by TUNEL staining and Nuclei staining (magnification 200×). B, Quantification of apoptosis was expressed by the percentage of TUNEL positive cells. *p < .05 versus Sham; †p < .05 versus Vehicle; #p < .01 versus LC I/R.
LC Ameliorates Contractile Function of Cardiomyocytes During Hypoxia
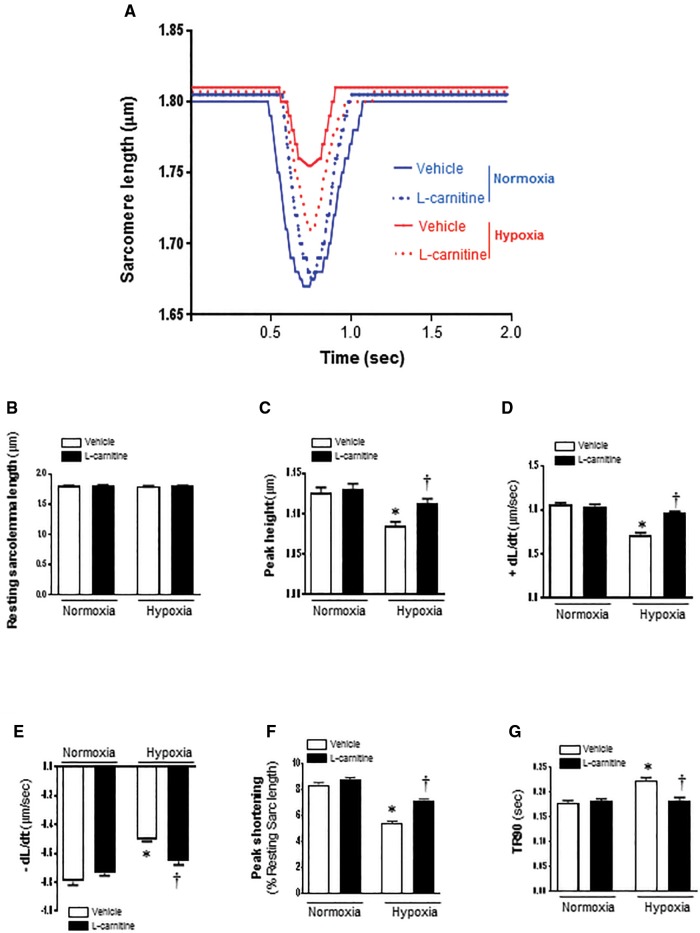
In order to determine whether LC protects cardiomyocytes against hypoxic injury, we investigated the cardiomyocyte contractility when they were exposed to hypoxia condition. The mechanical properties of cardiomyocyte contractility were obtained under extracellular Ca2+ of 1.0 mM and a stimulus frequency of 0.5 Hz (Figure 3A). As shown in Figure 3B, LC (5 mM) treatment did not affect resting cardiomyocyte contractile function under the normal or hypoxic condition (Figure 3B). However, during hypoxic condition, the cardiomyocytes displayed severe impaired PS (Figure 3C) and reduced maximal velocity of shortening/relengthening (+dL/dt, −dL/dt) (Figs. 3D and E), while LC treatment significantly ameliorates the contractile dysfunction of cardiomyocytes as reflected by both PS and maximal velocity of shortening/relengthening (Figs. 3D–F). Hypoxia also caused the prolonged time-to-90% relengthening (TR90) of cardiomyocytes; however, LC (5 mM) markedly inhibited the hypoxia-induced prolonged TR90 of cardiomyocytes (Figure 3G). From these results, we found that LC had no effect on the contractile function on normoxia cells, but it can protect cardiomyocytes from hypoxia-induced contractile dysfunction.
Figure 3.
Contractile properties of cardiomyocytes isolated from C57BL/6 mice with vehicle or LC treatment after being exposed to hypoxia. Recordings are shown from vehicle and 5 mM LC treatment for both normoxic and hypoxic treated cells. A, Representative sarcomeric length change recordings are shown during contractions stimulated at a frequency of 0.5 Hz. B, Resting sarcomere length; C, Peak sarcomeric length change during contraction; D, The maximal velocity of shortening (+dL/dt); E, The maximum velocity of relaxation (−dL/dt); F, PS (normalized to the resting sarcomere length); G, time-to-90% relengthening (TR90). Values are means ± SE, n = 75–127 cells per group derived from 3 to 4 mice each. *p < .05 versus normoxia vehicle; †p < .05 versus hypoxia vehicle.
The Intracellular Ca2+ Properties of Cardiomyocytes
In order to explore the potential mechanisms involved in the protection of LC against hypoxic cardiomyocyte contractile defect, intracellular Ca2+ homeostasis was evaluated using the fluorescence dye fura-2/AM (Zhao et al., 2009) (Figure 4A). We found that there were no significant differences on baseline Ca2+ signal (F340/380) between the 4 groups (Figs. 4A and B). The results also revealed that LC administration caused an significant elevation of the peak Ca2+ signal (△340/380) level, +dF/dt and −dF/dt in isolated cardiomyocytes (Figs. 4C–E) as compared with vehicle group under hypoxia condition. We also found that LC had no effect on the peak Ca2+ signal (△340/380) level, +dF/dt and −dF/dt on normoxia cells.
Figure 4.
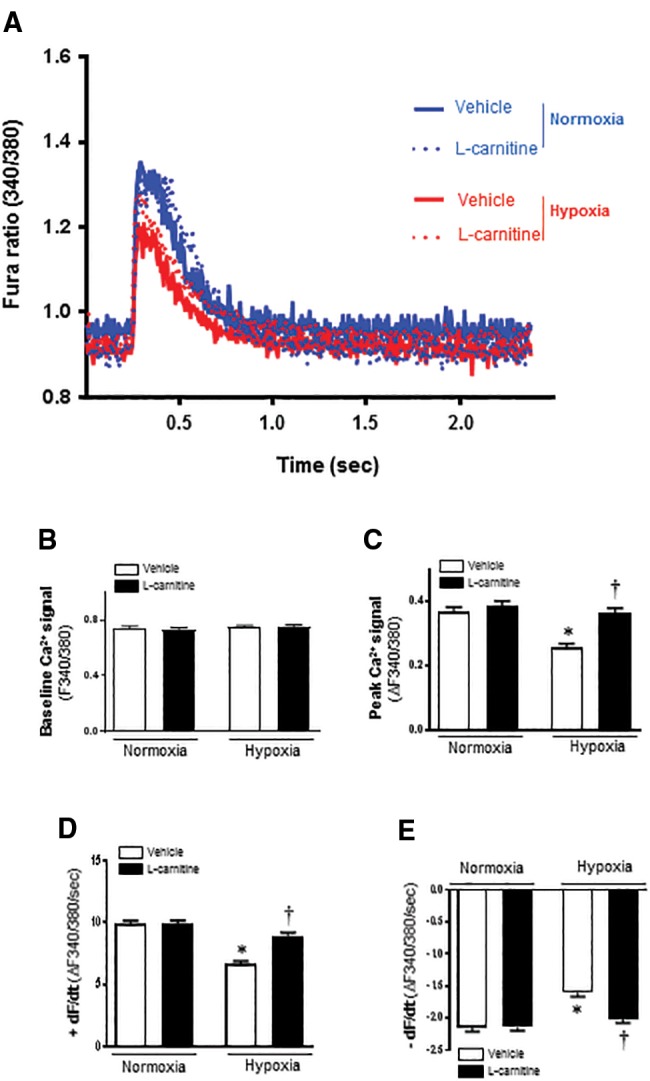
LC protects against hypoxia induced loss of intracellular Ca2+ release in cardiomyocytes. A, 340/380 ratio fluorescent imaging records of cardiomyocytes loaded with Fura2-AM Ca2+ indicator. Representative recordings of contraction induced intracellular Ca2+ changes are shown during contractions stimulated at a frequency of 0.5 Hz. B, Resting intracellular Ca2+; C, Peak Ca2+ changes during contraction. D, Maximum rate of Ca2+ change during contraction (+dF/dt); E, Maximum rate of Ca2+ change during relaxation (−dF/dt); Recordings are shown from vehicle and 5 mM LC treatment for both normoxic and hypoxic treated cells. Values are means ± SE, n = 75–127 cells per group derived from 3 to 4 mice each, *p < .05 versus normoxia vehicle; †p < .05 versus hypoxia vehicle.
Effects of LC on PI3K/Akt Signaling Pathway During I/R
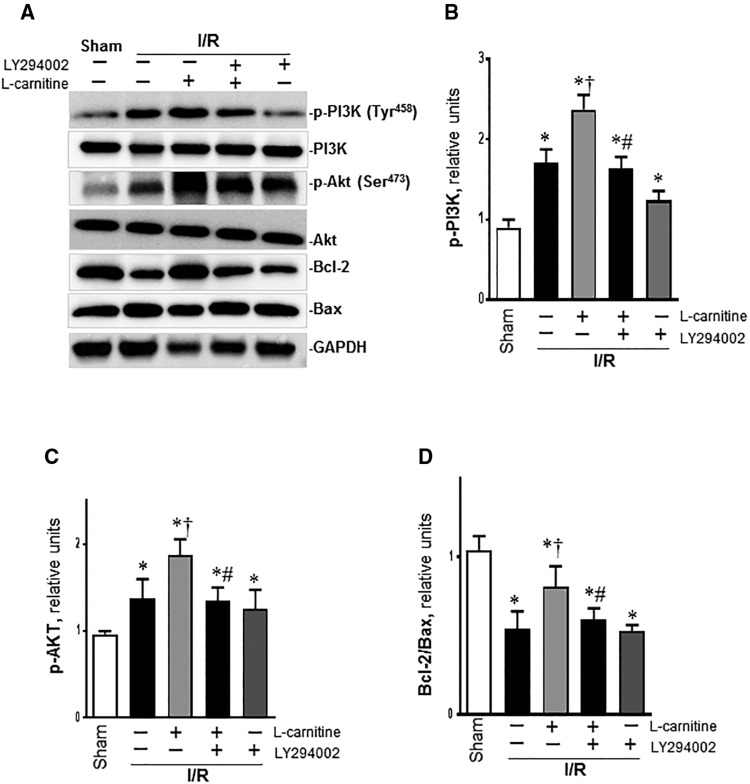
Western blot showed that I/R increased the p-PI3K in the vehicle group than the sham group, but it had no effect on the total PI3K. After LC administration, the p-PI3K was enhanced in the LC group than the vehicle group, and this was blocked in by LY294002, but the total PI3K was not affected (Figs. 5A and B). We also found that p-Akt was much higher in the vehicle group than the sham group, and this was enhanced in the LC group, but when treatment with LY294002, the p-Akt level was decreased in LC + LY group and LY group (Figs. 5A and C). Our results also showed that Bcl-2/Bax ratio was much lower in the vehicle group than the sham group (p < .01), it was significantly increased in the LC group than the vehicle group (Figs. 5A and D). However, compared with LC group, treatment with LC plus LY294002 or only with LY294002 both markedly decreased Bcl-2/Bax ratio (Figure 5D). These results demonstrated that LC reduced myocardial apoptosis following I/R probably through PI3K/Akt pathway.
Figure 5.
LC attenuates myocardial I/R injury by regulating the PI3K/Akt pathway. A, Representative western blot of different groups. B, The relative levels of p-PI3K with different conditions. C, The relative levels of p-Akt. D, The relative ratio of Bcl-2/Bax. Data are shown as the mean ± SE from independent experiments, n = 4–5, *p < .05 versus Sham; †p < .05 versus Vehicle I/R; #p < .05 versus LC I/R.
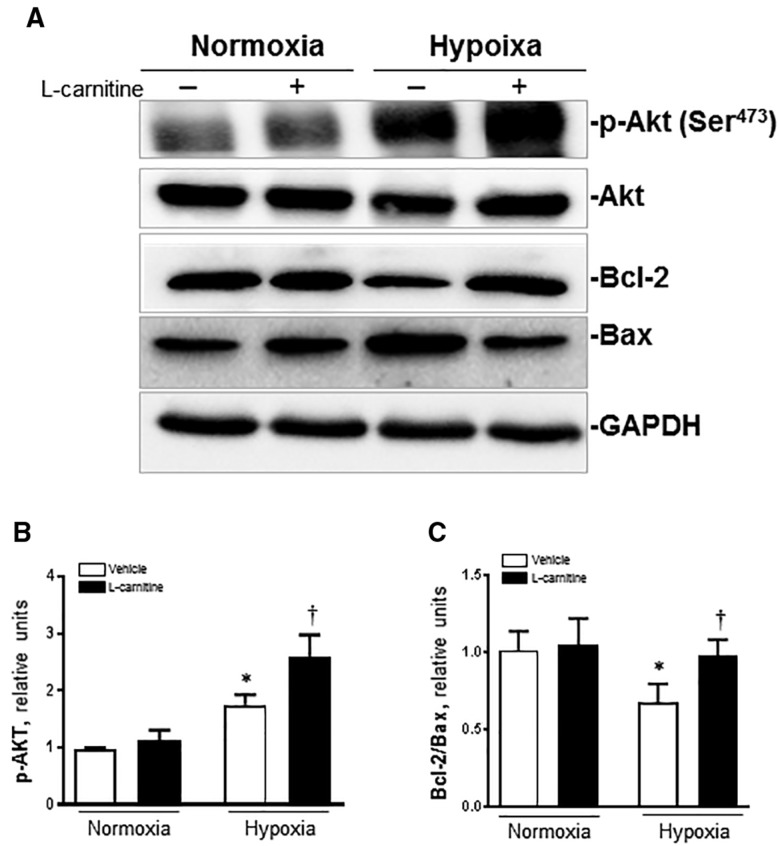
Effects of LC on Cardiomyocytes Akt Signaling Pathway During Hypoxia
There was no significant change in the expression of Akt protein in each group. Under normoxia condition, there is no significant difference on p-Akt protein between the vehicle and LC group, but under hypoxia condition, the expression of p-Akt protein was significantly enhanced in the vehicle group compared with the 2 normoxia groups (p < .01), and LC administration significantly increased the protein expression of p-Akt and the Akt phosphorylation level (p-Akt/Akt) in the LC group than the vehicle group (p < .01) under the hypoxia condition (Figs. 6A and B). We also found that under normoxia condition there was no significant difference about Bcl-2/Bax ratio between the vehicle and LC group. In the vehicle group under hypoxia, the Bcl-2/Bax ratio was much lower than the 2 normoxia groups, but it was markedly increased after LC administration (Figs. 6A and C).
Figure 6.
LC (5 mM) inhibits apoptotic signaling system hypoxia treatment. A, Representative immunoblots of different groups are shown from cells in normoxic and hypoxic conditions, and in the absence or presence of LC; B, The relative levels of p-Akt; C, The relative ratio of Bcl-2/Bax. Data are shown as the mean ± SE from independent experiments, n = 3–4 mice each group, *p < .05 versus normoxia vehicle; †p < .05 versus hypoxia vehicle.
DISCUSSION
During the MIRI, energy metabolism disorders, shrinkage protein degradation and other pathological changes will happen to the ischemic myocardium, myocardial ischemia initially causes contractile dysfunction and, when prolonged, cell death and necrosis (Opie, 1991) and lead to the systolic and diastolic dysfunction of ventricular. MIRI also causes cardiomyocyte apoptosis, which determines the degree of damage to cardiac function. How to reduce the number of apoptotic cardiomyocytes and protect cardiomyocytes become one of the important topics in the field of cardiovascular research (Gao et al., 2002; Song et al., 2013).
PI3K/Akt is an important pathway for intracellular transduction of membrane receptor signals, and it plays a key role in cell proliferation, metabolism and apoptosis. It is also essential for the cardiac physiology and protection against pathological remodeling and failure. When PI3K activated (Duan et al., 2015; Hong et al., 2016; Jiang et al., 2016) during the myocardial I/R injury, phosphatidylinositol (3, 4, 5)-trisphosphate activated phosphoinositide-dependent kinase-1, which in turn phosphorylates Akt at Thr308 and Ser473. The activated Akt produce anti-apoptotic effects by phosphorylation of 2 downstream substrates: Bcl-2 and Bax. The Bcl-2 gene is an anti-apoptotic protein, it can regulate the integrity of mitochondrial membrane involved in the regulation of apoptosis signal, promote myocardial cell survival, inhibits apoptosis. A high level of Bcl-2 expression prevents cells from apoptosis caused by cytotoxic factors or cellular stress (Tang et al., 2017). Bax is the opposite, it forms a heterodimer with Bcl2, inhibits Bcl2 and promotes apoptosis. When myocardial ischemia occurs, both Bcl2 and Bax are expressed in the cardiomyocytes. The Bax over expression can significantly inhibit the MIRI induced cardiomyocyte apoptosis, improve the cardiac function. The Bcl2 and Bax balance play an important role in the regulation of apoptosis, the decline of Bcl2/Bax can induce apoptosis, and the elevation of Bcl2/Bax can inhibit the apoptosis. LY294002 is an inhibitor of PI3K and able to inhibit the activation of downstream effectors including Akt (Tang et al., 2017). In our vivo study, the phosphorylated PI3K, p-Akt and Bcl-2 all increased when treated with LC compared with the vehicle group, but Bax decreased, all these were blocked by LY294002 pretreatment. In cell study, we also found LC administration could increase the level of p-Akt and Bcl-2, and decrease the Bax under hypoixa condition.
As a drug with cardioprotective effects on the heart, the clinical application of LC is very popular. Previous studies have made it clear that the essential physiological function of LC is to transport fatty acids into the cell mitochondria, through beta-oxidation into the tricarboxylic acid cycle, providing energy to cells. The previous study found that the cardioprotective effects of LC may be related to different mechanisms such as stimulating fatty acid oxidation during I/R, reducing toxic effects of long chain free fatty acids on cardiomyocytes, it can aslo has anti-arrhythmic effect (Najafi, 2013). There was also research found that LC can reduce myocardial cell apoptosis, which play a role in its cardioprotective effects (Chao et al., 2011). As mentioned earlier, LC can improve the cardiac function after I/R (Najafi, 2013), and its mechanism is not completely clear. In our study we found that administration of LC before the reperfusion significantly improved I/R induced cardiac function decrease. The EF and FS of the LC group significantly increased compared with the vehicle group. After using of PI3K blockers, the cardiac function improvement was blocked. Combined with western results, we concluded that LC improving cardiac function may related to the activation of the PI3K pathway, it up-regulated the expression of p-Akt and Bcl-2 protein and down-regulated the expression of Bax-2 protein, and inhibit the occurrence of apoptosis during I/R. Using TUNEL staining, we also directly found the pretreatment of LC before reperfusion markedly decreased the number of apoptotic cells, but when combined with the PI3K/Akt signaling pathway inhibitor (LY294002), the number of apoptotic cells significantly increased. These results agreed with the results of western blot. All our results demonstrated that LC reduced myocardial apoptosis following I/R probably through PI3K/Akt pathway.
In addition to the improvement on the cardiac function during the I/R, we also found that LC has significant effect on the contractile function of single cardiomyocyte under hypoxia. Reversible contractile dysfunction occurs in acutely hypoxia myocardium, a partial decrease in contractile function can be found during the hypoxia condition (Ross, 1991; Schulz et al., 1992). In the study of the single cardiomyocyte, we found that after the administration of LC under hypoxia condition, the contraction velocity of cardiomyocytes was significantly accelerated and the contraction amplitude increased, these indicated that LC exerted powerful positive inotropic effects in cardiomyocytes. Compared with vehicle group under hypoxia, the peak height, contraction amplitude and contraction velocity of LC group were significantly increased. The time to 90.0% of the peak was significantly lower than the vehicle group under hypoxia condition, which is near the pre-hypoxia level. The results also revealed that LC administration caused an significant elevation of the peak Ca2+ signal (△340/380) level, +dF/dt and −dF/dt in isolated cardiomyocytes compared with vehicle group under hypoxia conditions. All these results indicated that LC can improve the cadiomyocyte contractile function under hypoxia condition, this may related to the increase of peak Ca2+ signal, the results were consistent with previous studies (Kondo et al., 1998; Wang et al., 2016b). Combined the results of Western, LC’s cardioprotective effect on cadiomyocyte may also related to its activation of PI3K/Akt signaling pathway.
CONCLUSIONS
In summary, the administration of LC in MIRI can significantly improve the cardiac function. LC can also modulate the contractile function of single cardiomyocyte under hypoxic conditions. The cardioprotective effect of LC during the I/R and its cardioprotection against contractile dysfunction caused by hypoxia on single cardiomyocyte may related to the activation of PI3K/Akt signaling pathway.
FUNDING
American Diabetes Association (1-17-IBS-296); National Institutes of Health (R21AG044820, R01AG049835, P01HL051971, and P20GM104357); The Clinical Medical Science and Technology Innovation Plan of Ji Nan (201602173); National Natural Science Foundation of China (81370230 and 81570279); Science and Technology Program of Guangzhou, China (201508020107); Natural Science Foundation of Guangdong Province (2014A030311041).
REFERENCES
- Abe E., Fujiki M., Nagai Y., Shiqi K., Kubo T., Ishii K., Abe T., Kobayashi H. (2010). The phosphatidylinositol-3 kinase/Akt pathway mediates geranylgeranylacetone-induced neuroprotection against cerebral infarction in rats. Brain Res. 1330, 151–157. [DOI] [PubMed] [Google Scholar]
- Cantley L. C. (2002). The phosphoinositide 3-kinase pathway. Science 296, 1655–1657. [DOI] [PubMed] [Google Scholar]
- Chao H. H., Liu J. C., Hong H. J., Lin J. W., Chen C. H., Cheng T. H. (2011). L-carnitine reduces doxorubicin-induced apoptosis through a prostacyclin-mediated pathway in neonatal rat cardiomyocytes. Int. J. Cardiol. 146, 145–152. [DOI] [PubMed] [Google Scholar]
- Chen S., Li J., Liu H., Zeng J., Yang G., Wang J., Lu W., Yu N., Huang Z., Xu H., et al. (2014a). Esophagogastrostomy plus gastrojejunostomy: A novel reconstruction procedure after curative resection for proximal gastric cancer. J. Gastrointest. Surg. 18, 497–504. [DOI] [PubMed] [Google Scholar]
- Chen S., Zhu P., Guo H. M., Solis R. S., Wang Y., Ma Y., Wang J., Gao J., Chen J. M., Ge Y., et al. (2014b). Alpha1 catalytic subunit of AMPK modulates contractile function of cardiomyocytes through phosphorylation of troponin I. Life Sci. 98, 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Minno M. N., Dentali F., Veglia F., Russolillo A., Tremoli E., Ageno W. (2013). Antithrombin levels and the risk of a first episode of venous thromboembolism: A case-control study. Thromb. Haemost. 109, 167–169. [DOI] [PubMed] [Google Scholar]
- Din S., Konstandin M. H., Johnson B., Emathinger J., Volkers M., Toko H., Collins B., Ormachea L., Samse K., Kubli D. A., et al. (2014). Metabolic dysfunction consistent with premature aging results from deletion of Pim kinases. Circ. Res. 115, 376–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U., Iadecola C., Moskowitz M. A. (1999). Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 22, 391–397. [DOI] [PubMed] [Google Scholar]
- Duan Q., Madan N. D., Wu J., Kalisz J., Doshi K. Y., Haldar S. M., Liu L., Pierre S. V. (2015). Role of phosphoinositide 3-kinase IA (PI3K-IA) activation in cardioprotection induced by ouabain preconditioning. J. Mol. Cell. Cardiol. 80, 114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan T. T., Feng X. Y., Yang Y. Z., Gao F., Liu Q. (2017). Downregulation of PI3K-gamma in a mouse model of sepsis-induced myocardial dysfunction. Cytokine 96, 208–216. [DOI] [PubMed] [Google Scholar]
- Fresno Vara J. A., Casado E., de Castro J., Cejas P., Belda-Iniesta C., Gonzalez-Baron M. (2004). PI3K/Akt signalling pathway and cancer. Cancer Treatment Reviews 30, 193–204. [DOI] [PubMed] [Google Scholar]
- Furuno T., Kanno T., Arita K., Asami M., Utsumi T., Doi Y., Inoue M., Utsumi K. (2001). Roles of long chain fatty acids and carnitine in mitochondrial membrane permeability transition. Biochem. Pharmacol. 62, 1037–1046. [DOI] [PubMed] [Google Scholar]
- Gao F., Gao E., Yue T. L., Ohlstein E. H., Lopez B. L., Christopher T. A., Ma X. L. (2002). Nitric oxide mediates the antiapoptotic effect of insulin in myocardial ischemia-reperfusion: The roles of PI3-kinase, Akt, and endothelial nitric oxide synthase phosphorylation. Circulation 105, 1497–1502. [DOI] [PubMed] [Google Scholar]
- Hagen T. M., Moreau R., Suh J. H., Visioli F. (2002). Mitochondrial decay in the aging rat heart: Evidence for improvement by dietary supplementation with acetyl-L-carnitine and/or lipoic acid. Ann. N. Y. Acad. Sci. 959, 491–507. [DOI] [PubMed] [Google Scholar]
- Hong G. L., Liu J. M., Zhao G. J., Tan J. P., Wu B., Li M. F., Liang G., Qiu Q. M., Lu Z. Q. (2016). Cycloartenyl ferulate inhibits paraquat-induced apoptosis in HK-2 cells with the involvement of ABCC1. J. Cell. Biochem. 117, 872–880. [DOI] [PubMed] [Google Scholar]
- Jiang Y. Q., Chang G. L., Wang Y., Zhang D. Y., Cao L., Liu J. (2016). Geniposide prevents hypoxia/reoxygenation-induced apoptosis in H9c2 cells: Improvement of mitochondrial dysfunction and activation of GLP-1R and the PI3K/AKT signaling pathway. Cell. Physiol. Biochem. 39, 407–421. [DOI] [PubMed] [Google Scholar]
- Kalinec G. M., Fernandez-Zapico M. E., Urrutia R., Esteban-Cruciani N., Chen S., Kalinec F. (2005). Pivotal role of Harakiri in the induction and prevention of gentamicin-induced hearing loss. Proc. Natl. Acad. Sci. U.S.A. 102, 16019–16024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo R. P., Apstein C. S., Eberli F. R., Tillotson D. L., Suter T. M. (1998). Increased calcium loading and inotropy without greater cell death in hypoxic rat cardiomyocytes. Am. J. Physiol. 275, H2272–H2282. [DOI] [PubMed] [Google Scholar]
- Kuwajima M., Horiuchi M., Harashima H., Lu K., Hayashi M., Sei M., Ozaki K., Kudo T., Kamido H., Ono A., et al. (1999). Cardiomegaly in the juvenile visceral steatosis (JVS) mouse is reduced with acute elevation of heart short-chain acyl-carnitine level after L-carnitine injection. FEBS Lett. 443, 261–266. [DOI] [PubMed] [Google Scholar]
- Li X., Wu C., Chen N., Gu H., Yen A., Cao L., Wang E., Wang L. (2016). PI3K/Akt/mTOR signaling pathway and targeted therapy for glioblastoma. Oncotarget 7, 33440–33450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Wang H., Wang Y., Yin Y., Wang L., Liu Z., Yang J., Chen Y., Wang C. (2014). Exendin-4 pretreated adipose derived stem cells are resistant to oxidative stress and improve cardiac performance via enhanced adhesion in the infarcted heart. PloS One 9, e99756.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T., Tao J., del Monte F., Lee K. H., Li L., Picard M., Force T. L., Franke T. F., Hajjar R. J., Rosenzweig A. (2001). Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation 104, 330–335. [DOI] [PubMed] [Google Scholar]
- Najafi M. (2013). effects of postconditioning, preconditioning and perfusion of L-carnitine during whole period of ischemia/reperfusion on cardiac hemodynamic functions and myocardial infarction size in isolated rat heart. Iran. J. Basic Med. Sci. 16, 640–647. [PMC free article] [PubMed] [Google Scholar]
- Opie L. H. (1991). Role of calcium and other ions in reperfusion injury. Cardiovasc. Drugs Ther. 5(Suppl. 2), 237–247. [DOI] [PubMed] [Google Scholar]
- Oyanagi E., Uchida M., Miyakawa T., Miyachi M., Yamaguchi H., Nagami K., Utsumi K., Yano H. (2015). Palmitoleic acid induces the cardiac mitochondrial membrane permeability transition despite the presence of L-carnitine. Biochem. Biophys. Res. Commun. 463, 29–36. [DOI] [PubMed] [Google Scholar]
- Oyanagi E., Yano H., Uchida M., Utsumi K., Sasaki J. (2011). Protective action of L-carnitine on cardiac mitochondrial function and structure against fatty acid stress. Biochem. Biophys. Res. Commun. 412, 61–67. [DOI] [PubMed] [Google Scholar]
- Ren J., Privratsky J. R., Yang X., Dong F., Carlson E. C. (2008). Metallothionein alleviates glutathione depletion-induced oxidative cardiomyopathy in murine hearts. Crit Care Med 36, 2106–2116. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sinovas A., Garcia-Dorado D., Ruiz-Meana M., Soler-Soler J. (2006). Protective effect of gap junction uncouplers given during hypoxia against reoxygenation injury in isolated rat hearts. Am. J. Physiol. Heart Circ. Physiol. 290, H648–H656. [DOI] [PubMed] [Google Scholar]
- Ross J., Jr. (1991). Myocardial perfusion-contraction matching. Implications for coronary heart disease and hibernation. Circulation 83, 1076–1083. [DOI] [PubMed] [Google Scholar]
- Schulz R., Guth B. D., Pieper K., Martin C., Heusch G. (1992). Recruitment of an inotropic reserve in moderately ischemic myocardium at the expense of metabolic recovery. A model of short-term hibernation. Circ. Res. 70, 1282–1295. [DOI] [PubMed] [Google Scholar]
- Schulze C. J., Wang W., Suarez-Pinzon W. L., Sawicka J., Sawicki G., Schulz R. (2003). Imbalance between tissue inhibitor of metalloproteinase-4 and matrix metalloproteinases during acute myocardial [correction of myoctardial] ischemia-reperfusion injury. Circulation 107, 2487–2492. [DOI] [PubMed] [Google Scholar]
- Song M., Huang L., Zhao G., Song Y. (2013). Beneficial effects of a polysaccharide from Salvia miltiorrhiza on myocardial ischemia-reperfusion injury in rats. Carbohydr. Polym. 98, 1631–1636. [DOI] [PubMed] [Google Scholar]
- Tang L., Mo Y., Li Y., Zhong Y., He S., Zhang Y., Tang Y., Fu S., Wang X., Chen A. (2017). Urolithin A alleviates myocardial ischemia/reperfusion injury via PI3K/Akt pathway. Biochem. Biophys. Res. Commun. 486, 774–780. [DOI] [PubMed] [Google Scholar]
- Terragni J., Graham J. R., Adams K. W., Schaffer M. E., Tullai J. W., Cooper G. M. (2008). Phosphatidylinositol 3-kinase signaling in proliferating cells maintains an anti-apoptotic transcriptional program mediated by inhibition of FOXO and non-canonical activation of NFkappaB transcription factors. BMC Cell Biol. 9, 6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong C., Morrison A., Mattison S., Qian S., Bryniarski M., Rankin B., Wang J., Thomas D. P., Li J. (2013). Impaired SIRT1 nucleocytoplasmic shuttling in the senescent heart during ischemic stress. FASEB J. 27, 4332–4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tousson E., Hafez E., Zaki S., Gad A. (2016). The cardioprotective effects of L-carnitine on rat cardiac injury, apoptosis, and oxidative stress caused by amethopterin. Environ. Sci. Pollut. Res. Int. 23, 20600–20608. [DOI] [PubMed] [Google Scholar]
- Wang F. Y., Wang X. M., Wang C., Wang X. F., Zhang Y. Q., Wu J. D., Wu F., Zhang W. J., Zhang L. (2016a). Suppression of Mcl-1 induces apoptosis in mouse peritoneal macrophages infected with Mycobacterium tuberculosis. Microbiol. Immunol. 60, 215–227. [DOI] [PubMed] [Google Scholar]
- Wang H. Y., Wang G. L., Yu Y. H., Wang Y. (2009). The role of phosphoinositide-3-kinase/Akt pathway in propofol-induced postconditioning against focal cerebral ischemia-reperfusion injury in rats. Brain Res. 1297, 177–184. [DOI] [PubMed] [Google Scholar]
- Wang J., Ma Y., Sachs F., Li J., Suchyna T. M. (2016b). GsMTx4-D is a cardioprotectant against myocardial infarction during ischemia and reperfusion. J. Mol. Cell Cardiol. 98, 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wang Y., Wang J., Gao J., Tong C., Manithody C., Li J., Rezaie A. R. (2013). Antithrombin is protective against myocardial ischemia and reperfusion injury. J. Thromb. Haemost. 11, 1020–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang D., Sun Z., Xia J., Dong N., Du X., Chen X. (2005). Effect of L-carnitine on cardiomyocyte apoptosis and cardiac function in patients undergoing heart valve replacement operation. J. Huazhong Univ. Sci. Technol. Med. Sci. 25, 501–504. [DOI] [PubMed] [Google Scholar]
- Xu X., Chua C. C., Gao J., Chua K. W., Wang H., Hamdy R. C., Chua B. H. (2008). Neuroprotective effect of humanin on cerebral ischemia/reperfusion injury is mediated by a PI3K/Akt pathway. Brain Res. 1227, 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., Magishi K., Goh K., Sasajima T., Yamamoto F. (2009). Cardioprotective effects of normothermic reperfusion with oxygenated potassium cardioplegia: A possible mechanism. Interact. Cardiovasc. Thorac. Surg. 9, 598–604. [DOI] [PubMed] [Google Scholar]
- Yang H., Sun W., Quan N., Wang L., Chu D., Cates C., Liu Q., Zheng Y., Li J. (2016). Cardioprotective actions of Notch1 against myocardial infarction via LKB1-dependent AMPK signaling pathway. Biochem. Pharmacol. 108, 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P., Wang J., He L., Ma H., Zhang X., Zhu X., Dolence E. K., Ren J., Li J. (2009). Deficiency in TLR4 signal transduction ameliorates cardiac injury and cardiomyocyte contractile dysfunction during ischemia. J. Cell. Mol. Med. 13, 1513–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]