Abstract
Background
Socio-economic status from early life has been linked to cardiovascular disease risk, but the impact of life-course socio-economic trajectories, as well as the mechanisms underlying social inequalities in cardiovascular disease risk, is uncertain.
Objectives
We assessed the role of behavioural, psychosocial and physiological (including inflammatory) factors in the association between life-course socio-economic status and cardiovascular disease mortality in older adults.
Methods
Participants were 7846 individuals (44% women) from the English Longitudinal Study of Ageing, a representative study of individuals aged ≥ 50 years, established in 2002–03. Comprising four indicators of socio-economic status (father’s social class, own education, occupational position and wealth), we computed an index of socio-economic trajectory and a lifetime cumulative socio-economic score. Behavioural (smoking, physical activity, alcohol consumption, body mass index) and psychosocial (social relations, loneliness) factors, physiological (blood pressure, total cholesterol, triglycerides) and inflammatory markers (C-reactive protein, fibrinogen), measured repeatedly over time, were potential explanatory variables. Cardiovascular disease mortality was ascertained by linkage of study members to a national mortality register. Mediation was calculated using the traditional ‘change-in-estimate method’ and alternative approaches such as counterfactual mediation modelling could not be applied in this context.
Results
During the 8.4-year follow-up, 1301 study members died (438 from cardiovascular disease). A stable low-social-class trajectory was associated with around double the risk of cardiovascular disease mortality (hazard ratio; 95% confidence interval: 1.94, 1.37; 2.75) compared with a stable high social class across the life course. Individuals in the lowest relative to the highest life-course cumulative socio-economic status group were also more than twice as likely to die of cardiovascular disease (2.57, 1.81; 3.65). Behavioural factors and inflammatory markers contributed most to explaining this gradient, whereas the role of psychosocial and other physiological risk factors was modest.
Conclusions
In a population-based cohort of older individuals living in England, we provide evidence that disadvantage across the life course is linked to cardiovascular mortality. That behavioural factors and inflammatory markers partially explain this gradient may provide insights into the potential for intervention.
Keywords: Socioeconomic status, lifecourse, cardiovascular disease, cohort, mortality
Key Messages
The impact of life-course socio-economic trajectories on cardiovascular disease morbidity and mortality is uncertain.
In this study, low socio-economic status across the life course was strongly associated with a higher risk of cardiovascular mortality.
Behavioural factors and inflammatory markers appeared to explain a relatively large proportion of this association, providing insights into the potentials for intervention.
Introduction
Cardiovascular disease is a leading cause of death worldwide.1 It is now well established that, in high-income countries, the occurrence of cardiovascular disease (CVD) is greatest among individuals in poorer socio-economic circumstances.2–4 Socio-economic differentials also appear to have their origins in early life,5–12 and possibly even in prior generations. As highlighted in a recent position statement of the American Heart Association13 and other reviews,14 however, studies exploring the influence of life-course socio-economic status (SES) on CVD are very rare. Moreover, the few existent studies sample middle-aged populations, so whether life-course SES still exerts an impact on CVD mortality at older ages, when the burden of CVD is at its greatest, remains unknown.
Explaining socio-economic inequalities in CVD is key to the implementation of effective policies to reduce them,13 yet the physiological mechanisms underlying social inequalities in CVD at older ages are not well understood. Whereas it has been suggested that the association between life-course SES and adult chronic diseases may be explained, at least in part, by chronic inflammation,15 to the best of our knowledge, the contribution of inflammation to social inequalities in CVD mortality has yet to be examined.
At least three conceptual models describe the impact of life-course socio-economic circumstances on health in adulthood: (i) latent effects of early-life socio-economic circumstances on adult health; (ii) cumulative effect of exposure to adverse socio-economic circumstances from across the life course that affect health in a dose–response manner; and (iii) pathways effects of early-life socio-economic circumstances on individuals’ trajectories to SES in adulthood, that in turn have an impact on health.16,17
In a well-characterized study of individuals aged 50 years or older at study induction—the English Longitudinal Study on Ageing (ELSA)—our main objective was to assess the influence of life-course social trajectories on CVD risk. However, to address all the three conceptual models, we also compare different indicators of SES over the life course, as well as assess the impact of cumulative exposure to low SES across the life course for their effect on CVD mortality.
We also examine the potential mediator effect of inflammatory markers in the relationship between SES and risk of CVD, placing their role in context by comparing them with classic behavioural and psychosocial risk factors.
Data and methods
Study population
The ELSA is an ongoing, prospective cohort study of community-dwelling older people. Described in detail elsewhere,18 ELSA was established in 2002–03 with a core sample of 11 391 women and men aged 50 years and over. It is representative of the national population in this age range living in private addresses in England.18 Participants have been contacted every 2 years for an interview and every 4 years for a medical examination. For the present analysis, ‘baseline’ was fixed at the ELSA wave 2 (2004/5) when biological data were first collected (N = 8688).
Socio-economic factors
We used two indicators of life-course SES: a measure of life-course socio-economic trajectories and a cumulative life-course SES index. The trajectories index was computed using two socio-economic indicators chosen because they had the same scoring structure: paternal occupational social class (i.e. SES of origin) and study participant’s own occupational social class (i.e. attained SES). Paternal occupational social class was categorized as high (managerial, professional and administrative occupations or business owners), intermediate (trade and services related occupations) and low (manual and casual occupations and other occupations). Participants’ own occupational position was measured using the three-class version of the National Statistics—Socioeconomic Classification Scheme19 and was categorized as high (managerial and professional occupations), intermediate (intermediate occupations) and low (semi-routine and routine occupations). Participants who had never worked (n = 108) or were of unknown occupational status (n = 1) were excluded from the analyses. These participants tended to be older and had a lower educational level. However, given their very small number in relation to the total population (1%), it is unlikely that their exclusion has biased our results. This resulted in four combinations of possible occupational social class trajectories across the life course: ‘stable high’ (high paternal occupation and own occupation), ‘upward’ (low or intermediate paternal occupation and high own occupation), ‘downward’ (high or intermediate paternal occupation and low own occupation) and ‘stable low’ (low paternal occupation and own occupation).
A cumulative life-course SES status index was computed using information on four socio-economic indicators: paternal occupational position and participant’s own occupational position (described above) plus education, an indicator of SES in young adulthood, and wealth, measured at study induction and thus representing SES in early old life. Education was categorized according to the age at which participants finished full-time education [high (≥17 years), intermediate (15–16 years) and low (≤ 14 years or no education)]. Wealth, i.e. total net non-pension household wealth, was grouped into tertiles and based on an estimation of the assets of study members and their partners, including properties, businesses, other assets and any form of investments or savings (except for pension savings) less debts owed by them. The four individual SES indicators were each coded as 0–2, with higher values indicating greater disadvantage. To compute a cumulative life-course SES index, the four SES indicators were summed, resulting in a nine-value variable, with higher values again corresponding to greater life-course disadvantage.
Behavioural factors
Smoking status was self-reported and classified as current, former, never smoker. Leisure-time physical activity was assessed by asking participants how often they engaged in vigorous, moderate or mild physical activity. Three groups were then created: active (once/week), moderately active (one to three times/month) and inactive (hardly ever/never). Frequency of alcohol intake was self-reported and classified as less than daily or daily consumption. Height and weight were measured directly using standard procedures, and body mass index (BMI) then computed as weight in kilograms divided by the square of height in metres.20
Psychosocial factors
The self-completion questionnaire included a series of detailed items on the quality of the respondents’ social relationships, social networks and loneliness.21 Specifically, respondents were asked about the presence of support (positive/negative) from their spouse and children. Responses to positive and negative support items were summed to create positive aspects of social relations scale (total score ranges from 0 to 9, with higher scores indicating greater positive support) and negative aspects of social relations scale (total score ranges from 0 to 9, with higher scores indicating greater negative support).22 Only positive and negative support scores from the spouse were included in the present study. Low positive support was represented by the lowest tertile and high negative support as the highest tertile.
Respondents were also asked to indicate the number of family members and friends with whom they had a close relationship. From this question, a continuous variable was derived that indicated the number of close friends or family in the respondents’ social networks. High social network size was defined as the highest tertile. Loneliness was measured using an abridged version23 of the 20-item Revised UCLA loneliness scale.24 The dimensions of loneliness that this scale measures are self-perceived isolation, and relational and social connectedness. A summary score was created by summing up the responses on three of the items; the total score ranged from 0 to 6, with higher scores indicating greater perceived loneliness. High loneliness was represented by the highest tertile.
Physiological factors
During study waves 2 (2004/5) and 4 (2008/9), a nurse measured systolic (SBP) and diastolic blood pressure (DBP) (Omron HEM 907) on three occasions at 1-minute intervals with the subjects seated; SBP and DBP are derived as the mean of the first two readings. Hypertension was considered as SBP ≥ 140 or DBP ≥ 90 or taking anti-hypertensive drugs, whereas normotension was taken as SBP < 120 and DBP < 80. A blood sample was also drawn from consenting respondents in order to measure levels of fibrinogen, total cholesterol, triglycerides and C-reactive protein. High total cholesterol was defined for values ≥ 5.2 mmol/l and high triglycerides for values > 1.7 mmol/l.
High-sensitivity C-reactive protein (CRP) was analysed using the N Latex CRP mono Immunoassay on the Behring Nephelometer II Analyzer (Dade Behring, Milton Keynes, UK). In all analyses, CRP data, originally skewed, were normalized using log-transformation. Fibrinogen levels were ascertained using a modification of the Clauss thrombin clotting method on the Organon Teknika MDA 180 analyser. High CRP and high fibrinogen were represented by the highest tertiles of their distribution.
Prevalent chronic illness (coronary heart disease, stroke and/or cancer) at baseline, as well as age and sex, were considered as confounding factors in all analyses.
Mortality ascertainment
Study members were linked to the National Health Service’s Central Registry at Southport, UK, the procedures of which provide vital status data and, where applicable, cause of death. CVD death was coded according to International Classification of Disease chapters (version 10, codes I00–I99). Follow-up began on the date of study baseline (2004/5), with study members censored at date of death or end of follow-up (February 2013)—whichever came first.
Statistical analysis
Missing values for risk factors were replaced using information collected at the previous or successive wave. For those study members without information available at adjacent waves, multivariable imputation based on sex, age, ethnicity and lifestyle factors (Stata uvis procedure) was used. Missing values for the exposures (SES) and outcomes (CVD mortality) of interest were not imputed. Sensitivity analysis restricted to participants with no missing data yielded similar results to those presented here. Having ascertained that the proportional hazard assumption had not been violated, Cox regressions were used to compute hazard ratios (HRs) with accompanying 95% confidence intervals (CIs) for the association between life-course SES and mortality. With no evidence of effect modification by sex, data were pooled and sex-adjusted. Life-course SES trajectory was treated as a categorical variable (p for linear trend = 0.121). The life-course cumulative SES score was entered into the models as a continuous variable (p for linear trend = 0.014) and HRs are calculated for the highest vs the lowest category.25 Each life-course SES indicator was first entered into a basic model containing age, sex and prevalent disease conditions (Model 1). Then, behavioural factors, psychosocial factors, physiological factors and inflammatory markers (time-dependent covariates updated at waves 2 and 4) were added first individually and then simultaneously to the multivariable model. All covariates were entered as continuous variables, apart from smoking, physical activity and alcohol consumption. To account for long-term exposure to these risk factors, at each follow-up period, we controlled not only for the risk factor at the current wave, but also for the risk factors at previous waves, as we have previously.15 The contribution of risk factors to explaining the SES–mortality association was determined by the percent attenuation in the β coefficient for SES after inclusion of the risk factor in question to Model 1 (age and sex): ‘100 × (βModel 1 – βModel 1 + risk factor(s))/(βModel 1)’. We calculated a 95% CI around the percentage attenuation using a bootstrap method with 1000 re-samplings. All analyses were conducted using Stata (version 13.1, Stata Corp, College Station, TX, USA).
Results
A total of 8688 participants provided data at the baseline for the present analyses. Of these, 842 (9%) were excluded because of missing values for socio-economic indicators (N = 143), behavioural factors (N = 276), psychosocial factors (N = 17), physiological factors (N = 267) or inflammatory markers (N = 399) (categories are not mutually exclusive). Relative to study members included in the present analyses, those excluded were somewhat older (69.4 vs 65.9 years, p < .001) and were more likely to come from a disadvantaged socio-economic background (26% vs 22% from a ‘stable low’ SES trajectory, p < 0.001). Whereas these absolute differences were not considerable, they attained statistical significance owing to the large study numbers.
Table 1 shows the baseline characteristics of the sample according to occupational social class trajectories from childhood to adulthood. In general, study members whose father was from a low occupational group and those who also remained in this group as adults (‘stable low’) had the least favourable risk factor profile, such that there was a marked gradient in behavioural, psychological, physiological and inflammatory factors at baseline. In particular, relative to the ‘stable high’ group, people in the ‘stable low’ category were three times more likely to smoke cigarettes, twice as likely to be sedentary and have higher levels of systolic blood pressure, CRP and fibrinogen (p < 0.001). Whereas statistically significant differences were apparent for other characteristics, absolute differences between these groups were modest. In one of the few exceptions to the general unfavourable risk factor profile, people in the stable low category were less likely to consume alcohol frequently.
Table 1.
Study participant characteristics at baseline according to life-course occupational social class trajectories—the English Longitudinal Study of Ageing (N = 7846)
| Life-course social trajectoriesa |
Total | |||||
|---|---|---|---|---|---|---|
| Stable high | Upward | Downward | Stable low | Pb | ||
| N (%) | 1084 (13.8) | 2697 (34.4) | 2439 (31.1) | 1626 (20.7) | 7846 | |
| Age | 65.3 (9.5) | 65.9 (9.5) | 66.9 (9.7) | 67.1 (9.6) | <0.001 | 66.4 (9.6) |
| Men, N (%) | 508 (46.9) (481) | 1389 (51.5) | 1494 (61.3) | 900 (55.4) | <0.001 | 4291 (54.7) |
| CVD mortality (N, ratec) | 42 (5.1) | 136 (6.0) | 130 (6.4) | 130 (9.8) | 0.121 | 438 (6.7) |
| Behavioural factors | ||||||
| Current smoking, N (%) | 79 (7.3) | 352 (13.1) | 388 (15.9) | 347 (21.3) | <0.001 | 1166 (14.9) |
| Physical inactivity, N (%) | 98 (9.0) | 358 (13.3) | 411 (16.9) | 370 (22.8) | <0.001 | 1237 (15.8) |
| Daily alcohol consumption, N (%) | 409 (37.8) | 681 (25.3) | 510 (21.9) | 254 (15.6) | <0.001 | 1854 (23.6) |
| BMI | 27.1 (4.7) | 27.8 (4.7) | 27.9 (4.7) | 28.8 (5.3) | <0.001 | 27.9 (4.9) |
| Psychosocial factors | ||||||
| Loneliness score | 0.84 (1.3) | 1.04 (1.5) | 1.24 (1.6) | 1.42 (1.7) | <0.001 | 1.14 (1.5) |
| Social network size | 8.78 (5.6) | 8.54 (5.9) | 8.66 (5.9) | 8.35 (5.9) | 0.146 | 8.57 (5.8) |
| Positive support score | 7.88 (1.7) | 7.86 (1.7) | 7.74 (1.9) | 7.70 (1.8) | 0.001 | 7.80 (1.8) |
| Negative support score | 2.29 (1.7) | 2.38 (1.7) | 2.51 (1.9) | 2.48 (1.8) | 0.001 | 2.43 (1.8) |
| Physiological factors | ||||||
| Systolic blood pressure (mmHg) | 133.1 (18.2) | 134.9 (18.7) | 135.6 (9.0) | 136.2 (18.9) | <0.001 | 135.0 (18.7) |
| DBP (mmHg) | 74.6 (11.0) | 75.2 (11.4) | 74.6 (11.0) | 74.4 (11.3) | 0.110 | 74.8 (11.2) |
| Total cholesterol (mmol/l) | 5.95 (1.2) | 5.82 (1.2) | 5.85 (1.2) | 5.74 (1.2) | <0.001 | 5.83 (1.2) |
| Triglycerides (mmol/l) | 1.70 (1.1) | 1.81 (1.1) | 1.82 (1.2) | 1.95 (1.2) | <0.001 | 1.83 (1.2) |
| Inflammatory markers | ||||||
| CRP (mg/l) | 3.39 (7.6) | 4.10 (8.4) | 4.35 (7.8) | 4.93 (8.9) | <0.001 | 4.25 (8.2) |
| Fibrinogen (g/l) | 3.09 (0.6) | 3.23 (0.7) | 3.27 (0.7) | 3.33 (0.7) | <0.001 | 3.25 (0.7) |
Results are mean (SD) unless otherwise reported.
aLife-course social trajectories are based on trajectories from father’s occupational position to the participants’ own occupational class.
bp for linear trend across socio-economic categories.
cAge and sex-adjusted mortality rate per 1000 person-years.
A total of 1301 participants died during the 8.4-year follow-up, 438 from CVD. HRs (95% CI) for the association of individual behavioural, psychosocial, physiological and inflammatory risk factors at baseline and CVD mortality are shown in Table 2. For the purposes of comparison, here and throughout our analyses, we also include results for total mortality. As anticipated, smoking (HR; 95% CI: 2.01, 1.54; 2.63) and physical inactivity (2.64, 2.15; 3.24) were associated with CVD mortality, as well as loneliness (1.44, 1.19; 1.74) and small network size (1.31, 1.08; 1.57). High total cholesterol was related to lower CVD mortality risk (HR, 95% CI: 0.71, 0.58; 0.86), whereas high fibrinogen and CRP levels were related to higher CVD mortality risk (HR, 95% CI: 1.74, 1.44; 2.09 for high vs low CRP tertile). Alcohol consumption, obesity, positive support score, hypertension and triglycerides levels were not related to mortality. Results for all-cause mortality were similar to those for CVD mortality.
Table 2.
HRs (95% CI) for the association of risk factors at baseline with total and CVD mortality (N = 7846)—the English Longitudinal Study of Ageing
| Total mortality | CVD mortality | |
|---|---|---|
| Deaths | 1301 | 438 |
| Behavioural factors | ||
| Smoking | ||
| Never/Former | 1.00 (ref) | 1.00 (ref) |
| Current smoker | 2.10 (1.82; 2.43) | 2.01 (1.54; 2.63) |
| Physical activity | ||
| Active/ Moderately active | 1.00 | 1.00 |
| Inactive | 2.19 (1.94; 2.47) | 2.64 (2.15; 3.24) |
| Alcohol consumption | ||
| Less than daily | 1.00 | 1.00 |
| Daily | 0.98 (0.86; 1.11) | 1.00 (0.80; 1.25) |
| BMI | ||
| <30 kg/m2 | 1.00 | 1.00 |
| ≥30 kg/m2 | 1.09 (0.97; 1.23) | 1.21 (0.98; 1.49) |
| Psychosocial factors | ||
| Loneliness score | ||
| Low | 1.00 | 1.00 |
| High | 1.23 (1.10; 1.38) | 1.44 (1.19; 1.74) |
| Social network size | ||
| Large | 1.00 | 1.00 |
| Small | 1.23 (1.10; 1.37) | 1.31 (1.08; 1.57) |
| Positive support score | ||
| High | 1.00 | 1.00 |
| Low | 1.13 (1.01; 1.26) | 1.13 (0.93; 1.36) |
| Negative support score | ||
| Low | 1.00 | 1.00 |
| High | 1.12 (0.99; 1.28) | 1.13 (0.90; 1.42) |
| Physiological factors | ||
| Blood pressure | ||
| Normotensive | 1.00 | 1.00 |
| Hypertensive | 1.00 (0.94; 1.08) | 1.06 (0.96; 1.19) |
| Total cholesterol level | ||
| Low | 1.00 | 1.00 |
| High | 0.80 (0.71; 0.90) | 0.71 (0.58; 0.86) |
| Triglycerides level | ||
| Low | 1.00 | 1.00 |
| High | 1.00 (0.90; 1.12) | 1.01 (0.84; 1.23) |
| Inflammatory markers | ||
| Fibrinogen level | ||
| Low | 1.00 | 1.00 |
| High | 1.38 (1.24; 1.54) | 1.53 (1.26; 1.85) |
| CRP level | ||
| Low | 1.00 | 1.00 |
| High | 1.57 (1.41; 1.75) | 1.74 (1.44; 2.09) |
CVD, cardiovascular disease; SD, standard deviation; SBP, systolic blood pressure; DBP, diastolic blood pressure.
High CRP and high fibrinogen were represented by the highest tertiles of their distribution.
aHazard ratios adjusted for age, sex and prevalent conditions at baseline.
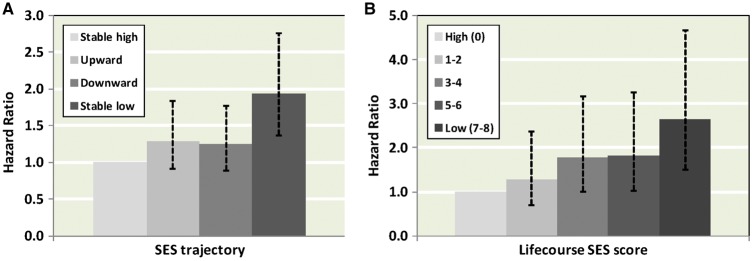
Table 3 and Figure 1 show results for the association of life-course social trajectories with total and CVD mortality. We also show the relative contribution of different explanatory risk factors to this association. In analyses featuring CVD death as the endpoint of interest, a stable low social class was associated with 94% higher risk (95% CI: 1.37; 2.75) as compared with a stable high social class. Upwardly and downwardly mobile individuals also experienced a higher risk of mortality for CVD but these effects were not statistically significant at conventional levels. All risk factors combined explained 37% of the association between stable low social trajectory and CVD mortality (95% CI: 7, 94), most of the contribution being from behavioural factors (24%, 95% CI: 3; 67) and inflammatory markers (16%, 95% CI: 6; 38). Results for total mortality were similar to those for CVD mortality, although the HRs were of lower magnitude. All risk factors combined explained 62% of the association between stable low social trajectory and total mortality (95% CI: 26; 159).
Table 3.
HRs (95% CI) for the association of life-course social trajectories with total and CVD mortality—the English Longitudinal Study of Ageing (N = 7846)
| Life-course social trajectories |
|||||||
|---|---|---|---|---|---|---|---|
| Stable high | Upward | Downward | Stable low | ||||
| Total mortality (1301 deaths) | HR (95% CI) | HR (95% CI) | %Δ | HR (95% CI) | %Δ | HR (95% CI) | %Δ (95% CI) |
| Model 1: Age, sex, & health conditions | 1.00 (ref) | 1.17 (0.97; 1.40) | 1.17 (0.97; 1.42) | 1.45 (1.20; 1.76) | |||
| Model 2: Model 1 + behavioural factors | 1.00 | 1.12 (0.93; 1.36) | – | 1.05 (0.87; 1.28) | – | 1.23 (1.01; 1.51) | –43 (–120; –17) |
| Model 3: Model 1 + psychosocial factors | 1.00 | 1.15 (0.95; 1.39) | – | 1.14 (0.94; 1.39) | – | 1.39 (1.14; 1.69) | –11 (–42; –4) |
| Model 4: Model 1 + physiological factors | 1.00 | 1.15 (0.95; 1.39) | – | 1.15 (0.95; 1.39) | – | 1.41 (1.15; 1.71) | –8 (–38; –1) |
| Model 5: Model 1 + inflammatory markers | 1.00 | 1.09 (0.91; 1.32) | – | 1.07 (0.87; 1.30) | – | 1.31 (1.08; 1.60) | –27 (–70; –11) |
| Model 6: Model 1 + all risk factors | 1.00 | 1.06 (0.88; 1.29) | – | 0.99 (0.81; 1.20) | – | 1.15 (0.94; 1.40) | –62 (–159; –26) |
| CVD mortality (438 deaths) | |||||||
| Model 1: Age, sex, & health conditions | 1.00 | 1.29 (0.92; 1.82) | 1.25 (0.88; 1.77) | 1.94 (1.37; 2.75) | |||
| Model 2: Model 1 + behavioural factors | 1.00 | 1.25 (0.88; 1.77) | – | 1.13 (0.79; 1.61) | – | 1.65 (1.16; 2.36) | –24 (–67; –3) |
| Model 3: Model 1 + psychosocial factors | 1.00 | 1.27 (0.90; 1.80) | – | 1.22 (0.86; 1.72) | – | 1.85 (1.30; 2.73) | –7 (–23; 1) |
| Model 4: Model 1 + physiological factors | 1.00 | 1.25 (0.88; 1.77) | – | 1.21 (0.85; 1.71) | – | 1.81 (1.27; 2.57) | –11 (–39; –2) |
| Model 5: Model 1 + inflammatory markers | 1.00 | 1.21 (0.85; 1.71) | – | 1.13 (0.80; 1.61) | – | 1.75 (1.23; 2.47) | –16 (–38; –6) |
| Model 6: Model 1 + all risk factors | 1.00 | 1.17 (0.82; 1.76) | – | 1.05 (0.73; 1.50) | – | 1.51 (1.05; 2.17) | –37 (–94; –7) |
CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; Δ, attenuation, representing the proportion of the SES–mortality association explained by the risk factor in question. % attenuation is calculated only for statistically significant associations.
Behavioural factors include current smoking, physical activity, alcohol consumption and BMI; psychosocial factors include loneliness score, social network size, negative and positive support from spouse; physiological factors include systolic and DBP, cholesterol and triglycerides levels; inflammatory markers include CRP and fibrinogen.
Figure 1.
Association of life-course SES trajectory (A) and life-course cumulative SES score (B) with CVD mortality.
Results for the association of life-course cumulative SES score with mortality are shown in Table 4 and Figure 1. Individuals with a low relative to high life-course cumulative SES score were 87% (95% CI: 1.40; 2.15) and 157% (95% CI: 1.66; 3.54) more likely to die of total or CVD mortality, respectively. As apparent for the analyses of life-course social trajectories and CVD mortality, of the study covariates, behavioural factors (38%) and inflammatory (18%) markers appeared to explain most of this gradient. Adjustment for all risk factors simultaneously attenuated the HR for lowest vs highest cumulative SES score by 55% (95% CI: 27; 104) for CVD mortality. Similar results were apparent when total mortality was the outcome of interest.
Table 4.
HRs (95% CI) for the association of life-course cumulative socio-economic score with total and CVD mortality—the English Longitudinal Study of Ageing (N = 7846)
| Life-course cumulative SES scorea |
||
|---|---|---|
| HR (95% CI) | %Δ (95% CI) | |
| Total mortality (1301 deaths) | ||
| Model 1: Age, sex and health conditions | 1.87 (1.53; 2.28) | |
| Model 2: Model 1 + behavioural factors | 1.35 (1.09; 1.66) | –52 (–93; –31) |
| Model 3: Model 1 + psychosocial factors | 1.74 (1.42; 2.13) | –11 (–29; –7) |
| Model 4: Model 1 + physiological factors | 1.78 (1.45; 2.18) | –7 (–24; –2) |
| Model 5: Model 1 + inflammatory markers | 1.59 (1.29; 1.94) | –26 (–38; –12) |
| Model 6: Model 1 + all risk factors | 1.18 (0.95; 1.46) | –73 (–126; –46) |
| CVD mortality (438 deaths) | ||
| Model 1: Age, sex and health conditions | 2.57 (1.81; 3.65) | |
| Model 2: Model 1 + behavioural factors | 1.79 (1.24; 2.58) | –38 (–76; –19) |
| Model 3: Model 1 + psychosocial factors | 2.38 (1.67; 3.40) | –8 (–24; –1) |
| Model 4: Model 1 + physiological factors | 2.33 (1.63; 3.33) | –10 (–29; –3) |
| Model 5: Model 1 + inflammatory markers | 2.17 (1.52; 3.09) | –18 (–33; –6) |
| Model 6: Model 1 + all risk factors | 1.53 (1.05; 2.23) | –55 (–104; –27) |
CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; SES, socio-economic status; Δ, attenuation, representing the proportion of the SES–mortality association explained by the risk factor in question. % attenuation is calculated only for statistically significant associations.
Behavioural factors include current smoking, physical activity, alcohol consumption and BMI; psychosocial factors include loneliness score, social network size, negative and positive support from spouse; physiological factors include systolic and DBP, cholesterol and triglycerides levels; inflammatory markers include CRP and fibrinogen.
aHR is for the lowest vs highest score.
Associations between the four individual SES indicators and mortality are shown in supplementary materials, available as Supplementary Data at IJE online. Low father’s social class (Supplementary Table 1, available as Supplementary Data at IJE online), low education (Supplementary Table 2, available as Supplementary Data at IJE online), low adult social class (Supplementary Table 3, available as Supplementary Data at IJE online) and low adult wealth (Supplementary Table 4, available as Supplementary Data at IJE online) were related to higher total and CVD mortality risk. All risk factors combined explained between 62 and 85% of the SES gradient in total mortality, with corresponding values of 40–66% for CVD mortality. As per the analyses of these SES indices in combination, most of the explanatory contributions came from behavioural factors and inflammatory markers. Finally, we conducted additional sensitivity analyses to assess whether an existing acute infection at study induction altered our results. To do so, we repeated our analyses of cumulative SES score and CVD mortality after an incremental exclusion of people with raised CRP levels (Supplementary Table 5, available as Supplementary Data at IJE online). Our overall conclusions were unchanged; if anything, the SES-CVD relation strengthened the lower the CRP levels at which exclusions of study members took place.
Formal tests did not reveal a significantly different effect by age group (p for interaction between age group and life-course SES = 0.404), although, in sensitivity analyses stratified by age group (≤65 years and >65 years, Supplementary Table 8, available as Supplementary Data at IJE online), the association between life-course SES indicators and CVD mortality tended to be somewhat stronger in the younger compared with the older age group.
Discussion
Our main finding was that, in a population of older people, SES across the life course was a strong predictor of CVD mortality, such that individuals who had a stable low socio-economic trajectory or were cumulatively exposed to poor socio-economic circumstances from childhood to adulthood experienced the greatest risk. Moreover, we showed that up to half of the socio-economic gradient in CVD mortality was explained by socio-economic variations in behavioural, psychosocial, physiological and inflammatory risk factors, the major contribution being from behavioural factors and inflammatory markers. To our knowledge, our study represents the first evidence of a contribution of inflammation to life-course social differences in mortality.
In this study, we used two indicators of life-course SES: a measure of social trajectories from childhood to adulthood and a summary measure of four individual socio-economic indicators spanning across the lifetime (father’s social class, education, occupational position and wealth). We showed that individuals who were in the low SES group across the life course had a greater mortality risk than those people who were in the higher SES category throughout this period. As our study focuses on older ages, comparing with previous studies, we choose to compute a life-course SES score using four indicators from early life to early old age. We found that the longer the duration of exposure to disadvantaged SES, the higher the CVD mortality risk, supporting the accumulation hypothesis of disease risk across the life course.16,26
A systematic review that evaluated the evidence for models of life-course socio-economic factors and cardiovascular outcomes concluded that there is little support for a unique influence of social mobility on CVD risk.26 In addition, a recent scientific statement from the American Heart Association on the social determinants of risk and outcomes for cardiovascular disease concluded that evidence on the role of upward or downward socio-economic mobility in generating CVD differences is limited.13 In our study, although individuals with an upward or downward social trajectory had a slightly higher mortality risk than those with a ‘stable high’ trajectory, this was not statistically significant at conventional levels. This result supports the ‘health constraint’ hypothesis according to which socially mobile individuals possess health characteristics of both the SES group that they leave and the one that they join, placing them at an intermediate risk.27 This has been reported in other studies focusing on younger populations.26,28–30
We examine the contribution of several factors to the association between life-course SES and CVD mortality. Existing studies have shown that behavioural factors are major contributors to the association between adult SES and mortality, at least in populations with a strong social patterning of these behaviours.25,31,32 Our study confirms that this is also the case for life-course SES in older populations, although the proportion of the gradient explained by behavioural factors seemed to be lower than in studies focusing on adult SES.25,32 Even in our study, behavioural factors explained a larger proportion of the association of adult SES with mortality than that seen for early-life SES. This may be related to the fact that we captured health behaviours covered in older age as opposed to earlier in the life course.
Comparison with existing studies
Our study pointed to a role for chronic inflammation in explaining social differences in total and CVD mortality. Recent evidence suggests that individuals who experience early-life socio-economic adversity are characterized by exaggerated inflammatory responses that potentially put them at increased risk of inflammation-related diseases in later life.33,34 In a report in the British Whitehall II study, chronic inflammation explained about one-third of the association between life-course SES and the incidence of type 2 diabetes.15 Life-course socio-economic differences in chronic inflammation may be related to, first, the impact of exposure to adversity in early life on the regulation of the inflammatory response33 and, second, to insults across the life course that are themselves related to inflammation, such as chronic stress or health-risk behaviours.35–38 Further research is needed to disentangle the impact of different exposures across the life course on chronic inflammation.
In our study, psychosocial and physiological factors, such as social relations, blood pressure, total cholesterol and triglycerides, contributed little to life-course socio-economic differentials in total and CVD mortality. This was to be expected given their weaker associations than those evident for behavioural factors and inflammatory markers. Estimates for the contribution of psychosocial factors to social inequalities in mortality vary across studies,39–43 partly because studies generally differ in the type of psychosocial factors assessed, so rendering any comparison problematic.
In our study, blood cholesterol concentration was slightly higher in the higher SES group, as commonly observed in England.44 Further, high cholesterol was protective against total and CVD mortality in our sample. This may in part be related to our failure to account for cholesterol-lowering drugs, information on which was not collected in the present study. On the other hand, there is evidence that total cholesterol is less strongly related to all-cause and CVD mortality,45 especially stroke mortality,46 in older age groups. A recent review, for instance, concluded that low-density-lipoprotein cholesterol is inversely associated with mortality risk in people over 60 years.45
It is likely that some of the mediators being compared (e.g. behavioural and physiological variables) lie at different points on the same causal pathway rather than being on separate causal pathways. For example, psychosocial factors like negative support and loneliness might influence behaviours, such as smoking intensity and alcohol consumption,47 whereas other behaviours such as physical activity are likely to have a causal effect on physiological indices, such as blood pressure and inflammation.48 On the other hand, SES may have a direct effect on these factors, so that it is difficult to establish the order of the effects. In this study, we decided to test the independent effect of each set of potential mediating factors and then combine all factors in a joint model, the overall objective being to examine the mediating effect of inflammatory markers, if any, in the association between life-course SES and CVD mortality. Further, we calculated mediation using the traditional ‘change-in-estimate method’, i.e. the quantification of the percentage reduction in estimate after controlling for a potential mediating factor. This method, although widely used,15,25,49 has been criticized.50,51 However, alternative approaches, such as counterfactual mediation modelling,52–54 that facilitate disentanglement of the complex pathways leading from SES to health outcomes, as well as accounting for some of the limitations of the traditional approach, are difficult to apply to time to event data with time varying mediators and complicate interpretation. We attempted to control for some of the limitations of the traditional method by controlling not only for exposure-outcome, but also for mediator-outcome confounding.
After accounting for an array of factors such as health behaviours, psychosocial conditions, inflammatory and physiological factors, 50% of the association between life-course SES and CVD mortality remained unexplained. Other unmeasured factors that may contribute to this association are the long-term anatomical and physiological effects of socio-economic disadvantage in utero and in early childhood, such as intrauterine growth retardation, low birth weight or epigenetic modifications,13,55,56 exposure to occupational or environmental risk factors,57 failure to adhere to medication,58 as well as dietary factors59 and psychological factors (i.e. anxiety, anger/hostility).60
Strengths and limitations
Our population of individuals in early old age is broadly representative of the general population of England, suggesting a high degree of generalisability. The rich phenotyping of this cohort also allowed us to examine the contribution of an unusually wide array of factors including behavioural, psychosocial and physiological factors. Finally, these factors were assessed on repeat occasions, thus allowing changes over time to be taken into account. This study has some limitations that should be considered. SES in early life was collected retrospectively and thus may be subject to recall bias, potentially leading to misclassification and, if not systematic, to a weakening of associations with mortality.61 For reasons of study-member anonymity, users of the ELSA data are only provided with broad causes of death and we could therefore not examine specific cardiovascular outcomes separately.
Conclusions
In a population-based cohort of older individuals living in England, low life-course SES was strongly associated with a higher risk of CVD and total mortality. Behavioural factors and inflammatory markers contribute to explain a relatively large proportion of this association.
Supplementary Data
Supplementary data are available at IJE online.
Funding
Silvia Stringhini is supported by the Swiss National Science Foundation (Ambizione Grant no. PZ00P3_167732) and by the European Commission and the Swiss State Secretariat for Education, Research and Innovation SERI (Horizon 2020 grant no. 633666). Mika Kivimäki is supported by the UK Medical Research Council (K013351) and NordForsk, the Nordisk Programme on Health and Welfare.
Conflict of interest: The authors have no conflicts of interest to declare.
Supplementary Material
References
- 1. World Health Organisation. The top 10 causes of death. Geneva, Switzerland: World Health Organisation, 2014, Fact Sheet No. 310. http://www.who.int/mediacentre/factsheets/fs310/en/ (25 May 2017, date last accessed).
- 2. Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation 1993;88:1973–98. [DOI] [PubMed] [Google Scholar]
- 3. Kilander L, Berglund L, Boberg M, et al. Education, lifestyle factors and mortality from cardiovascular disease and cancer: a 25-year follow-up of Swedish 50-year-old men. Int J Epidemiol 2001;30:1119–26. [DOI] [PubMed] [Google Scholar]
- 4. Kivimaki M, Lawlor DA, Davey Smith G, et al. Socioeconomic position, co-occurrence of behavior-related risk factors, and coronary heart disease: the Finnish Public Sector study. Am J Public Health 2007;97:874–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frankel S, Smith GD, Gunnell D. Childhood socioeconomic position and adult cardiovascular mortality: the Boyd Orr Cohort. Am J Epidemiol 1999;150:1081–4. [DOI] [PubMed] [Google Scholar]
- 6. Strand BH, Kunst A. Childhood socioeconomic position and cause-specific mortality in early adulthood. Am J Epidemiol 2007;165:85–93. [DOI] [PubMed] [Google Scholar]
- 7. Lawlor DA, Sterne JAC, Tynelius P, et al. Association of childhood socioeconomic position with cause-specific mortality in a prospective record linkage study of 1,839,384 individuals. Am J Epidemiol 2006;164:907–15. [DOI] [PubMed] [Google Scholar]
- 8. Galobardes B, Lynch JW, Smith GD. Childhood socioeconomic circumstances and cause-specific mortality in adulthood: systematic review and interpretation. Epidemiol Rev 2004;26:7–21. [DOI] [PubMed] [Google Scholar]
- 9. Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986;1:1077–81. [DOI] [PubMed] [Google Scholar]
- 10. Eriksson JG, Forsen T, Tuomilehto J, et al. Fetal and childhood growth and hypertension in adult life. Hypertension 2000;36:790–4. [DOI] [PubMed] [Google Scholar]
- 11. Forsdahl A. Living conditions in childhood and subsequent development of risk factors for arteriosclerotic heart disease: the cardiovascular survey in Finnmark 1974–75. J Epidemiol Community Health 1978;32:34–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elo IT, Martikainen P, Myrskyla M. Socioeconomic status across the life course and all-cause and cause-specific mortality in Finland. Soc Sci Med 2014;119:198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Havranek EP, Mujahid MS, Barr DA, et al. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2015;132(9):873–98. [DOI] [PubMed] [Google Scholar]
- 14. Harper S, Lynch J, Smith GD. Social determinants and the decline of cardiovascular diseases: understanding the links. Annu Rev Public Health 2011;32:39–69. [DOI] [PubMed] [Google Scholar]
- 15. Stringhini S, Batty GD, Bovet P, et al. Association of lifecourse socioeconomic status with chronic inflammation and type 2 diabetes risk: the Whitehall II prospective cohort study. PLoS Med 2013;10:e1001479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol 2002;31:285–93. [PubMed] [Google Scholar]
- 17. Smith BT, Lynch JW, Fox CS, et al. Life-course socioeconomic position and type 2 diabetes mellitus: the Framingham Offspring Study. Am J Epidemiol 2011;173:438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Steptoe A, Breeze E, Banks J, et al. Cohort profile: the English longitudinal study of ageing. Int J Epidemiol 2013;42:1640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Office for National Statistics. National Statistics—Socioeconomic Classification Scheme. London, UK: Office for National Statistics, 2010. [Google Scholar]
- 20. World Health Organisation. The Challenge of Obesity in the WHO European Region and the Strategies for Response. 1st edn Copenhagen Ø, Denmark:WHO, 2007. [Google Scholar]
- 21. Shankar A, McMunn A, Banks J, et al. Loneliness, social isolation, and behavioral and biological health indicators in older adults. Health Psychol 2011;30:377–85. [DOI] [PubMed] [Google Scholar]
- 22. Stafford M, McMunn A, Zaninotto P, et al. Positive and negative exchanges in social relationships as predictors of depression: evidence from the English Longitudinal Study of Aging. J Aging Health 2011;23:607–28. [DOI] [PubMed] [Google Scholar]
- 23. Hughes ME, Waite LJ, Hawkley LC, et al. A short scale for measuring loneliness in large surveys: results from two population-based studies. Res Aging 2004;26:655–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Russell DW. UCLA Loneliness Scale (Version 3): reliability, validity, and factor structure. J Pers Assess 1996;66:20–40. [DOI] [PubMed] [Google Scholar]
- 25. Stringhini S, Sabia S, Shipley M, et al. Association of socioeconomic position with health behaviors and mortality. JAMA 2010;303:1159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pollitt RA, Rose KM, Kaufman JS. Evaluating the evidence for models of life course socioeconomic factors and cardiovascular outcomes: a systematic review. BMC Public Health 2005;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blane D, Smith GD, Bartley M. Social selection—what does it contribute to social-class differences in health. Sociology of Health & Illness 1993;15:2–15. [Google Scholar]
- 28. Melchior M, Berkman LF, Kawachi I, et al. Lifelong socioeconomic trajectory and premature mortality (35–65 years) in France: findings from the GAZEL Cohort Study. J Epidemiol Community Health 2006;60:937–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hart CL, Smith GD, Blane D. Social mobility and 21 year mortality in a cohort of Scottish men. Soc Sci Med 1998;47:1121–30. [DOI] [PubMed] [Google Scholar]
- 30. Tiikkaja S, Hemstrom O. Does intergenerational social mobility among men affect cardiovascular mortality? A population-based register study from Sweden. Scand J Public Health 2008;36:619–28. [DOI] [PubMed] [Google Scholar]
- 31. Stringhini S, Dugravot A, Shipley M, et al. Health behaviours, socioeconomic status, and mortality: further analyses of the British Whitehall II and the French GAZEL prospective cohorts. PLoS Med 2011;8:e1000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nandi A, Glymour MM, Subramanian SV. Association among socioeconomic status, health behaviors, and all-cause mortality in the United States. Epidemiology 2014;25:170–7. [DOI] [PubMed] [Google Scholar]
- 33. Miller GE, Chen E, Fok AK, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci U S A 2009;106:14716–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Slopen N, Koenen KC, Kubzansky LD. Childhood adversity and immune and inflammatory biomarkers associated with cardiovascular risk in youth: a systematic review. Brain Behav Immun 2012;26:239–50. [DOI] [PubMed] [Google Scholar]
- 35. Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm 2010;2010 See https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2913796/pdf/MI2010-289645.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hermsdorff HH, Angeles Zulet M, Bressan J, et al. Effect of diet on the low-grade and chronic inflammation associated with obesity and metabolic syndrome. Endocrinologia y nutricion: organo de la Sociedad Espanola de Endocrinologia y Nutricion 2008;55:409–19. [DOI] [PubMed] [Google Scholar]
- 37. Cohen S, Janicki-Deverts D, Doyle WJ, et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci U S A 2012;109:5995–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Black PH, Garbutt LD. Stress, inflammation and cardiovascular disease. J Psychosom Res 2002;52:1–23. [DOI] [PubMed] [Google Scholar]
- 39. Stringhini S, Berkman L, Dugravot A, et al. Socioeconomic status, structural and functional measures of social support, and mortality: The British Whitehall II Cohort Study, 1985–2009. Am J Epidemiol 2012;175:1275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Oort FV, van Lenthe FJ, Mackenbach JP. Material, psychosocial, and behavioural factors in the explanation of educational inequalities in mortality in The Netherlands. J Epidemiol Community Health 2005;59:214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Skalicka V, Ringdal K, Witvliet MI. Socioeconomic inequalities in mortality and repeated measurement of explanatory risk factors in a 25 years follow-up. PLoS ONE 2015;10:e0124690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kamphuis CB, Turrell G, Giskes K, et al. Socioeconomic inequalities in cardiovascular mortality and the role of childhood socioeconomic conditions and adulthood risk factors: a prospective cohort study with 17-years of follow up. BMC Public Health 2012;12:1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lynch JW, Smith GD, Kaplan GA, et al. Income inequality and mortality: importance to health of individual income, psychosocial environment, or material conditions. Br Med J 2000;320:1200–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Townsend N, Wickramasinghe K, Bhatnagar P, et al. Coronary Heart Disease Statistics. 2012 Edition London: British Heart Foundation, 2012. [Google Scholar]
- 45. Ravnskov U, Diamond DM, Hama R, et al. Lack of an association or an inverse association between low-density-lipoprotein cholesterol and mortality in the elderly: a systematic review. BMJ Open 2016;6:e010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Prospective Studies C, Lewington S, Whitlock G, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 2007;370:1829–39. [DOI] [PubMed] [Google Scholar]
- 47. Elovainio M, Hakulinen C, Pulkki-Råback L, et al. The contribution of risk factors to excess mortality in isolated and lonely individuals: the prospective UK Biobank cohort study. Lancet Public Health 2017; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mora S, Cook N, Buring JE, et al. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation 2007;116:2110–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stringhini S, Tabak AG, Akbaraly TN, et al. Contribution of modifiable risk factors to social inequalities in type 2 diabetes: prospective Whitehall II cohort study. BMJ 2012;345:e5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lange T, Starkopf L. Commentary: Mediation analyses in the real world. Epidemiology 2016;27:677–81. [DOI] [PubMed] [Google Scholar]
- 51. Richiardi L, Bellocco R, Zugna D. Mediation analysis in epidemiology: methods, interpretation and bias. Int J Epidemiol 2013;42:1511–19. [DOI] [PubMed] [Google Scholar]
- 52. Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods 2013; 18(2):137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. VanderWeele TJ. Marginal structural models for the estimation of direct and indirect effects. Epidemiology 2009;20:18–26. [DOI] [PubMed] [Google Scholar]
- 54. Keil AP, Edwards JK, Richardson DB, et al. The parametric g-formula for time-to-event data: intuition and a worked example. Epidemiology 2014;25:889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Joseph KS, Liston RM, Dodds L, et al. Socioeconomic status and perinatal outcomes in a setting with universal access to essential health care services. CMAJ 2007;177:583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Borghol N, Suderman M, McArdle W, et al. Associations with early-life socio-economic position in adult DNA methylation. Int J Epidemiol 2012;41:62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Evans GW, Kantrowitz E. Socioeconomic status and health: the potential role of environmental risk exposure. Annu Rev Public Health 2002;23:303–31. [DOI] [PubMed] [Google Scholar]
- 58. Wamala S, Merlo J, Bostrom G, et al. Socioeconomic disadvantage and primary non-adherence with medication in Sweden. Int J Qual Health Care 2007;19:134–40. [DOI] [PubMed] [Google Scholar]
- 59. Darmon N, Drewnowski A. Does social class predict diet quality? Am J Clin Nutr 2008;87:1107–17. [DOI] [PubMed] [Google Scholar]
- 60. Gallo LC, Matthews KA. Understanding the association between socioeconomic status and physical health: do negative emotions play a role? Psychol Bull 2003;129:10–51. [DOI] [PubMed] [Google Scholar]
- 61. Batty GD, Lawlor DA, Macintyre S, et al. Accuracy of adults’ recall of childhood social class: findings from the Aberdeen children of the 1950s study. J Epidemiol Community Health 2005;59:898–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.