Abstract
Aims
Dual antiplatelet therapy reduces non-fatal ischaemic events after acute coronary syndrome (ACS) but increases bleeding to a similar extent. We sought to determine the prognostic impact of myocardial infarction (MI) vs. bleeding during an extended follow-up period to gain insight into the trade-off between efficacy and safety among patients after ACS.
Methods and results
In 12 944 patients with non-ST-segment elevation ACS from the Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) trial, we investigated the relative impact of MI and bleeding occurring >30 days post-ACS and subsequent all-cause mortality. Bleeding was graded according to Bleeding Academic Research Consortium (BARC) criteria. MI was associated with a five-fold increase in mortality. BARC type 2 and 3, but not type 1, bleeding had a significant impact on mortality. MI was associated with a greater risk of mortality compared with BARC 2 [relative risk (RR) 3.5; 95% confidence interval (CI) 2.08–4.77; P < 0.001] and BARC 3a bleeding (RR 2.23; 95% CI 1.36–3.64; P = 0.001), and a risk similar to BARC 3b bleeding (RR 1.37; 95% CI 0.81–2.30; P = 0.242). Risk of death after MI was significantly lower than after BARC 3c bleeding (RR 0.22; 95% CI 0.13–0.36; P < 0.001). MI and bleeding had similar time-associations with mortality, which remained significant for several months, still being higher early after the event.
Conclusion
In patients treated with antiplatelet therapy after ACS, both MI and bleeding significantly impacted mortality with similar time-dependency. Although BARC 2 and 3a bleeding were less prognostic for death than MI, the risk of mortality was equivalent between BARC 3b bleeding and MI, and was higher following BARC 3c bleeding.
Keywords: Bleeding , Myocardial infarction , DAPT , Acute coronary syndrome
Introduction
Dual antiplatelet therapy (DAPT) reduces the occurrence of both stent-related and spontaneous myocardial infarction (MI) after acute coronary syndrome (ACS).1–3 However, this benefit is counterbalanced by an increase in bleeding.2–4 Bleeding, which was historically considered an acceptable price to pay for antithrombotic therapy, has been recently shown to independently impact mortality, and a causal relationship is generally accepted, although mechanisms are not fully understood.5–8 International guidelines not only recommend at least 12 months of DAPT after ACS or coronary stent implantation, but they also encourage considering bleeding risk when selecting treatment duration.9–12Adequately accounting for the efficacy on coronary thrombotic events and safety in medical decision-making on type and duration of antiplatelet therapy is challenging.13
In light of recent clinical trial data with DAPT regimens beyond 1 year showing further reduction of ischaemic events at the price of a similar increase in bleeding, the number of patients who may qualify for longer-term DAPT is going to increase.14,15 Therefore, it is critical to understand the prognostic implications of MI, especially spontaneous MI, relative to bleeding in order to assist clinicians in selecting patients for more potent or prolonged antiplatelet treatment. Previous studies reported on the prognostic implications of in-hospital—largely procedural—bleeding vs. ischaemic events.16,17 However, the impact of spontaneous (i.e. non-procedural) bleeding occurring later during treatment has been less extensively investigated,18,19 and its effect on mortality, depending on the severity and how it compares with the mortality risks following an MI, remains unclear.
In this analysis, using a large randomized clinical trial of patients with non-ST-segment elevation (NSTE) ACS with long-term follow-up, we aimed to assess the relative impact on all-cause mortality of MI and bleeding occurring late after the initial ACS presentation on all-cause mortality.
Methods
Patient population
The Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) trial design and inclusion and exclusion criteria have been described previously.20 In brief, TRACER was an international, prospective, randomized, double-blind trial of vorapaxar vs. placebo in patients hospitalized for NSTE ACS managed according to contemporary practice. A total of 12 944 patients from 37 countries and 818 sites were enrolled, and 12 702 who were alive and free from recurrent MI at 30 days after randomization were considered in the current analysis.
All enrolled patients had acute symptoms of coronary ischaemia within 24 h before hospital presentation and at least one of the following findings: a cardiac troponin (I or T) or creatine kinase-MB level that was higher than the upper limit of the normal range or a new ST-segment depression of >0.1 mV or transient ST-segment elevation (<30 min) of >0.1 mV in at least two contiguous leads. Also, at least two of the following criteria were required: age ≥55 years; previous MI, percutaneous coronary intervention (PCI), or coronary artery bypass grafting (CABG); or presence of diabetes mellitus or peripheral arterial disease.
Study procedures
Patients were randomly assigned in a 1:1 ratio to receive vorapaxar (a loading dose of 40 mg and a daily maintenance dose of 2.5 mg thereafter), which is an oral protease-activated receptor 1 antagonist, or matching placebo. Type and timing of revascularization were at the discretion of the operating physician. Concomitant antiplatelet treatments were also decided by the treating physician and according to international guidelines; >90% of patients were treated with clopidogrel during the index ACS hospitalization.
Endpoints
The endpoint of interest for this analysis was all-cause death. We aimed to assess the association of mortality with (i) any MI and (ii) bleeding that was not related to CABG. We only included MI and bleeding events that occurred at least 30 days after randomization. This time window was justified by the need to focus only on late events occurring in patients already stabilized post-ACS, excluding early events that are largely influenced by in-hospital interventional or surgical procedures. Bleeding events were graded according to the Bleeding Academic Research Consortium (BARC) criteria.21 In brief, BARC bleeding was defined as follows: BARC type 1, any bleeding that is not actionable; type 2, any overt, actionable sign of bleeding; type 3a, overt bleeding with a haemoglobin drop of 3–5 g/dL or any transfusion; type 3b, overt bleeding with a haemoglobin drop >5 g/dL, requiring vasopressors, surgical intervention, or due to cardiac tamponade; type 3c, any intracranial or intraocular bleeding; type 4, any bleeding that was CABG-related; and finally type 5, any bleeding resulting in death. MI was classified according to the Third Universal Definition of Myocardial Infarction.22
An independent clinical events committee (CEC) adjudicated all events. Definitions were previously described.20 Bleeding events were classified by the CEC according to the TIMI (Thrombolysis In Myocardial Infarction) and GUSTO (Global Use of Strategies to Open Occluded Coronary Arteries) definitions. The BARC classification was derived with an algorithm on data points adjudicated by the CEC. The ethics committee or institutional review board of each participating institution approved the study protocol, and written informed consent was required prior to study inclusion. The study was conducted in accordance with the principles of the Declaration of Helsinki. The Duke University Institutional Review Board approved the use of the TRACER database for secondary analyses.
Statistical analysis
Demographic and baseline variables were summarized by the worst BARC bleeding event experienced from 30 days postrandomization (see Supplementary material online, Table S1) and by whether an MI occurred during this period (see Supplementary material online, Table S2). Continuous variables were presented as medians (inter-quartile ranges), and categorical variables were presented as counts (proportions).
All-cause mortality risk was investigated using Cox proportional hazards models. All models described below included covariates for MI and BARC bleeding during the first 30 days postrandomization, as well as age, body mass index, female sex, Killip class ≤2 at enrolment, history of peripheral arterial disease, prior stroke, prior MI, hypertension, hyperlipidaemia, diabetes mellitus, smoker at enrolment, and systolic blood pressure at enrolment. Randomized treatment was not included as a covariate in the models because of the lack of association with all-cause mortality.20 MI and BARC bleeding events after 30 days were included as time-dependent binary indicators. Risk of recurrent events has been modelled taking into account the first occurrence of such an event, whereas in case of events of different severity (i.e. BARC 2 followed by BARC 3 bleeding), these have been considered as separate covariates. Hazard ratios (HRs) and P-values for the risk of all-cause death associated with post-30-day bleeding and post-30-day MI events were obtained from Model 1—BARC 3a, 3b, and 3c bleeding events were treated the same as BARC 3 bleeding events (Table 1)—and from Model 2, where they were evaluated separately (Table 2). The relative hazard of MI vs. bleeding events was estimated from the same models. Additional analyses investigated the time-dependent nature of the risk of death associated with post-30-day bleeding and MI events as a function of the time elapsed since the event. In these analyses, the natural log of the hazard of death was modelled as a function of (i) natural log, (ii) square root, (iii) quadratic, and (iv) piecewise linear function (with separate slopes for 0–30 days and after 30 days) of time elapsed since the bleeding or MI event. The best-fitting model was selected using the Akaike Information Criterion. For patients who experienced both a bleeding event and an MI, both events were counted and each was considered individually as predictors of death (i.e. the models included covariates for MI and bleeding events).
Table 1.
Model 1: Association between risk of mortality and BARC 1, 2, and 3 (any 3a, 3b, 3c pooled) bleeding events occurring >30 days after randomization (adjusted for known mortality risk factors)
| Covariate | Definition | Death for no event | Death for event | Adjusteda HR (95% CI) | P-value for covariate risk (HR) |
|---|---|---|---|---|---|
| MI | — | 3.19% | 16.43% | 5.36 | <0 .001 |
| (382/11 984) | (118/718) | (4.26–6.74) | |||
| Any BARC bleeding | <0 .001 | ||||
| Nuisance (BARC 1) | Non-actionable bleeding | 3.95% | 3.76% | 0.89 | 0 .551 |
| (467/11 824) | (33/878) | (0.61–1.31) | |||
| Minor (BARC 2) | Any overt, actionable sign of bleeding | 3.79% | 6.32% | 1.70 | 0 .001 |
| (455/11 990) | (45/712) | (1.23–2.36) | |||
| Major (BARC 3a–c) | Any overt, actionable bleeding associated with ≥3 g/dL hgb drop, transfusion, or more severe bleeding | 3.41% | 22.83% | 5.73 | <0 .001 |
| (421/12 356) | (79/346) | (4.32–7.59) |
CI, confidence interval; hgb, haemoglobin; HR, hazard ratio; MI, myocardial infarction; BARC, Bleeding Academic Research Consortium.
Adjustment covariates include: MI and BARC bleeding through Day 30 post-randomization, age, body mass index, female sex, Killip class ≤2 at enrolment, history of peripheral arterial disease, prior stroke, prior MI, hypertension, hyperlipidaemia, diabetes mellitus, smoker at enrolment, and systolic blood pressure at enrolment.
Table 2.
Model 2: Association between risk of mortality and BARC 1, 2, 3a, 3b, and 3c bleeding events occurring more than 30 days after randomization (adjusted for known mortality risk factors)
| Covariate | Definition | Death for no event | Death for event | Adjusteda HR (95% CI) | P-value for covariate risk (HR) |
|---|---|---|---|---|---|
| MI | — | 3.19% | 16.43% | 6.15 | <0.001 |
| (382/11 984) | (118/718) | (4.90–7.74) | |||
| Major bleeding | |||||
| BARC 3a | Overt bleeding with hgb drop 3–5 g/dL or any transfusion | 3.75% | 18.29% | 2.77 | <0.001 |
| (470/12 538) | (30/164) | (1.86–4.12) | |||
| BARC 3b | Overt bleeding with hgb drop > 5 g/dL, requiring vasopressors, surgical intervention, or due to cardiac tamponade | 3.78% | 20.00% | 4.51 | <0.001 |
| (475/12 577) | (25/125) | (2.86–7.10) | |||
| BARC 3c | Intracranial or intraocular bleeding | 3.76% | 42.11% | 28.2 | <0.001 |
| (476/12 645) | (24/57) | (17.5–45.7) |
CI, confidence interval; hgb, haemoglobin; HR, hazard ratio; MI, myocardial infarction; BARC, Bleeding Academic Research Consortium.
Adjustment covariates include: MI and BARC bleeding through Day 30 post-randomization, age, body mass index, female sex, Killip class ≤2 at enrolment, history of peripheral arterial disease, prior stroke, prior MI, hypertension, hyperlipidaemia, diabetes mellitus, smoker at enrolment, and systolic blood pressure at enrolment.
All statistical tests were performed at a significance level of 0.05. All analyses were performed at the Duke Clinical Research Institute (Durham, NC, USA) using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
In the TRACER trial, a total of 12 702 patients were alive and free from any recurrent MI at 30 days. A total of 718 patients (5.6%) suffered a recurrent MI 30 days after the index event, most of which were spontaneous (Table 3). Bleeding events occurring during study follow-up were distributed as follows: BARC 1 occurred in 878 patients (6.9%); BARC 2 occurred in 712 patients (5.6%); BARC 3 occurred in 346 patients (2.7%) (Table 1). More than one post-30-day MI occurred in 0.98% of patients during follow-up, whereas multiple BARC 2 or 3 bleeding events occurred in 1.39%. The proportion of patients experiencing both bleeding and MI during follow-up was 1.46%, with similar rates of patients experiencing an MI first (0.72%) or bleeding first (0.75%) (Figure 1). Demographic and clinical characteristics of patients experiencing bleeding and MI events are presented in the Supplementary materia online, Tables S1 and S2, respectively. Patients with vs. without bleeding were older; more often affected by dyslipidaemia or diabetes; and more often had a history of stroke, peripheral vascular disease, and atrial fibrillation. Patients with vs. without MI were older and more often affected by hypertension, dyslipidaemia, diabetes, and other comorbidities.
Table 3.
Myocardial infarction occurring >30 days after randomization in the TRACER trial, stratified according to the Third Universal Definition of Myocardial Infarction
| Event | Definition | Frequency | Valid per cent (%) |
|---|---|---|---|
| Myocardial infarction | 718 | — | |
| Type 1 | Spontaneous | 594 | 82.7 |
| Type 2 | Secondary to ischaemic imbalance | 46 | 6.41 |
| Type 3 | Resulting in SCD | 0 | 0.0 |
| Type 4a | Related to PCI | 34 | 6.69 |
| Type 4b | Related to stent thrombosis | 35 | 4.87 |
| Type 5 | Related to CABG | 7 | 5.07 |
| STEMI | 77 | 10.75 | |
| New Q-wave | 31 | 5.18 |
CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention; SCD, sudden cardiac death; STEMI, ST-segment elevation myocardial infarction.
Figure 1.
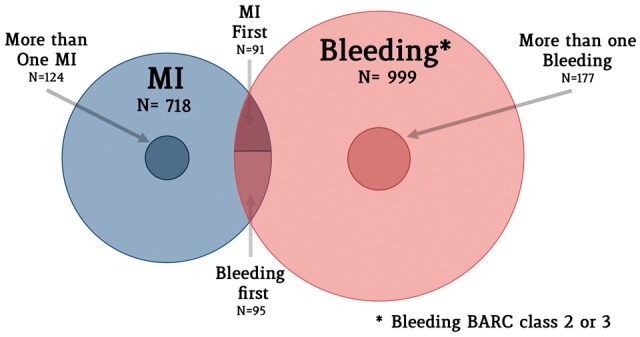
Distribution of minor and major bleeding and myocardial infarction in the TRACER trial. The Venn diagram shows patients experiencing minor or major bleeding (in red) and myocardial infarction (in blue). Smaller circles represent patients experiencing more than one event during follow-up. The intersection represents patients experiencing both myocardial infarction and bleeding during follow-up. MI, myocardial infarction.
Mortality following bleeding or myocardial infarction
Death occurred in 500 patients (3.9%) at the end of follow-up. Table 1 reports the number of patients who died following MI or bleeding events. When considered as time-dependent covariates in the multivariable-adjusted model, both post-30-day bleeding and MI were associated with mortality (Table 1). Patients with an MI ≥30 days after randomization had a five-fold increase in the hazard of death [adjusted HR 5.36; 95% confidence interval (CI) 4.26–6.74; P < 0.001]. BARC 1 bleeding did not affect survival (adjusted HR 0.89; 95% CI 0.61–1.31; P = 0.551), whereas BARC 2 and 3 bleeding types were associated with a significant increase in the risk of mortality, with a progressive increase in risk with more severe categories of bleeding (Table 1). When BARC 3 major bleeding subcategories (BARC 3a, 3b, and 3c) were separately included in a second multivariate-adjusted model for mortality, a consistent risk progression among more severe bleeding events was also noted (Table 2). The prognostic impact was independent from the randomized treatment with vorapaxar for both MI (Pint = 0.19) and different bleeding types (BARC 1: Pint = 0.61; BARC 2: Pint = 0.20; BARC 3: Pint = 0.17).
Relative impact of bleeding vs. myocardial infarction on mortality
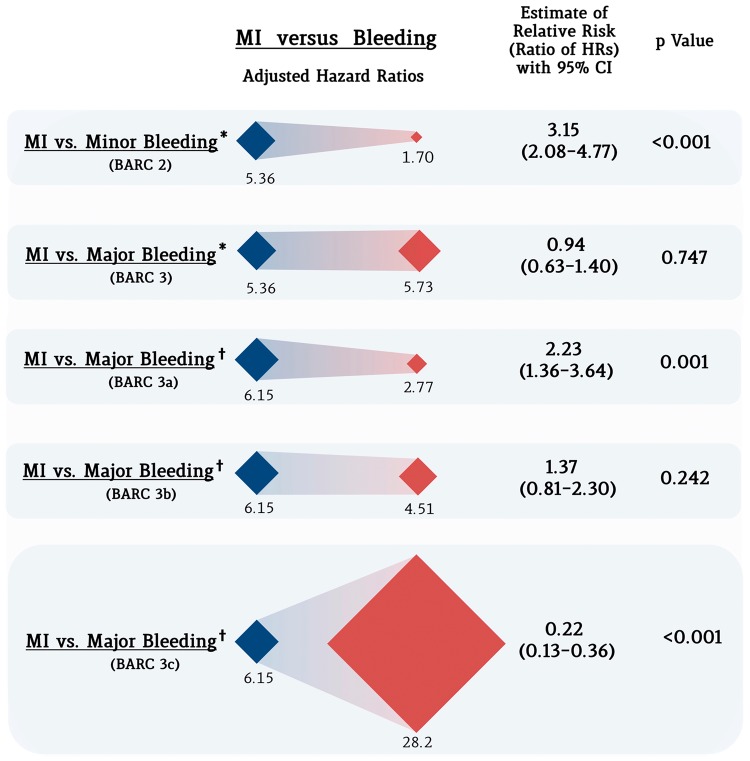
The relative impact on mortality of an MI vs. a bleeding event is displayed graphically in Figure 2. The relative hazard of death was three-fold higher in patients experiencing an MI vs. a BARC 2 bleeding event [5.36 vs. 1.70; relative risk (RR) 3.15; 95% CI 2.08–4.77; P < 0.001], whereas no significant difference was noted for BARC 3 bleeding (5.36 vs. 5.73; RR 0.94; 95% CI 0.63–1.40; P = 0.747). In a second adjusted model for mortality that accounted for the BARC 3 subcategories (BARC 3a, 3b, and 3c) separately, the risk of mortality associated with MI was significantly higher than for BARC 3a bleeding (6.15 vs. 2.77; RR 2.23; 95% CI 1.36–3.64; P = 0.001). MI was associated with a non-significant increase in the risk of death compared with BARC 3b bleeding (6.15 vs. 4.51; RR 1.37; 95% CI 0.81–2.30; P = 0.242). Mortality following MI was significantly lower than following a BARC 3c bleeding event (6.15 vs. 28.2; RR 0.22; 95% CI 0.13–0.36; P < 0.001) (Figure 2).
Figure 2.
Differential impact of myocardial infarction vs. bleeding on mortality. Blue rhombuses represent the magnitude (adjusted hazard ratio) of the impact on mortality of late myocardial infarction, whereas red rhombuses represent that of bleeding of different severity. On the right part of the figure, the estimate of the relative risk (ratio of the hazard ratios) for each category is presented. *The estimates of the impact of events on mortality is derived from Model 1, including BARC 3 bleeding as a single category. †The estimates of the impact of events on mortality is derived from Model 2, including BARC 3 bleeding subcategories separately. MI, myocardial infarction.
Time relation of bleeding and myocardial infarction with hazard of mortality
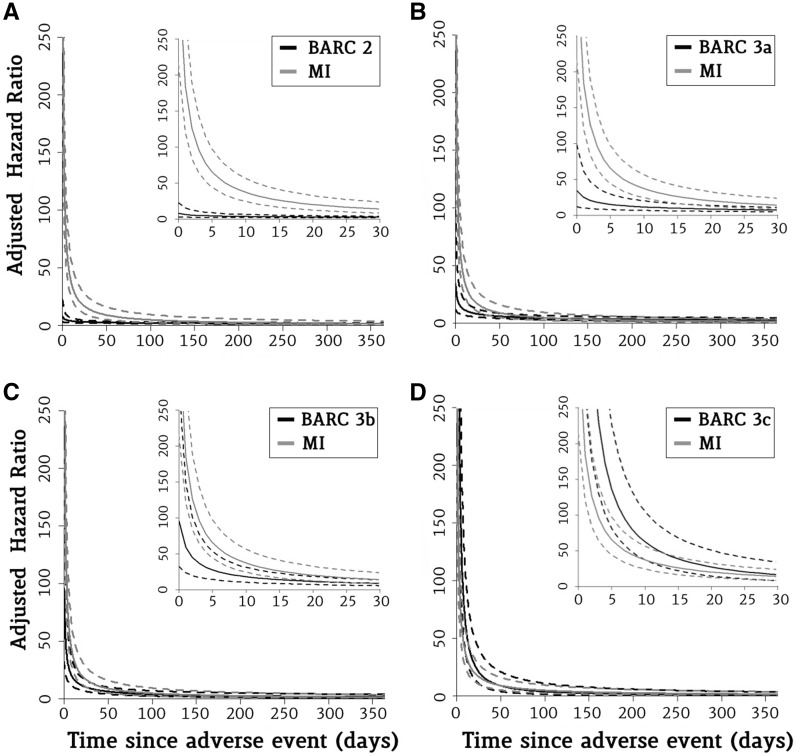
The time-pattern of the associated hazard of mortality was similar between MI and bleeding events. For both MI and bleeding, the risk of death was higher early after the event; it rapidly dissipated in the subsequent days, but it still kept a significant prognostic impact for several months thereafter. The mortality risk was no longer significantly different from that in patients without an MI or bleeding event 215 days after an MI; 183 days after a BARC 2 minor bleeding event; and 538, 239, and 113 days for BARC 3a, 3b, and 3c bleeding, respectively (Figure 3).
Figure 3.
Evolution of the prognostic impact of minor and major bleeding vs. myocardial infarction over time. This figure shows the adjusted hazard ratio for mortality of myocardial infarction vs. minor (BARC 2) and major (BARC 3a–c) bleeding as a function of time elapsed after the event. Inside graph: The decline in hazard ratio as an exponential function of time in the first 30 days. Outside graph: The decline in hazard ratio for the first year after the event. Solid lines represent point estimates; dashed lines represent 95% confidence intervals. MI, myocardial infarction; BARC, Bleeding Academic Research Consortium.
Discussion
We have confirmed the prognostic significance of MI and bleeding occurring late after hospitalization, and found that the relative prognostic impact of MI compared with bleeding markedly varied based on the severity of bleeding. The risk of mortality following an MI was three-fold higher compared with that of BARC 2 bleeding, whereas it was similar to that of BARC 3 bleeding. When BARC 3 subcategories were separately appraised, MI had a higher mortality risk than BARC 3a bleeding, and was substantially equivalent to the risk of mortality following BARC 3b bleeding. Importantly, the risk of death was highest after intracranial or intraocular haemorrhages (captured in the BARC 3c category), and it was ∼4.5-fold higher than the risk of bleeding following MI.
Our analysis was based on centrally adjudicated events and analysed bleeding according to a standardized, widely accepted and reproducible bleeding definition. Prior analyses have observed a similarly increased risk of mortality between spontaneous MI and bleeding during a 4 year follow-up.23 However, this consideration might largely depend on bleeding definition and the type of bleeding explored.24 In fact, accounting for bleeding severity, we found that recurrent MI had higher prognostic impact on mortality than bleeding, except for BARC 3c categories. This information is of major importance, as it indicates that it might be fair to pursue a more potent antiplatelet regimen to avoid an MI even at the expense of mild-to-moderate bleeding in patients with high ischaemic risk. In addition, our data suggest that combining all bleeding, including minor bleeds that are more frequent but less prognostically significant, into a safety endpoint or net clinical outcome including MI may pose significant challenges in interpreting the clinical benefit of drugs.
The temporal association with mortality was similar between MI and minor or major bleeding. Although the highest risk of mortality was present in the first week after the event, its magnitude rapidly decreased thereafter, despite remaining elevated for several months. Prior studies assessing the temporal association of ischaemia and bleeding with mortality have had contrasting results.16,18 In one report based on an NSTE MI population, the impact of bleeding was sustained over time up to 1 year after the event, whereas the impact of MI rapidly dissipated and was no longer significant after 30 days.16 In a second report focusing on ST-segment elevation MI patients, recurrent MI had a more long-lasting effect on mortality (>1 year), whereas severe bleeding did not have a significant impact on mortality as early as 30 days after the event.18 A similar result in an all-ACS population was observed by Hochholzer et al.,19 who found the risk of bleeding being no more significantly elevated 40 days after the event. Reconciling these inconsistent results is challenging as they concern different populations, different types and definitions of events, and different statistical methods. Accordingly, further studies are needed to confirm the time-association pattern between MI and bleeding with mortality.
The findings of our analysis might also help explain the efficacy/safety balance of recent clinical trials exploring newer strategies of prolonged DAPT, which have demonstrated an ischaemic benefit obtained at the expense of increased bleeding complications.14,15,25,26 The PEGASUS-TIMI 54 (Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin–Thrombolysis in Myocardial Infarction 54) trial tested the effect of a long course of treatment with two different doses of ticagrelor (90 and 60 mg) vs. placebo in patients with an MI that occurred 1–3 years earlier.15 Ticagrelor reduced the primary efficacy endpoint by an absolute 1.2%, mainly by reducing MIs and stroke, but with no significant impact on mortality. Interestingly, the treatment with ticagrelor also increased major bleeding to a similar extent. The DAPT trial included patients treated with drug-eluting stents, who after a 12 month run-in phase of standard DAPT with thienopyridines were randomized to stop or continue the P2Y12 inhibitor.14 After 30 months, patients randomized to an extended DAPT course showed a reduction of ischaemic events at the expense of a similar increase. Taken together, these trials and our current findings strongly suggest that DAPT duration should be weighed considering the ischaemic vs. bleeding risk profile of the patient, as both complications may concur to significantly increase mortality, with comparative effects that largely depend on the bleeding severity.14,15,27–29
Net clinical benefit outcomes have become a popular endpoint to account for both efficacy and bleeding effect. However, there is an intrinsic risk of misinterpretation when heterogeneity among components exists with respect to either importance, number of events, or magnitude of treatment effect.30 In this scenario, one can imagine that if the directions of minor bleeding and MI are different, but minor bleeding occurs more frequently, the net clinical benefit will be pushed towards the treatment with fewer events, irrespective of their clinical significance. To overcome this limitation of the classic time-to-event analysis, alternative statistical approaches have been proposed.31–33 These methods rank or weigh events according to their clinical significance, minimizing imbalances from differences in direction and magnitude of single components of the endpoint. However, the evidence regarding how various bleeding types should be weighed in combined endpoints against MI is so far limited. Hence, our study could be useful, informing a more objective way to rank/weigh ischaemic and bleeding events.
The limitations of our study must be acknowledged. This is a posthoc analysis, and the interplay between MI, bleeding, and mortality is very complex; we could not account for all possible factors implicated in their causal relationship. Second, we did not evaluate the prognostic impact of MI subcategories; however, because we excluded events occurring during the first 30 days, PCI- and CABG-related MI were observed rarely. Finally, although bleeding events were stratified by severity, we did not have a systematic measure of infarct severity or size. For example, imaging assessing infarct size or changes in ejection fraction are typically not systematically performed in large phase 3 randomized clinical trials and were not available in TRACER. It is likely to expect a different impact on mortality of events related with larger areas of myocardium at risk and/or with proximal segments of the coronary arteries.34,35
Conclusions
Recurrent MI occurring 30 days after hospitalization for NSTE ACS appeared to be associated with a higher risk of mortality compared with mild-to-moderate bleeding, and had a similar prognostic implication compared with severe, non-intracranial bleeding. Intracranial and intraocular bleeding were associated with a mortality risk that was higher than for MI. These findings may help interpret the risk-and-benefit profile of antithrombotic medications.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The TRACER trial was supported by Merck & Co., Inc. However, this work was supported internally by the Duke Clinical Research Institute.
Conflict of interest: M.V. has received research grants from The Medicines Company, Terumo, and AstraZeneca. L.W. has received research grants from AstraZeneca, Merck & Co, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, and GlaxoSmithKline; speakers’ bureau: lecture fees from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, GlaxoSmithKline, and Merck & Co.; honoraria from Boehringer Ingelheim, AstraZeneca, Bristol-Myers Squibb/Pfizer, GlaxoSmithKline, and Merck & Co.; consultant/advisory board from Merck & Co, Regado Biosciences, Evolva, Portola, C.S.L. Behring, Athera Biotechnologies, Boehringer-Ingelheim, AstraZeneca, GlaxoSmithKline, and Bristol-Myers Squibb/Pfizer; travel support from Bristol-Myers Squibb/Pfizer. P.W.A. has disclosures listed at http://www.vigour.ualberta.ca/en/About/ConflictofInterest.aspx. H.D.W. has received research grants from Sanofi Aventis, Eli Lilly, NIH, Merck Sharpe & Dohme, AstraZeneca, GSK, and Daiichi Sankyo Pharma Development; consulting fees from AstraZeneca. C.H. has received institutional research grants from AstraZeneca, GlaxoSmithKline, Pfizer/Bristol Myers Squibb, Roche, and Schering-Plough (now Merck); consulting fees from AstraZeneca. P.E.A. has received research grant from Merck & Company, AstraZeneca, Sanofi, and GSK; honoraria for speaker bureau and advisory board from AstraZeneca, Eli Lilly, Boehringer Ingelheim, Bayer J&J, Servier, and Bristol Myer Squibb. F.V.d.W. has received research grant from Merck; honoraria for lectures and advisory board membership from Merck. R.A.H. has received consultant fees/honoraria from Adverse Events, Amgen Inc., Daiichi-Lilly, Gilead Sciences, Janssen Research and Development, Medtronic, Merck, Novartis Corporation, The Medicines Company, Vida Health, Vox Media, and WebMD; research/research grants from AstraZeneca, BMS, CSL Behring, GSK, Merck, Portola, Sanofi-Aventis, and The Medicines Company; ownership interest/partnership/principal for Element Science and MyoKardia; Officer, Director, Trustee, or other Fiduciary Role for Evidint and Scanadu; Data Safety Monitoring Board: Regado; other: American Heart Association. K.W.M. has full disclosures prior to 1 August 2013 available at www.dcri.org; disclosures after 1 August 2013 available at https://med.stanford.edu/profiles/47970?tab=research-and-scholarship. P.T. has received consultant agreement and research grant from Merck and CSL; research grant from Sanofi-Aventis. The other authors declared no conflict of interest.
Supplementary Material
References
- 1. Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK.. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494–502. [DOI] [PubMed] [Google Scholar]
- 2. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM.. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001–2015. [DOI] [PubMed] [Google Scholar]
- 3. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA.. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045–1057. [DOI] [PubMed] [Google Scholar]
- 4. Becker RC, Bassand JP, Budaj A, Wojdyla DM, James SK, Cornel JH, French J, Held C, Horrow J, Husted S, Lopez-Sendon J, Lassila R, Mahaffey KW, Storey RF, Harrington RA, Wallentin L.. Bleeding complications with the P2Y12 receptor antagonists clopidogrel and ticagrelor in the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J 2011;32:2933–2944. [DOI] [PubMed] [Google Scholar]
- 5. Rao SV, O'Grady K, Pieper KS, Granger CB, Newby LK, Van de Werf F, Mahaffey KW, Califf RM, Harrington RA.. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol 2005;96:1200–1206. [DOI] [PubMed] [Google Scholar]
- 6. Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S.. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 2006;114:774–782. [DOI] [PubMed] [Google Scholar]
- 7. Manoukian SV, Feit F, Mehran R, Voeltz MD, Ebrahimi R, Hamon M, Dangas GD, Lincoff AM, White HD, Moses JW, King SB 3rd, Ohman EM, Stone GW.. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes. an analysis from the ACUITY Trial. J Am Coll Cardiol 2007;49:1362–1368. [DOI] [PubMed] [Google Scholar]
- 8. Ndrepepa G, Berger PB, Mehilli J, Seyfarth M, Neumann FJ, Schomig A, Kastrati A.. Periprocedural bleeding and 1-year outcome after percutaneous coronary interventions: appropriateness of including bleeding as a component of a quadruple end point. J Am Coll Cardiol 2008;51:690–697. [DOI] [PubMed] [Google Scholar]
- 9. Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, Ohman M, Petrie MC, Sonntag F, Uva MS, Storey RF, Wijns W, Zahger D.. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2999–3054. [DOI] [PubMed] [Google Scholar]
- 10. Steg PG, Huber K, Andreotti F, Arnesen H, Atar D, Badimon L, Bassand JP, De Caterina R, Eikelboom JA, Gulba D, Hamon M, Helft G, Fox KA, Kristensen SD, Rao SV, Verheugt FW, Widimsky P, Zeymer U, Collet JP.. Bleeding in acute coronary syndromes and percutaneous coronary interventions: position paper by the Working Group on Thrombosis of the European Society of Cardiology. Eur Heart J 2011;32:1854–1864. [DOI] [PubMed] [Google Scholar]
- 11. Authors/Task Force members, Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A.. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541–2619. [DOI] [PubMed] [Google Scholar]
- 12. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH.. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011;124:e574–e651. [DOI] [PubMed] [Google Scholar]
- 13. Valgimigli M, Ariotti S, Costa F.. Duration of dual antiplatelet therapy after drug-eluting stent implantation: will we ever reach a consensus? Eur Heart J 2015;36:1219–1222. [DOI] [PubMed] [Google Scholar]
- 14. Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, Normand SL, Braunwald E, Wiviott SD, Cohen DJ, Holmes DR Jr, Krucoff MW, Hermiller J, Dauerman HL, Simon DI, Kandzari DE, Garratt KN, Lee DP, Pow TK, Ver Lee P, Rinaldi MJ, Massaro JM.. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014;371:2155–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K, Bengtsson O, Oude Ophuis T, Budaj A, Theroux P, Ruda M, Hamm C, Goto S, Spinar J, Nicolau JC, Kiss RG, Murphy SA, Wiviott SD, Held P, Braunwald E, Sabatine MS.. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015;372:1791–1800. [DOI] [PubMed] [Google Scholar]
- 16. Mehran R, Pocock SJ, Stone GW, Clayton TC, Dangas GD, Feit F, Manoukian SV, Nikolsky E, Lansky AJ, Kirtane A, White HD, Colombo A, Ware JH, Moses JW, Ohman EM.. Associations of major bleeding and myocardial infarction with the incidence and timing of mortality in patients presenting with non-ST-elevation acute coronary syndromes: a risk model from the ACUITY trial. Eur Heart J 2009;30:1457–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pocock SJ, Mehran R, Clayton TC, Nikolsky E, Parise H, Fahy M, Lansky AJ, Bertrand ME, Lincoff AM, Moses JW, Ohman EM, White HD, Stone GW.. Prognostic modeling of individual patient risk and mortality impact of ischemic and hemorrhagic complications: assessment from the Acute Catheterization and Urgent Intervention Triage Strategy trial. Circulation 2010;121:43–51. [DOI] [PubMed] [Google Scholar]
- 18. Kikkert WJ, Zwinderman AH, Vis MM, Baan J Jr, Koch KT, Peters RJ, de Winter RJ, Piek JJ, Tijssen JG, Henriques JP.. Timing of mortality after severe bleeding and recurrent myocardial infarction in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv 2013;6:391–398. [DOI] [PubMed] [Google Scholar]
- 19. Hochholzer W, Wiviott SD, Antman EM, Contant CF, Guo J, Giugliano RP, Dalby AJ, Montalescot G, Braunwald E.. Predictors of bleeding and time dependence of association of bleeding with mortality: insights from the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel–Thrombolysis in Myocardial Infarction 38 (TRITON-TIMI 38). Circulation 2011;123:2681–2689. [DOI] [PubMed] [Google Scholar]
- 20. Tricoci P, Huang Z, Held C, Moliterno DJ, Armstrong PW, Van de Werf F, White HD, Aylward PE, Wallentin L, Chen E, Lokhnygina Y, Pei J, Leonardi S, Rorick TL, Kilian AM, Jennings LH, Ambrosio G, Bode C, Cequier A, Cornel JH, Diaz R, Erkan A, Huber K, Hudson MP, Jiang L, Jukema JW, Lewis BS, Lincoff AM, Montalescot G, Nicolau JC, Ogawa H, Pfisterer M, Prieto JC, Ruzyllo W, Sinnaeve PR, Storey RF, Valgimigli M, Whellan DJ, Widimsky P, Strony J, Harrington RA, Mahaffey KW.. Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med 2012;366:20–33. [DOI] [PubMed] [Google Scholar]
- 21. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H.. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011;123:2736–2747. [DOI] [PubMed] [Google Scholar]
- 22. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasché P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S.. Third universal definition of myocardial infarction. Circulation 2012;126:2020–2035.22923432 [Google Scholar]
- 23. Kazi DS, Leong TK, Chang TI, Solomon MD, Hlatky MA, Go AS.. Association of spontaneous bleeding and myocardial infarction with long-term mortality after percutaneous coronary intervention. J Am Coll Cardiol 2015;65:1411–1420. [DOI] [PubMed] [Google Scholar]
- 24. Vranckx P, White HD, Huang Z, Mahaffey KW, Armstrong PW, Van de Werf F, Moliterno DJ, Wallentin L, Held C, Aylward PE, Cornel JH, Bode C, Huber K, Nicolau JC, Ruzyllo W, Harrington RA, Tricoci P.. Validation of BARC Bleeding Criteria in Patients With Acute Coronary Syndromes: The TRACER Trial. J Am Coll Cardiol 2016;67:2135–2144. [DOI] [PubMed] [Google Scholar]
- 25. Valgimigli M, Costa F, Byrne R, Haude M, Baumbach A, Windecker S.. Dual antiplatelet therapy duration after coronary stenting in clinical practice: results of an EAPCI survey. EuroIntervention 2015;11:68–74. [DOI] [PubMed] [Google Scholar]
- 26. Navarese EP, Andreotti F, Schulze V, Kolodziejczak M, Buffon A, Brouwer M, Costa F, Kowalewski M, Parati G, Lip GY, Kelm M, Valgimigli M.. Optimal duration of dual antiplatelet therapy after percutaneous coronary intervention with drug eluting stents: meta-analysis of randomised controlled trials. BMJ 2015;350:h1618.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Costa F, Vranckx P, Leonardi S, Moscarella E, Ando G, Calabro P, Oreto G, Zijlstra F, Valgimigli M.. Impact of clinical presentation on ischaemic and bleeding outcomes in patients receiving 6- or 24-month duration of dual-antiplatelet therapy after stent implantation: a pre-specified analysis from the PRODIGY (Prolonging Dual-Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia) trial. Eur Heart J 2015;36:1242–1251. [DOI] [PubMed] [Google Scholar]
- 28. Udell JA, Bonaca MP, Collet JP, Lincoff AM, Kereiakes DJ, Costa F, Lee CW, Mauri L, Valgimigli M, Park SJ, Montalescot G, Sabatine MS, Braunwald E, Bhatt DL.. Long-term dual antiplatelet therapy for secondary prevention of cardiovascular events in the subgroup of patients with previous myocardial infarction: a collaborative meta-analysis of randomized trials. Eur Heart J 2016;37:390–399. [DOI] [PubMed] [Google Scholar]
- 29. Costa F, Tijssen JG, Ariotti S, Giatti S, Moscarella E, Guastaroba P, De Palma R, Ando G, Oreto G, Zijlstra F, Valgimigli M.. Incremental value of the CRUSADE, ACUITY, and HAS-BLED risk scores for the prediction of hemorrhagic events after coronary stent implantation in patients undergoing long or short duration of dual antiplatelet therapy. J Am Heart Assoc 2015;4:pii: e002524.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Freemantle N, Calvert M, Wood J, Eastaugh J, Griffin C.. Composite outcomes in randomized trials: greater precision but with greater uncertainty? JAMA 2003;289:2554–2559. [DOI] [PubMed] [Google Scholar]
- 31. Armstrong PW, Westerhout CM, Van de Werf F, Califf RM, Welsh RC, Wilcox RG, Bakal JA.. Refining clinical trial composite outcomes: an application to the Assessment of the Safety and Efficacy of a New Thrombolytic-3 (ASSENT-3) trial. Am Heart J 2011;161:848–854. [DOI] [PubMed] [Google Scholar]
- 32. Pocock SJ, Ariti CA, Collier TJ, Wang D.. The win ratio: a new approach to the analysis of composite endpoints in clinical trials based on clinical priorities. Eur Heart J 2012;33:176–182. [DOI] [PubMed] [Google Scholar]
- 33. Bakal JA, Roe MT, Ohman EM, Goodman SG, Fox KA, Zheng Y, Westerhout CM, Hochman JS, Lokhnygina Y, Brown EB, Armstrong PW.. Applying novel methods to assess clinical outcomes: insights from the TRILOGY ACS trial. Eur Heart J 2015;36:385–92a. [DOI] [PubMed] [Google Scholar]
- 34. Campos CM, Costa F, Garcia-Garcia HM, Bourantas C, Suwannasom P, Valgimigli M, Morel MA, Windecker S, Serruys PW.. Anatomic characteristics and clinical implications of angiographic coronary thrombus: insights from a patient-level pooled analysis of SYNTAX, RESOLUTE, and LEADERS Trials. Circ Cardiovasc Interv 2015;8:pii: e002279.. [DOI] [PubMed] [Google Scholar]
- 35. Costa F, Adamo M, Ariotti S, Ferrante G, Navarese EP, Leonardi S, Garcia-Garcia H, Vranckx P, Valgimigli M.. Left main or proximal left anterior descending coronary artery disease location identifies high-risk patients deriving potentially greater benefit from prolonged dual antiplatelet therapy duration. EuroIntervention 2016;11:e1222–e1230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.